Improving Total Mixed Ration Silage: Effects of Lactic Acid Bacteria Inoculants and Antimicrobial Additives on Fermentation Quality and Aerobic Stability
Abstract
:1. Introduction
2. Material and Methods
2.1. Total Mixed Ration (TMR) Silage Preparation
- (1)
- Deionized water (control, CON).
- (2)
- Lactic acid bacteria (Lactiplantibacillus plantarum + Lentilactobacillus buchneri, LPB).
- (3)
- Natamycin (NT).
- (4)
- Hexanoic acid (HA).
- (5)
- Lactic acid bacteria + natamycin (SLNT).
- (6)
- Lactic acid bacteria + hexanoic acid (SLHA).
| Items 1 | Mean |
|---|---|
| Chemical compositions (g/kg DM) | |
| Dry matter (g/kg FW) | 585 |
| Crude protein | 141 |
| WSC | 83.9 |
| Neutral-detergent fiber | 462 |
| Acid-detergent fiber | 243 |
| Ash | 100 |
| Ether extract | 60.2 |
| BC (mEq/kg DM) | 186 |
| Microbial compositions (log10 cfu/g FW) | |
| Lactic acid bacteria | 6.51 |
| Aerobic bacteria | 6.31 |
| Yeasts | 5.09 |
2.2. Chemical Composition and Fermentation Quality Analysis
2.3. Aerobic Stability Test
2.4. Statistical Analyses
3. Results
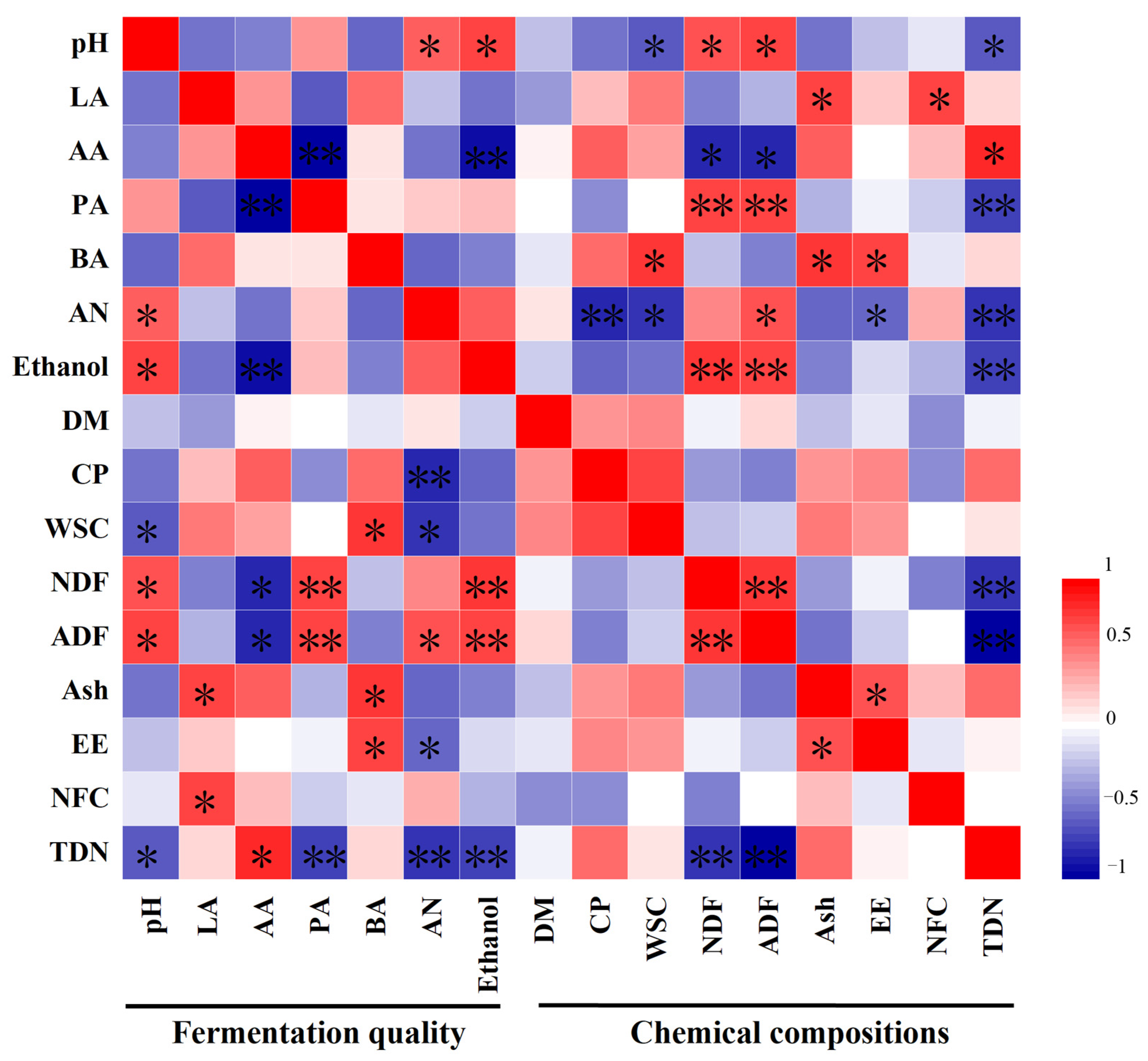
3.1. Fermentation Quality and Chemical and Microbial Compositions of Total Mixed Ration Silage
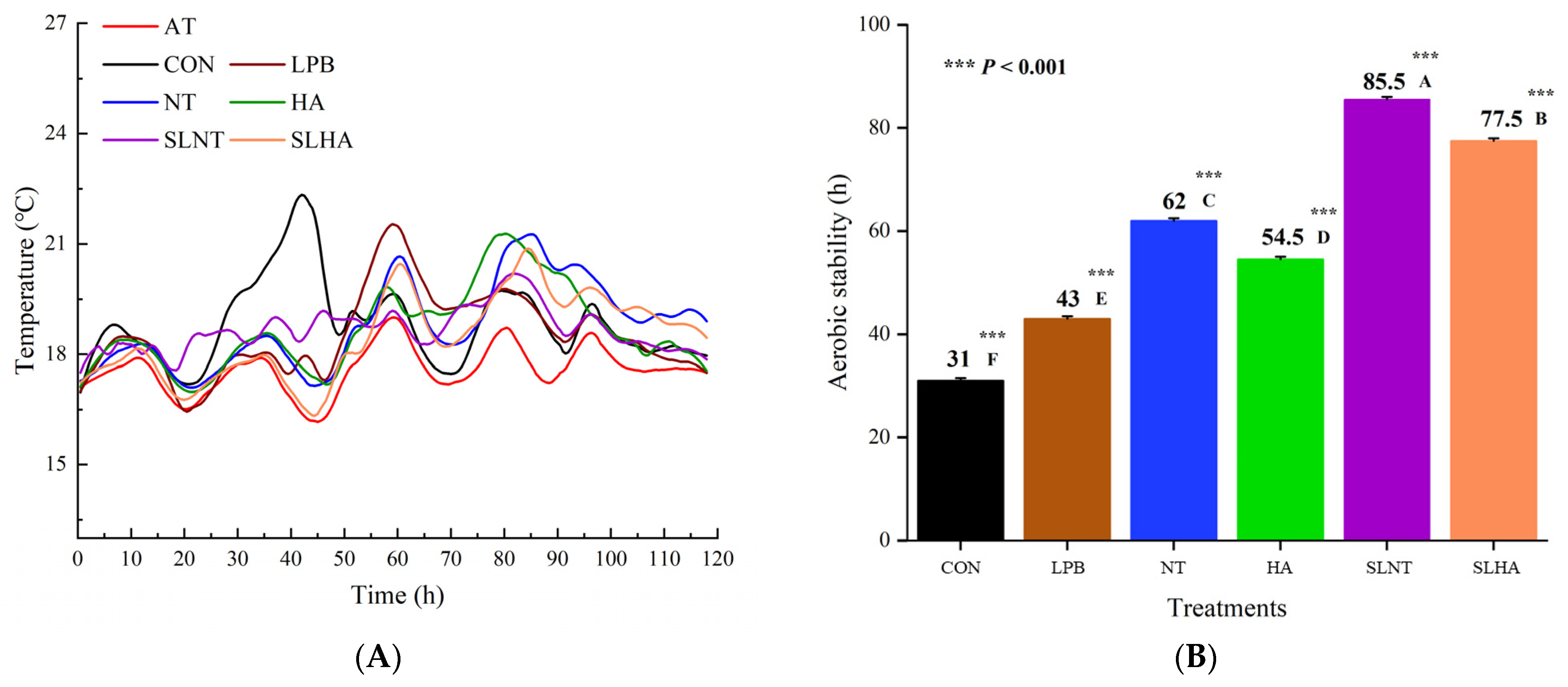
3.2. Aerobic Stability of Total Mixed Ration Silage
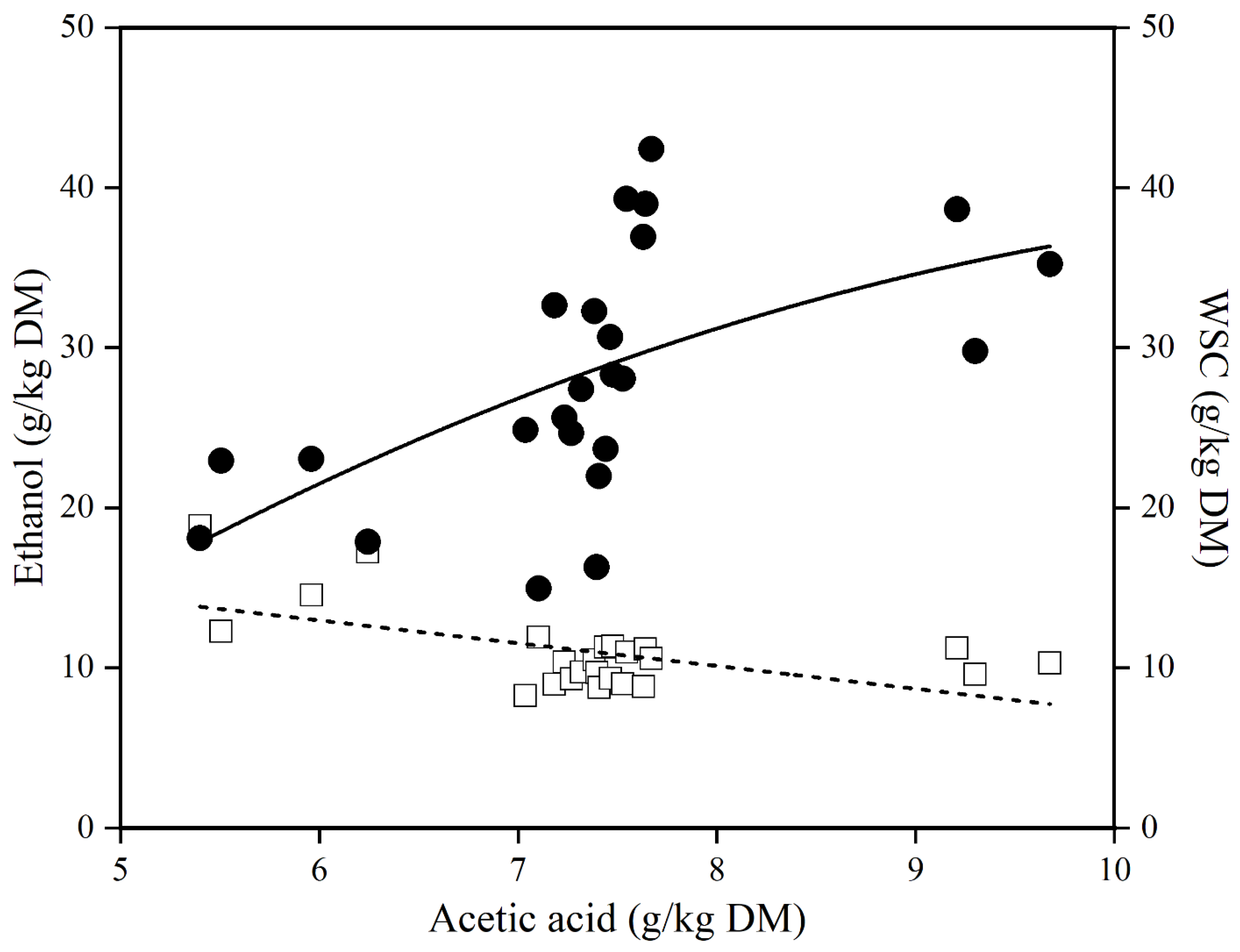
3.3. Relationship between Acetic Acid with Water-Soluble Carbohydrate and Ethanol during Aerobic Exposure
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bueno, A.V.I.; Lazzari, G.; Jobim, C.C.; Daniel, J.L.P. Ensiling Total Mixed Ration for Ruminants: A Review. Agronomy 2020, 10, 879. [Google Scholar] [CrossRef]
- Schingoethe, D.J. A 100-Year Review: Total mixed ration feeding of dairy cows. J. Dairy Sci. 2017, 100, 10143–10150. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Wang, L.; Li, W.; Xu, S.; Bao, J.; Deng, J.; Wu, Z.; Yu, Z. Fermentation Quality, In Vitro Digestibility, and Aerobic Stability of Total Mixed Ration Silage in Response to Varying Proportion Alfalfa Silage. Animals 2022, 12, 1039. [Google Scholar] [CrossRef] [PubMed]
- Yanti, Y.; Kawai, S.; Yayota, M. Effect of total mixed ration silage containing agricultural by-products with the fermented juice of epiphytic lactic acid bacteria on rumen fermentation and nitrogen balance in ewes. Trop. Anim. Health Pro. 2019, 51, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Muck, R.E.; Nadeau, E.M.G.; McAllister, T.A.; Contreras-Govea, F.E.; Santos, M.C.; Kung, L. Silage review: Recent advances and future uses of silage additives. J. Dairy Sci. 2018, 101, 3980–4000. [Google Scholar] [CrossRef] [PubMed]
- Ogunade, I.M.; Martinez-Tuppia, C.; Queiroz, O.C.M.; Jiang, Y.; Drouin, P.; Wu, F.; Vyas, D.; Adesogan, A.T. Silage review: Mycotoxins in silage: Occurrence, effects, prevention, and mitigation. J. Dairy Sci. 2018, 101, 4034–4059. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.S.; Weinberg, Z.G.; Ogunade, I.M.; Cervantes, A.A.P.; Arriola, K.G.; Jiang, Y.; Kim, D.; Li, X.; Gonçalves, M.C.M.; Vyas, D.; et al. Meta-analysis of effects of inoculation with homofermentative and facultative heterofermentative lactic acid bacteria on silage fermentation, aerobic stability, and the performance of dairy cows. J. Dairy Sci. 2017, 100, 4587–4603. [Google Scholar] [CrossRef] [PubMed]
- Kung, L., Jr.; Shaver, R.D.; Grant, R.J.; Schmidt, R.J. Silage review: Interpretation of chemical, microbial, and organoleptic components of silages. J. Dairy Sci. 2018, 101, 4020–4033. [Google Scholar] [CrossRef] [PubMed]
- Kung, L., Jr.; Savage, R.M.; da Silva, E.B.; Polukis, S.A.; Smith, M.L.; Johnson, A.C.B.; Miller, M.A. The effects of air stress during storage and low packing density on the fermentation and aerobic stability of corn silage inoculated with Lactobacillus buchneri 40788. J. Dairy Sci. 2021, 104, 4206–4222. [Google Scholar] [CrossRef]
- da Silva, N.C.; Nascimento, C.F.; Nascimento, F.A.; de Resende, F.D.; Daniel, J.L.P.; Siqueira, G.R. Fermentation and aerobic stability of rehydrated corn grain silage treated with different doses of Lactobacillus buchneri or a combination of Lactobacillus plantarum and Pediococcus acidilactici. J. Dairy Sci. 2018, 101, 4158–4167. [Google Scholar] [CrossRef]
- Okoye, C.O.; Wu, Y.; Wang, Y.; Gao, L.; Li, X.; Jiang, J. Fermentation profile, aerobic stability, and microbial community dynamics of corn straw ensiled with Lactobacillus buchneri PC-C1 and Lactobacillus plantarum PC1-1. Microbiol. Res. 2023, 270, 127329. [Google Scholar] [CrossRef]
- Ollé Resa, C.P.; Jagus, R.J.; Gerschenson, L.N. Natamycin efficiency for controlling yeast growth in models systems and on cheese surfaces. Food Control 2014, 35, 101–108. [Google Scholar] [CrossRef]
- Bueno, A.V.I.; Vigne, G.L.D.; Novinski, C.O.; Bayer, C.; Jobim, C.C.; Schmidt, P. Natamycin as a potential silage additive: A lab trial using sugarcane to assess greenhouse gas emissions. Rev. Bras. Zootecn. 2020, 49, e20200017. [Google Scholar] [CrossRef]
- Woolford, M.K.; Cook, J.E.; Hall, D.M.; Bonis, A. The use of pimaricin as an additive to improve the aerobic stability of silage. J. Sci. Food Agric. 1980, 31, 558–566. [Google Scholar] [CrossRef]
- Mugabe, W.; Yuan, X.; Li, J.; Dong, Z.; Shao, T. Effects of hexanoic acid, Lactobacillus plantarum and their combination on the fermentation characteristics of Napier grass. J. Anim. Sci. 2019, 253, 135–140. [Google Scholar] [CrossRef]
- Dong, D.; Lin, Z.; Dai, T.; Dong, Z.; Li, J.; Shao, T. Dynamics associated with fermentation and aerobic deterioration of high-moisture Italian ryegrass silage made using Lactobacillus plantarum and caproic acid. J. Appl. Microbiol. 2023, 134, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Playne, M.J.; McDonald, P. The buffering constituents of herbage and of silage. J. Sci. Food Agric. 1966, 17, 264–268. [Google Scholar] [CrossRef]
- Broderick, G.A.; Kang, J.H. Automated Simultaneous Determination of Ammonia and Total Amino Acids in Ruminal Fluid and In Vitro Media1. J. Dairy Sci. 1980, 63, 64–75. [Google Scholar] [CrossRef]
- Harlan, D.W.; Holter, J.B.; Hayes, H.H. Detergent fiber traits to predict productive energy of forages fed free choice to nonlactating dairy cattle. J. Dairy Sci. 1991, 74, 1337–1353. [Google Scholar] [CrossRef]
- Wang, S.; Liu, H.; Zhao, J.; Dong, Z.; Li, J.; Shao, T. Influences of Organic Acid Salts and Bacterial Additives on Fermentation Profile, Aerobic Stability, and In Vitro Digestibility of Total Mixed Ration Silage Prepared with Wet Hulless Barley Distillers’ Grains. Agronomy 2023, 13, 672. [Google Scholar] [CrossRef]
- Arthur Thomas, T. An automated procedure for the determination of soluble carbohydrates in herbage. J. Sci. Food Agric. 1977, 28, 639–642. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Association of Official and Analytical Chemist (AOAC). Official Methods of Analysis, 18th ed.; AOAC: Washington, DC, USA, 2000. [Google Scholar]
- Council, N.R. Nutrient Requirements of Beef Cattle: Seventh Revised Edition: Update 2000; The National Academies Press: Washington, DC, USA, 2000; p. 248. [Google Scholar]
- Wilkinson, J.M.; Davies, D.R. The aerobic stability of silage: Key findings and recent developments. Grass Forage Sci. 2013, 68, 1–19. [Google Scholar] [CrossRef]
- Ferrero, F.; Tabacco, E.; Piano, S.; Casale, M.; Borreani, G. Temperature during conservation in laboratory silos affects fermentation profile and aerobic stability of corn silage treated with Lactobacillus buchneri, Lactobacillus hilgardii, and their combination. J. Dairy Sci. 2021, 104, 1696–1713. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.A.; Qian, C.; Liu, Z.; Wu, J.; Sultana, N.; Mobashar, M.; Wanapat, M.; Zhong, X. Evaluation of biological and chemical additives on microbial community, fermentation characteristics, aerobic stability, and in vitro gas production of SuMu No. 2 elephant grass. J. Sci. Food Agric. 2021, 101, 5429–5436. [Google Scholar] [CrossRef]
- Zhao, J.; Yin, X.-J.; Wang, S.-R.; Li, J.-F.; Dong, Z.-H.; Shao, T. Changes in the fermentation products, taxonomic and functional profiles of microbiota during high-moisture sweet sorghum silage fermentation. Front. Microbiol. 2022, 13, 967624. [Google Scholar] [CrossRef]
- Kamphayae, S.; Kumagai, H.; Angthong, W.; Narmseelee, R.; Bureenok, S. Effects of different ratios and storage periods of liquid brewer’s yeast mixed with cassava pulp on chemical composition, fermentation quality and in vitro ruminal fermentation. Asian Australas. J. Anim. Sci. 2017, 30, 470–478. [Google Scholar] [CrossRef]
- Pinto, S.; Warth, J.F.G.; Schmidt, P. Natamycin added to maize silage does not adversely affect performance and voluntary feed intake of lambs. J. Anim. Sci. 2022, 31, 352–359. [Google Scholar] [CrossRef]
- Koç, F.; Özkan Ünal, E.; Okuyucu, B.; Esen, S.; Işık, R. Effect of Different Kefir Source on Fermentation, Aerobic Stability, and Microbial Community of Alfalfa Silage. Animals 2021, 11, 2096. [Google Scholar] [CrossRef]
- Shah, A.A.; Qian, C.; Wu, J.; Liu, Z.; Khan, S.; Tao, Z.; Zhang, X.; Khan, I.U.; Zhong, X. Effects of natamycin and Lactobacillus plantarum on the chemical composition, microbial community, and aerobic stability of Hybrid pennisetum at different temperatures. RSC Adv. 2020, 10, 8692–8702. [Google Scholar] [CrossRef]
- Nurdianti, R.R.; Nuryana, R.S.; Handoko, A.; Hernaman, I.; Ramdani, D.; Jayanegara, A.; Dickhoefer, U.; Böttger, C.; Südekum, K.H. Nutritional compositions of Katuk leaves and their supplementation to hays of different quality: An in vitro study. J. Agric. Sci. 2023, 161, 428–437. [Google Scholar] [CrossRef]
- Villalba, J.J.; Ates, S.; MacAdam, J.W. Non-fiber Carbohydrates in Forages and Their Influence on Beef Production Systems. Front. Sustain. Food Syst. 2021, 5, 566338. [Google Scholar] [CrossRef]
- Xu, G.; Han, Z.; Wang, S.; Dai, T.; Dong, D.; Zong, C.; Yin, X.; Jia, Y.; Shao, T. Soy sauce residue in total mixed ration silage: Fermentation characteristics, chemical compositions, in vitro digestibility and gas production. J. Anim. Sci. 2022, 21, 1058–1066. [Google Scholar] [CrossRef]
- Nascimento Agarussi, M.; Pereira, O.; da Silva, L.; da Silva, V.; de Paula, R.; Fonseca e Silva, F.; Guimarães Ribeiro, K. Effect of Various Strains of Lactobacillus buchneri on the Fermentation Quality and Aerobic Stability of Corn Silage. Agriculture 2022, 12, 95. [Google Scholar] [CrossRef]
- Nanda, K.; Taniguchi, M.; Ujike, S.; Ishihara, N.; Mori, H.; Ono, H.; Murooka, Y. Characterization of acetic acid bacteria in traditional acetic acid fermentation of rice vinegar (komesu) and unpolished rice vinegar (kurosu) produced in Japan. Appl. Env. Environ. Microbiol. 2001, 67, 986–990. [Google Scholar] [CrossRef]
- Nair, J.; Huaxin, N.; Andrada, E.; Yang, H.E.; Chevaux, E.; Drouin, P.; McAllister, T.A.; Wang, Y. Effects of inoculation of corn silage with Lactobacillus hilgardii and Lactobacillus buchneri on silage quality, aerobic stability, nutrient digestibility, and growth performance of growing beef cattle. J. Anim. Sci. 2020, 98, skaa267. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yang, X.; Li, J.; Dong, Z.; Zhao, J.; Shao, T.; Yuan, X. Effects of hexanoic acid on microbial communities, fermentation, and hygienic quality of corn silages infested with toxigenic fungi. J. Sci. Food AGR. 2021, 102, 3522–3534. [Google Scholar] [CrossRef]
- Garnier, L.; Valence, F.; Mounier, J. Diversity and Control of Spoilage Fungi in Dairy Products: An Update. Microorganisms 2017, 5, 42. [Google Scholar] [CrossRef]
- Te Welscher, Y.M.; Ten Napel, H.H.; Balagué, M.M.; Souza, C.M.; Riezman, H.; de Kruijff, B.; Breukink, E. Natamycin Blocks Fungal Growth by Binding Specifically to Ergosterol without Permeabilizing the Membrane. J. Biol. Chem. 2008, 283, 6393–6401. [Google Scholar] [CrossRef]
- Kleinschmit, D.H.; Kung, L., Jr. A meta-analysis of the effects of Lactobacillus buchneri on the fermentation and aerobic stability of corn and grass and small-grain silages. J. Dairy Sci. 2006, 89, 4005–4013. [Google Scholar] [CrossRef]
- Schmidt, R.J.; Kung, L. The effects of Lactobacillus buchneri with or without a homolactic bacterium on the fermentation and aerobic stability of corn silages made at different locations. J. Dairy Sci. 2010, 93, 1616–1624. [Google Scholar] [CrossRef] [PubMed]
| Items 1 | Treatments 2 | SEM 3 | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| CON | LPB | NT | HA | SLNT | SLHA | |||
| Fermentation characteristics (g/kg DM) | ||||||||
| pH | 5.61 A | 4.80 B | 5.26 A | 5.30 A | 4.71 B | 4.76 B | 0.121 | <0.001 |
| Lactic acid | 27.7 C | 37.1 B | 29.2 C | 28.0 C | 44.4 A | 41.7 AB | 1.561 | <0.001 |
| Acetic acid | 5.64 C | 18.1 A | 6.08 C | 5.90 C | 19.5 A | 13.6 B | 0.400 | <0.001 |
| Propionic acid | 4.03 | 2.63 | 2.30 | 2.19 | 2.19 | 2.20 | 0.253 | 0.521 |
| Propionic acid | ND | ND | ND | ND | ND | ND | 0.064 | 0.119 |
| Volatile fatty acids | 9.67 C | 20.73 A | 8.38 C | 8.09 C | 21.7 A | 15.8 B | 0.329 | <0.001 |
| AN/TN (g/kg TN) | 79.0 A | 70.4 B | 62.7 C | 68.7 B | 60.2 C | 62.8 B | 4.079 | <0.001 |
| Ethanol | 12.7 | 8.70 | 8.98 | 9.03 | 8.17 | 8.49 | 2.263 | 0.132 |
| Chemical compositions (g/kg DM) | ||||||||
| Dry matter (g/kg FW) | 557 | 561 | 569 | 566 | 571 | 568 | 6.222 | 0.653 |
| Crude protein | 230 | 235 | 245 | 240 | 255 | 251 | 5.871 | 0.712 |
| Water-soluble carbohydrates | 32.9 D | 39.9 C | 42.3 B | 40.2 B | 48.8 A | 45.0 AB | 1.736 | <0.001 |
| Neutral-detergent fiber | 349 A | 321 B | 321 B | 325 B | 294 C | 301 C | 7.523 | <0.001 |
| Acid-detergent fiber | 210 | 211 | 215 | 216 | 197 | 203 | 7.149 | 0.811 |
| Ash | 87.3 | 86.2 | 86.1 | 85.7 | 86.0 | 86.6 | 1.363 | 0.641 |
| Ether extract | 40.4 | 41.9 | 42.4 | 41.1 | 44.7 | 43.6 | 1.202 | 0.081 |
| Non-fibrous carbohydrate | 291 C | 316 AB | 304 B | 308 B | 321A | 321 A | 8.633 | 0.005 |
| TDN (%DM) | 68.0 | 67.6 | 67.9 | 67.7 | 68.9 | 68.5 | 1.066 | 0.079 |
| Microbial compositions (log10 cfu/g FW) | ||||||||
| Lactic acid bacteria | 6.90 C | 8.44 A | 7.56 B | 7.19 B | 8.49 A | 7.90 B | 0.349 | <0.001 |
| Aerobic bacteria | 5.87 A | 3.47 B | <2.00 | <2.00 | <2.00 | <2.00 | 1.132 | <0.001 |
| Yeasts | 3.13 A | <2.00 | <2.00 | <2.00 | <2.00 | <2.00 | 0.833 | <0.001 |
| Items | Treatments 1 | Aerobic Exposure Days (d) | SEM 2 | p-Value 3 | Model Construction p 4 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | T | D | T × D | L | Q | |||
| pH | CON | 5.61 Ac | 6.27 Ab | 7.35 Aa | 7.50 Aa | 0.091 | <0.001 | <0.001 | <0.001 | <0.001 | 0.005 |
| LPB | 4.80 Bc | 4.86 Dc | 5.21 Db | 6.77 Ca | 0.046 | <0.001 | <0.001 | ||||
| NT | 5.26 Ac | 5.39 Cc | 6.48 Cb | 7.23 Ba | 0.067 | <0.001 | <0.001 | ||||
| HA | 5.30 Ac | 5.55 Bc | 6.88 Bb | 7.48 Aa | 0.109 | <0.001 | 0.008 | ||||
| SLNT | 4.71 Bc | 4.73 Dc | 4.98 Db | 6.37 Da | 0.080 | <0.001 | <0.001 | ||||
| SLHA | 4.76 Bb | 4.81 Db | 5.05 Db | 6.48 Da | 0.037 | <0.001 | <0.001 | ||||
| SEM 2 | 0.121 | 0.133 | 0.059 | 0.063 | |||||||
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | |||||||
| Lactic acid (g/kg DM) | CON | 27.7 Ca | 18.1 Db | 10.7 Dc | 5.41 Dd | 0.695 | <0.001 | <0.001 | <0.001 | <0.001 | 0.002 |
| LPB | 37.1 Ba | 33.6 Ba | 25.8 Bb | 13.1 Bc | 0.999 | <0.001 | <0.001 | ||||
| NT | 29.2 Ca | 25.2 Ca | 19.4 Cb | 8.86 Cc | 1.059 | <0.001 | <0.001 | ||||
| HA | 28.0 Ca | 21.4 CDa | 12.3 Dc | 8.36 CDc | 1.267 | <0.001 | 0.066 | ||||
| SLNT | 44.4 Aa | 39.9 Aab | 32.9 Ab | 24.9 Ac | 1.895 | <0.001 | 0.011 | ||||
| SLHA | 41.7 ABa | 38.6 Aa | 31.2 ABb | 21.0 Ac | 2.043 | <0.001 | 0.175 | ||||
| SEM 2 | 1.561 | 1.406 | 1.636 | 0.940 | |||||||
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | |||||||
| Acetic acid (g/kg DM) | CON | 5.64 C | 4.19 C | 5.35 B | 4.44 B | 0.278 | <0.001 | <0.001 | <0.001 | 0.313 | 0.201 |
| LPB | 18.1 Aa | 14.6 Ab | 10.3 Abc | 11.3 Ac | 0.420 | 0.708 | <0.001 | ||||
| NT | 6.08 C | 5.34 C | 6.24 B | 6.58 B | 0.219 | 0.001 | 0.305 | ||||
| HA | 5.90 C | 5.06 Ca | 5.46 B | 5.44 B | 0.196 | 0.049 | 0.061 | ||||
| SLNT | 19.5 Aa | 15.4 Ab | 11.6 Ac | 12.7 Abc | 0.291 | <0.001 | 0.001 | ||||
| SLHA | 13.6 Ba | 10.4 Ba | 8.23 Ab | 10.5 Aa | 0.249 | <0.001 | <0.001 | ||||
| SEM 2 | 0.400 | 0.348 | 0.131 | 0.150 | |||||||
| p-value | <0.001 | <0.001 | 0.090 | 0.204 | |||||||
| Propionic acid (g/kg DM) | CON | 4.03 | 5.85 | 6.82 | 5.88 | 0.094 | 0.272 | 0.241 | 0.379 | 0.389 | 0.052 |
| LPB | 2.63 | 3.89 | 4.83 | 4.77 | 0.310 | 0.567 | 0.653 | ||||
| NT | 2.30 | 3.97 | 4.77 | 5.03 | 0.136 | 0.763 | 0.305 | ||||
| HA | 2.19 | 3.97 | 4.86 | 4.89 | 0.079 | 0.614 | 0.609 | ||||
| SLNT | 2.19 | 3.74 | 4.66 | 4.93 | 0.089 | 0.189 | 0.077 | ||||
| SLHA | 2.20 | 3.73 | 4.62 | 4.98 | 0.177 | 0.213 | |||||
| SEM 2 | 0.253 | 0.123 | 0.061 | 0.127 | |||||||
| p-value | 0.521 | 0.263 | 0.109 | 0.446 | |||||||
| Ethanol (g/kg DM) | CON | 12.7 | 11.4 | 8.34 | 5.97 | 1.051 | 0.371 | 0.315 | 0.235 | 0.301 | 0.086 |
| LPB | 8.70 | 6.93 | 5.23 | 3.19 | 1.458 | 0.547 | 0.819 | ||||
| NT | 8.98 | 7.04 | 4.10 | 2.60 | 0.609 | 0.261 | 0.632 | ||||
| HA | 9.03 | 7.61 | 4.98 | 3.20 | 0.612 | 0.182 | 0.451 | ||||
| SLNT | 8.17 | 6.60 | 3.38 | 3.00 | 0.519 | 0.781 | 0.503 | ||||
| SLHA | 9.49 | 7.65 | 4.95 | 3.31 | 0.541 | 0.605 | 0.737 | ||||
| SEM 2 | 2.264 | 0.600 | 0.241 | 0.330 | |||||||
| p-value | 0.134 | 0.238 | 0.301 | 0.712 | |||||||
| Items 1 | Treatments 2 | Aerobic Exposure Days (d) | SEM 3 | p-Value 4 | Model Construction p 5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | T | D | T × D | L | Q | |||
| WSC (g/kg DM) | CON | 32.9 Ca | 27.0 Da | 20.4 Cb | 13.4 Bd | 1.301 | <0.001 | <0.001 | <0.001 | <0.001 | 0.653 |
| LPB | 39.9 Ca | 30.6 CDb | 25.0 BCc | 17.6 Bc | 1.614 | <0.001 | 0.036 | ||||
| NT | 42.3 ABa | 35.4 BCa | 27.1 BCb | 20.6 Bc | 1.571 | <0.001 | 0.133 | ||||
| HA | 40.3 BCa | 32.6 CDb | 26.6 BCc | 19.4 Bc | 1.402 | <0.001 | 0.073 | ||||
| SLNT | 48.8 Aa | 43.0 Aab | 39.6 Ab | 32.8 Ac | 1.425 | <0.001 | 0.046 | ||||
| SLHA | 45.0 ABa | 37.8 Bb | 31.6 Bb | 25.3 Bc | 2.189 | <0.001 | 0.042 | ||||
| SEM 3 | 1.733 | 1.308 | 1.737 | 1.628 | |||||||
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | |||||||
| AN/TN (g/kg TN) | CON | 79.0 Ac | 82.6 Abc | 87.9 Ab | 108 Aa | 5.073 | 0.005 | <0.001 | <0.001 | <0.001 | 0.395 |
| LPB | 70.4 ABa | 74.4 Bab | 80.6 Bb | 89.2 Ba | 6.016 | <0.001 | 0.013 | ||||
| NT | 62.7 Bb | 66.8 Cab | 75.4 Ca | 86.4 Ba | 4.664 | <0.001 | 0.661 | ||||
| HA | 68.7 Bc | 73.1 Ac | 78.7 Ab | 87.5 Ba | 5.535 | <0.001 | 0.938 | ||||
| SLNT | 60.2 Bb | 62.3 Cab | 66.4 Dab | 72.8 Da | 5.490 | 0.001 | 0.014 | ||||
| SLHA | 62.8 Cc | 63.4 Cc | 70.0 Cb | 81.1 Ca | 5.400 | 0.012 | 0.034 | ||||
| SEM 3 | 4.079 | 3.116 | 3.116 | 5.557 | |||||||
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | |||||||
| LAB (log10 cfu/g FW) | CON | 6.90 Ca | 6.51 Cb | 5.75 Cc | 4.35 Cd | 0.933 | <0.001 | <0.001 | <0.001 | <0.001 | 0.001 |
| LPB | 8.44 Aa | 8.18 Aa | 7.75 Ab | 6.86 Ac | 0.230 | <0.001 | 0.001 | ||||
| NT | 7.56 Ba | 7.51 Bb | 6.75 Bc | 5.65 Bd | 0.170 | 0.002 | 0.261 | ||||
| HA | 7.19 Ba | 6.82 Cb | 6.29 Bc | 5.18 Bd | 0.764 | <0.001 | 0.061 | ||||
| SLNT | 8.56 Aa | 8.43 Aa | 7.84 Ab | 7.09 Ac | 0.194 | 0.004 | 0.056 | ||||
| SLHA | 7.90 Ba | 7.71 Aa | 7.01 Ab | 6.27 Ac | 0.303 | 0.031 | 0.753 | ||||
| SEM 3 | 1.349 | 1.530 | 1.347 | 1.333 | |||||||
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | |||||||
| AB (log10 cfu/g FW) | CON | 5.87 Ad | 6.36 Ac | 7.13 Ab | 8.06 Aa | 0.442 | <0.001 | <0.001 | <0.001 | <0.001 | 0.036 |
| LPB | 3.47 Cc | 5.01 Bb | 5.38 Bb | 6.08 Ba | 0.510 | 0.004 | 1.198 | ||||
| NT | <2.00 | 4.60 Bb | 5.05 Cb | 5.53 Ca | 0.612 | <0.001 | 0.031 | ||||
| HA | <2.00 | 4.81 Cc | 5.31 Ba | 5.66 Ba | 0.510 | <0.001 | 0.163 | ||||
| SLNT | <2.00 | 4.47 Bc | 4.89 Cb | 5.29 Ca | 0.476 | 0.001 | 0.754 | ||||
| SLHA | <2.00 | 4.53 Dc | 4.99 Cb | 5.42 Ba | 0.748 | <0.001 | 0.147 | ||||
| SEM 3 | 1.132 | 0.526 | 0.485 | 0.404 | |||||||
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | |||||||
| Yeasts (log10 cfu/g FW) | CON | 3.13 Ad | 3.45 Ac | 3.96 Ab | 4.75 Aa | 1.012 | 0.008 | <0.001 | <0.001 | <0.001 | 0.044 |
| LPB | <2.00 | 2.3 Bb | 3.07 Bb | 3.52 Ba | 0.408 | <0.001 | 0.791 | ||||
| NT | <2.00 | <2.00 | 2.47 Cb | 3.13 Ca | 0.348 | <0.001 | 0.358 | ||||
| HA | <2.00 | <2.00 | 2.83 Bb | 3.69 Ba | 0.650 | <0.001 | 0.736 | ||||
| SLNT | <2.00 | <2.00 | <2.00 | 2.60 Ca | 0.363 | 0.001 | 0.075 | ||||
| SLHA | <2.00 | <2.00 | 2.53 Bb | 3.69 Ba | 0.801 | 0.002 | 0.244 | ||||
| SEM 3 | 0.833 | 0.572 | 0.653 | 0.539 | |||||||
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Cheng, Y.; Yang, F.; Hu, J.; Ma, R.; Liu, H.; Shao, T. Improving Total Mixed Ration Silage: Effects of Lactic Acid Bacteria Inoculants and Antimicrobial Additives on Fermentation Quality and Aerobic Stability. Agronomy 2024, 14, 1602. https://doi.org/10.3390/agronomy14081602
Li X, Cheng Y, Yang F, Hu J, Ma R, Liu H, Shao T. Improving Total Mixed Ration Silage: Effects of Lactic Acid Bacteria Inoculants and Antimicrobial Additives on Fermentation Quality and Aerobic Stability. Agronomy. 2024; 14(8):1602. https://doi.org/10.3390/agronomy14081602
Chicago/Turabian StyleLi, Xinbao, Yuanzhen Cheng, Feifei Yang, Junfeng Hu, Rui Ma, Haopeng Liu, and Tao Shao. 2024. "Improving Total Mixed Ration Silage: Effects of Lactic Acid Bacteria Inoculants and Antimicrobial Additives on Fermentation Quality and Aerobic Stability" Agronomy 14, no. 8: 1602. https://doi.org/10.3390/agronomy14081602





