Recovering Alpine Secale cereale (Rye) Varieties: Insights from Genetic, Agronomic, and Phytochemical Analyses to Support Sustainable Mountain Agriculture Economy
Abstract
1. Introduction
- -
- Characterize the genetic structure through varietal genetic analyses based on codominant microsatellite (SSR) markers of 12 local landraces of rye collected from small-scale farmers in Valtellina (Central Alps), one alpine accession, and two commercial modern accessions from Poland and Russia;
- -
- Characterize the rye landraces from a nutraceutical point of view to explore the possibility of enhancing their value in the framework of the local economy.
- -
- Carry out agronomic analyses to estimate the productivity of the landraces and improve their short food supply chain.
2. Materials and Methods
2.1. The Study Species
2.2. Plant Materials
- -
- Ten at the village of Teglio in Valtellina (T_1–T_10);
- -
- One in upper Valtellina (Up_V);
- -
- One population of alpine origin certified at the Laimburg Research Centre, an experimental station devoted to mountain agriculture in the Bolzano province (LAI);
- -
- One commercial accession from Poland (C_POL_P1) that was already analyzed in the study of Targońska et al. [12] (i.e., Danko farm, accession SZK44) where it was included in the group of the ancestry population P1 clustering accession from Central and Western Europe. The second cluster (ancestry population P2) of the study [12] included accessions from Southwest Asia, South Europe, and in the Balkans;
- -
- Another is from a local mill of Teglio (C_UN); about 20 years ago, a local farmer started producing rye from a commercial seed stock, originating from Russia.
| Population | Abbreviation | Sample Size (n) | Farmer | Origin |
|---|---|---|---|---|
| Teglio_1 | T_1 | 20 | Motalli | Teglio, Valtellina, Italy |
| Teglio_2 | T_2 | 20 | Mazzucchelli | Triangia (SO), Valtellina, Italy |
| Teglio_3 | T_3 | 14 | Finotti | Teglio, Valtellina, Italy |
| Teglio_4 | T_4 | 20 | Fanchi | Teglio, Valtellina, Italy |
| Teglio_5 | T_5 | 20 | Fanchetti | Teglio, Valtellina, Italy |
| Teglio_6 | T_6 | 19 | Marchetti | Teglio, Valtellina, Italy |
| Teglio_7 | T_7 | 20 | Arrondini | Teglio, Valtellina, Italy |
| Teglio_8 | T_8 | 20 | Saini | Teglio, Valtellina, Italy |
| Teglio_9 | T_9 | 16 | Pelacchi | Teglio, Valtellina, Italy |
| Teglio_10 | T_10 | 20 | De Filippi | Teglio, Valtellina, Italy |
| Upper_Valley | Up_V | 20 | Martinelli | Valdidentro, Valtellina, Italy |
| Laimburg | LAI | 20 | Experimental Centre | Laimburg, Adige Valley, Italy |
| Comm_Poland_P1 | C_POL_P1 | 19 | Commercial seeds | Poland (Danko farm, accession SZK44) and analyzed by Targońska et al. (2016) [12] |
| Comm_Uncertain | C_UN | 20 | Maxenti | Russia, (not coded) uncertain |
2.3. Genetic Analysis
2.4. Seed Characteristics and Agronomic Analysis
- -
- Plant height (cm);
- -
- Seed weight of 1000 seeds (g);
- -
- Seed color;
- -
- Crop yield (kg/ha).
2.5. Phytochemical Analysis
2.6. Data Analyses
3. Results
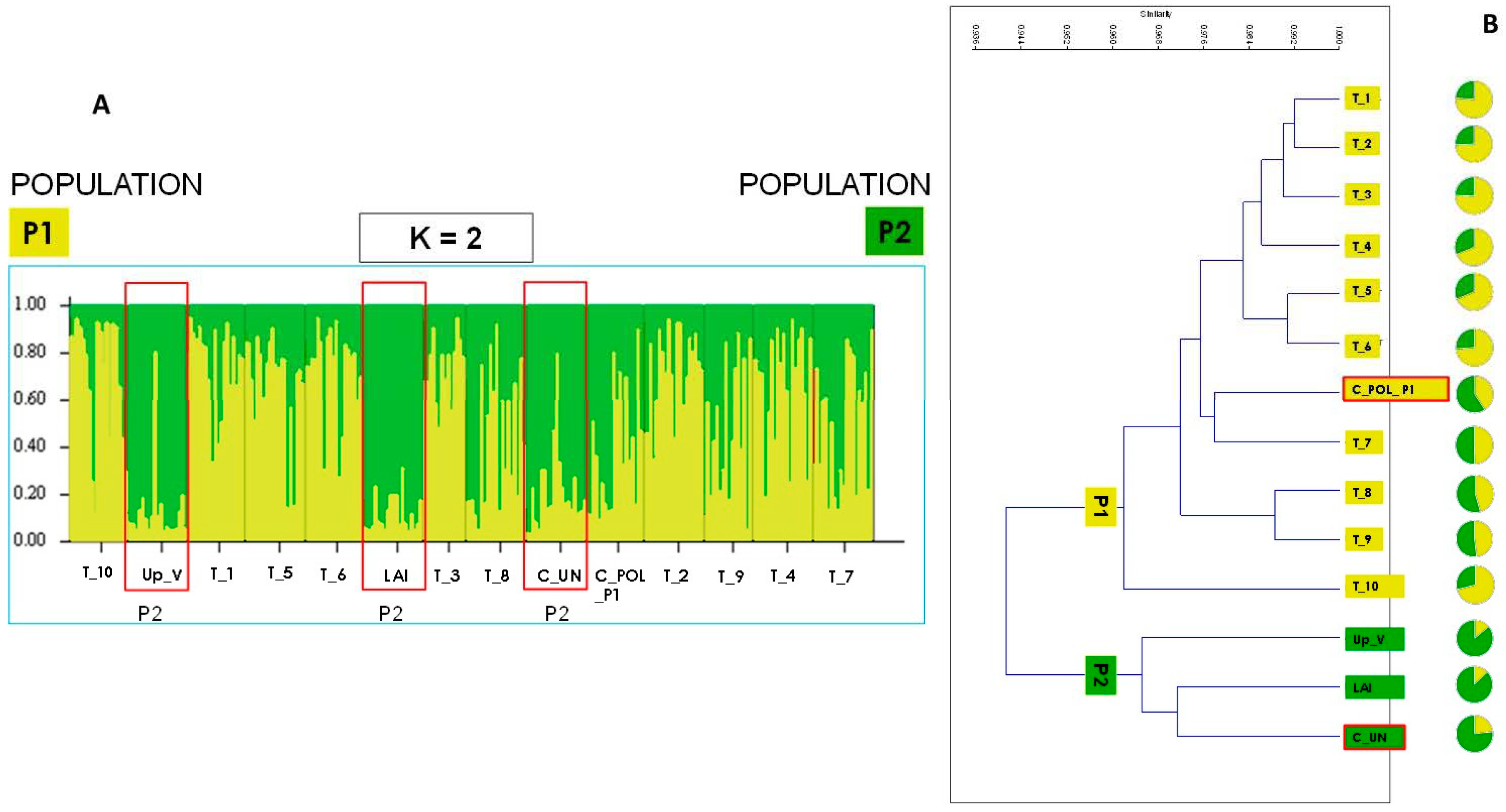
3.1. Genetic Analysis and Population Structure
3.2. Seed Characteristics and Agronomic Analysis
3.3. Phytochemical Analysis
4. Discussion
Implication for the Rural Mountain Economy
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Canella, M.; Ardenghi, N.M.G.; Müller, J.V.; Rossi, G.; Guzzon, F. An updated checklist of plant agrobiodiversity of northern Italy. Genet. Resour. Crop Evol. 2022, 69, 2159–2178. [Google Scholar] [CrossRef]
- Sardella, C.; Capo, L.; Adamo, M.; Donna, M.; Ravetto Enri, S.; Vanara, F.; Lonati, M.; Mucciarelli, M.; Blandino, M. The cultivation of rye in marginal Alpine environments: A comparison of the agronomic, technological, health and sanitary traits of local landraces and commercial cultivars. Front. Plant Sci. 2023, 14, 1130543. [Google Scholar] [CrossRef]
- Zohary, D.; Hopf, M.; Weiss, E. Domestication of Plants in the Old World: The Origin and Spread of Domesticated Plants in Southwest Asia, Europe, and the Mediterranean Basin; Oxford University Press on Demand: Oxford, UK, 2012. [Google Scholar]
- Hillman, G. On the Origins of Domestic Rye—Secale cereale: The Finds from Aceramic Can Hasan III in Turkey. Anatol. Stud. 1978, 28, 157–174. [Google Scholar] [CrossRef]
- Schreiber, M.; Himmelbach, A.; Börner, A.; Mascher, M. Genetic diversity and relationship between domesticated rye and its wild relatives as revealed through genotyping-by-sequencing. Evol. Appl. 2019, 12, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Behre, K.-E. The history of rye cultivation in Europe. Veget. Hist. Archaeobot. 1992, 1, 141–156. [Google Scholar] [CrossRef]
- Ma, R.; Yli-Mattila, T.; Pulli, S. Phylogenetic relationships among genotypes of worldwide collection of spring and winter ryes (Secale cereale L.) determined by RAPD-PCR markers. Hereditas 2004, 140, 210–221. [Google Scholar] [CrossRef]
- Preece, C.; Livarda, A.; Christin, P.-A.; Wallace, M.; Martin, G.; Charles, M.; Jones, G.; Rees, M.; Osborne, C.P. How did the domestication of fertile crescent grain crops increase their yields? Funct. Ecol. 2017, 31, 387–397. [Google Scholar] [CrossRef]
- Mucciarelli, M.; Blandino, M.; Capo, L.; Adamo, M. La Segale in Piemonte. Storia di Una Rinascita; Ecomuseo della Segale, Tipolito Europa, Cuneo: Valdieri, Italy, 2022. [Google Scholar]
- Festi, D.; Putzer, A.; Oeggl, K. Mid and late Holocene land-use changes in the Ötztal Alps, territory of the Neolithic Iceman “Ötzi”. Quat. Int. 2014, 353, 17–33. [Google Scholar] [CrossRef]
- Bolibok-Brągoszewska, H.; Targońska, M.; Bolibok, L.; Kilian, A.; Rakoczy-Trojanowska, M. Genome-wide characterization of genetic diversity and population structure in Secale. BMC Plant Biol. 2014, 14, 184. [Google Scholar] [CrossRef]
- Targońska, M.; Bolibok-Brągoszewska, H.; Rakoczy-Trojanowska, M. Assessment of Genetic Diversity in Secale cereale Based on SSR Markers. Plant Mol. Biol. Rep. 2016, 34, 37–51. [Google Scholar] [CrossRef]
- Hagenblad, J.; Oliveira, H.R.; Forsberg, N.E.G.; Leino, M.W. Geographical Distribution of Genetic Diversity in Secale Landrace and Wild Accessions. BMC Plant Biol. 2016, 16, 23. [Google Scholar] [CrossRef] [PubMed]
- Khoury, C.K.; Brush, S.; Costich, D.E.; Curry, H.A.; de Haan, S.; Engels, J.M.M.; Guarino, L.; Hoban, S.; Mercer, K.L.; Miller, A.J.; et al. Crop Genetic Erosion: Understanding and Responding to Loss of Crop Diversity. New Phytol. 2022, 233, 84–118. [Google Scholar] [CrossRef] [PubMed]
- Leoni, V.; Pedrali, D.; Zuccolo, M.; Rodari, A.; Giupponi, L.; Giorgi, A. The Importance of Technical Support in the Return of Traditional Crops in the Alps: The Case of Rye in Camonica Valley. Sustainability 2021, 13, 13818. [Google Scholar] [CrossRef]
- Giupponi, L.; Borgonovo, G.; Panseri, S.; Giorgi, A. Multidisciplinary Study of a Little Known Landrace of Fagopyrum tataricum Gaertn. of Valtellina (Italian Alps). Genet. Resour. Crop Evol. 2019, 66, 783–796. [Google Scholar] [CrossRef]
- Begna, T. Role and economic importance of crop genetic diversity in food security. J. Agric. Sci. Food Technol. 2021, 7, 164–169. [Google Scholar] [CrossRef]
- Ramirez-Villegas, J.; Khoury, C.K.; Achicanoy, H.A.; Diaz, M.V.; Mendez, A.C.; Sosa, C.C.; Kehel, Z.; Guarino, L.; Abberton, M.; Aunario, J.; et al. State of ex situ conservation of landrace groups of 25 major crops. Nat. Plants 2022, 8, 491–499. [Google Scholar] [CrossRef]
- Giupponi, L.; Pilu, R.; Scarafoni, A.; Giorgi, A. Plant Agro-biodiversity Needs Protection, Study and Promotion: Results of Research Conducted in Lombardy Region (Northern Italy). Biodivers. Conserv. 2020, 29, 409–430. [Google Scholar] [CrossRef]
- Singh, S.; Singh, L.; Singh, H.; Sangwan, S. Nutraceutical Potential of Seed and Grain Proteins in Health Promotion. In Grain and Seed Proteins Functionality; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Bondia-Pons, I.; Aura, A.-M.; Vuorela, S.; Kolehmainen, M.; Mykkänen, H.; Poutanen, K. Rye phenolics in nutrition and health. J. Cereal Sci. 2009, 49, 323–336. [Google Scholar] [CrossRef]
- Garutti, M.; Nevola, G.; Mazzeo, R.; Cucciniello, L.; Totaro, F.; Bertuzzi, C.A.; Caccialanza, R.; Pedrazzoli, P.; Puglisi, F. The Impact of Cereal Grain Composition on the Health and Disease Outcomes. Front. Nutr. 2022, 9, 888974. [Google Scholar] [CrossRef]
- Ortman, T.; Sandström, E.; Bengtsson, J.; Watson, C.A.; Bergkvist, G. Farmers’ motivations for landrace cereal cultivation in Sweden. Biol. Agric. Hort. 2023, 39, 247–268. [Google Scholar] [CrossRef]
- Batur, F.; Bocci, R.; Bartha, B. Marketing farmers’ varieties in Europe: Encouraging pathways with missing links for the recognition and support of farmer seed systems. Agronomy 2021, 11, 2159. [Google Scholar] [CrossRef]
- Wendin, K.; Mustafa, A.; Ortman, T.; Gerhardt, K. Consumer awareness, attitudes and preferences towards heritage cereals. Foods 2020, 9, 742. [Google Scholar] [CrossRef] [PubMed]
- Magnani, N.; Struffi, L. Translation sociology and social capital in rural development initiatives. A case study from the Italian Alps. J. Rural Stud. 2009, 25, 231–238. [Google Scholar] [CrossRef]
- de Pasquale, G.; Spinelli, E. The alpine rural landscape as a cultural reserve: The case study of Teglio in Valtellina. Biodivers. Conserv. 2022, 31, 2397–2420. [Google Scholar] [CrossRef]
- Prończuk, M.; Madej, L.; Kolasińska, I. Research for resistance to Microdochium nivale among inbred lines of rye. Plant Breed. Seed Sci. 2003, 48, 83–86. [Google Scholar]
- Clark, A. (Ed.) Managing Cover Crops Profitably, 3rd ed.; Sustainable Agriculture Research and Education (SARE) Program; Diane Publishing: Collingdale, PA, USA, 2007. [Google Scholar]
- Whan, A.P.; Smith, A.B.; Cavanagh, C.R.; Ral, J.-P.F.; Shaw, L.M.; Howitt, C.A.; Bischof, L. GrainScan: A low cost, fast method for grain size and colour measurements. Plant Methods 2014, 10, 23. [Google Scholar] [CrossRef]
- Weselake, R.J.; MacGregor, A.W.; Hill, R.D. Endogenous alpha-amylase inhibitor in various cereals. Cereal Chem. 1985, 62, 120–123. [Google Scholar]
- Kulichová, K.; Sokol, J.; Nemeček, P.; Maliarová, M.; Maliar, T.; Havrlentová, M.; Kraic, J. Phenolic Compounds and Biological Activities of Rye (Secale cereale L.) Grains. Open Chem. 2019, 17, 988–999. [Google Scholar] [CrossRef]
- Shi, L.; Zhao, W.; Yang, Z.; Subbiah, V.; Suleria, H.A.R. Extraction and characterization of phenolic compounds and their potential antioxidant activities. Environ. Sci. Pollut. Res. 2022, 29, 81112–81129. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Hosu, A.; Cristea, V.M.; Cimpoiu, C. Analysis of Total Phenolic, Flavonoids, Anthocyanins and Tannins Content in Romanian Red Wines: Prediction of Antioxidant Activities and Classification of Wines Using Artificial Neural Networks. Food Chem. 2014, 150, 113–118. [Google Scholar] [CrossRef]
- Krošlák, E.; Maliar, T.; Nemeček, P.; Viskupičová, J.; Maliarová, M.; Havrlentová, M.; Kraic, J. Antioxidant and Proteinase Inhibitory Activities of Selected Poppy (Papaver somniferum L.) Genotypes. Chem. Biodivers. 2017, 14, e1700176. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GENALEX 6: Genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Notes 2006, 6, 288–295. [Google Scholar] [CrossRef]
- Wright, S. The genetical structure of populations. Ann. Eugen. 1951, 15, 323–354. [Google Scholar] [CrossRef]
- Goudet, J. FSTAT (Version 1.2): A Computer Program to Calculate F-Statistics. J. Hered. 1995, 86, 485–486. [Google Scholar] [CrossRef]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef]
- Falush, D.; Stephens, M.; Pritchard, J.K. Inference of population structure using multilocus genotype data: Dominant markers and null alleles. Mol. Ecol. Notes 2007, 7, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Earl, D.A.; vonHoldt, B.M. STRUCTURE HARVESTER: A website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv. Genet. Resour. 2012, 4, 359–361. [Google Scholar] [CrossRef]
- Besta, E. Bormio Antica e Medievale, e le Sue Relazioni Con le Potenze Finitime; Edizioni Giuffrè: Milano, Italy, 1945. [Google Scholar]
- Della Misericordia, M. Giustizia, identità locale ed esclusione a Bormio nel Quattrocento. Boll. Stor. Alta Valtellina 2010, 13, 79–126. [Google Scholar]
- Antonioli, G. Una relazione ottocentesca sullo stato dell’ex contado di Bormio. Boll. Stor. Dell’alta Valtellina 2001, 4, 133–149. [Google Scholar]
- Scaramellini, G. Coltura della vite, produzione e commercio del vino in Valtellina (secoli IX-XVIII). Territ. du vin 2014. Available online: http://preo.u-bourgogne.fr/territoiresduvin/index.php?id=829 (accessed on 22 December 2023). [CrossRef]
- Marques, I.; Shiposha, V.; López-Alvarez, D.; Manzaneda, A.J.; Hernandez, P.; Olonova, M.; Catálan, P. Environmental isolation explains Iberian genetic diversity in the highly homozygous model grass Brachypodium distachyon. BMC Evol. Biol. 2017, 17, 139. [Google Scholar] [CrossRef] [PubMed]
- Adamo, M.; Blandino, M.; Capo, L.; Ravetto Enri, S.; Fusconi, A.; Lonati, M.; Mucciarelli, M.A. ddRADseq Survey of the Genetic Diversity of Rye (Secale cereale L.) Landraces from the Western Alps Reveals the Progressive Reduction of the Local Gene Pool. Plants 2021, 10, 2415. [Google Scholar] [CrossRef] [PubMed]
- Lopes, M.S.; El-Basyoni, I.; Baenziger, P.S.; Singh, S.; Royo, C.; Ozbek, K.; Aktas, H.; Ozer, E.; Ozdemir, F.; Manickavelu, A.; et al. Exploiting genetic diversity from landraces in wheat breeding for adaptation to climate change. J. Exp. Bot. 2015, 66, 3477–3486. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, D.; Yu, K.; Ji, J.; Liu, N.; Zhang, Y.; Xu, M.; Zhang, Y.-J.; Ma, X.; Liu, S.; et al. Frequent germplasm exchanges drive the high genetic diversity of Chinese-cultivated common apricot germplasm. Hortic. Res. 2021, 8, 215. [Google Scholar] [CrossRef] [PubMed]
- Stetter, M.G.; Vidal-Villarejo, M.; Schmid, K.J. Parallel Seed Color Adaptation during Multiple Domestication Attempts of an Ancient New World Grain. Mol. Biol. Evol. 2020, 37, 1407–1419. [Google Scholar] [CrossRef] [PubMed]
- Giraldo, P.; Royo, C.; González, M.; Carrillo, J.M.; Ruiz, M. Genetic diversity and association mapping for agromorphological and grain quality traits of a structured collection of durum wheat landraces including subsp. durum, turgidum and diccocon. PLoS ONE 2016, 11, e0166577. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Moreno, F.; Giraldo, P.; Cátedra, M.d.M.; Ruiz, M. Evaluation of Leaf Rust Resistance in the Spanish Core Collection of Tetraploid Wheat Landraces and Association with Ecogeographical Variables. Agriculture 2021, 11, 277. [Google Scholar] [CrossRef]
- Tao, Y.; Mace, E.S.; Tai, S.; Cruickshank, A.; Campbell, B.C.; Zhao, X.; Van Oosterom, E.J.; Godwin, I.D.; Botella, J.R.; Jordan, D.R. Whole-Genome Analysis of Candidate genes Associated with Seed Size and Weight in Sorghum bicolor Reveals Signatures of Artificial Selection and Insights into Parallel Domestication in Cereal Crops. Front. Plant Sci. 2017, 8, 1237. [Google Scholar] [CrossRef]
- Olejniczak, P.; Czarnoleski, M.; Delimat, A.; Majcher, B.M.; Szczepka, K. Seed size in mountain herbaceous plants changes with elevation in a species-specific manner. PLoS ONE 2018, 13, e0199224. [Google Scholar] [CrossRef]
- Wang, X.; Alvarez, M.; Donohue, K.; Ge, W.; Cao, Y.; Liu, K.; Du, G.; Bu, H. Elevation filters seed traits and germination strategies in the eastern Tibetan Plateau. Ecography 2021, 44, 242–254. [Google Scholar] [CrossRef]
- Laurent, A.; Pelzer, E.; Loyce, C.; Makowski, D. Ranking yields of energy crops: A meta-analysis using direct and indirect comparisons. Renew. Sustain. Energy Rev. 2015, 46, 41–50. [Google Scholar] [CrossRef]
- Brown, D.; Van den Bergh, I.; de Bruin, S.; Machida, L.; van Etten, J. Data synthesis for crop variety evaluation. A review. Agron. Sustain. Dev. 2020, 40, 25. [Google Scholar] [CrossRef] [PubMed]
- Peratoner, G.; Seling, S.; Klotz, C.; Florian, C.; Figl, U.; Schmitt, A.O. Variation of agronomic and qualitative traits and local adaptation of mountain landraces of winter rye (Secale cereale L.) from Val Venosta/Vinschgau (South Tyrol). Genet. Resour. Crop Evol. 2016, 63, 261–273. [Google Scholar] [CrossRef]
- Mattila, P.; Pihlava, J.M.; Hellström, J. Contents of phenolic acids, alkyl- and alkenylresorcinols, and avenanthramides in commercial grain products. J. Agric. Food Chem. 2005, 53, 8290–8295. [Google Scholar] [CrossRef] [PubMed]
- Gliwa, J.; Gunenc, A.; Ames, N.; Willmore, W.G.; Hosseinian, F.S. Antioxidant Activity of Alkylresorcinols from Rye Bran and Their Protective Effects on Cell Viability of PC-12 AC Cells. J. Agric. Food Chem. 2011, 59, 11473–11482. [Google Scholar] [CrossRef] [PubMed]
- Łukaszewicz, M.; Szopa, J. Pleiotropic effect of flavonoid biosynthesis manipulation in transgenic potato plants. Acta Physiol. Plant 2005, 27, 221–228. [Google Scholar] [CrossRef]
- Barcaccia, G.; Volpato, M.; Gentili, R.; Abeli, T.; Galla, G.; Orsenigo, S.; Citterio, S.; Sgorbati, S.; Rossi, G. Genetic identity of common buckwheat (Fagopyrum esculentum Moench) landraces locally cultivated in the Alps. Genet. Resour. Crop Evol. 2016, 63, 639–651. [Google Scholar] [CrossRef]
- Giupponi, L.; Pedrali, D.; Leoni, V.; Rodari, A.; Giorgi, A. The Analysis of Italian Plant Agrobiodiversity Databases Reveals That Hilly and Sub-Mountain Areas Are Hotspots of Herbaceous Landraces. Diversity 2021, 13, 70. [Google Scholar] [CrossRef]
- Akhalkatsi, M.; Otte, A.; Togonidze, N.; Bragvadze, T.; Asanidze, Z.; Arabuli, G.; Chikhelidze, N.; Mazanishvili, L. Agrobiodiversity and genetic erosion of crop varieties and plant resources in the Central Great Caucasus. Ann. Agrar. Sci. 2017, 15, 11–16. [Google Scholar] [CrossRef]
- Wan, J.; Li, R.; Wang, W.; Liu, Z.; Chen, B. Income Diversification: A Strategy for Rural Region Risk Management. Sustainability 2016, 8, 1064. [Google Scholar] [CrossRef]
- Colombo, F.; Franguelli, N.; Licheri, G.; Ghidoli, M.; Cassani, E.; Castelli, L.; Pasquali, M.; Bresciani, A.; Marti, A.; Dell’Anno, M.; et al. Agriculture in Marginal Areas: Reintroduction of Rye and Wheat Varieties for Breadmaking in the Antrona Valley. Agronomy 2022, 12, 1695. [Google Scholar] [CrossRef]
- Limpo, S.Y.; Fahmid, I.M.; Fattah, A.; Rauf, A.W.; Surmaini, E.; Muslimin; Saptana; Syahbuddin, H.; Andri, K.B. Integrating Indigenous and Scientific Knowledge for Decision Making of Rice Farming in South Sulawesi, Indonesia. Sustainability 2022, 14, 2952. [Google Scholar] [CrossRef]
- Rasul, G.; Hussain, A. Sustainable Food Security in the Mountains of Pakistan: Towards a Policy Framework. Ecol. Food Nutr. 2015, 54, 625–643. [Google Scholar] [CrossRef] [PubMed]
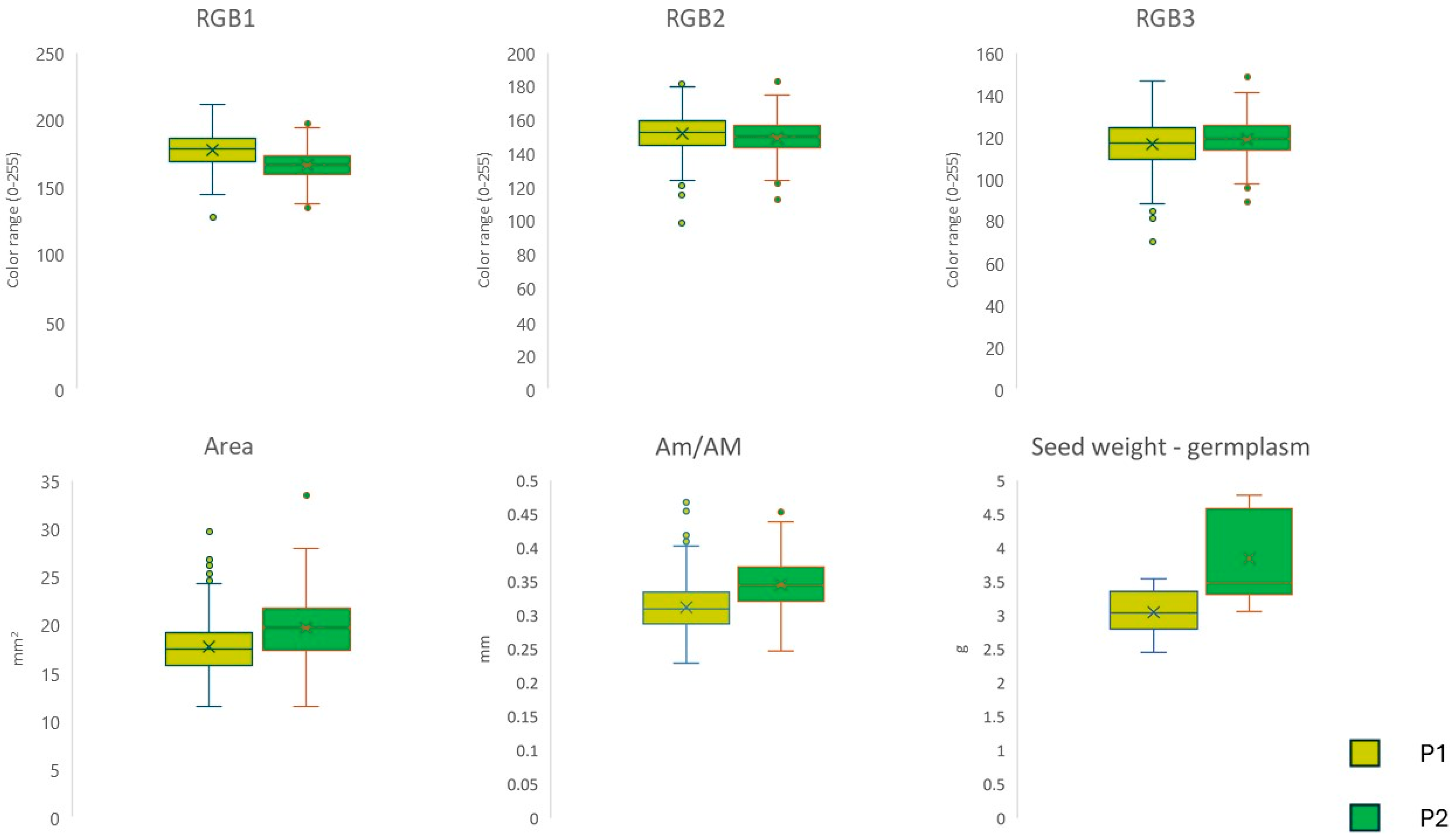
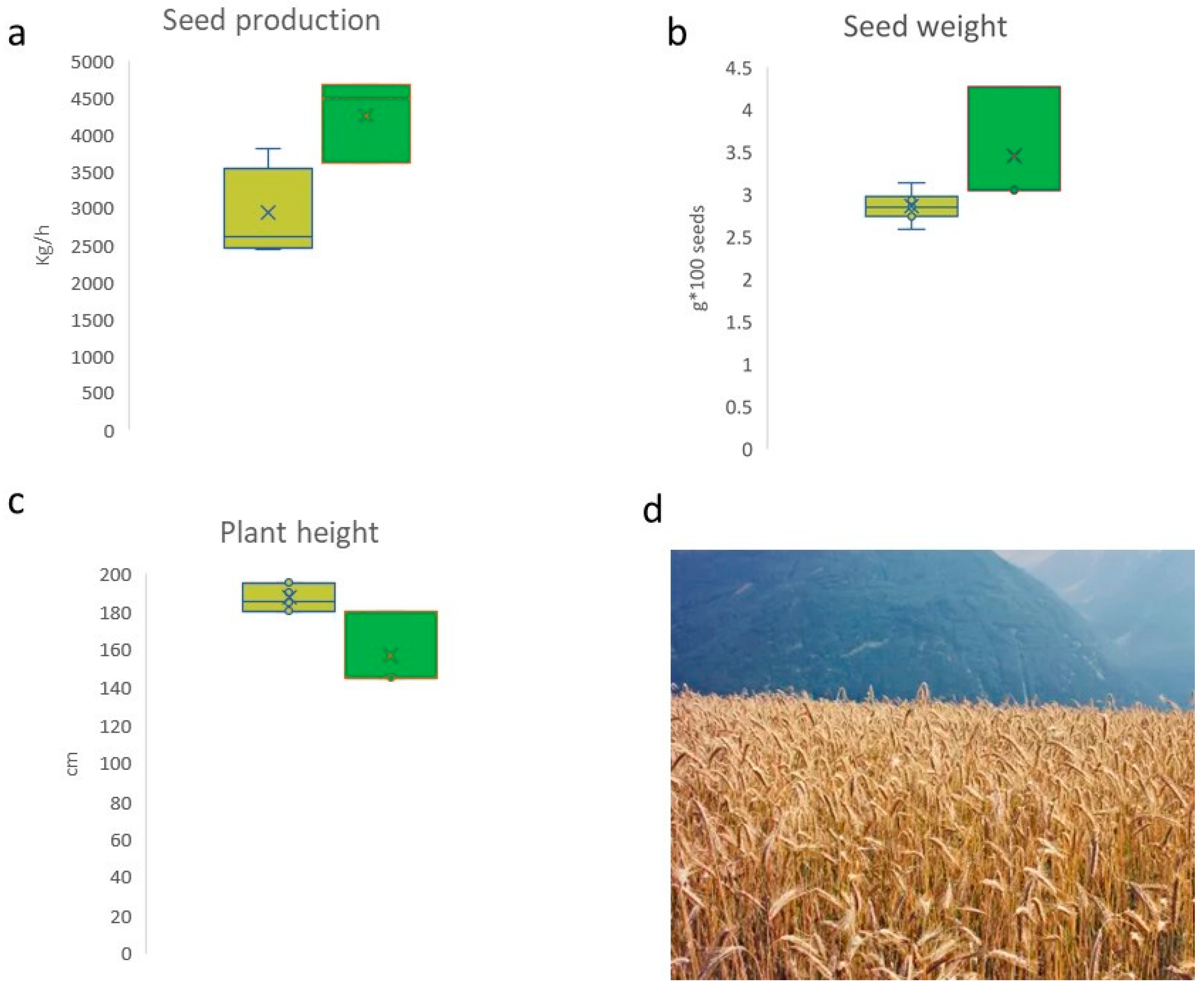
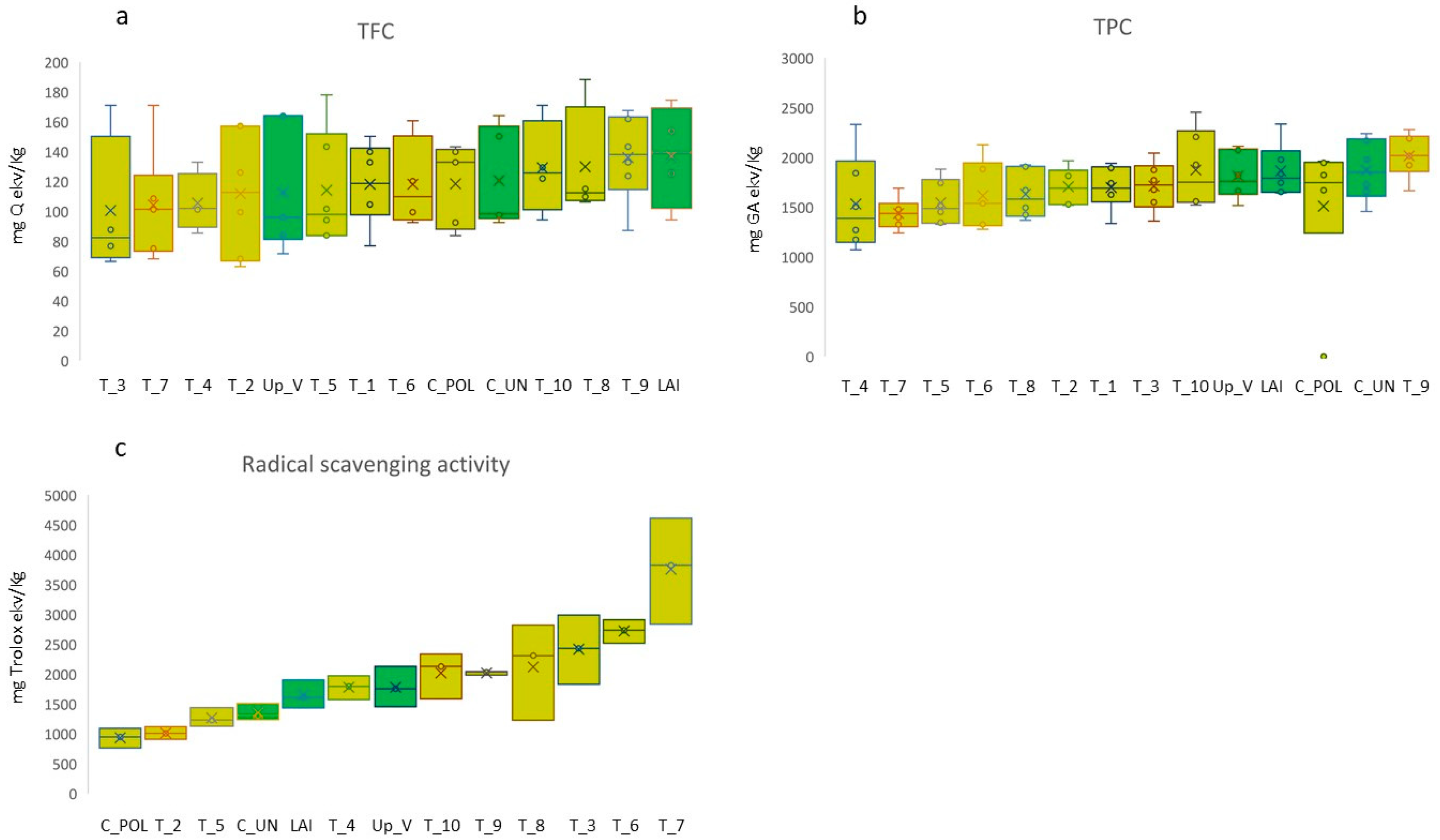
| Population | Na | Ne | I | Ho | uHe | F |
|---|---|---|---|---|---|---|
| T_1 | 5.25 | 2.94 | 1.25 | 0.60 | 0.66 | 0.06 |
| T_2 | 5.50 | 3.03 | 1.27 | 0.61 | 0.67 | 0.07 |
| T_3 | 4.50 | 2.74 | 1.16 | 0.56 | 0.63 | 0.09 |
| T_4 | 5.37 | 3.22 | 1.31 | 0.62 | 0.68 | 0.08 |
| T_5 | 5.00 | 3.06 | 1.23 | 0.62 | 0.66 | 0.02 |
| T_6 | 5.25 | 3.03 | 1.29 | 0.67 | 0.68 | −0.02 |
| T_7 | 4.75 | 3.28 | 1.26 | 0.61 | 0.67 | 0.06 |
| T_8 | 4.87 | 3.25 | 1.25 | 0.66 | 0.65 | −0.033 |
| T_9 | 4.87 | 3.19 | 1.24 | 0.69 | 0.65 | −0.063 |
| T_10 | 5.15 | 2.88 | 1.22 | 0.48 | 0.64 | 0.22 |
| Up_V | 4.12 | 2.83 | 1.13 | 0.51 | 0.62 | 0.16 |
| LAI | 4.5 | 2.56 | 1.07 | 0.59 | 0.58 | −0.05 |
| C_POL_P1 | 4.5 | 3.19 | 1.22 | 0.68 | 0.65 | −0.02 |
| C_RUS | 4.62 | 3.04 | 1.21 | 0.58 | 0.65 | 0.08 |
| Gene Flow (Nm) = 3.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gentili, R.; La Ferla, B.; Cardarelli, E.; Gusmeroli, F.; Della Marianna, G.; Parolo, G.; Maestroni, G.; Citterio, S. Recovering Alpine Secale cereale (Rye) Varieties: Insights from Genetic, Agronomic, and Phytochemical Analyses to Support Sustainable Mountain Agriculture Economy. Agronomy 2024, 14, 1605. https://doi.org/10.3390/agronomy14081605
Gentili R, La Ferla B, Cardarelli E, Gusmeroli F, Della Marianna G, Parolo G, Maestroni G, Citterio S. Recovering Alpine Secale cereale (Rye) Varieties: Insights from Genetic, Agronomic, and Phytochemical Analyses to Support Sustainable Mountain Agriculture Economy. Agronomy. 2024; 14(8):1605. https://doi.org/10.3390/agronomy14081605
Chicago/Turabian StyleGentili, Rodolfo, Barbara La Ferla, Elisa Cardarelli, Fausto Gusmeroli, Gianpaolo Della Marianna, Gilberto Parolo, Giancarla Maestroni, and Sandra Citterio. 2024. "Recovering Alpine Secale cereale (Rye) Varieties: Insights from Genetic, Agronomic, and Phytochemical Analyses to Support Sustainable Mountain Agriculture Economy" Agronomy 14, no. 8: 1605. https://doi.org/10.3390/agronomy14081605
APA StyleGentili, R., La Ferla, B., Cardarelli, E., Gusmeroli, F., Della Marianna, G., Parolo, G., Maestroni, G., & Citterio, S. (2024). Recovering Alpine Secale cereale (Rye) Varieties: Insights from Genetic, Agronomic, and Phytochemical Analyses to Support Sustainable Mountain Agriculture Economy. Agronomy, 14(8), 1605. https://doi.org/10.3390/agronomy14081605








