Co-Application of Coated Phosphate Fertilizer and Humic Acid for Wheat Production and Soil Nutrient Transport
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Design
2.2.1. Pot Experiment
2.2.2. Leaching Experiment
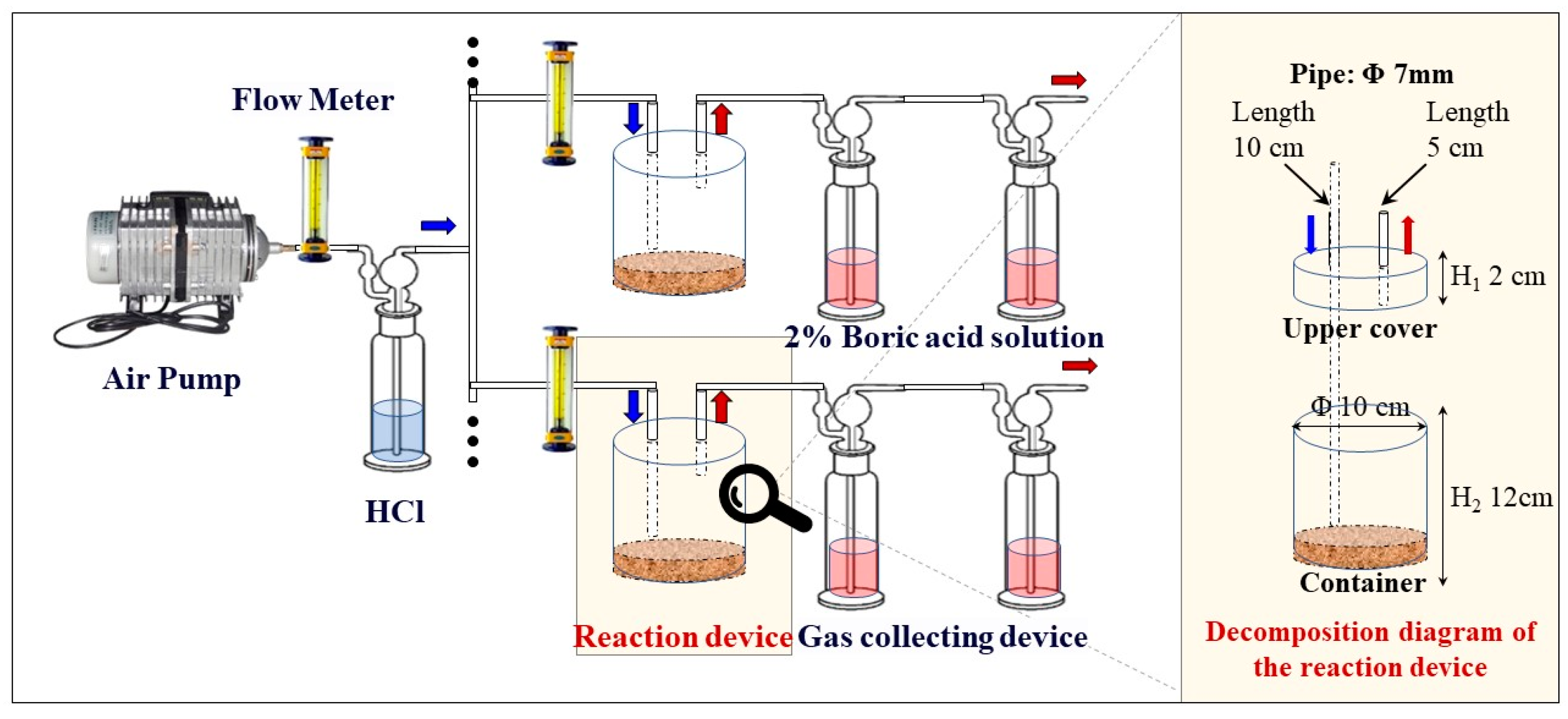
2.2.3. Ammonia Volatilization Experiment
2.3. Sample Collection
2.4. Sample Measurement and Analysis Methods
2.5. Statistical Analyses
3. Results
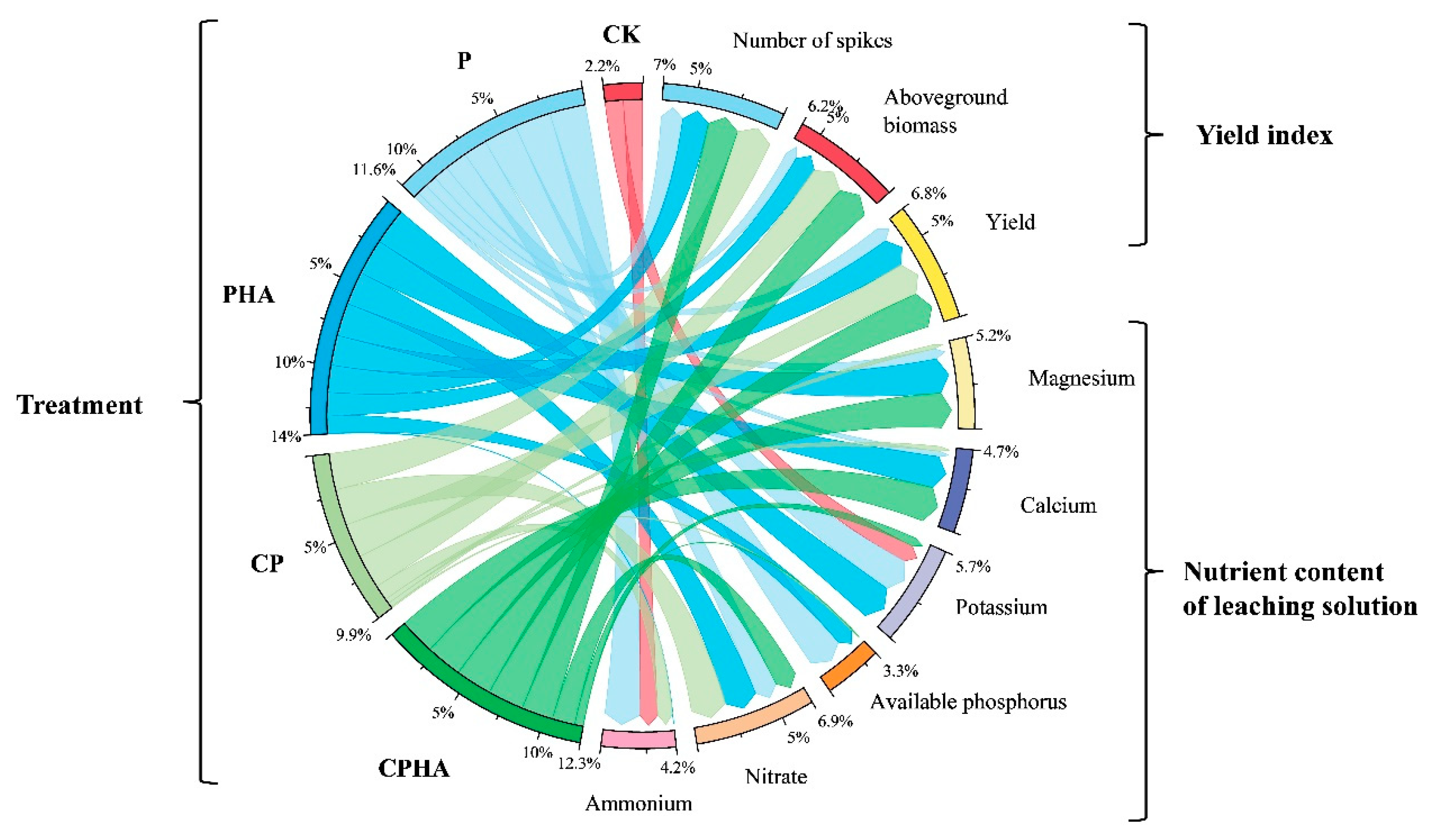
3.1. Winter Wheat Yield under Different Treatments
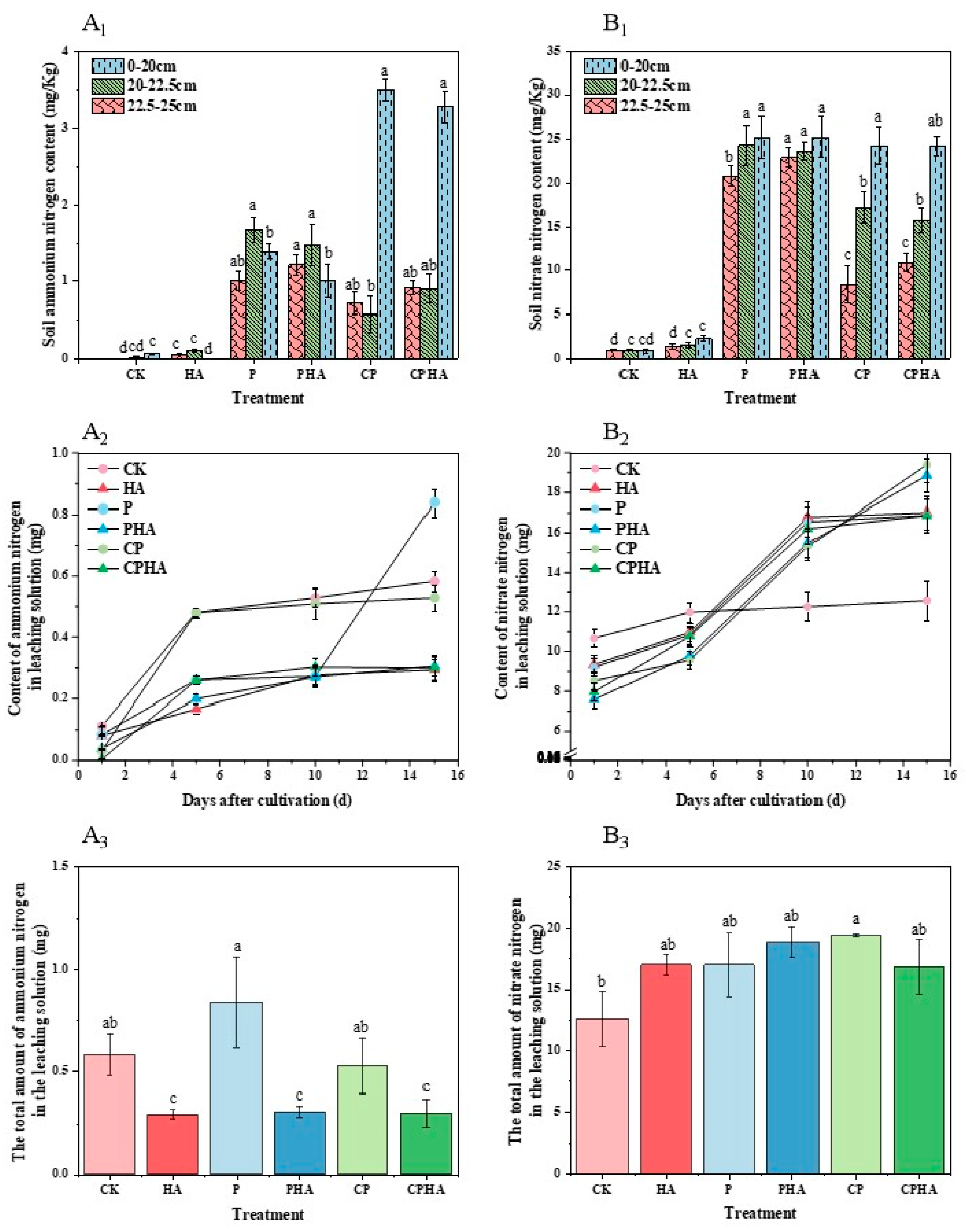
3.2. Different Fertilization Treatments on Soil Ammonia Volatilization and Soil Mineral Nitrogen Loss
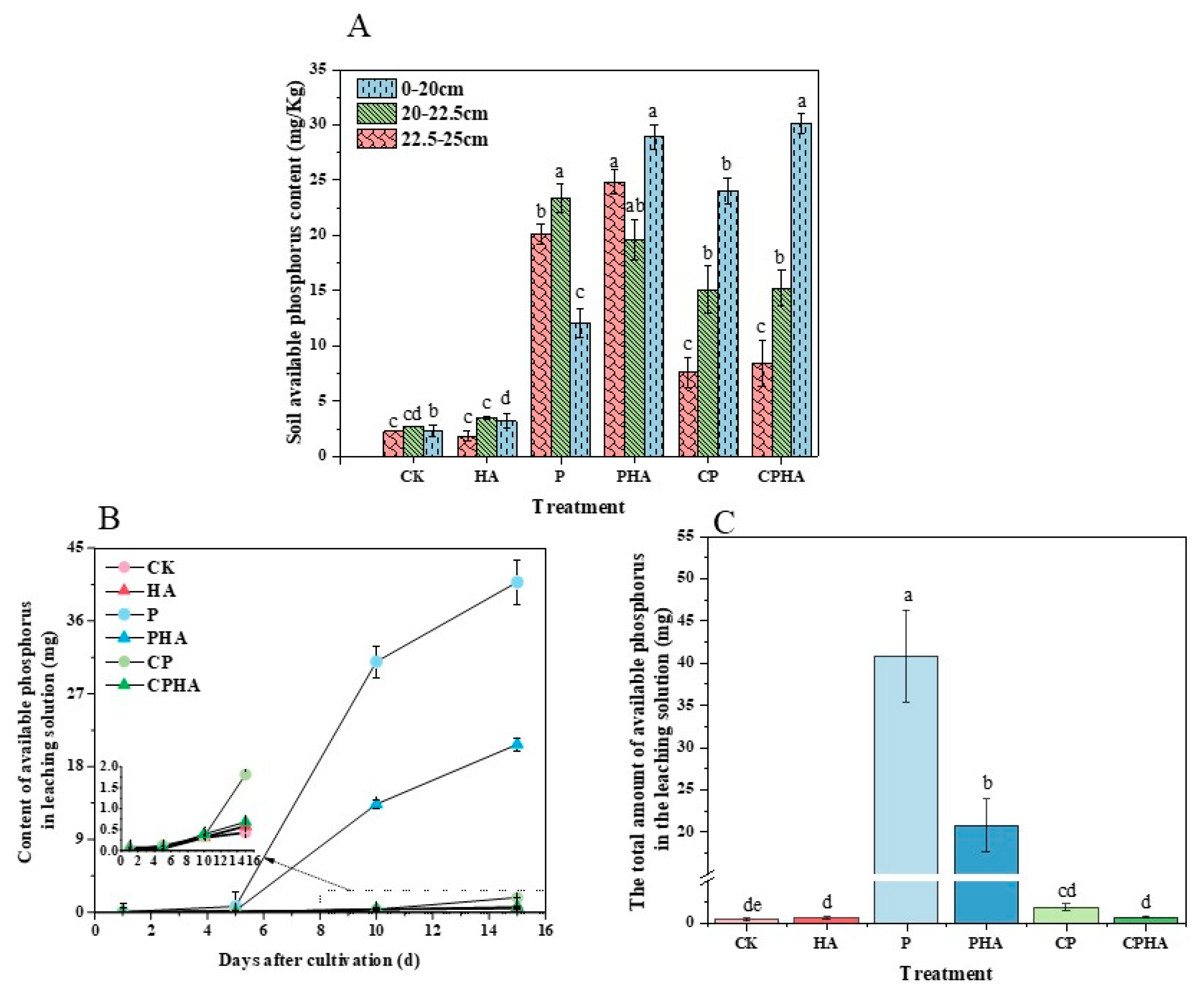
3.3. Different Fertilization Treatments on Phosphorus Content in Soil and Leachate in Leaching Experiments
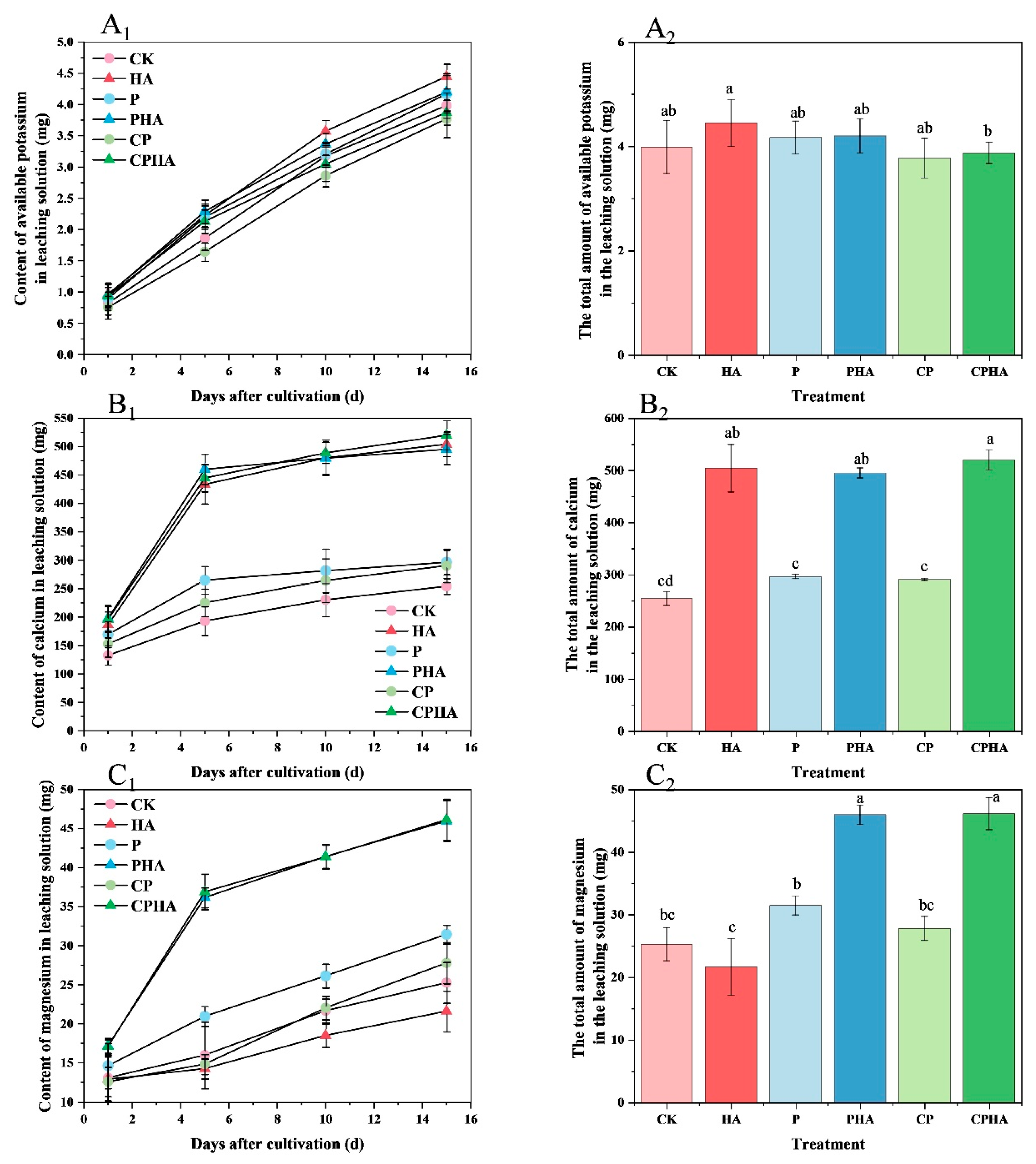
3.4. Different Fertilization Treatments on the Content of Potassium, Calcium, and Magnesium in Leachate during Leaching Experiments
4. Discussion
4.1. Coated Diammonium Phosphate on Wheat Yield and Soil Nutrient Transport
4.2. Humic Acid on Wheat Yield and Soil Nutrient Transport
4.3. Coated Diammonium Phosphate Combined with Humic Acid on Wheat Yield and Soil Nutrient Transport
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, X.; Ren, H.; Zhang, J.; Zhao, B.; Ren, B.; Wan, Y.; Liu, P. Deep phosphorus fertilizer placement increases maize productivity by improving root-shoot coordination and photosynthetic performance. Soil Tillage Res. 2024, 235, 105915. [Google Scholar] [CrossRef]
- Enzai, D.; César, T.; Adam, F.A.P.; Anders, A.; Caspar, J.; Zhao, X.; Xia, N.; Wu, X.; Robert, B.J. Global patterns of terrestrial nitrogen and phosphorus limitation. Nat. Geosci. 2020, 13, 221–226. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, W.; Torsten, M.; Prakash, L.; Liu, Y.; Liang, T.; Wang, L.; Yang, H.; Chen, X. Soil phosphorus availability and fractionation in response to different phosphorus sources in alkaline and acid soils: A short-term incubation study. Sci. Rep. 2023, 13, 5677. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Steven, G.P.; Li, H.; Geng, J.; Diao, Y.; David, W.H. Modeling phosphorus sources and transport in a headwater catchment with rapid agricultural expansion. Environ. Pollut. 2019, 255, 113273. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Peng, S.; Chen, Y.; Cao, Y. Changes in soil phosphorus and its influencing factors following afforestation in Northern China. Land Degrad. Dev. 2019, 30, 1655–1666. [Google Scholar] [CrossRef]
- Lu, H.; Tian, H.; Zhang, M.; Liu, Z.; Chen, Q.; Guan, R.; Wang, H. Water Polishing improved controlled-release characteristics and fertilizer efficiency of castor oil-based polyurethane coated diammonium phosphate. Sci. Rep. 2020, 10, 5763. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Liu, Z.; Zhang, M.; Li, Z.; Qu, Z.; Yang, M.; Sun, L. Enhancement of wheat yield and soil nutrient supply intensity by coating diammonium phosphate with fulvic acid. Acta Pedol. Sin. 2018, 55, 1472–1484. [Google Scholar] [CrossRef]
- Diego, F.; Ricardo, B.; Gelton, G.; Wagner, L.; Caue, R. Role of polymeric coating on the phosphate availability as a fertilizer: Insight from phosphate release by castor polyurethane coatings. J. Agric. Food Chem. 2017, 65, 5890–5895. [Google Scholar] [CrossRef]
- Abhijit, S.; Dipak, R.; Samar, C.; Trisha, R.; Pravash, C.; Siddhartha, S.; Avijit, G. Polymer coated novel controlled release rock phosphate formulations for improving phosphorus use efficiency by wheat in an Inceptisol. Soil Tillage Res. 2018, 180, 48–62. [Google Scholar] [CrossRef]
- Chen, Q.; Qu, Z.; Li, Z.; Zhang, Z.; Ma, G.; Liu, Z.; Wang, Y.; Wu, L.; Fang, F.L.; Wei, Z.; et al. Coated Diammonium Phosphate Combined with Humic Acid Improves Soil Phosphorus Availability and Photosynthesis and the Yield of Maize. Front. Plant Sci. 2021, 12, 759929. [Google Scholar] [CrossRef]
- Zhu, J.; Li, M.; Whelan, M. Phosphorus activators contribute to legacy phosphorus availability in agricultural soils: A review. Sci. Total Environ. 2018, 612, 522–537. [Google Scholar] [CrossRef]
- Newcomb, C.J. Humic matter in soil and the environment, principles and controversies. Soil Sci. Soc. Am. J. 2015, 79, 1520. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, X.; Lv, J. Molecular Characterization of Size-Fractionated Humic Acids Derived from Lignite and Its Activation of Soil Legacy Phosphorus and Lactuca sativa Growth-Promoting Performances. ACS Omega 2023, 8, 6838–6846. [Google Scholar] [CrossRef]
- Ge, X.; Wang, L.; Zhang, W.; Christine, V.P. Molecular Understanding of Humic Acid-Limited Phosphate Precipitation and Transformation. Environ. Sci. Technol. 2020, 54, 207–215. [Google Scholar] [CrossRef]
- Luciano, P.C.; Fábio, L.O.; Natália, O.A.; Davey, L.J.; Antonio, N.; Pierluigi, M.; Alessandro, P. Humic and fulvic acids as biostimulants in horticulture. Sci. Hortic. 2015, 196, 15–27. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, K.; Qiu, L.; Ding, S.; Wang, H.; Liu, Z.; Zhang, M.; Wei, Z. Soil microbial co-occurrence patterns under controlled-release urea and fulvic acid applications. Microorganisms 2022, 10, 1823. [Google Scholar] [CrossRef] [PubMed]
- Sara, D.; Carlos, A.; Henrique, J.; Moreira, M. Wheat nutrition and growth as affected by humic acid-phosphate interaction. J. Soil Sci. Plant Nutr. 2018, 181, 870–877. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Q.; Xu, K.; Zhang, K.; Zhang, M.; Qi, Y.; Zhang, W.; Liu, Y.; Wei, Z.; Liu, Z. Selective modifier-assisted humic acid extraction: Implications for soil quality enhancement. Environ. Sci. Technol. 2024, 58, 9896–9907. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Liu, D.; Liu, X.; Liang, F.; Liu, M.; Li, Q.; Wang, G.; Liu, P.; Jia, H. Effects of different water-soluble phosphorus on the distribution and utilization of phosphorus in maize in Xinjiang, China. Soil Use Manag. 2024, 40, e13010. [Google Scholar] [CrossRef]
- Huang, T.; Yang, N.; Lu, C.; Qin, X.; Kadambot, H. Soil organic carbon, total nitrogen, available nutrients, and yield under different straw returning methods. Soil Tillage Res. 2021, 214, 105171. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Z.; Zhang, M.; Chen, Q.; Zheng, L.; Li, Y.; Sun, L. The combined application of controlled-release urea and fulvic acid improved the soil nutrient supply and maize yield. Arch. Agron. Soil Sci. 2021, 67, 633–646. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Y.; Song, L.; Yuan, J.; Li, W.; Zhu, Y.; Scott, X.C.; Luo, Y.; Philippe, C.; Josep, P.; et al. Reduced phosphorus availability in paddy soils under atmospheric CO2 enrichment. Nat. Geosci. 2023, 16, 162–168. [Google Scholar] [CrossRef]
- Li, S.; Chen, X.; Wang, Z.; Wu, D.; Wang, M.; Müeller, T.; Zou, C.; Chen, X.; Zhang, W. Phosphorus fertilizer management for high yields in intensive winter wheat-summer maize rotation system: Integrating phosphorus budget and soil available phosphorus. Field Crops Res. 2024, 313, 109410. [Google Scholar] [CrossRef]
- Balemi, T.; Negisho, K. Management of soil phosphorus and plant adaptation mechanisms to phosphorus stress for sustainable crop production: A review. J. Soil Sci. Plant Nutr. 2012, 12, 547–562. [Google Scholar] [CrossRef]
- Chen, Q.; Qu, Z.; Zhang, Z.; Ma, G.; Zhu, M.; Dan, J.; Wang, J.; Zhang, S.; Ding, X.; Zhang, M.; et al. Coated diammonium phosphate combined with Paecilomyces variotii extracts improves root architecture, enhances spring low temperature tolerance, and increases wheat yield. Soil Tillage Res. 2023, 227, 105613. [Google Scholar] [CrossRef]
- Whitehead, D.C.; Qi, L. Soil surface was applied to the ammonia volatilization of five nitrogen compounds. Prog. Soil Sci. 1990, 41, 387–389. [Google Scholar]
- Qin, Q.; Zhang, Z.; Song, Z. Research progress on sources, characteristics of humus and mechanism of interaction with heavy metals in soil. Environ. Chem. 2023, 42, 1899–1910. [Google Scholar] [CrossRef]
- Susic, M. Replenishing Humic Acids in Agricultural Soils. Agronomy 2016, 6, 45. [Google Scholar] [CrossRef]
- Liu, X.; Yang, J.; Tao, J.; Yao, R.; Wang, X.; Xie, W.; Zhu, H. Elucidating the effect and interaction mechanism of fulvic acid and nitrogen fertilizer application on phosphorus availability in a salt-affected soil. J. Soils Sediments 2021, 21, 2525–2539. [Google Scholar] [CrossRef]
- Ileana, P.; Laura, B. Adsorption and surhace precipitation of phosphate onto CaCO3–montmorillonite: Effect of pH, ionic strength and competition with humic acid. Geoderma 2014, 232–234, 600–608. [Google Scholar] [CrossRef]
- Mayakaduwege, A.; Yusop, M.; Roslan, I.; Liyana, R.; Minninga, G.; Mohamed, M. Identification of Phosphate Sorption-Desorption and Accumulation Characteristics to Reduce Environmental Consequences. Commun. Soil Sci. Plant Anal. 2022, 54, 926–944. [Google Scholar] [CrossRef]
- George, T.S.; Hinsinger, P.; Turner, B.L. Phosphorus in soils and plants – facing phosphorus scarcity. Plant Soil 2016, 401, 1–6. [Google Scholar] [CrossRef]
- Delgado, A.; Madrid, A.; Kassem, S.; Andreu, L.; del Carmen del Campillo, M. Phosphorus fertilizer recovery from calcareous soils amended with humic and fulvic acids. Plant Soil 2002, 245, 277–286. [Google Scholar] [CrossRef]
- Ma, G.; Chen, Q.; Shi, R.; Kong, B.; Chen, D.; Zhang, Z.; Li, X.; Qu, Z.; Li, M.; Zhang, M.; et al. Effect of Coated Diammonium Phosphate Combined with Paecilomyces variotii Extracts on Soil Available Nutrients, Photosynthesis-Related Enzyme Activities, Endogenous Hormones, and Maize Yield. ACS Omega 2022, 7, 23566–23575. [Google Scholar] [CrossRef]
- Daniel, C.O.; Dana, L.D.; Rene Scoresby, J.; Chad, R.C.; Jerald, W.D. Humic products in agriculture: Potential benefits and research challenges—A review. J. Soils Sediments 2018, 18, 2881–2891. [Google Scholar] [CrossRef]
- Hans, D.; Eric, J.W.V.; Heidrun, H.; Liesje, M.; Michael, J.H. A modular concept of plant foraging behaviour: The interplay between local responses and systemic control. Plant Cell Environ. 2009, 32, 704–712. [Google Scholar] [CrossRef]
- Wang, X.; Whalley, W.R.; Anthony, J.M.; Philip, J.W.; Zhang, F.; Shen, J. Sustainable Cropping Requires Adaptation to a Heterogeneous Rhizosphere. Trends Plant Sci. 2020, 25, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Mehtab, M.; José, J.P.; Pang, J.; Yang, J.; Chen, W.; Chen, H.; Muhammad, W.; Li, Y.; Zhang, J.; Xu, W. Root acid phosphatases and rhizobacteria synergistically enhance white lupin and rice phosphorus acquisition. Plant Physiol. 2022, 190, 2449–2465. [Google Scholar] [CrossRef]

| Treatment | Description | Quantity | |||||
|---|---|---|---|---|---|---|---|
| Diammonium Phosphate (g/pot) | Coated Diammonium Phosphate (g/pot) | Coated Urea (g/pot) | Urea (g/pot) | Potassium Chloride (g/pot) | Humic Acid (g/pot) | ||
| CK | Phosphorus blank control treatment | - | - | 7.53 | 4.70 | 3.00 | - |
| P | Conventional diammonium phosphate | 7.83 | - | 7.44 | 1.60 | 3.00 | - |
| CP | Coated diammonium phosphate | - | 8.18 | 4.26 | 4.70 | 3.00 | - |
| PHA | Conventional diammonium phosphate with humic acid | 7.83 | - | 7.53 | 1.40 | 2.73 | 5.40 |
| CPHA | Coated diammonium phosphate with humic acid | - | 8.18 | 4.26 | 4.46 | 2.73 | 5.40 |
| Treatment | Yield (g/pot) | Increase Yield Compared to P Treatment (%) | Aboveground Biomass (g/pot) | Number of Spikes (p−1) |
|---|---|---|---|---|
| CK | 44.2 c | −13.84 | 79.3 d | 44.8 c |
| P | 51.3 b | - | 93.0 c | 57.3 b |
| CP | 58.2 a | 13.45 | 106.2 b | 59.8 ab |
| PHA | 60.1 a | 17.15 | 119.7 a | 64.8 a |
| CPHA | 61.1 a | 19.10 | 122.3 a | 62.3 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Ma, Y.; Tian, Y.; Liu, P.; Zhang, M.; Liu, Z.; Zhu, X.; Wang, C.; Zhuang, Y.; Zhang, W.; et al. Co-Application of Coated Phosphate Fertilizer and Humic Acid for Wheat Production and Soil Nutrient Transport. Agronomy 2024, 14, 1621. https://doi.org/10.3390/agronomy14081621
Zhang Z, Ma Y, Tian Y, Liu P, Zhang M, Liu Z, Zhu X, Wang C, Zhuang Y, Zhang W, et al. Co-Application of Coated Phosphate Fertilizer and Humic Acid for Wheat Production and Soil Nutrient Transport. Agronomy. 2024; 14(8):1621. https://doi.org/10.3390/agronomy14081621
Chicago/Turabian StyleZhang, Zixin, Yutong Ma, Ye Tian, Pingan Liu, Min Zhang, Zhiguang Liu, Xiaofan Zhu, Conghui Wang, Yuezhuo Zhuang, Wenrui Zhang, and et al. 2024. "Co-Application of Coated Phosphate Fertilizer and Humic Acid for Wheat Production and Soil Nutrient Transport" Agronomy 14, no. 8: 1621. https://doi.org/10.3390/agronomy14081621





