Wheat Response to Foliar-Applied Phosphorus Is Determined by Soil Phosphorus Buffering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Selection and Experimental Details
| No. | Year | Experiment ID | Date of Sowing | Date of Harvesting | Wheat Variety | Seeding Rate (kg/ha) | Row Spacing (cm) | Seeding Depth (cm) | N Applied (kg/ha) | P Applied (kg/ha) | K Applied (kg/ha) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2021 | Bannister | 22/05/2021 | 13/12/2021 | Sceptre | 120 | 23 | 2.5 | 124 | 19 | 31 |
| 2 | 2021 | Kojonup S | 18/05/2021 | 24/12/2021 | Sceptre | 110 | 28 | 2.5 | 63 | 19 | 1 |
| 3 | 2022 | Gunwarrie 1 | 7/05/2022 | 16/12/2022 | Kinsei | 130 | 25 | 2.5 | 121 | 21 | 40 |
| 4 | 2022 | Frankland | 12/05/2022 | 16/12/2022 | Kinsei | 130 | 25 | 2.5 | 121 | 21 | 40 |
| 5 | 2022 | Gunwarrie 2 | 5/05/2022 | 15/12/2022 | Kinsei | 130 | 25 | 2.5 | 121 | 21 | 40 |
| 6 | 2022 | Kojonup E | 23/05/2022 | 18/12/2022 | Chief | 100 | 22.5 | 2.5 | 72 | 18 | 10 |
| 7 | 2022 | Qualeup 1 | 20/05/2022 | 13/12/2022 | Rockstar | 110 | 22.5 | 2.5 | 82 | 20 | 35 |
| 8 | 2022 | Qualeup 2 | 20/05/2022 | 13/12/2022 | Rockstar | 110 | 22.5 | 2.5 | 82 | 20 | 35 |
| 9 | 2022 | Wandering | 28/05/2022 | 17/12/2022 | Rockstar | 75 | 22.5 | 2 | 79 | 15 | 7 |
2.2. Experimental Design
| No. | Experiment ID | Gravel (%) | Texture | ASC | pH (CaCl2) | SOC (%) | * PBI | ** DGT-P (μg/L) | *** PCol (mg/kg) | Inorg-N (mg/kg) | KCol (mg/kg) | S (mg/kg) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Bannister | 51 | Loamy sand | Chromosol | 6.2 | 4.39 | 175.6 | 15 | 50 | 71 | 89 | 7.1 |
| 2 | Kojonup S | 51 | Loamy sand | Chromosol | 5.7 | 3.91 | 110.1 | 19 | 42 | 106 | 226 | 21.8 |
| 3 | Gunwarrie 1 | 63 | Loamy sand | Chromosol | 5.6 | 4.31 | 165.5 | 22 | 61 | 65 | 103 | 18 |
| 4 | Frankland | 51 | Loamy sand | Chromosol | 6.2 | 3.43 | 145.6 | 20 | 39 | 59 | 89 | 14.2 |
| 5 | Gunwarrie 2 | 33 | Loamy sand | Sodosol | 5.6 | 3.65 | 218.5 | 15 | 43 | 43 | 103 | 16.5 |
| 6 | Kojonup E | 53 | Loam | Sodosol | 4.8 | 3.13 | 79.9 | 24 | 21 | 51 | 193 | 16.2 |
| 7 | Qualeup 1 | 59 | Clay loam | Tenosol | 6.5 | 4.32 | 176.3 | 24 | 93 | 39 | 146 | 8.6 |
| 8 | Qualeup 2 | 53 | Clay loam | Chromosol | 6.1 | 4.72 | 202 | 27 | 73 | 56 | 91 | 3.3 |
| 9 | Wandering | 30 | Clay loam | Chromosol | 5.6 | 3.65 | 168.4 | 22 | 48 | 15 | 82 | 4.9 |
2.3. In-Season Measurements, Plant and Grain Analysis, P Uptake and Efficiencies
2.4. P Efficiency Indices
2.5. Statistical Analysis
3. Results
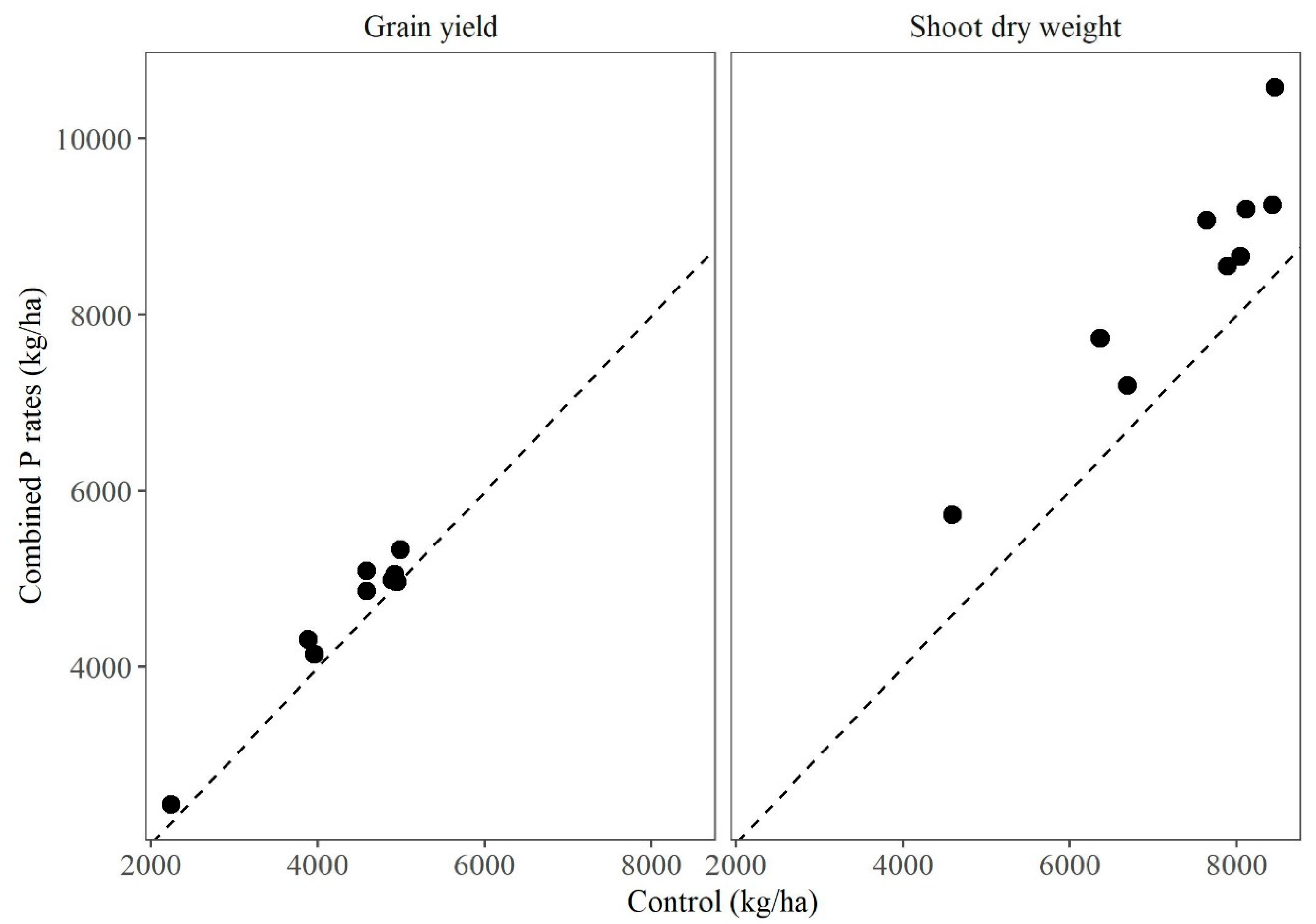
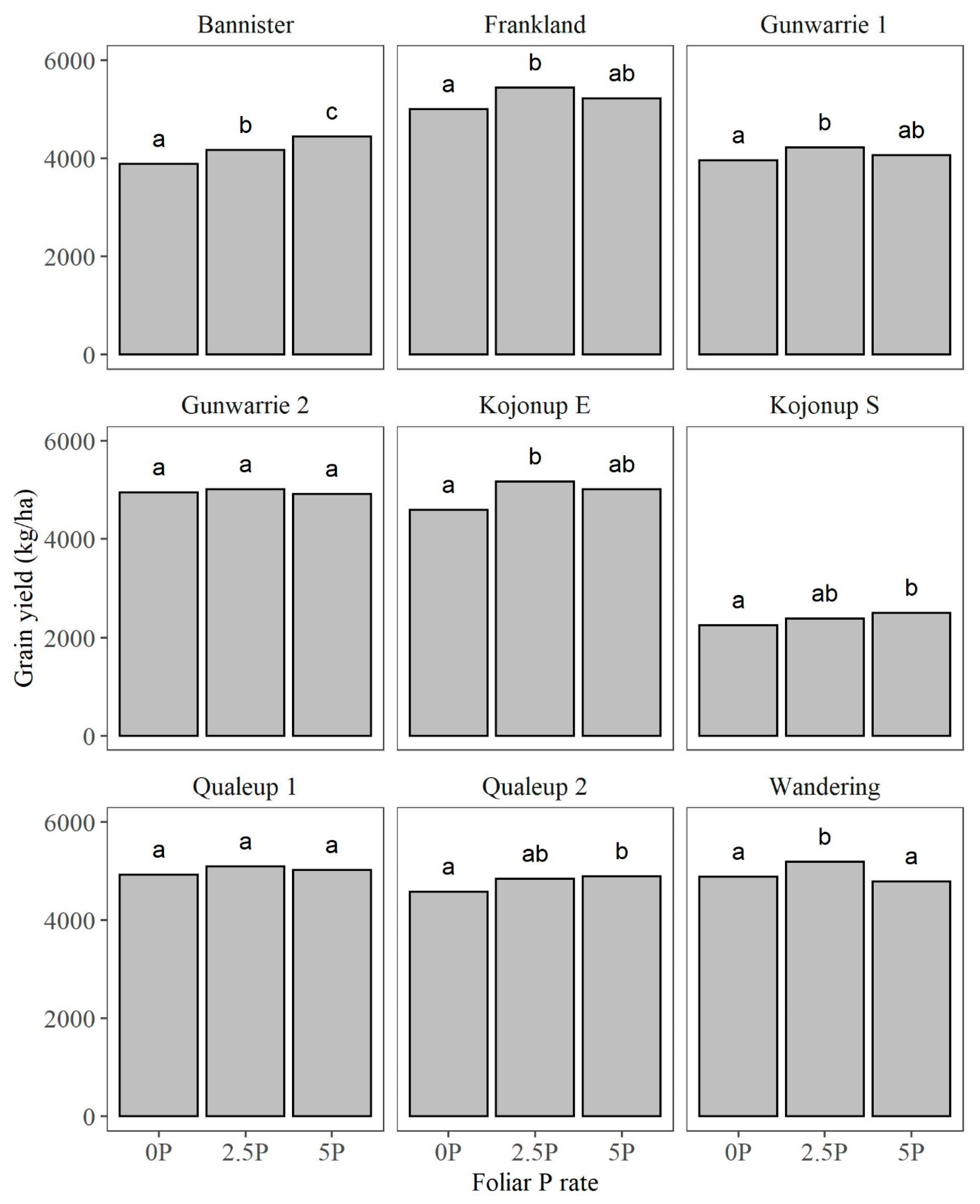
3.1. Grain Yield (GY)
| Site | Treatments | GY | SDW | % Shoot P | Shoot P Uptake | % Grain P | Grain P Uptake | % Leaf Scorching | AE | RE | PUE | Protein | Hectolitre wt | Av Grain wt |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bannister | Control vs. applied P | *** | ** | NS | ** | NS | NS | *** | - | - | - | * | * | NS |
| P Rates (2.5 P vs. 5 P) | ** | NS | ** | ** | NS | NS | ** | NS | NS | NS | NS | NS | NS | |
| P Timings | NS | NS | NS | NS | NS | NS | *** | NS | NS | NS | NS | NS | NS | |
| P Rates × P Timings | NS | NS | NS | NS | NS | NS | *** | NS | NS | NS | NS | NS | NS | |
| Kojonup S | Control vs. applied P | ** | ** | NS | NS | NS | NS | *** | - | - | - | NS | *** | NS |
| P Rates (2.5 P vs. 5 P) | NS | NS | NS | * | NS | NS | *** | NS | NS | NS | NS | * | NS | |
| P Timings | ** | *** | * | NS | NS | NS | *** | NS | NS | NS | NS | NS | NS | |
| P Rates × P Timings | NS | NS | ** | NS | ** | ** | *** | NS | NS | ** | NS | NS | ** | |
| Gunwarrie 1 | Control vs. applied P | ** | ** | NS | NS | *** | ** | *** | - | - | - | NS | NS | * |
| P Rates (2.5 P vs. 5 P) | NS | NS | NS | NS | ** | NS | *** | ** | NS | NS | NS | NS | NS | |
| P Timings | ** | NS | NS | NS | NS | NS | *** | * | NS | NS | NS | NS | NS | |
| P Rates × P Timings | NS | NS | NS | NS | NS | NS | *** | NS | NS | NS | NS | NS | NS | |
| Frankland | Control vs. applied P | ** | ** | * | ** | *** | *** | *** | - | - | - | NS | NS | NS |
| P Rates (2.5 P vs. 5 P) | NS | ** | ** | NS | NS | NS | *** | ** | NS | ** | NS | NS | NS | |
| P Timings | NS | ** | NS | NS | NS | NS | *** | NS | NS | NS | * | NS | NS | |
| P Rates × P Timings | NS | NS | NS | ** | NS | NS | *** | NS | NS | NS | NS | NS | NS | |
| Gunwarrie 2 | Control vs. applied P | NS | * | NS | ** | * | NS | *** | - | - | - | NS | NS | * |
| P Rates (2.5 P vs. 5 P) | NS | NS | ** | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | |
| P Timings | NS | NS | *** | ** | ** | NS | * | NS | ** | NS | ** | NS | NS | |
| P Rates × P Timings | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | |
| Kojonup E | Control vs. applied P | ** | ** | NS | * | *** | ** | *** | - | - | - | NS | NS | NS |
| P Rates (2.5 P vs. 5 P) | NS | NS | NS | NS | ** | NS | *** | ** | NS | * | NS | NS | NS | |
| P Timings | NS | NS | NS | NS | *** | NS | *** | NS | NS | NS | NS | NS | NS | |
| P Rates × P Timings | NS | NS | NS | NS | NS | NS | ** | NS | NS | NS | ** | NS | NS | |
| Qualeup 1 | Control vs. applied P | NS | ** | ** | *** | ** | ** | *** | NS | NS | NS | |||
| P Rates (2.5 P vs. 5 P) | NS | NS | ** | NS | ** | NS | *** | NS | NS | NS | NS | NS | NS | |
| P Timings | ** | ** | ** | NS | NS | NS | *** | ** | NS | NS | NS | NS | NS | |
| P Rates × P Timings | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | |
| Qualeup 2 | Control vs. applied P | ** | NS | *** | ** | *** | *** | *** | - | - | - | NS | NS | NS |
| P Rates (2.5 P vs. 5 P) | NS | NS | NS | NS | NS | NS | ** | NS | * | ** | NS | * | NS | |
| P Timings | ** | * | NS | NS | ** | NS | *** | ** | NS | NS | NS | NS | NS | |
| P Rates × P Timings | NS | NS | NS | NS | NS | NS | *** | NS | NS | NS | NS | NS | NS | |
| Wandering | Control vs. applied P | NS | ** | ** | ** | *** | ** | *** | - | - | - | NS | NS | NS |
| P Rates (2.5 P vs. 5 P) | ** | ** | ** | NS | NS | NS | *** | ** | NS | NS | ** | NS | NS | |
| P Timings | NS | NS | NS | * | * | NS | *** | NS | * | NS | NS | NS | NS | |
| P Rates × P Timings | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | |
| Combined analysis | Control vs. applied P | *** | *** | ** | *** | *** | *** | *** | - | - | - | NS | NS | ** |
| P Rates (2.5 P vs. 5 P) | NS | ** | *** | ** | ** | NS | *** | *** | NS | *** | NS | NS | NS | |
| P Timings | *** | ** | *** | NS | ** | NS | *** | ** | NS | NS | ** | NS | NS | |
| P Rates × P Timings | NS | NS | ** | NS | ** | ** | *** | NS | NS | ** | NS | NS | NS |
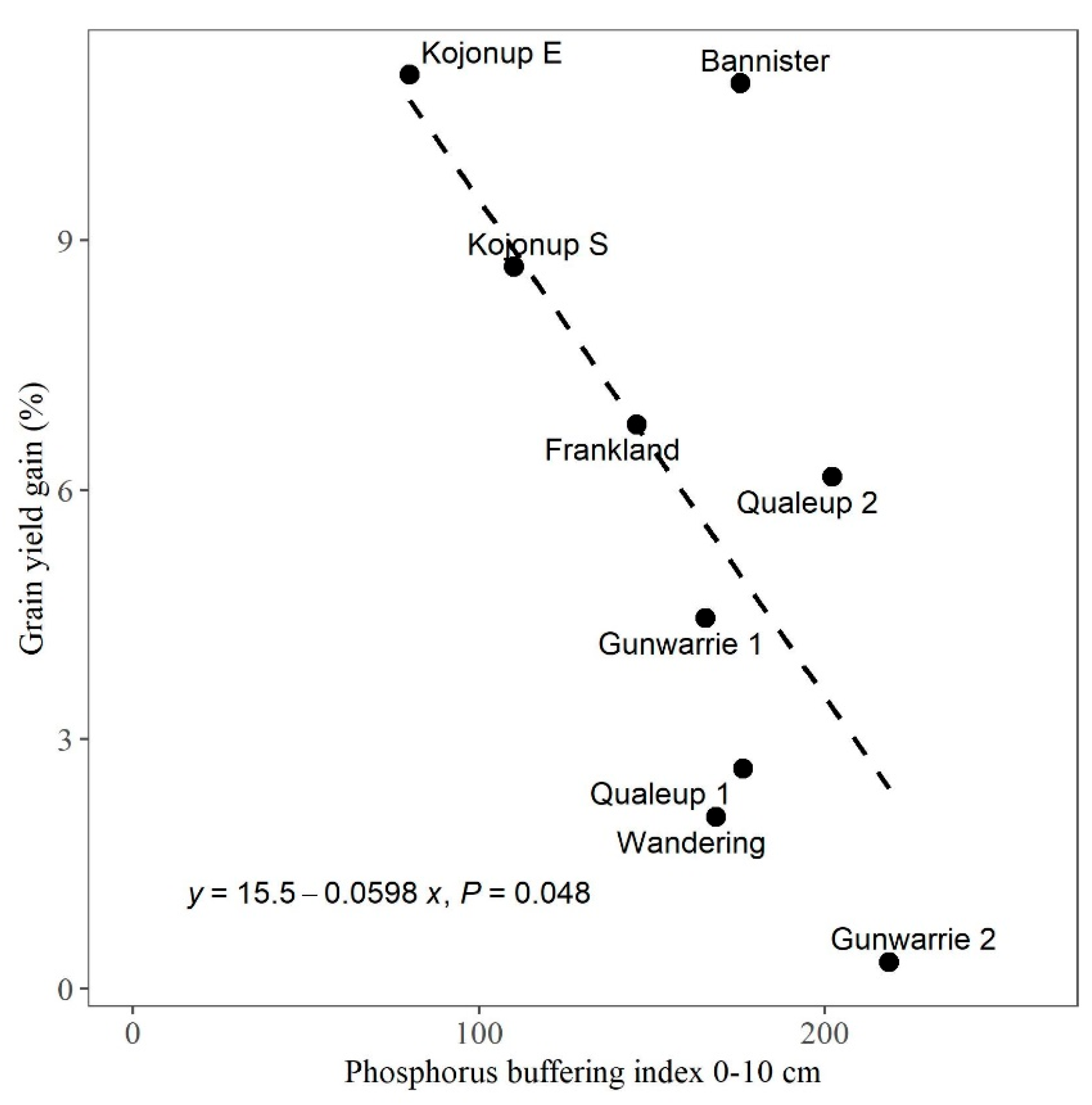
3.2. Relationships between Percent Yield Gain (% Response) Due to Foliar P Application and Site Factors
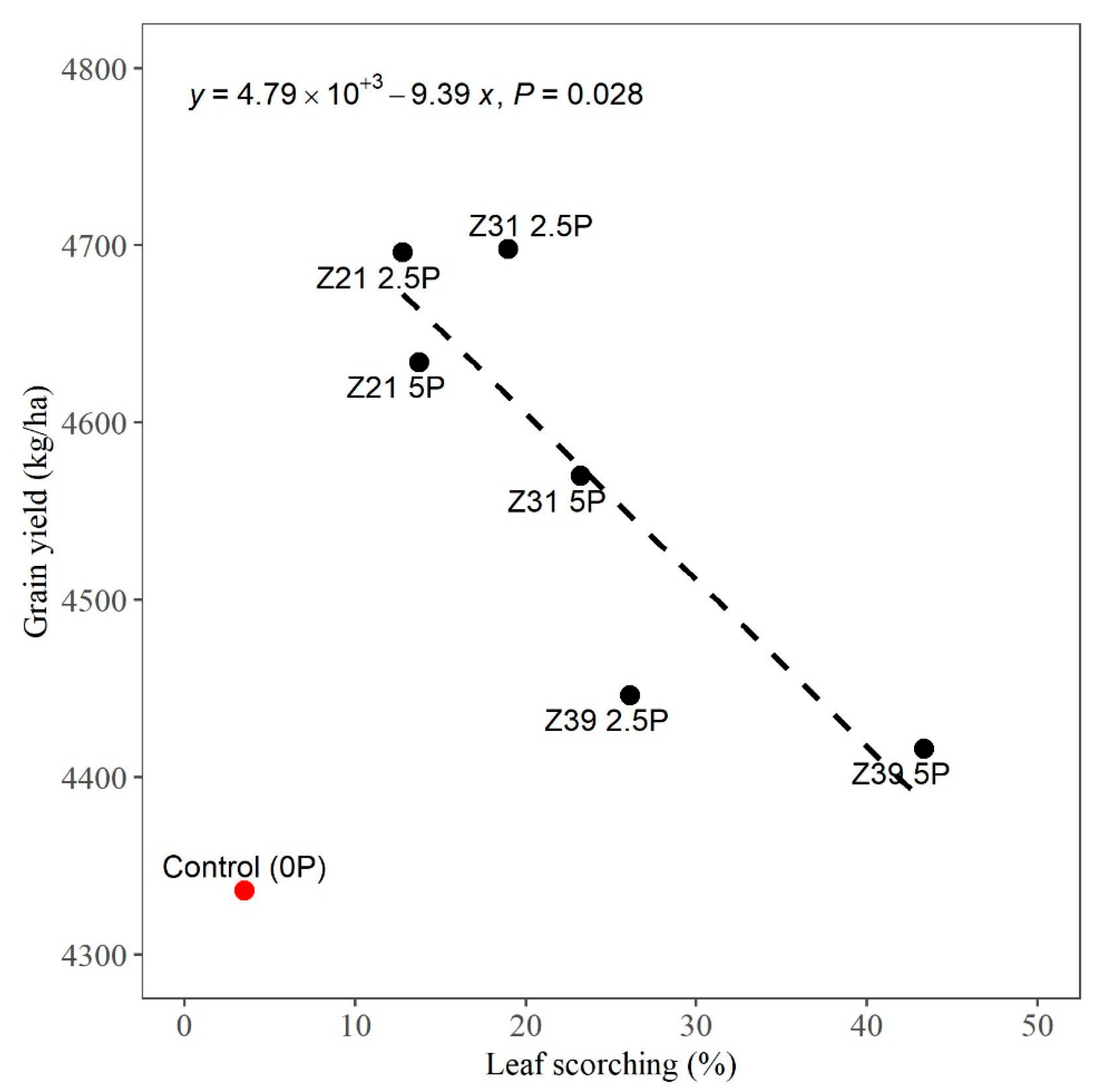
3.3. Relationship between Grain Yield and Leaf Scorching
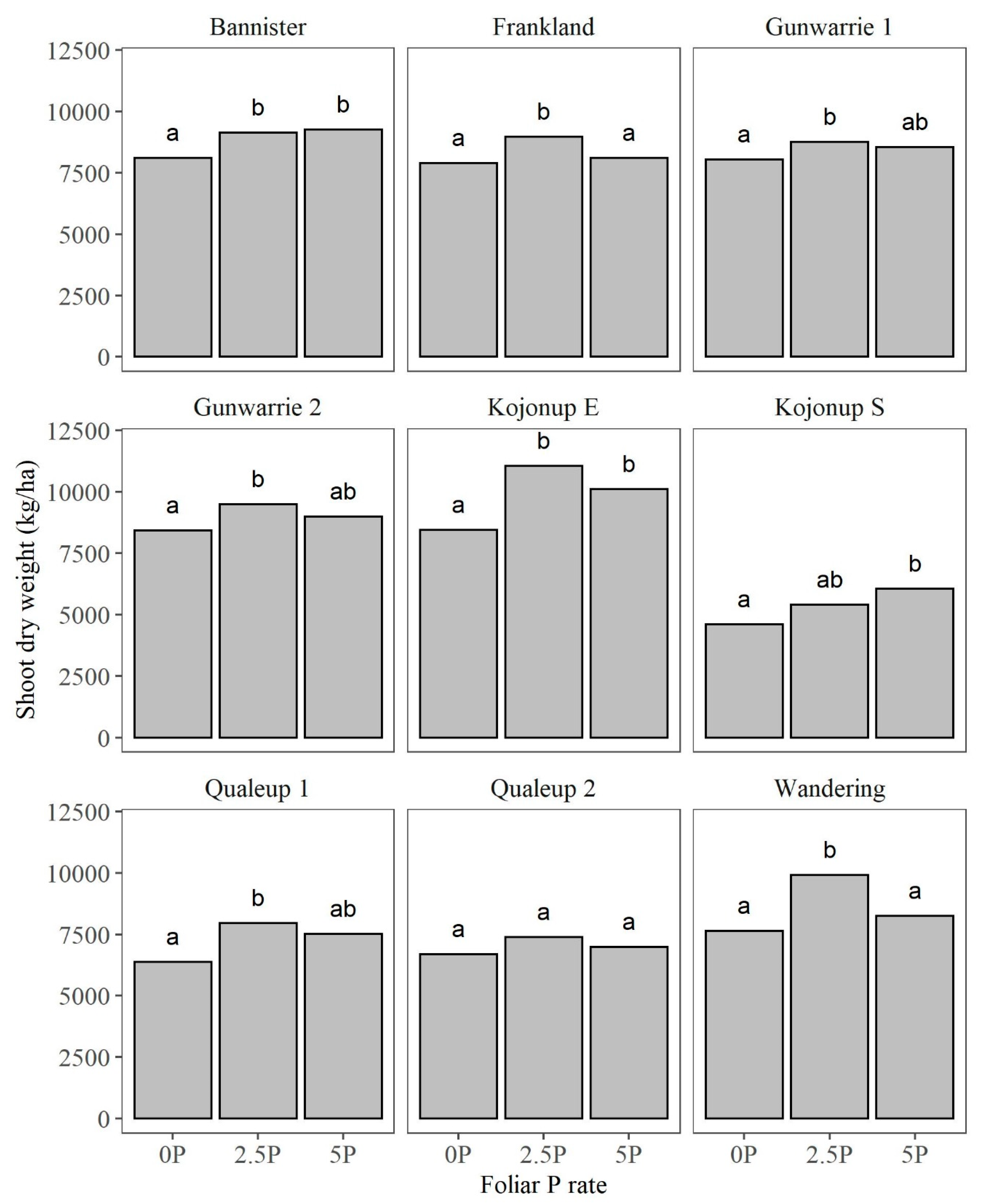
3.4. Shoot Dry Weight (SDW)
3.5. Shoot P Concentration and Uptake at Anthesis
| Experiment ID | Treatments | Shoot P (%) | Shoot P Uptake (kg/ha) | Grain P (%) | Grain P Uptake (kg/ha) |
|---|---|---|---|---|---|
| Bannister | Control | 0.12 | 9.84 | 0.28 | 12.13 |
| 2.5 P | 0.12 | 11.42 | 0.28 | 12.13 | |
| 5 P | 0.15 | 14.02 | 0.29 | 12.38 | |
| Mean | 0.13 | 11.76 | 0.29 | 12.21 | |
| LSD (p < 0.05) | 0.02 | 2.34 | 0.01 | 0.54 | |
| Z21 | 0.14 | 13.63 | 0.28 | 12.09 | |
| Z31 | 0.13 | 11.59 | 0.28 | 12.15 | |
| Z39 | 0.15 | 12.93 | 0.29 | 12.52 | |
| LSD (p < 0.05) | 0.03 | 3.17 | 0.01 | 0.55 | |
| Kojonup S | Control | 0.18 | 7.99 | 0.31 | 8.35 |
| 2.5 P | 0.15 | 8.14 | 0.31 | 8.23 | |
| 5 P | 0.17 | 9.65 | 0.30 | 8.21 | |
| Mean | 0.17 | 8.59 | 0.31 | 8.26 | |
| LSD (p < 0.05) | 0.04 | 1.77 | 0.03 | 0.70 | |
| Z21 | 0.14 | 9.35 | 0.30 | 8.20 | |
| Z31 | 0.15 | 9.05 | 0.30 | 8.17 | |
| Z39 | 0.19 | 8.28 | 0.31 | 8.30 | |
| LSD (p < 0.05) | 0.04 | 2.01 | 0.03 | 0.74 | |
| Gunwarrie 1 | Control | 0.12 | 9.28 | 0.24 | 9.50 |
| 2.5 P | 0.12 | 10.59 | 0.25 | 10.44 | |
| 5 P | 0.12 | 10.66 | 0.26 | 10.69 | |
| Mean | 0.12 | 10.18 | 0.25 | 10.21 | |
| LSD (p < 0.05) | 0.02 | 2.07 | 0.01 | 0.70 | |
| Z21 | 0.12 | 10.34 | 0.25 | 10.87 | |
| Z31 | 0.12 | 9.95 | 0.25 | 10.48 | |
| Z39 | 0.14 | 11.58 | 0.26 | 10.35 | |
| LSD (p < 0.05) | 0.04 | 2.01 | 0.03 | 0.74 | |
| Frankland | Control | 0.15 | 11.46 | 0.23 | 11.23 |
| 2.5 P | 0.15 | 13.55 | 0.25 | 13.43 | |
| 5 P | 0.18 | 14.34 | 0.25 | 13.15 | |
| Mean | 0.16 | 13.12 | 0.24 | 12.60 | |
| LSD (p < 0.05) | 0.03 | 2.47 | 0.01 | 0.98 | |
| Z21 | 0.15 | 13.62 | 0.24 | 12.88 | |
| Z31 | 0.17 | 14.45 | 0.25 | 13.63 | |
| Z39 | 0.17 | 13.76 | 0.25 | 13.35 | |
| LSD (p < 0.05) | 0.04 | 3.74 | 0.01 | 1.19 | |
| Gunwarrie 2 | Control | 0.11 | 9.07 | 0.22 | 10.79 |
| 2.5 P | 0.11 | 10.11 | 0.23 | 11.59 | |
| 5 P | 0.13 | 11.32 | 0.23 | 11.02 | |
| Mean | 0.11 | 10.17 | 0.22 | 11.13 | |
| LSD (p < 0.05) | 0.02 | 1.90 | 0.02 | 1.08 | |
| Z21 | 0.10 | 8.83 | 0.22 | 10.97 | |
| Z31 | 0.12 | 11.18 | 0.23 | 11.51 | |
| Z39 | 0.13 | 12.15 | 0.24 | 11.43 | |
| LSD (p < 0.05) | 0.02 | 2.36 | 0.02 | 1.61 | |
| Kojonup E | Control | 0.13 | 11.53 | 0.21 | 9.79 |
| 2.5 P | 0.13 | 14.26 | 0.23 | 11.70 | |
| 5 P | 0.16 | 15.49 | 0.24 | 11.85 | |
| Mean | 0.14 | 13.76 | 0.23 | 11.11 | |
| LSD (p < 0.05) | 0.03 | 4.21 | 0.01 | 1.29 | |
| Z21 | 0.14 | 15.30 | 0.22 | 11.11 | |
| Z31 | 0.13 | 13.00 | 0.24 | 12.40 | |
| Z39 | 0.16 | 16.30 | 0.24 | 11.81 | |
| LSD (p < 0.05) | 0.03 | 5.86 | 0.01 | 1.45 | |
| Qualeup 1 | Control | 0.13 | 8.06 | 0.16 | 8.03 |
| 2.5 P | 0.14 | 10.88 | 0.17 | 8.86 | |
| 5 P | 0.16 | 11.50 | 0.19 | 9.50 | |
| Mean | 0.14 | 10.15 | 0.18 | 8.80 | |
| LSD (p < 0.05) | 0.02 | 1.84 | 0.01 | 0.99 | |
| Z21 | 0.13 | 11.75 | 0.18 | 9.33 | |
| Z31 | 0.15 | 11.50 | 0.18 | 9.44 | |
| Z39 | 0.16 | 10.31 | 0.19 | 8.77 | |
| LSD (p < 0.05) | 0.03 | 2.82 | 0.02 | 1.37 | |
| Qualeup 2 | Control | 0.13 | 8.83 | 0.16 | 7.40 |
| 2.5 P | 0.17 | 12.47 | 0.18 | 8.56 | |
| 5 P | 0.18 | 12.79 | 0.18 | 8.84 | |
| Mean | 0.16 | 11.36 | 0.17 | 8.27 | |
| LSD (p < 0.05) | 0.02 | 2.44 | 0.01 | 0.49 | |
| Z21 | 0.17 | 13.73 | 0.18 | 8.85 | |
| Z31 | 0.17 | 11.95 | 0.17 | 8.59 | |
| Z39 | 0.19 | 12.21 | 0.19 | 8.67 | |
| LSD (p < 0.05) | 0.02 | 3.54 | 0.01 | 0.72 | |
| Wandering | Control | 0.14 | 10.61 | 0.15 | 7.37 |
| 2.5 P | 0.14 | 13.87 | 0.17 | 8.81 | |
| 5 P | 0.18 | 15.27 | 0.18 | 8.47 | |
| Mean | 0.15 | 13.25 | 0.17 | 8.22 | |
| LSD (p < 0.05) | 0.03 | 3.26 | 0.01 | 0.87 | |
| Z21 | 0.14 | 12.04 | 0.16 | 8.10 | |
| Z31 | 0.17 | 15.23 | 0.18 | 8.73 | |
| Z39 | 0.17 | 16.46 | 0.18 | 9.10 | |
| LSD (p < 0.05) | 0.03 | 4.13 | 0.02 | 1.28 |
3.6. Grain P Concentration and Uptake
3.7. P Efficiency Indices—Agronomic Efficiency (AE), P Use Efficiency (PUE) and Recovery Efficiency (RE)
| P Rates (kg/ha) | P Rates (kg/ha) | |||||||
|---|---|---|---|---|---|---|---|---|
| Experiment ID | 2.5 P | 5 P | Mean | LSD (p ≤ 0.05) 2.5 P vs. 5 P | 2.5 P | 5 P | Mean | LSD (p ≤ 0.05) 2.5 P vs. 5 P |
| AE | PUE | |||||||
| Bannister | 114 | 112 | 113 | NS | 0.00 | 0.05 | 0.03 | NS |
| Kojonup S | 56 | 50 | 53 | NS | −0.04 | −0.03 | −0.04 | NS |
| Gunwarrie 1 | 102 | 20 | 61 | 74 | 0.38 | 0.24 | 0.31 | NS |
| Frankland | 179 | 46 | 113 | 127 | 0.88 | 0.38 | 0.63 | 0.33 |
| Gunwarrie 2 | 25 | −6 | 9 | NS | 0.32 | 0.05 | 0.19 | NS |
| Kojonup E | 232 | 86 | 159 | 146 | 0.76 | 0.41 | 0.59 | NS |
| Qualeup 1 | 68 | 18 | 43 | NS | 0.33 | 0.29 | 0.31 | NS |
| Qualeup 2 | 103 | 61 | 82 | NS | 0.46 | 0.29 | 0.38 | 0.17 |
| Wandering | 121 | −20 | 51 | 95 | 0.14 | 0.01 | 0.08 | NS |
| Combined analysis | 111 | 41 | 76 | 36 | 0.36 | 0.19 | 0.27 | 0.10 |
3.8. Grain Quality Response to Foliar P
4. Discussion
4.1. Grain Yield Response to Foliar P in Relation to Soil PBI
4.2. Grain Yield in Relation to Foliar-Applied Foliar P Rates, Timing, and Leaf Scorching
4.3. P Efficiency Indices
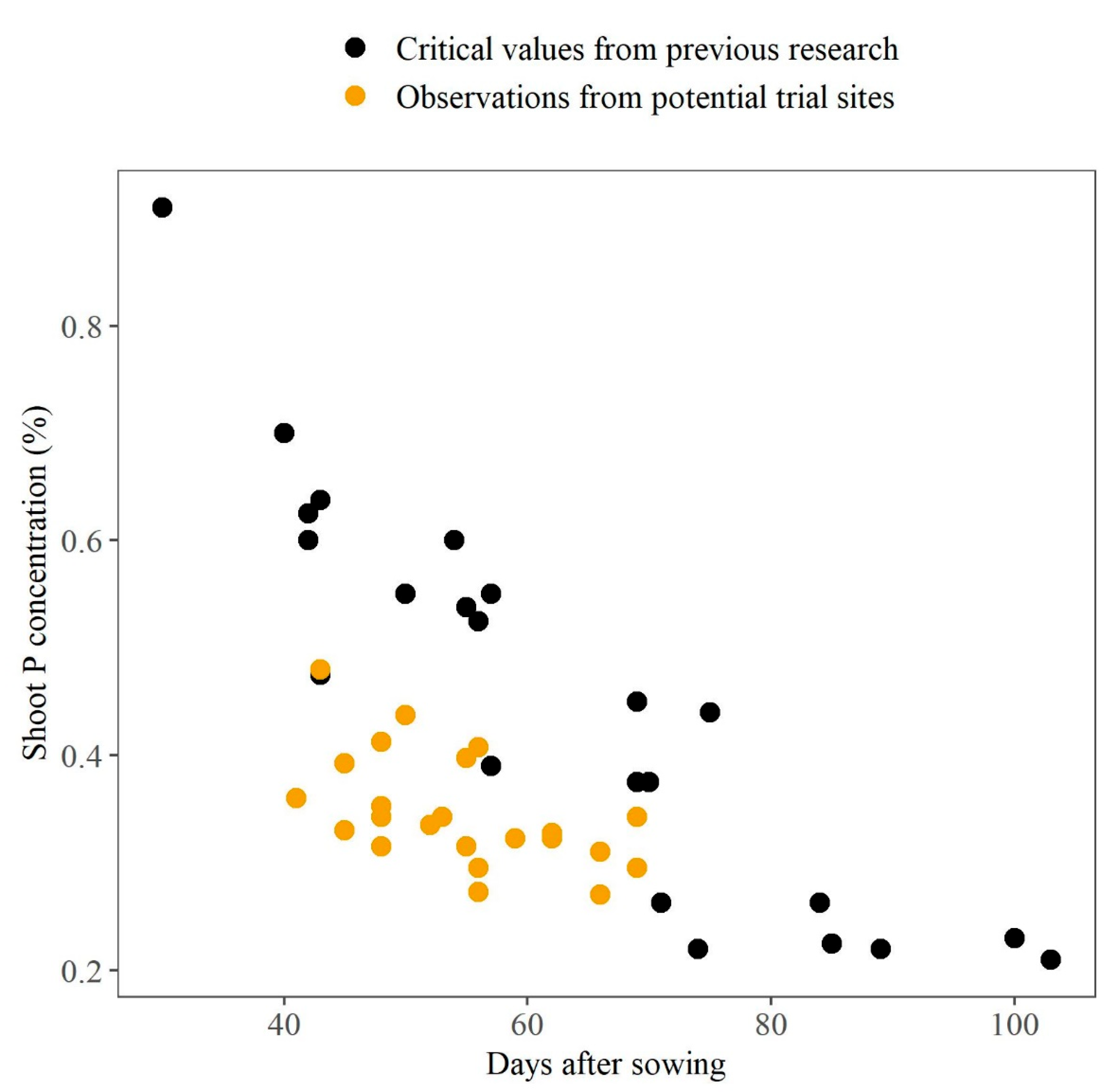
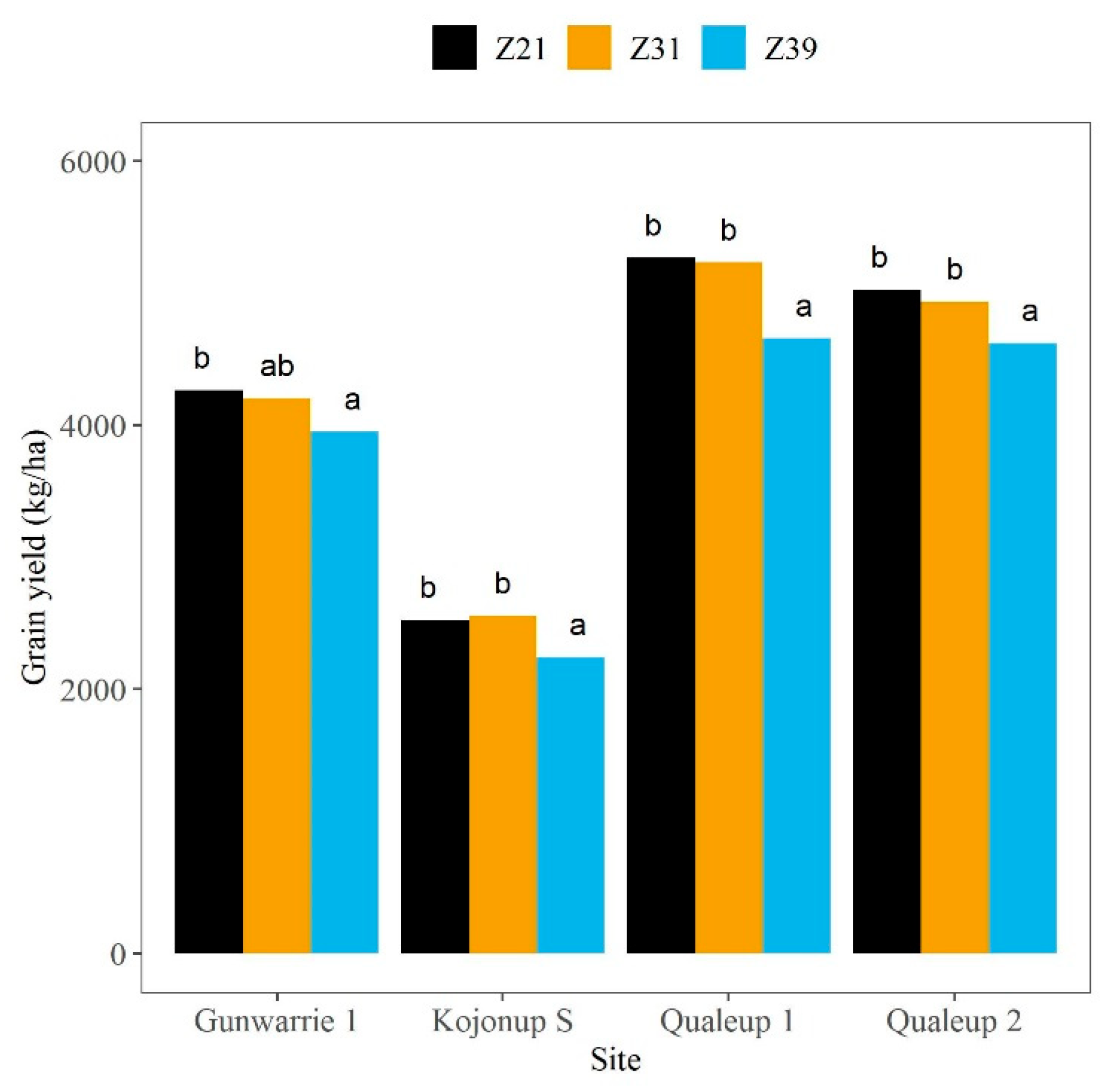
4.4. Value of Tissue Testing in Early Crop Growth
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nkebiwe, P.M.; Weinmann, M.; Bar-Tal, A.; Müller, T. Fertilizer placement to improve crop nutrient acquisition and yield: A review and meta-analysis. Field Crops Res. 2016, 196, 389–401. [Google Scholar] [CrossRef]
- Rodríguez, D.; Andrade, F.H.; Goudriaan, J. Effects of phosphorus nutrition on tiller emergence in wheat. Plant Soil. 1999, 209, 283–295. [Google Scholar] [CrossRef]
- Rodriguez, D.; Maria, G.S.; Pomar, M. Phosphorus deficiency affects the early development of wheat plants. J. Agron. Crop Sci. 1994, 173, 69–72. [Google Scholar] [CrossRef]
- Valle, S.R.; Calderini, D.F. Phyllochron and tillering of wheat in response to soil aluminum toxicity and phosphorus deficiency. Crop Pasture Sci. 2010, 61, 863–872. [Google Scholar] [CrossRef]
- Jacob, J.; Lawlor, D.W. Stomatal and mesophyll limitations of photosynthesis in phosphate deficient sunflower, maize and wheat Plants. J. Exppt. Bot. 1991, 42, 1003–1011. [Google Scholar] [CrossRef]
- Ali, M.S.; Sutradhar, A.; Edano, M.L.; Edwards, J.T.; Girma, K. Response of winter wheat grain yield and phosphorus uptake to foliar phosphite fertilization. Int. J. Agron. 2014, 2014, 801626. [Google Scholar] [CrossRef]
- Alston, A.M. Effects of soil water content and foliar fertilization with nitrogen and phosphorus in late season on the yield and composition of wheat. Aust. J. Agric. Res. 1979, 30, 577. [Google Scholar] [CrossRef]
- McBeath, T.M.; McLaughlin, M.J.; Noack, S.R. Wheat grain yield response to and translocation of foliar-applied phosphorus. Crop Pasture Sci. 2011, 62, 58–65. [Google Scholar] [CrossRef]
- McBeath, T.M.; Facelli, E.; Peirce, C.A.E.; Arachchige, V.K.; McLaughlin, M.J. Assessment of foliar-applied phosphorus fertiliser formulations to enhance phosphorus nutrition and grain production in wheat. Crop Pasture Sci. 2020, 71, 795–806. [Google Scholar] [CrossRef]
- Sherchand, K.; Paulsen, G.M. Response of wheat to foliar phosphorus treatments under field and high temperature regimes. J. Plant Nutr. 1985, 8, 1171–1181. [Google Scholar] [CrossRef]
- Torres, G.M. Foliar Phosphorus Fertilization and the Effect of Surfactants on Winter Wheat (Triticum aestivum L.). Master’s Thesis, Oklahoma State University, Stillwater, OK, USA, 2011. Available online: https://hdl.handle.net/11244/9374 (accessed on 6 May 2024).
- Harbi, S.F.A.; Ghoneim, A.M.; Modaihsh, A.S.; Mahjoub, M.O. Effect of foliar and soil application of phosphorus on phosphorus uptake, use efficiency and wheat grain yield in calcareous soil. J. Appl. Sci. 2013, 13, 188–192. [Google Scholar] [CrossRef]
- Mosali, J.; Desta, K.; Teal, R.K.; Freeman, K.W.; Martin, K.L.; Lawles, J.W.; Raun, W.R. Effect of foliar application of phosphorus on winter wheat grain yield, phosphorus uptake, and use efficiency. J. Plant Nutr. 2006, 29, 2147–2163. [Google Scholar] [CrossRef]
- Green, D.R.; Racz, G.J. The effect of foliar phosphate solution application on wheat yield and quality. In Proceedings of the Manitoba Soil Science Society Annual General Meeting, University of Manitoba, Winnipeg, Manitoba, 2 February 1999; pp. 90–96. [Google Scholar]
- Planfarm. Planfarm Benchmarks 2022; Planfarm Pty Ltd.; Bankwest Agribusiness Centre: Perth, WA, Australia, 2022; Available online: https://planfarm.com.au/resources/#benchmark (accessed on 8 May 2024).
- Mason, S.; McNeill, A.; McLaughlin, M.J.; Zhang, H. Prediction of wheat response to an application of phosphorus under field conditions using diffusive gradients in thin-films (DGT) and extraction methods. Plant Soil. 2010, 337, 243–258. [Google Scholar] [CrossRef]
- McQuaker, N.R.; Kluckner, P.D.; Chang, G.N. Calibration of an inductively coupled plasma-atomic emission spectrometer for the analysis of environmental materials. Anal. Chem. 1979, 51, 888–895. [Google Scholar] [CrossRef]
- Bolland, M.D.A.; Paynter, B.H. Critical phosphorus concentrations for burr medic, yellow serradella, subterranean clover, and wheat. Commun. Soil Sci. Plant Anal. 1994, 25, 385–394. [Google Scholar] [CrossRef]
- Elliott, D.E.; Reuter, D.J.; Reddy, G.D.; Abbott, R.J. Phosphorus nutrition of spring wheat (Triticum aestivum L.). 4. * Calibration of plant phosphorus test criteria from rain-fed field experiments. Aust. J. Agric. Res. 1997, 48, 899–912. [Google Scholar] [CrossRef]
- Rayment, G.E.; Lyons, D.J. Soil Chemical Methods—Australasia; CSIRO Publishing: Collingwood, Australia, 2011. [Google Scholar] [CrossRef]
- McDonald, R.C.; Isbell, R.F.; Speight, J.G.; Walker, J.; Hopkins, M.S. Department of Primary Industries and Energy and CSIRO Australia. In Australian Soil and Land Survey Field Handbook; CSIRO Publishing: Collingwood, Australia, 1998. [Google Scholar]
- Isbell, R.F. National Committee on Soil and Terrain. In The Australian Soil Classification; CSIRO Publishing: Collingwood, Australia, 2021; Available online: https://www.publish.csiro.au/ebook/download/pdf/8016 (accessed on 14 May 2024).
- Zadoks, J.C.; Chang, T.T.; Konzak, C.P. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Patrignani, A.; Ochsner, T.E. Canopeo: A powerful new tool for measuring fractional green canopy cover. Agron. J. 2015, 107, 2312–2320. [Google Scholar] [CrossRef]
- Moody, P.W. Interpretation of a single-point P buffering index for adjusting critical levels of the Colwell soil P test. Soil Res. 2007, 45, 55–62. [Google Scholar] [CrossRef]
- Mason, S. Soil Phosphorus Availability by DGT. 2024. Available online: https://www.soilquality.org.au/factsheets/dgt-phosphorus (accessed on 14 May 2024).
- Peverill, K.I.; Sparrow, L.A.; Reuter, D.J. Soil Analysis—An Interpretation Manual; CSIRO Publishing: Collingwood, Australia, 2001. [Google Scholar] [CrossRef]
- Bell, R.; Reuter, D.; Scott, B.; Sparrow, L.; Strong, W.; Chen, W. Soil phosphorus–crop response calibration relationships and criteria for winter cereal crops grown in Australia. Crop Pasture Sci. 2013, 64, 480–498. [Google Scholar] [CrossRef]
- Chien, S.H.; Sikora, F.J.; Gilkes, R.J.; McLaughlin, M.J. Comparing of the difference and balance methods to calculate percent recovery of fertilizer phosphorus applied to soils: A critical discussion. Nutr. Cycl. Agroecosyst. 2012, 92, 1–8. [Google Scholar] [CrossRef]
- Hedley, M.; McLaughlin, M.J. Reactions of phosphate fertilizers and by-products in soils. In Phosphorus: Agriculture and the Environment; Sims, G.T., Sharpley, A.N., Eds.; ASA; CSSA; SSSA: Madison, WI, USA, 2005; Volume 46, pp. 181–252. [Google Scholar] [CrossRef]
- McLaughlin, M.J.; McBeath, T.M.; Smernik, R.; Stacey, S.P.; Ajiboye, B.; Guppy, C. The chemical nature of P accumulation in agricultural soils—Implications for fertiliser management and design: An Australian perspective. Plant Soil. 2011, 349, 69–87. [Google Scholar] [CrossRef]
- Kirby, J.M.E.; Appleyard, M.; Fellowes, G. Leaf emergence and tillering in barley and wheat. Agronomie 1985, 5, 193–200. Available online: https://hal.science/hal-00884750 (accessed on 4 June 2024). [CrossRef]
- Grey, D. Crop Growth Stages Refresher. Available online: https://grdc.com.au/resources-and-publications/grdc-update-papers/tab-content/grdc-update-papers/2023/02/crop-growth-stages-refresher (accessed on 4 June 2024).
- Henningsen, J.N.; Görlach, B.M.; Quintero, J.M.; Garrido, R.R.; Mühling, K.H.; Fernández, V. Leaf wettability is the main driver for foliar P uptake in P-deficient maize. Plant Physiol. Biochem. 2023, 205, 108170. [Google Scholar] [CrossRef] [PubMed]
- Peirce, C.A.E.; McBeath, T.M.; Fernández, V.; McLaughlin, M.J. Wheat leaf properties affecting the absorption and subsequent translocation of foliar-applied phosphoric acid fertiliser. Plant Soil 2014, 384, 37–51. [Google Scholar] [CrossRef]
- Castro, S.A.Q.d.; Kichey, T.; Persson, D.P.; Schjoerring, J.K. Leaf Scorching following Foliar Fertilization of Wheat with Urea or Urea–Ammonium Nitrate Is Caused by Ammonium Toxicity. Agronomy 2022, 12, 1405. [Google Scholar] [CrossRef]
- Zabkiewicz. Adjuvants and herbicidal efficacy—Present status and future prospects. Weed Res. 2000, 40, 139–149. [Google Scholar] [CrossRef]
- Henningsen, J.N.; Görlach, B.M.; Fernández, V.; Dölger, J.L.; Buhk, A.; Mühling, K.H. Foliar P application cannot fully restore photosynthetic capacity, P nutrient status, and growth of P deficient maize (Zea mays L.). Plants 2022, 11, 2986. [Google Scholar] [CrossRef]
- Peirce, C.A.; McBeath, T.M.; Priest, C.; McLaughlin, M.J. The timing of application and inclusion of a surfactant are important for absorption and translocation of foliar phosphoric acid by wheat leaves. Front. Plant Sci. 2019, 10, 1532. [Google Scholar] [CrossRef]
- Haloi, B. Effect of foliar application of phosphorus salt on yellowing of wheat seedlings. J. Res. Assam Agric. Uni. 1980, 1, 108–109. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/19840765920 (accessed on 14 May 2024).
- Lopes, M.S.; Reynolds, M.P. Stay-green in spring wheat can be determined by spectral reflectance measurements (normalized difference vegetation index) independently from phenology. J. Exp. Bot. 2012, 63, 3789–3798. [Google Scholar] [CrossRef] [PubMed]
- Chuan, L.; He, P.; Pampolino, M.F.; Johnston, A.M.; Jin, J.; Xu, X.; Zhao, S.; Qiu, S.; Zhou, W. Establishing a scientific basis for fertilizer recommendations for wheat in China: Yield response and agronomic efficiency. Field Crops Res. 2013, 140, 1–8. [Google Scholar] [CrossRef]
- Holford, I.C.R.; Doyle, A.S. The recovery of fertilizer phosphorus by wheat, its agronomic efficiency, and their relationship to soil phosphorus. Aust. J. Agric. Res. 1993, 44, 1745–1756. [Google Scholar] [CrossRef]
- Limon-Ortega, A.; Baez-Perez, A. Maize yield and grain quality response to foliar-applied phosphorus in a soil testing high in P. Span. J. Agric. Res. 2024, 22, 1–11. [Google Scholar] [CrossRef]
- Yu, X.; Keitel, C.; Dijkstra, F.A. Global analysis of phosphorus fertilizer use efficiency in cereal crops. Glob. Food Secur. 2021, 29, 100545. [Google Scholar] [CrossRef]
- McLaughlin, M.J.; Alston, A.M.; Martin, J.K. Phosphorus cycling in wheat pasture rotations. I. The source of phosphorus taken up by wheat. Soil Res. 1988, 26, 323–331. [Google Scholar] [CrossRef]
- McLaughlin, M.J.; Alston, A.M. The relative contribution of plant residues and fertilizer to the phosphorus nutrition of wheat in a pasture cereal system. Soil Res. 1986, 24, 517–526. [Google Scholar] [CrossRef]
- Harries, M.; Flower, K.C.; Scanlan, C.A. Sustainability of nutrient management in grain production systems of south-west Australia. Crop Pasture Sci. 2021, 72, 197–212. [Google Scholar] [CrossRef]
- Malik, R. (DPIRD Katanning, W., Australia); Scanlan, C.A. (DPIRD Northam, W., Australia). Nutrient status of wheat and canola plants at early growth stages—A plant analysis survey in acid gravelly soils of south-west Western Australia. Personal communication. 2023. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malik, R.; Scanlan, C.; van Burgel, A.; Singh, B. Wheat Response to Foliar-Applied Phosphorus Is Determined by Soil Phosphorus Buffering. Agronomy 2024, 14, 1630. https://doi.org/10.3390/agronomy14081630
Malik R, Scanlan C, van Burgel A, Singh B. Wheat Response to Foliar-Applied Phosphorus Is Determined by Soil Phosphorus Buffering. Agronomy. 2024; 14(8):1630. https://doi.org/10.3390/agronomy14081630
Chicago/Turabian StyleMalik, Raj, Craig Scanlan, Andrew van Burgel, and Balwinder Singh. 2024. "Wheat Response to Foliar-Applied Phosphorus Is Determined by Soil Phosphorus Buffering" Agronomy 14, no. 8: 1630. https://doi.org/10.3390/agronomy14081630






