Fertilizers Based on Nanoparticles as Sources of Macro- and Microelements for Plant Crop Growth: A Review
Abstract
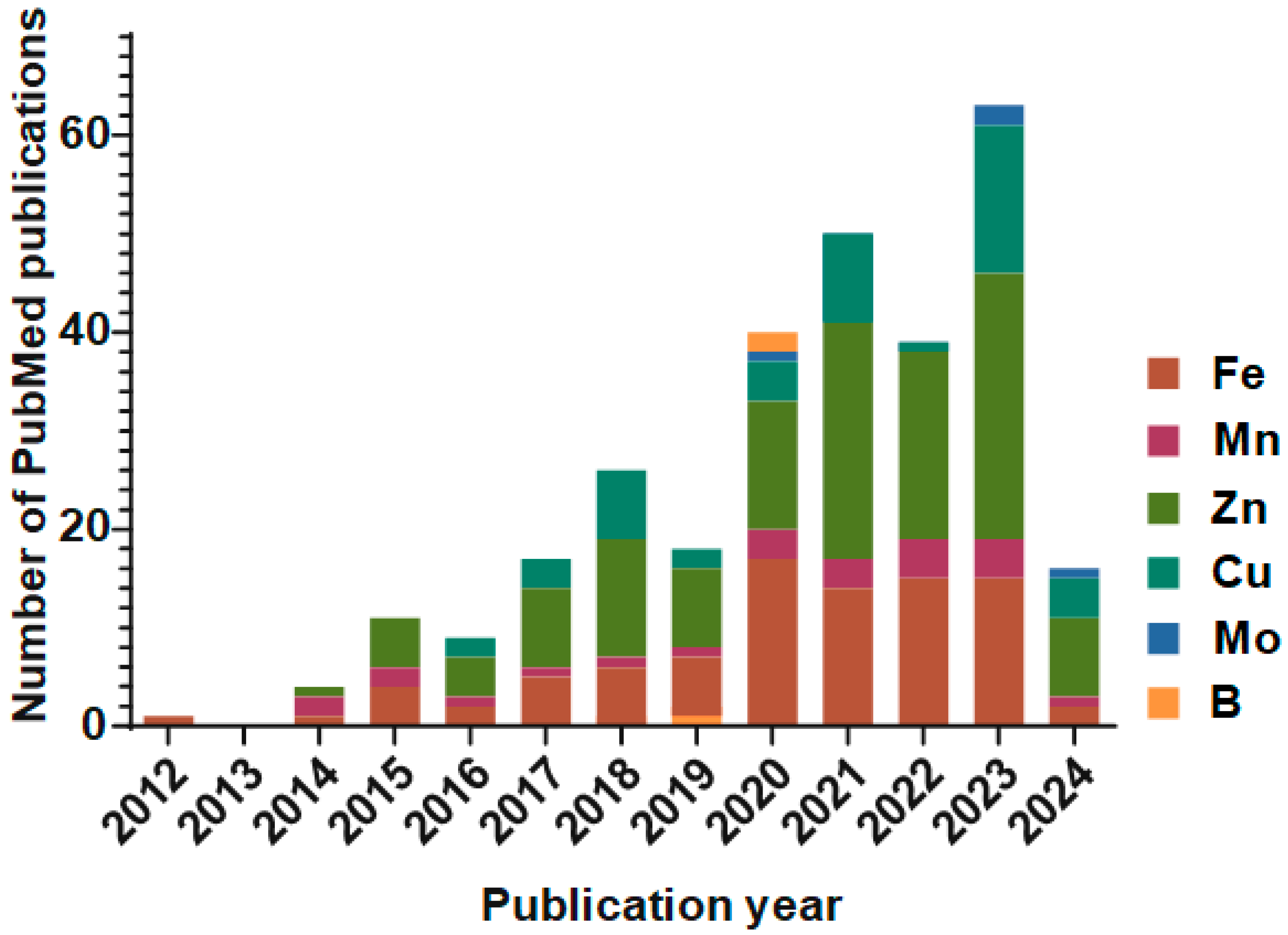
1. Introduction
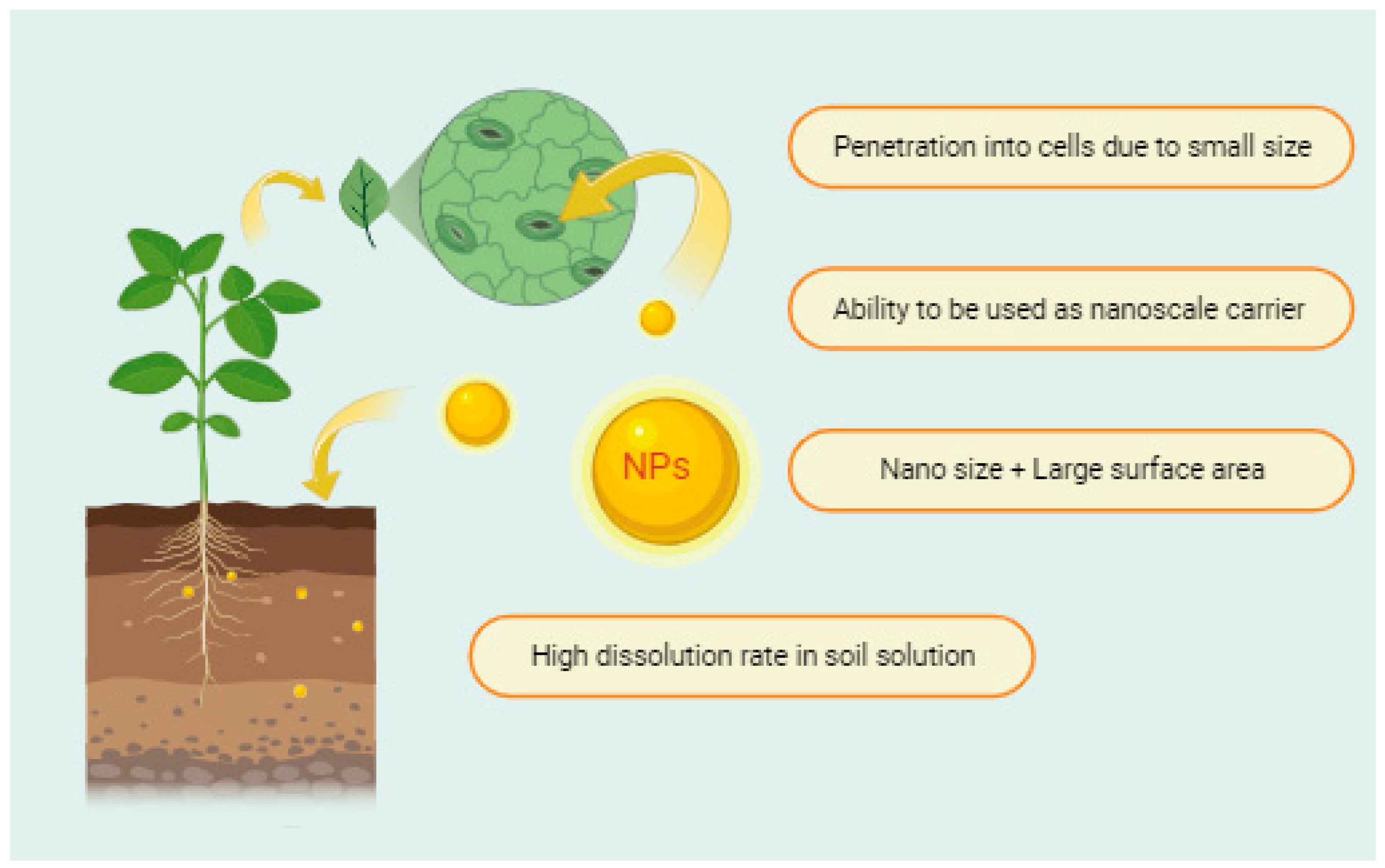
2. Nanoparticles in Agriculture
3. Nanofertilizers
3.1. Nanofertilizers: Sources of Macronutrients
3.1.1. NFs: Nitrogen Sources
3.1.2. NFs: Phosphorus Sources
3.1.3. NFs: Potassium Sources
3.1.4. NFs: Calcium Sources
3.1.5. NFs: Magnesium Sources
3.1.6. NFs: Sulfur Sources
3.2. Nanofertilizers: Sources of Micronutrients
3.2.1. NFs: Iron Sources
3.2.2. NFs: Manganese Sources
3.2.3. NFs: Zinc Sources
3.2.4. NFs: Copper Sources
3.2.5. NFs: Molybdenum Sources
3.2.6. NFs: Boron Sources
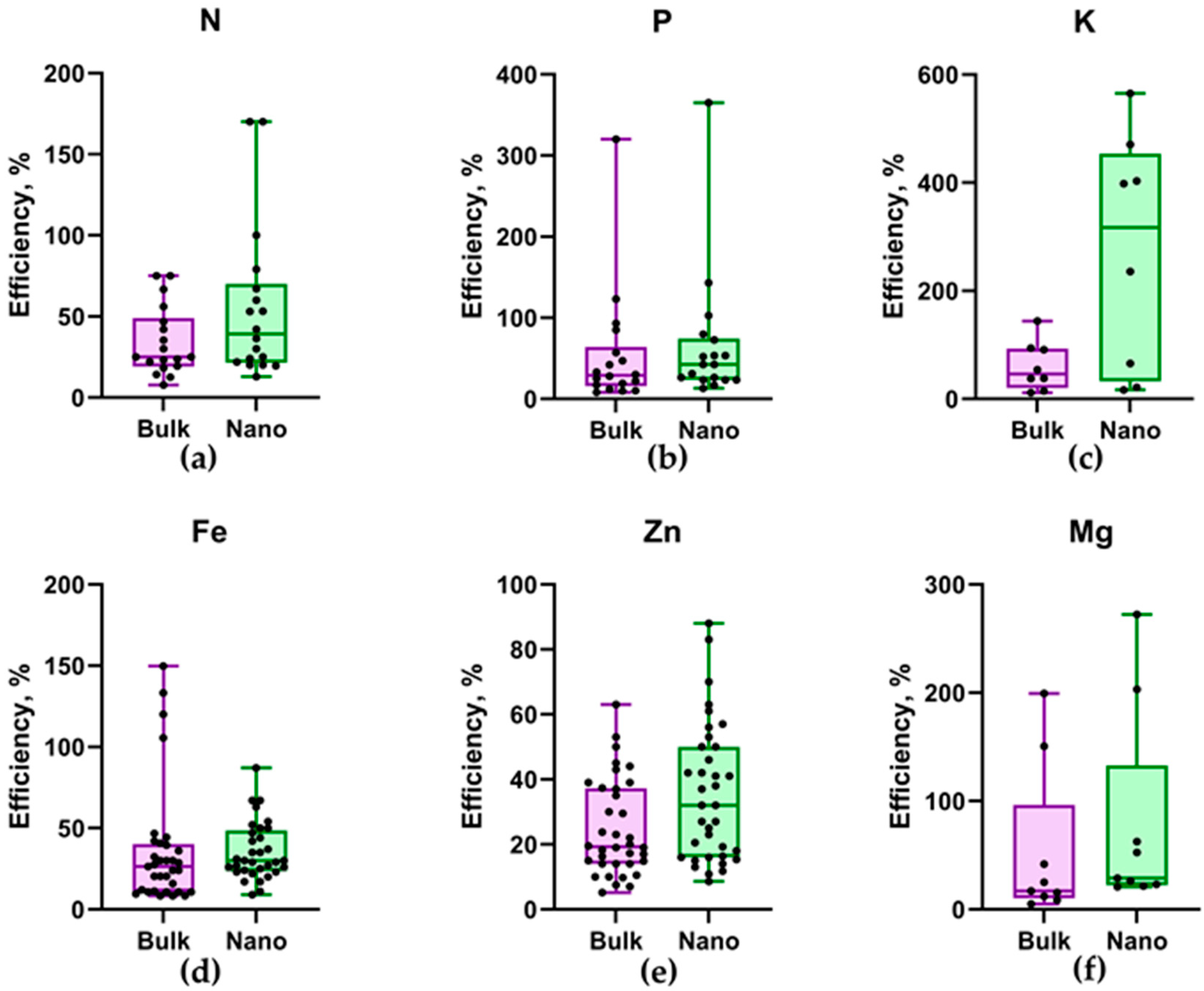
3.3. Prospects for Further Research and the Application of Nanofertilizers in Agriculture
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gupta, G.S. Land Degradation and Challenges of Food Security. Rev. Eur. Stud. 2019, 11, 63. [Google Scholar] [CrossRef]
- Gu, D.; Andreev, K.; Dupre, M.E. Major Trends in Population Growth Around the World. China CDC Wkly. 2021, 3, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Global Nanotechnology Market by Type (Nano Device, Nano Materials, Nano Sensors), End-User (Aerospace & Defense, Agriculture, Automotive & Transportation)—Forecast 2024–2030. Available online: https://www.researchandmarkets.com/report/nanotechnology#tag-pos-10 (accessed on 3 July 2024).
- Alexandratos, N. World food and agriculture to 2030/2050: Highlights and views from mid-2009. In Proceedings of the Proc. FAO Expert Meeting on How to Feed the World in 2050, Rome, Italy, 24–26 June 2009. [Google Scholar]
- Ram, P.; Vivek, K.; Kumar, S.P. Nanotechnology in sustainable agriculture: Present concerns and future aspects. Afr. J. Biotechnol. 2014, 13, 705–713. [Google Scholar] [CrossRef]
- Ditta, A.; Arshad, M.; Ibrahim, M. Nanoparticles in sustainable agricultural crop production: Applications and perspectives. In Nanotechnology and Plant Sciences: Nanoparticles and Their Impact on Plants; Springer: Cham, Switzerland, 2015; pp. 55–75. [Google Scholar]
- Tarafdar, J.C.; Sharma, S.; Raliya, R. Nanotechnology: Interdisciplinary science of applications. Afr. J. Biotechnol. 2013, 12, 219–226. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Sarimov, R.M.; Astashev, M.E.; Pishchalnikov, R.Y.; Yanykin, D.V.; Simakin, A.V.; Shkirin, A.V.; Serov, D.A.; Konchekov, E.M.; Gusein-zade, N.G.; et al. Modern physical methods and technologies in agriculture. Physics-Uspekhi 2024, 67, 194–210. [Google Scholar] [CrossRef]
- Verma, K.K.; Song, X.-P.; Joshi, A.; Tian, D.-D.; Rajput, V.D.; Singh, M.; Arora, J.; Minkina, T.; Li, Y.-R. Recent Trends in Nano-Fertilizers for Sustainable Agriculture under Climate Change for Global Food Security. Nanomaterials 2022, 12, 173. [Google Scholar] [CrossRef]
- Perfileva, A.I.; Kharasova, A.R.; Nozhkina, O.A.; Sidorov, A.V.; Graskova, I.A.; Krutovsky, K.V. Effect of Nanopriming with Selenium Nanocomposites on Potato Productivity in a Field Experiment, Soybean Germination and Viability of Pectobacterium carotovorum. Horticulturae 2023, 9, 458. [Google Scholar] [CrossRef]
- Khutsishvili, S.S.; Perfileva, A.I.; Kon’kova, T.V.; Lobanova, N.A.; Sadykov, E.K.; Sukhov, B.G. Copper-Containing Bionanocomposites Based on Natural Raw Arabinogalactan as Effective Vegetation Stimulators and Agents against Phytopathogens. Polymers 2024, 16, 716. [Google Scholar] [CrossRef] [PubMed]
- Shafeev, G.A.; Barmina, E.V.; Pimpha, N.; Rakov, I.I.; Simakin, A.V.; Sharapov, M.G.; Uvarov, O.V.; Gudkov, S.V. Laser generation and fragmentation of selenium nanoparticles in water and their testing as an additive to fertilisers. Quantum Electron. 2021, 51, 615–618. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Shafeev, G.A.; Glinushkin, A.P.; Shkirin, A.V.; Barmina, E.V.; Rakov, I.I.; Simakin, A.V.; Kislov, A.V.; Astashev, M.E.; Vodeneev, V.A.; et al. Production and Use of Selenium Nanoparticles as Fertilizers. ACS Omega 2020, 5, 17767–17774. [Google Scholar] [CrossRef]
- Xin, X.; Judy, J.D.; Sumerlin, B.B.; He, Z. Nano-enabled agriculture: From nanoparticles to smart nanodelivery systems. Environ. Chem. 2020, 17, 413–425. [Google Scholar] [CrossRef]
- Bashir, I.; Lone, F.A.; Bhat, R.A.; Mir, S.A.; Dar, Z.A.; Dar, S.A. Concerns and Threats of Contamination on Aquatic Ecosystems. In Bioremediation and Biotechnology; Springer: Cham, Switzerland, 2020; pp. 1–26. [Google Scholar]
- Dubrovsky, N.; Hamilton, P. The Quality of Our Nation’s Water: Nutrients in the Nation’s Streams and Groundwater; U.S. Geological Survey: Reston, VA, USA, 2010.
- Raliya, R.; Saharan, V.; Dimkpa, C.; Biswas, P. Nanofertilizer for Precision and Sustainable Agriculture: Current State and Future Perspectives. J. Agric. Food Chem. 2017, 66, 6487–6503. [Google Scholar] [CrossRef] [PubMed]
- Aziz, T.; Rahmatullah, M.A.; Maqsood, M.A.; Tahir, I.A.; Cheema, M.A. Phosphorus utilization by six Brassica cultivars (Brassica juncea L.) from tri-calcium phosphate; a relatively insoluble P compound. Pak. J. Bot. 2006, 38, 1529–1538. [Google Scholar]
- Morales-Díaz, A.B.; Ortega-Ortíz, H.; Juárez-Maldonado, A.; Cadenas-Pliego, G.; González-Morales, S.; Benavides-Mendoza, A. Application of nanoelements in plant nutrition and its impact in ecosystems. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 8, e013001. [Google Scholar] [CrossRef]
- Andrews, D.; Nann, T.; Lipson, R.H. Comprehensive Nanoscience and Nanotechnology; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Chhipa, H. Nanofertilizers and nanopesticides for agriculture. Environ. Chem. Lett. 2016, 15, 15–22. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; Bindraban, P.S. Nanofertilizers: New Products for the Industry? J. Agric. Food Chem. 2017, 66, 6462–6473, Erratum in J. Agric. Food Chem. 2018, 66, 9158–9158. [Google Scholar] [CrossRef]
- Mohd Noor, N.; Elgharbawy, A.A.M. Fate of nanofertilizer in agroecosystem. In Nanotoxicology for Agricultural and Environmental Applications; Academic Press: Cambridge, MA, USA, 2024; pp. 281–295. [Google Scholar]
- Biju, V.; Itoh, T.; Anas, A.; Sujith, A.; Ishikawa, M. Semiconductor quantum dots and metal nanoparticles: Syntheses, optical properties, and biological applications. Anal. Bioanal. Chem. 2008, 391, 2469–2495. [Google Scholar] [CrossRef]
- Feil, S.B.; Rodegher, G.; Gaiotti, F.; Alzate Zuluaga, M.Y.; Carmona, F.J.; Masciocchi, N.; Cesco, S.; Pii, Y. Physiological and Molecular Investigation of Urea Uptake Dynamics in Cucumis sativus L. Plants Fertilized with Urea-Doped Amorphous Calcium Phosphate Nanoparticles. Front. Plant Sci. 2021, 12, 745581. [Google Scholar] [CrossRef]
- Sharma, B.; Afonso, L.O.B.; Singh, M.P.; Soni, U.; Cahill, D.M. Zinc- and magnesium-doped hydroxyapatite-urea nanohybrids enhance wheat growth and nitrogen uptake. Sci. Rep. 2022, 12, 19506. [Google Scholar] [CrossRef]
- Tombuloglu, H.; Slimani, Y.; Tombuloglu, G.; Almessiere, M.; Sozeri, H.; Demir-Korkmaz, A.; AlShammari, T.M.; Baykal, A.; Ercan, I.; Hakeem, K.R. Impact of calcium and magnesium substituted strontium nano-hexaferrite on mineral uptake, magnetic character, and physiology of barley (Hordeum vulgare L.). Ecotoxicol. Environ. Saf. 2019, 186, 109751. [Google Scholar] [CrossRef]
- Dontsova, T.A.; Nahirniak, S.V.; Astrelin, I.M. Metaloxide nanomaterials and nanocomposites of ecological purpose. J. Nanomater. 2019, 2019, 5942194. [Google Scholar] [CrossRef]
- Li, J.; Chang, P.R.; Huang, J.; Wang, Y.; Yuan, H.; Ren, H. Physiological Effects of Magnetic Iron Oxide Nanoparticles Towards Watermelon. J. Nanosci. Nanotechnol. 2013, 13, 5561–5567. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Kreslavski, V.D.; Shmarev, A.N.; Ivanov, A.A.; Zharmukhamedov, S.K.; Kosobryukhov, A.; Yu, M.; Allakhverdiev, S.I.; Shabala, S. Effects of Iron Oxide Nanoparticles (Fe3O4) on Growth, Photosynthesis, Antioxidant Activity and Distribution of Mineral Elements in Wheat (Triticum aestivum) Plants. Plants 2022, 11, 1894. [Google Scholar] [CrossRef]
- Shebl, A.; Hassan, A.A.; Salama, D.M.; Abd El-Aziz, M.E.; Abd Elwahed, M.S.A. Template-free microwave-assisted hydrothermal synthesis of manganese zinc ferrite as a nanofertilizer for squash plant (Cucurbita pepo L.). Heliyon 2020, 6, e03596. [Google Scholar] [CrossRef]
- Saad, A.M.; Alabdali, A.Y.M.; Ebaid, M.; Salama, E.; El-Saadony, M.T.; Selim, S.; Safhi, F.A.; Alshamrani, S.M.; Abdalla, H.; Mahdi, A.H.A.; et al. Impact of Green Chitosan Nanoparticles Fabricated from Shrimp Processing Waste as a Source of Nano Nitrogen Fertilizers on the Yield Quantity and Quality of Wheat (Triticum aestivum L.) Cultivars. Molecules 2022, 27, 5640. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Rodríguez, G.B.; Miguel-Rojas, C.; Montanha, G.S.; Carmona, F.J.; Dal Sasso, G.; Sillero, J.C.; Skov Pedersen, J.; Masciocchi, N.; Guagliardi, A.; Pérez-de-Luque, A.; et al. Reducing Nitrogen Dosage in Triticum durum Plants with Urea-Doped Nanofertilizers. Nanomaterials 2020, 10, 1043. [Google Scholar] [CrossRef] [PubMed]
- El-Ganainy, S.M.; El-Bakery, A.M.; Hafez, H.M.; Ismail, A.M.; El-Abdeen, A.Z.; Ata, A.A.E.; Elraheem, O.A.Y.A.; El Kady, Y.M.Y.; Hamouda, A.F.; El-Beltagi, H.S.; et al. Humic Acid-Coated Fe3O4 Nanoparticles Confer Resistance to Acremonium Wilt Disease and Improve Physiological and Morphological Attributes of Grain Sorghum. Polymers 2022, 14, 3099. [Google Scholar] [CrossRef] [PubMed]
- Elmer, W.H.; White, J.C. The use of metallic oxide nanoparticles to enhance growth of tomatoes and eggplants in disease infested soil or soilless medium. Environ. Sci. Nano 2016, 3, 1072–1079. [Google Scholar] [CrossRef]
- Tan, J.; Zhao, S.; Chen, J.; Pan, X.; Li, C.; Liu, Y.; Wu, C.; Li, W.; Zheng, M. Preparation of nitrogen-doped carbon dots and their enhancement on lettuce yield and quality. J. Mater. Chem. B 2023, 11, 3113–3123. [Google Scholar] [CrossRef]
- Liu, R.; Lal, R. Synthetic apatite nanoparticles as a phosphorus fertilizer for soybean (Glycine max). Sci. Rep. 2014, 4, 5686. [Google Scholar] [CrossRef]
- Salama, D.M.; Osman, S.A.; Shaaban, E.A.; Abd Elwahed, M.; Abd El-Aziz, M.E. Effect of foliar application of phosphorus nanoparticles on the performance and sustainable agriculture of sweet corn. Plant Physiol. Biochem. 2023, 203, 108058. [Google Scholar] [CrossRef] [PubMed]
- Abdelghany, A.M.; El-Banna, A.A.A.; Salama, E.A.A.; Ali, M.M.; Al-Huqail, A.A.; Ali, H.M.; Paszt, L.S.; El-Sorady, G.A.; Lamlom, S.F. The Individual and Combined Effect of Nanoparticles and Biofertilizers on Growth, Yield, and Biochemical Attributes of Peanuts (Arachis hypogea L.). Agronomy 2022, 12, 398. [Google Scholar] [CrossRef]
- Subramanian, K.S.; Rajeswari, R.; Yuvaraj, M.; Pradeep, D.; Guna, M.; Yoganathan, G. Synthesis and Characterization of Nano-Sulfur and Its Impact on Growth, Yield, and Quality of Sunflower (Helianthus annuus L.). Commun. Soil Sci. Plant Anal. 2022, 53, 2700–2709. [Google Scholar] [CrossRef]
- Kottegoda, N.; Munaweera, I.; Madusanka, N.; Karunaratne, V. A green slow-release fertilizer composition based on urea-modified hydroxyapatite nanoparticles encapsulated wood. Curr. Sci. 2011, 101, 73–78. [Google Scholar]
- Saleem, I.; Maqsood, M.A.; Rehman, M.Z.u.; Aziz, T.; Bhatti, I.A.; Ali, S. Potassium ferrite nanoparticles on DAP to formulate slow release fertilizer with auxiliary nutrients. Ecotoxicol. Environ. Saf. 2021, 215, 112148. [Google Scholar] [CrossRef]
- Ramírez-Rodríguez, G.B.; Dal Sasso, G.; Carmona, F.J.; Miguel-Rojas, C.; Pérez-de-Luque, A.; Masciocchi, N.; Guagliardi, A.; Delgado-López, J.M. Engineering Biomimetic Calcium Phosphate Nanoparticles: A Green Synthesis of Slow-Release Multinutrient (NPK) Nanofertilizers. ACS Appl. Bio Mater. 2020, 3, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Álvarez, E.P.; Ramírez-Rodríguez, G.B.; Carmona, F.J.; Martínez-Vidaurre, J.M.; Masciocchi, N.; Guagliardi, A.; Garde-Cerdán, T.; Delgado-López, J.M. Towards a more sustainable viticulture: Foliar application of N-doped calcium phosphate nanoparticles on Tempranillo grapes. J. Sci. Food Agric. 2020, 101, 1307–1313. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Zhao, Y.; Lou, W.; Su, J.; Wei, S.; Yang, X.; Wang, R.; Guan, R.; Pu, H.; Shen, W. Nitrate reductase-dependent nitric oxide is crucial for multi-walled carbon nanotube-induced plant tolerance against salinity. Nanoscale 2019, 11, 10511–10523. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhu, L.; Zhao, F.; Li, J.; Zhang, X.; Kong, X.; Wu, H.; Zhang, Z. Plant salinity stress response and nano-enabled plant salt tolerance. Front. Plant Sci. 2022, 13, 843994. [Google Scholar] [CrossRef]
- Shoukat, A.; Saqib, Z.A.; Akhtar, J.; Aslam, Z.; Pitann, B.; Hossain, M.S.; Mühling, K.H. Zinc and Silicon Nano-Fertilizers Influence Ionomic and Metabolite Profiles in Maize to Overcome Salt Stress. Plants 2024, 13, 1224. [Google Scholar] [CrossRef]
- Rodríguez-Seijo, A.; Soares, C.; Ribeiro, S.; Amil, B.F.; Patinha, C.; Cachada, A.; Fidalgo, F.; Pereira, R. Nano-Fe2O3 as a tool to restore plant growth in contaminated soils–Assessment of potentially toxic elements (bio) availability and redox homeostasis in Hordeum vulgare L. J. Hazard. Mater. 2022, 425, 127999. [Google Scholar] [CrossRef]
- Umair Hassan, M.; Huang, G.; Haider, F.U.; Khan, T.A.; Noor, M.A.; Luo, F.; Zhou, Q.; Yang, B.; Ul Haq, M.I.; Iqbal, M.M. Application of Zinc Oxide Nanoparticles to Mitigate Cadmium Toxicity: Mechanisms and Future Prospects. Plants 2024, 13, 1706. [Google Scholar] [CrossRef] [PubMed]
- Raliya, R.; Tarafdar, J.C.; Mahawar, H.; Kumar, R.; Gupta, P.; Mathur, T.; Kaul, R.K.; Praveen, K.; Kalia, A.; Gautam, R.; et al. ZnO nanoparticles induced exopolysaccharide production by B. subtilis strain JCT1 for arid soil applications. Int. J. Biol. Macromol. 2014, 65, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, X.; Shi, Z.; Tong, R.; Adams, C.A.; Shi, X. Arbuscular mycorrhizae alleviate negative effects of zinc oxide nanoparticle and zinc accumulation in maize plants-A soil microcosm experiment. Chemosphere 2016, 147, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Achari, G.A.; Kowshik, M. Recent Developments on Nanotechnology in Agriculture: Plant Mineral Nutrition, Health, and Interactions with Soil Microflora. J. Agric. Food Chem. 2018, 66, 8647–8661. [Google Scholar] [CrossRef] [PubMed]
- Rhaman, M.S.; Tania, S.S.; Imran, S.; Rauf, F.; Kibria, M.G.; Ye, W.; Hasanuzzaman, M.; Murata, Y. Seed Priming with Nanoparticles: An Emerging Technique for Improving Plant Growth, Development, and Abiotic Stress Tolerance. J. Soil Sci. Plant Nutr. 2022, 22, 4047–4062. [Google Scholar] [CrossRef]
- Santás-Miguel, V.; Arias-Estévez, M.; Rodríguez-Seijo, A.; Arenas-Lago, D. Use of metal nanoparticles in agriculture. A review on the effects on plant germination. Environ. Pollut. 2023, 334, 122222. [Google Scholar] [CrossRef] [PubMed]
- Fatima, F.; Hashim, A.; Anees, S. Efficacy of nanoparticles as nanofertilizer production: A review. Environ. Sci. Pollut. Res. 2020, 28, 1292–1303. [Google Scholar] [CrossRef] [PubMed]
- Garza-García, J.J.O.; Hernández-Díaz, J.A.; Zamudio-Ojeda, A.; León-Morales, J.M.; Guerrero-Guzmán, A.; Sánchez-Chiprés, D.R.; López-Velázquez, J.C.; García-Morales, S. The Role of Selenium Nanoparticles in Agriculture and Food Technology. Biol. Trace Elem. Res. 2021, 200, 2528–2548. [Google Scholar] [CrossRef]
- Liu, R.; Lal, R. Potentials of engineered nanoparticles as fertilizers for increasing agronomic productions. Sci. Total Environ. 2015, 514, 131–139. [Google Scholar] [CrossRef]
- Kah, M.; Kookana, R.S.; Gogos, A.; Bucheli, T.D. A critical evaluation of nanopesticides and nanofertilizers against their conventional analogues. Nat. Nanotechnol. 2018, 13, 677–684. [Google Scholar] [CrossRef]
- Kopittke, P.M.; Lombi, E.; Wang, P.; Schjoerring, J.K.; Husted, S. Nanomaterials as fertilizers for improving plant mineral nutrition and environmental outcomes. Environ. Sci. Nano 2019, 6, 3513–3524. [Google Scholar] [CrossRef]
- Sonawane, H.; Shelke, D.; Chambhare, M.; Dixit, N.; Math, S.; Sen, S.; Borah, S.N.; Islam, N.F.; Joshi, S.J.; Yousaf, B.; et al. Fungi-derived agriculturally important nanoparticles and their application in crop stress management—Prospects and environmental risks. Environ. Res. 2022, 212, 113543. [Google Scholar] [CrossRef] [PubMed]
- Altammar, K.A. A review on nanoparticles: Characteristics, synthesis, applications, and challenges. Front. Microbiol. 2023, 14, 1155622. [Google Scholar] [CrossRef] [PubMed]
- Currall, S.C.; King, E.B.; Lane, N.; Madera, J.; Turner, S. What drives public acceptance of nanotechnology? Nat. Nanotechnol. 2006, 1, 153–155. [Google Scholar] [CrossRef]
- Maysinger, D. Nanoparticles and cells: Good companions and doomed partnerships. Org. Biomol. Chem. 2007, 5, 2335–2342. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.S. Nanotechnology in agriculture: Prospects and constraints. Nanotechnol. Sci. Appl. 2014, 7, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Grodetskaya, T.A.; Evlakov, P.M.; Fedorova, O.A.; Mikhin, V.I.; Zakharova, O.V.; Kolesnikov, E.A.; Evtushenko, N.A.; Gusev, A.A. Influence of Copper Oxide Nanoparticles on Gene Expression of Birch Clones In Vitro under Stress Caused by Phytopathogens. Nanomaterials 2022, 12, 864. [Google Scholar] [CrossRef]
- Babu, S.; Singh, R.; Yadav, D.; Rathore, S.S.; Raj, R.; Avasthe, R.; Yadav, S.K.; Das, A.; Yadav, V.; Yadav, B.; et al. Nanofertilizers for agricultural and environmental sustainability. Chemosphere 2022, 292, 133451. [Google Scholar] [CrossRef]
- Rajput, V.D.; Singh, A.; Minkina, T.M.; Shende, S.S.; Kumar, P.; Verma, K.K.; Bauer, T.; Gorobtsova, O.; Deneva, S.; Sindireva, A. Potential applications of nanobiotechnology in plant nutrition and protection for sustainable agriculture. In Nanotechnology in Plant Growth Promotion and Protection: Recent Advances and Impacts; Wiley: Hoboken, NJ, USA, 2021; pp. 79–92. [Google Scholar]
- Rajput, V.D.; Minkina, T.; Kumari, A.; Harish; Singh, V.K.; Verma, K.K.; Mandzhieva, S.; Sushkova, S.; Srivastava, S.; Keswani, C. Coping with the challenges of abiotic stress in plants: New dimensions in the field application of nanoparticles. Plants 2021, 10, 1221. [Google Scholar] [CrossRef]
- Verma, K.K.; Song, X.-P.; Degu, H.D.; Guo, D.-J.; Joshi, A.; Huang, H.-R.; Xu, L.; Singh, M.; Huang, D.-L.; Rajput, V.D.; et al. Recent advances in nitrogen and nano-nitrogen fertilizers for sustainable crop production: A mini-review. Chem. Biol. Technol. Agric. 2023, 10, 111. [Google Scholar] [CrossRef]
- Ditta, A.; Arshad, M. Applications and perspectives of using nanomaterials for sustainable plant nutrition. Nanotechnol. Rev. 2016, 5, 209–229. [Google Scholar] [CrossRef]
- Brackhage, C.; Schaller, J.; Bäucker, E.; Dudel, E.G. Silicon Availability Affects the Stoichiometry and Content of Calcium and Micro Nutrients in the Leaves of Common Reed. Silicon 2013, 5, 199–204. [Google Scholar] [CrossRef]
- DeRosa, M.C.; Monreal, C.; Schnitzer, M.; Walsh, R.; Sultan, Y. Nanotechnology in fertilizers. Nat. Nanotechnol. 2010, 5, 91. [Google Scholar] [CrossRef]
- Wu, M.-y. Effects of Incorporation of Nano-carbon into Slow-released Fertilizer on Rice Yield and Nitrogen Loss in Surface Water of Paddy Soil. Adv. J. Food Sci. Technol. 2013, 5, 398–403. [Google Scholar] [CrossRef]
- Prasad, R.; Jain, V.; Varma, A. Role of nanomaterials in symbiotic fungus growth enhancement. Curr. Sci. 2010, 99, 1189–1191. [Google Scholar]
- Corradini, E.; de Moura, M.R.; Mattoso, L.H.C. A preliminary study of the incorparation of NPK fertilizer into chitosan nanoparticles. Express Polym. Lett. 2010, 4, 509–515. [Google Scholar] [CrossRef]
- Mahiwal, S.; Pandey, G.K. Potassium: A vital nutrient mediating stress tolerance in plants. J. Plant Biochem. Biotechnol. 2022, 31, 705–719. [Google Scholar] [CrossRef]
- Hawkesford, M.J.; Cakmak, I.; Coskun, D.; De Kok, L.J.; Lambers, H.; Schjoerring, J.K.; White, P.J. Functions of macronutrients. In Marschner’s Mineral Nutrition of Plants; Academic Press: Cambridge, MA, USA, 2023; pp. 201–281. [Google Scholar]
- Abd El-Aziz, M.; Saber, E.; El-Khateeb, M. Preparation and characterization of CMC/HA-NPs/pulp nanocomposites for the removal of heavy metal ions. KGK-Kautsch. Gummi Kunststoffe 2019, 72, 36–41. [Google Scholar]
- Xiong, L.; Wang, P.; Hunter, M.N.; Kopittke, P.M. Bioavailability and movement of hydroxyapatite nanoparticles (HA-NPs) applied as a phosphorus fertiliser in soils. Environ. Sci. Nano 2018, 5, 2888–2898. [Google Scholar] [CrossRef]
- Xiong, L.; Wang, P.; Kopittke, P.M. Tailoring hydroxyapatite nanoparticles to increase their efficiency as phosphorus fertilisers in soils. Geoderma 2018, 323, 116–125. [Google Scholar] [CrossRef]
- Sheoran, P.; Goel, S.; Boora, R.; Kumari, S.; Yashveer, S.; Grewal, S. Biogenic synthesis of potassium nanoparticles and their evaluation as a growth promoter in wheat. Plant Gene 2021, 27, 100310. [Google Scholar] [CrossRef]
- Liu, X.-M.; Zhang, F.-D.; Zhang, S.-Q.; He, X.-S.; Wang, R.-F.; Feng, Z.-B.; Wang, Y.-J. Responses of peanut to nano-calcium carbonate. J. Plant Nutr. Fertil. 2005, 11, 385–389. [Google Scholar]
- Ranjbar, S.; Rahemi, M.; Ramezanian, A. Comparison of nano-calcium and calcium chloride spray on postharvest quality and cell wall enzymes activity in apple cv. Red Delicious. Sci. Hortic. 2018, 240, 57–64. [Google Scholar] [CrossRef]
- Delfani, M.; Baradarn Firouzabadi, M.; Farrokhi, N.; Makarian, H. Some Physiological Responses of Black-Eyed Pea to Iron and Magnesium Nanofertilizers. Commun. Soil Sci. Plant Anal. 2014, 45, 530–540. [Google Scholar] [CrossRef]
- Amaya-Olivas, N.I.; SÁNchez, E.; HernÁNdez-Ochoa, L.; Ojeda-Barrios, D.L.; ÁVila-Quezada, G.D.; Flores-CÓRdova, M.A.; ChÁVez-Flores, D.; Ayala-Soto, J.G.; Salcido-MartÍNez, A.; RamÍRez-Estrada, C.A. Biofortification with magnesium nanofertilizer on bioactive compounds and antioxidant capacity in green beans. Not. Bot. Hort. Agrobot. 2023, 51, 12830. [Google Scholar] [CrossRef]
- Kurmanbayeva, M.; Sekerova, T.; Tileubayeva, Z.; Kaiyrbekov, T.; Kusmangazinov, A.; Shapalov, S.; Madenova, A.; Burkitbayev, M.; Bachilova, N. Influence of new sulfur-containing fertilizers on performance of wheat yield. Saudi J. Biol. Sci. 2021, 28, 4644–4655. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, C.; Zhao, L.; Dimkpa, C.O.; Elmer, W.H.; Wang, B.; Sharma, S.; Wang, Z.; Dhankher, O.P.; Xing, B.; et al. Time-Dependent and Coating Modulation of Tomato Response upon Sulfur Nanoparticle Internalization and Assimilation: An Orthogonal Mechanistic Investigation. ACS Nano 2024, 18, 11813–11827. [Google Scholar] [CrossRef]
- Alexandratos, N.; Bruinsma, J. World Agriculture Towards 2030/2050: The 2012 Revision; FAO: Rome, Italy, 2012. [Google Scholar]
- Lassaletta, L.; Billen, G.; Grizzetti, B.; Anglade, J.; Garnier, J. 50 year trends in nitrogen use efficiency of world cropping systems: The relationship between yield and nitrogen input to cropland. Environ. Res. Lett. 2014, 9, 105011. [Google Scholar] [CrossRef]
- Davidson, E.A. The contribution of manure and fertilizer nitrogen to atmospheric nitrous oxide since 1860. Nat. Geosci. 2009, 2, 659–662. [Google Scholar] [CrossRef]
- Malekian, R.; Abedi-Koupai, J.; Eslamian, S.S. Influences of clinoptilolite and surfactant-modified clinoptilolite zeolite on nitrate leaching and plant growth. J. Hazard. Mater. 2011, 185, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Perrin, T.S.; Drost, D.T.; Boettinger, J.L.; Norton, J.M. Ammonium-loaded clinoptilolite: A slow-release nitrogen fertilizer for sweet corn. J. Plant Nutr. 1998, 21, 515–530. [Google Scholar] [CrossRef]
- Millán, G.; Agosto, F.; Vázquez, M.; Botto, L.; Lombardi, L.; Juan, L. Use of clinoptilolite as a carrier for nitrogen fertilizers in soils of the Pampean regions of Argentina. Cien. Inv. Agric. 2008, 35, 293–302. [Google Scholar]
- Ferrante, A.; Savin, R.; Slafer, G.A. Floret development and grain setting differences between modern durum wheats under contrasting nitrogen availability. J. Exp. Bot. 2013, 64, 169–184. [Google Scholar] [CrossRef] [PubMed]
- Carmona, F.J.; Dal Sasso, G.; Ramírez-Rodríguez, G.B.; Pii, Y.; Delgado-López, J.M.; Guagliardi, A.; Masciocchi, N. Urea-functionalized amorphous calcium phosphate nanofertilizers: Optimizing the synthetic strategy towards environmental sustainability and manufacturing costs. Sci. Rep. 2021, 11, 3419. [Google Scholar] [CrossRef] [PubMed]
- Marchiol, L.; Filippi, A.; Adamiano, A.; Degli Esposti, L.; Iafisco, M.; Mattiello, A.; Petrussa, E.; Braidot, E. Influence of Hydroxyapatite Nanoparticles on Germination and Plant Metabolism of Tomato (Solanum lycopersicum L.): Preliminary Evidence. Agronomy 2019, 9, 161. [Google Scholar] [CrossRef]
- Olad, A.; Zebhi, H.; Salari, D.; Mirmohseni, A.; Reyhani Tabar, A. Slow-release NPK fertilizer encapsulated by carboxymethyl cellulose-based nanocomposite with the function of water retention in soil. Mater. Sci. Eng. C 2018, 90, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Mejias, J.H.; Salazar, F.; Pérez Amaro, L.; Hube, S.; Rodriguez, M.; Alfaro, M. Nanofertilizers: A Cutting-Edge Approach to Increase Nitrogen Use Efficiency in Grasslands. Front. Environ. Sci. 2021, 9, 635114. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; Fugice, J.; Singh, U.; Lewis, T.D. Development of fertilizers for enhanced nitrogen use efficiency—Trends and perspectives. Sci. Total Environ. 2020, 731, 139113. [Google Scholar] [CrossRef]
- Ahmed, N.; Zhang, B.; Bozdar, B.; Chachar, S.; Rai, M.; Li, J.; Li, Y.; Hayat, F.; Chachar, Z.; Tu, P. The power of magnesium: Unlocking the potential for increased yield, quality, and stress tolerance of horticultural crops. Front. Plant Sci. 2023, 14, 1285512. [Google Scholar] [CrossRef]
- Aamir Iqbal, M. Nano-Fertilizers for Sustainable Crop Production under Changing Climate: A Global Perspective. In Sustainable Crop Production; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Hube, S.; Salazar, F.; Rodríguez, M.; Mejías, J.; Ramírez, L.; Alfaro, M. Dynamics of Nitrogen Gaseous Losses Following the Application of Foliar Nanoformulations to Grasslands. J. Soil Sci. Plant Nutr. 2022, 22, 1758–1767. [Google Scholar] [CrossRef]
- Roy, R.N.; Finck, A.; Blair, G.; Tandon, H. Plant nutrition for food security. A guide for integrated nutrient management. FAO Fertil. Plant Nutr. Bull. 2006, 16, 201–214. [Google Scholar]
- Cordell, D.; Drangert, J.-O.; White, S. The story of phosphorus: Global food security and food for thought. Glob. Environ. Change 2009, 19, 292–305. [Google Scholar] [CrossRef]
- Carmona, F.J.; Guagliardi, A.; Masciocchi, N. Nanosized Calcium Phosphates as Novel Macronutrient Nano-Fertilizers. Nanomaterials 2022, 12, 2709. [Google Scholar] [CrossRef] [PubMed]
- Tarafdar, J.; Raliya, R.; Rathore, I. Microbial synthesis of phosphorous nanoparticle from tri-calcium phosphate using Aspergillus tubingensis TFR-5. J. Bionanoscience 2012, 6, 84–89. [Google Scholar] [CrossRef]
- Kottegoda, N.; Sandaruwan, C.; Priyadarshana, G.; Siriwardhana, A.; Rathnayake, U.A.; Berugoda Arachchige, D.M.; Kumarasinghe, A.R.; Dahanayake, D.; Karunaratne, V.; Amaratunga, G.A.J. Urea-Hydroxyapatite Nanohybrids for Slow Release of Nitrogen. ACS Nano 2017, 11, 1214–1221. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.; Vishwakarma, K.; Hossen, M.S.; Kumar, V.; Shackira, A.M.; Puthur, J.T.; Abdi, G.; Sarraf, M.; Hasanuzzaman, M. Potassium in plants: Growth regulation, signaling, and environmental stress tolerance. Plant Physiol. Biochem. 2022, 172, 56–69. [Google Scholar] [CrossRef] [PubMed]
- White, P.J. Calcium in Plants. Ann. Bot. 2003, 92, 487–511. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.U.; Hussain, T.; Abdullah; Khan, M.A.; Almostafa, M.M.; Younis, N.S.; Yahya, G. Antibacterial and Antibiofilm Activity of Ficus carica-Mediated Calcium Oxide (CaONPs) Phyto-Nanoparticles. Molecules 2023, 28, 5553. [Google Scholar] [CrossRef]
- Luan, S.; Wang, C. Calcium Signaling Mechanisms Across Kingdoms. Annu. Rev. Cell Dev. Biol. 2021, 37, 311–340. [Google Scholar] [CrossRef]
- Hamedeh, H.; Antoni, S.; Cocciaglia, L.; Ciccolini, V. Molecular and Physiological Effects of Magnesium–Polyphenolic Compound as Biostimulant in Drought Stress Mitigation in Tomato. Plants 2022, 11, 586. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.T.T.; Nguyen, L.M.; Nguyen, T.T.T.; Tran, U.P.N.; Nguyen, D.T.C.; Tran, T.V. A critical review on the bio-mediated green synthesis and multiple applications of magnesium oxide nanoparticles. Chemosphere 2023, 312, 137301. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Gao, Y.; Yang, A. Sulfur Homeostasis in Plants. Int. J. Mol. Sci. 2020, 21, 8926. [Google Scholar] [CrossRef] [PubMed]
- Nakai, Y.; Maruyama-Nakashita, A. Biosynthesis of Sulfur-Containing Small Biomolecules in Plants. Int. J. Mol. Sci. 2020, 21, 3470. [Google Scholar] [CrossRef] [PubMed]
- Wawrzyńska, A.; Sirko, A. The Role of Selective Protein Degradation in the Regulation of Iron and Sulfur Homeostasis in Plants. Int. J. Mol. Sci. 2020, 21, 2771. [Google Scholar] [CrossRef] [PubMed]
- Alvi, A.F.; Iqbal, N.; Albaqami, M.; Khan, N.A. The emerging key role of reactive sulfur species in abiotic stress tolerance in plants. Physiol. Plant. 2023, 175, e13945. [Google Scholar] [CrossRef] [PubMed]
- Griffith, C.M.; Woodrow, J.E.; Seiber, J.N. Environmental behavior and analysis of agricultural sulfur. Pest Manag. Sci. 2015, 71, 1486–1496. [Google Scholar] [CrossRef] [PubMed]
- Singh, B. Sulfur and crop quality-agronomical strategies for crop improvement. In Proceedings of the COST Action 829 Meetings, Braunschweig, Germany, 15–18 May 2003; pp. 15–18. [Google Scholar]
- Karimi, J.; Mohsenzadeh, S. Rapid, Green, and Eco-Friendly Biosynthesis of Copper Nanoparticles Using Flower Extract of Aloe Vera. Synth. React. Inorg. Met. Org. Nano-Met. Chem. 2015, 45, 895–898. [Google Scholar] [CrossRef]
- Solberg, E.D.; Malhi, S.S.; Nyborg, M.; Gill, K.S. Fertilizer Type, Tillage, and Application Time Effects on Recovery of Sulfate-S from Elemental Sulfur Fertilizers in Fallow Field Soils. Commun. Soil Sci. Plant Anal. 2011, 34, 815–830. [Google Scholar] [CrossRef]
- Aula, L.; Dhillon, J.S.; Omara, P.; Wehmeyer, G.B.; Freeman, K.W.; Raun, W.R. World Sulfur Use Efficiency for Cereal Crops. Agron. J. 2019, 111, 2485–2492. [Google Scholar] [CrossRef]
- Zhang, Z.; He, X.; Zhang, H.; Ma, Y.; Zhang, P.; Ding, Y.; Zhao, Y. Uptake and distribution of ceria nanoparticles in cucumber plants. Metallomics 2011, 3, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Jiang, Y.; Li, Y.; Wang, Q.; Zhu, G.; Yi, T.; Wang, Q.; Wang, Y.; Dhankher, O.P.; Tan, Z.; et al. Unlocking the potential of nanoscale sulfur in sustainable agriculture. Chem. Sci. 2024, 15, 4709–4722. [Google Scholar] [CrossRef] [PubMed]
- Cape, J.N.; Fowler, D.; Davison, A. Ecological effects of sulfur dioxide, fluorides, and minor air pollutants: Recent trends and research needs. Environ. Int. 2003, 29, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Deng, C.; Shen, Y.; Borgatta, J.; Dimkpa, C.O.; Xing, B.; Dhankher, O.P.; Wang, Z.; White, J.C.; Elmer, W.H. Surface Coated Sulfur Nanoparticles Suppress Fusarium Disease in Field Grown Tomato: Increased Yield and Nutrient Biofortification. J. Agric. Food Chem. 2022, 70, 14377–14385. [Google Scholar] [CrossRef] [PubMed]
- Yazhini, R.I.; Latha, M.R.; Rajeswari, R.; Marimuthu, S.; Lakshmanan, A.; Subramanian, K.S. Synthesis and characterization of Nano sulphur: Exploring its potential as slow release fertilizer. J. Appl. Nat. Sci. 2023, 15, 937–944. [Google Scholar] [CrossRef]
- Yuan, H.; Liu, Q.; Guo, Z.; Fu, J.; Sun, Y.; Gu, C.; Xing, B.; Dhankher, O.P. Sulfur nanoparticles improved plant growth and reduced mercury toxicity via mitigating the oxidative stress in Brassica napus L. J. Clean. Prod. 2021, 318, 128589. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, G.; Wang, Y.; White, J.C.; Xing, B.; Dhankher, O.P. Nanoscale sulfur alleviates silver nanoparticle toxicity and improves seed and oil yield in Soybean (Glycine max). Environ. Pollut. 2023, 336, 122423. [Google Scholar] [CrossRef] [PubMed]
- Meselhy, G.; Sharma, S.; Guo, Z.; Singh, G.; Yuan, H.; Tripathi, R.D.; Xing, B.; Musante, C.; White, J.C.; Dhankher, O.P. Nanoscale Sulfur Improves Plant Growth and Reduces Arsenic Toxicity and Accumulation in Rice (Oryza sativa L.). Environ. Sci. Technol. 2021, 55, 13490–13503. [Google Scholar] [CrossRef]
- Sidhu, M.K.; Raturi, H.C.; Kachwaya, D.S.; Sharma, A. Role of micronutrients in vegetable production: A review. J. Pharmacogn. Phytochem. 2019, 8, 332–340. [Google Scholar]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circular. Calif. Agric. Exp. Stn. 1950, 347, 39. [Google Scholar]
- Fageria, N.K. The Use of Nutrients in Crop Plants; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Ghafariyan, M.H.; Malakouti, M.J.; Dadpour, M.R.; Stroeve, P.; Mahmoudi, M. Effects of Magnetite Nanoparticles on Soybean Chlorophyll. Environ. Sci. Technol. 2013, 47, 10645–10652. [Google Scholar] [CrossRef]
- Mahmoud, A.W.M.; Ayad, A.A.; Abdel-Aziz, H.S.; Williams, L.L.; El-Shazoly, R.M.; Abdel-Wahab, A.; Abdeldaym, E.A. Foliar application of different iron sources improves morpho-physiological traits and nutritional quality of broad bean grown in sandy soil. Plants 2022, 11, 2599. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Xu, M.; Xiao, L.; Dai, Z.; Li, J. The impacts of γ-Fe2O3 and Fe3O4 nanoparticles on the physiology and fruit quality of muskmelon (Cucumis melo) plants. Environ. Pollut. 2019, 249, 1011–1018. [Google Scholar] [CrossRef]
- Al-Amri, N.; Tombuloglu, H.; Slimani, Y.; Akhtar, S.; Barghouthi, M.; Almessiere, M.; Alshammari, T.; Baykal, A.; Sabit, H.; Ercan, I.; et al. Size effect of iron (III) oxide nanomaterials on the growth, and their uptake and translocation in common wheat (Triticum aestivum L.). Ecotoxicol. Environ. Saf. 2020, 194, 110377. [Google Scholar] [CrossRef]
- Palmqvist, N.G.M.; Seisenbaeva, G.A.; Svedlindh, P.; Kessler, V.G. Maghemite Nanoparticles Acts as Nanozymes, Improving Growth and Abiotic Stress Tolerance in Brassica napus. Nanoscale Res. Lett. 2017, 12, 631. [Google Scholar] [CrossRef]
- Hu, J.; Guo, H.; Li, J.; Wang, Y.; Xiao, L.; Xing, B. Interaction of γ-Fe2O3 nanoparticles with Citrus maxima leaves and the corresponding physiological effects via foliar application. J. Nanobiotechnol. 2017, 15, 51. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Guo, H.; Li, J.; Gan, Q.; Wang, Y.; Xing, B. Comparative impacts of iron oxide nanoparticles and ferric ions on the growth of Citrus maxima. Environ. Pollut. 2017, 221, 199–208. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, J.; Dai, Z.; Li, J.; Huang, J. In Vitro assessment of physiological changes of watermelon (Citrullus lanatus) upon iron oxide nanoparticles exposure. Plant Physiol. Biochem. 2016, 108, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Alidoust, D.; Isoda, A. Effect of γFe2O3 nanoparticles on photosynthetic characteristic of soybean (Glycine max (L.) Merr.): Foliar spray versus soil amendment. Acta Physiol. Plant. 2013, 35, 3365–3375. [Google Scholar] [CrossRef]
- Yoon, H.; Kang, Y.-G.; Chang, Y.-S.; Kim, J.-H. Effects of Zerovalent Iron Nanoparticles on Photosynthesis and Biochemical Adaptation of Soil-Grown Arabidopsis thaliana. Nanomaterials 2019, 9, 1543. [Google Scholar] [CrossRef]
- Kim, J.-H.; Oh, Y.; Yoon, H.; Hwang, I.; Chang, Y.-S. Iron Nanoparticle-Induced Activation of Plasma Membrane H+-ATPase Promotes Stomatal Opening in Arabidopsis thaliana. Environ. Sci. Technol. 2014, 49, 1113–1119. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, A.D.; Yoon, H.; Singh, J.P.; Chae, K.H.; Rho, S.-c.; Hwang, D.S.; Chang, Y.-S. Uptake, Distribution, and Transformation of Zerovalent Iron Nanoparticles in the Edible Plant Cucumis sativus. Environ. Sci. Technol. 2018, 52, 10057–10066. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, P.; Adeel, M.; Guo, Z.; Chetwynd, A.J.; Ma, C.; Bai, T.; Hao, Y.; Rui, Y. Physiological impacts of zero valent iron, Fe3O4 and Fe2O3 nanoparticles in rice plants and their potential as Fe fertilizers. Environ. Pollut. 2021, 269, 116134. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, S.; Patra, P.; Das, S.; Chandra, S.; Mitra, S.; Dey, K.K.; Akbar, S.; Palit, P.; Goswami, A. Photochemical Modulation of Biosafe Manganese Nanoparticles on Vigna radiata: A Detailed Molecular, Biochemical, and Biophysical Study. Environ. Sci. Technol. 2013, 47, 13122–13131. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, S.; Patra, P.; Mitra, S.; Dey, K.K.; Jain, S.; Sarkar, S.; Roy, S.; Palit, P.; Goswami, A. Manganese Nanoparticles: Impact on Non-nodulated Plant as a Potent Enhancer in Nitrogen Metabolism and Toxicity Study both In Vivo and In Vitro. J. Agric. Food Chem. 2014, 62, 8777–8785. [Google Scholar] [CrossRef] [PubMed]
- Haydar, M.S.; Ali, S.; Mandal, P.; Roy, D.; Roy, M.N.; Kundu, S.; Kundu, S.; Choudhuri, C. Fe–Mn nanocomposites doped graphene quantum dots alleviate salt stress of Triticum aestivum through osmolyte accumulation and antioxidant defense. Sci. Rep. 2023, 13, 11040. [Google Scholar] [CrossRef] [PubMed]
- Raliya, R.; Tarafdar, J.C.; Biswas, P. Enhancing the Mobilization of Native Phosphorus in the Mung Bean Rhizosphere Using ZnO Nanoparticles Synthesized by Soil Fungi. J. Agric. Food Chem. 2016, 64, 3111–3118. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, P.; Dhoke, S.; Khanna, A. Effect of nano-ZnO particle suspension on growth of mung (Vigna radiata) and gram (Cicer arietinum) seedlings using plant agar method. J. Nanotechnol. 2011, 2011, 696535. [Google Scholar] [CrossRef]
- Zhao, L.; Peralta-Videa, J.R.; Rico, C.M.; Hernandez-Viezcas, J.A.; Sun, Y.; Niu, G.; Servin, A.; Nunez, J.E.; Duarte-Gardea, M.; Gardea-Torresdey, J.L. CeO2 and ZnO Nanoparticles Change the Nutritional Qualities of Cucumber (Cucumis sativus). J. Agric. Food Chem. 2014, 62, 2752–2759. [Google Scholar] [CrossRef]
- Zhao, L.; Sun, Y.; Hernandez-Viezcas, J.A.; Servin, A.D.; Hong, J.; Niu, G.; Peralta-Videa, J.R.; Duarte-Gardea, M.; Gardea-Torresdey, J.L. Influence of CeO2 and ZnO Nanoparticles on Cucumber Physiological Markers and Bioaccumulation of Ce and Zn: A Life Cycle Study. J. Agric. Food Chem. 2013, 61, 11945–11951. [Google Scholar] [CrossRef]
- García, L.; Niño, M.; Olivares, S.; Lira, S.; Barriga, C.; Vázquez, A.; Rodríguez, S.; Zavala, G. Foliar Application of Zinc Oxide Nanoparticles and Zinc Sulfate Boosts the Content of Bioactive Compounds in Habanero Peppers. Plants 2019, 8, 254. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Xing, B. Phytotoxicity of nanoparticles: Inhibition of seed germination and root growth. Environ. Pollut. 2007, 150, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Prasad, T.N.V.K.V.; Sudhakar, P.; Sreenivasulu, Y.; Latha, P.; Munaswamy, V.; Reddy, K.R.; Sreeprasad, T.S.; Sajanlal, P.R.; Pradeep, T. Effect of Nanoscale Zinc Oxide Particles on the Germination, Growth and Yield of Peanut. J. Plant Nutr. 2012, 35, 905–927. [Google Scholar] [CrossRef]
- Sedghi, M.; Hadi, M.; Toluie, S.G. Effect of nano zinc oxide on the germination parameters of soybean seeds under drought stress. Ann. West Univ. Timisoara Ser. Biol. 2013, 16, 73. [Google Scholar]
- Yusefi-Tanha, E.; Fallah, S.; Rostamnejadi, A.; Pokhrel, L.R. Responses of soybean (Glycine max [L.] Merr.) to zinc oxide nanoparticles: Understanding changes in root system architecture, zinc tissue partitioning and soil characteristics. Sci. Total Environ. 2022, 835, 155348. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, M.; Palanisamy, K.; Babu, K.; Sharma, N.K. Effects of bulk & nano-titanium dioxide and zinc oxide on physio-morphological changes in Triticum aestivum Linn. J. Glob. Biosci. 2014, 3, 415–422. [Google Scholar]
- Tarafdar, J.C.; Raliya, R.; Mahawar, H.; Rathore, I. Development of Zinc Nanofertilizer to Enhance Crop Production in Pearl Millet (Pennisetum americanum). Agric. Res. 2014, 3, 257–262. [Google Scholar] [CrossRef]
- Raliya, R.; Nair, R.; Chavalmane, S.; Wang, W.-N.; Biswas, P. Mechanistic evaluation of translocation and physiological impact of titanium dioxide and zinc oxide nanoparticles on the tomato (Solanum lycopersicum L.) plant. Metallomics 2015, 7, 1584–1594. [Google Scholar] [CrossRef] [PubMed]
- Raskar, S.; Laware, S. Effect of zinc oxide nanoparticles on cytology and seed germination in onion. Int. J. Curr. Microbiol. App. Sci. 2014, 3, 467–473. [Google Scholar]
- Raliya, R.; Tarafdar, J.C. ZnO Nanoparticle Biosynthesis and Its Effect on Phosphorous-Mobilizing Enzyme Secretion and Gum Contents in Clusterbean (Cyamopsis tetragonoloba L.). Agric. Res. 2013, 2, 48–57. [Google Scholar] [CrossRef]
- Poornima, R.; Koti, R. Effect of nano zinc oxide on growth, yield and grain zinc content of sorghum (Sorghum bicolor). J. Pharmacogn. Phytochem. 2019, 8, 727–731. [Google Scholar]
- de la Rosa, G.; López-Moreno, M.L.; de Haro, D.; Botez, C.E.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Effects of ZnO nanoparticles in alfalfa, tomato, and cucumber at the germination stage: Root development and X-ray absorption spectroscopy studies. Pure Appl. Chem. 2013, 85, 2161–2174. [Google Scholar] [CrossRef]
- Reshma, Z.; Meenal, K. Foliar application of biosynthesised zinc nanoparticles as a strategy for ferti-fortification by improving yield, zinc content and zinc use efficiency in amaranth. Heliyon 2022, 8, e10912. [Google Scholar] [CrossRef]
- Sharma, P.; Urfan, M.; Anand, R.; Sangral, M.; Hakla, H.R.; Sharma, S.; Das, R.; Pal, S.; Bhagat, M. Green synthesis of zinc oxide nanoparticles using Eucalyptus lanceolata leaf litter: Characterization, antimicrobial and agricultural efficacy in maize. Physiol. Mol. Biol. Plants 2022, 28, 363–381. [Google Scholar] [CrossRef] [PubMed]
- Beig, B.; Niazi, M.B.K.; Jahan, Z.; Zia, M.; Shah, G.A.; Iqbal, Z.; Douna, I. Facile coating of micronutrient zinc for slow release urea and its agronomic effects on field grown wheat (Triticum aestivum L.). Sci. Total Environ. 2022, 838, 155965. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, S.; Varma, A.; Rao, M.; Prasad, R.; Goel, A. Deciphering the fertilizing and disease suppression potential of phytofabricated zinc oxide nanoparticles on Brassica juncea. Environ. Res. 2023, 231, 116276. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, S.; Varma, A.; Prasad, R.; Goel, A.; Velmurugan, P. Mechanistic Insight of the Antifungal Potential of Green Synthesized Zinc Oxide Nanoparticles against Alternaria brassicae. J. Nanomater. 2022, 2022, 7138843. [Google Scholar] [CrossRef]
- Lin, D.; Xing, B. Root Uptake and Phytotoxicity of ZnO Nanoparticles. Environ. Sci. Technol. 2008, 42, 5580–5585. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.M.; An, Y.J.; Yoon, H.; Kweon, H.S. Toxicity and bioavailability of copper nanoparticles to the terrestrial plants mung bean (Phaseolus radiatus) and wheat (Triticum aestivum): Plant agar test for water-insoluble nanoparticles. Environ. Toxicol. Chem. 2009, 27, 1915–1921. [Google Scholar] [CrossRef]
- Musante, C.; White, J.C. Toxicity of silver and copper to Cucurbita pepo: Differential effects of nano and bulk-size particles. Environ. Toxicol. 2010, 27, 510–517. [Google Scholar] [CrossRef]
- Shah, V.; Belozerova, I. Influence of Metal Nanoparticles on the Soil Microbial Community and Germination of Lettuce Seeds. Water Air Soil Pollut. 2008, 197, 143–148. [Google Scholar] [CrossRef]
- Nekrasova, G.F.; Ushakova, O.S.; Ermakov, A.E.; Uimin, M.A.; Byzov, I.V. Effects of copper(II) ions and copper oxide nanoparticles on Elodea densa Planch. Russ. J. Ecol. 2011, 42, 458–463. [Google Scholar] [CrossRef]
- Taran, N.Y.; Gonchar, O.M.; Lopatko, K.G.; Batsmanova, L.M.; Patyka, M.V.; Volkogon, M.V. The effect of colloidal solution of molybdenum nanoparticles on the microbial composition in rhizosphere of Cicer arietinum L. Nanoscale Res. Lett. 2014, 9, 289. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yin, Y.; Zhu, Y.; Song, K.; Ding, W. Favorable physiological and morphological effects of molybdenum nanoparticles on tobacco (Nicotiana tabacum L.): Root irrigation is superior to foliar spraying. Front. Plant Sci. 2023, 14, 1220109. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, P.; Guo, Z.; Zhao, W.; Li, Y.; Yi, T.; Cao, W.; Gao, L.; Tian, C.F.; Chen, Q.; et al. Dynamic Transformation of Nano-MoS2 in a Soil–Plant System Empowers Its Multifunctionality on Soybean Growth. Environ. Sci. Technol. 2024, 58, 1211–1222. [Google Scholar] [CrossRef] [PubMed]
- Meier, S.; Moore, F.; Morales, A.; González, M.-E.; Seguel, A.; Meriño-Gergichevich, C.; Rubilar, O.; Cumming, J.; Aponte, H.; Alarcón, D.; et al. Synthesis of calcium borate nanoparticles and its use as a potential foliar fertilizer in lettuce (Lactuca sativa) and zucchini (Cucurbita pepo). Plant Physiol. Biochem. 2020, 151, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Kasozi, N.; Tandlich, R.; Fick, M.; Kaiser, H.; Wilhelmi, B. Iron supplementation and management in aquaponic systems: A review. Aquac. Rep. 2019, 15, 100221. [Google Scholar] [CrossRef]
- Rout, G.R.; Sahoo, S. Role of Iron in Plant Growth and Metabolism. Rev. Agric. Sci. 2015, 3, 1–24. [Google Scholar] [CrossRef]
- Korcak, R.F. Iron deficiency chlorosis. In Horticultural Reviews; Wiley: Hoboken, NJ, USA, 1987; pp. 133–186. [Google Scholar]
- Cieschi, M.T.; Polyakov, A.Y.; Lebedev, V.A.; Volkov, D.S.; Pankratov, D.A.; Veligzhanin, A.A.; Perminova, I.V.; Lucena, J.J. Eco-Friendly Iron-Humic Nanofertilizers Synthesis for the Prevention of Iron Chlorosis in Soybean (Glycine max) Grown in Calcareous Soil. Front. Plant Sci. 2019, 10, 413. [Google Scholar] [CrossRef]
- Wang, H.; Kou, X.; Pei, Z.; Xiao, J.Q.; Shan, X.; Xing, B. Physiological effects of magnetite (Fe3O4) nanoparticles on perennial ryegrass (Lolium perenne L.) and pumpkin (Cucurbita mixta) plants. Nanotoxicology 2010, 5, 30–42. [Google Scholar] [CrossRef]
- Zimbovskaya, M.M.; Polyakov, A.Y.; Volkov, D.S.; Kulikova, N.A.; Lebedev, V.A.; Pankratov, D.A.; Konstantinov, A.I.; Parfenova, A.M.; Zhilkibaev, O.T.; Perminova, I.V. Foliar Application of Humic-Stabilized Nanoferrihydrite Resulted in an Increase in the Content of Iron in Wheat Leaves. Agronomy 2020, 10, 1891. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, H.; Lal, R. Effects of Stabilized Nanoparticles of Copper, Zinc, Manganese, and Iron Oxides in Low Concentrations on Lettuce (Lactuca sativa) Seed Germination: Nanotoxicants or Nanonutrients? Water Air Soil Pollut. 2016, 227, 1–14. [Google Scholar] [CrossRef]
- Bibi, H.; Haroon, U.; Farhana; Kamal, A.; Akbar, M.; Anar, M.; Batool, S.S.; Bilal, A.; Jabeen, H.; Ahmed, J.; et al. Impact of bacterial synthesized nanoparticles on quality attributes and postharvest disease control efficacy of apricot and loquat. J. Food Sci. 2023, 88, 3920–3934. [Google Scholar] [CrossRef] [PubMed]
- Elbasuney, S.; El-Sayyad, G.S.; Attia, M.S.; Abdelaziz, A.M. Ferric Oxide Colloid: Towards Green Nano-Fertilizer for Tomato Plant with Enhanced Vegetative Growth and Immune Response Against Fusarium Wilt Disease. J. Inorg. Organomet. Polym. Mater. 2022, 32, 4270–4283. [Google Scholar] [CrossRef]
- Millaleo, R.; Reyes- Diaz, M.; Ivanov, A.G.; Mora, M.L.; Alberdi, M. Manganese as Essential and Toxic Element for Plants: Transport, Accumulation and Resistance Mechanisms. J. Soil Sci. Plant Nutr. 2010, 10, 470–481. [Google Scholar] [CrossRef]
- Ducic, T.; Polle, A. Transport and detoxification of manganese and copper in plants. Braz. J. Plant Physiol. 2005, 17, 103–112. [Google Scholar] [CrossRef]
- Sharma, A.; Patni, B.; Shankhdhar, D.; Shankhdhar, S.C. Zinc—An Indispensable Micronutrient. Physiol. Mol. Biol. Plants 2012, 19, 11–20. [Google Scholar] [CrossRef]
- Sturikova, H.; Krystofova, O.; Huska, D.; Adam, V. Zinc, zinc nanoparticles and plants. J. Hazard. Mater. 2018, 349, 101–110. [Google Scholar] [CrossRef]
- Liu, L.; Nian, H.; Lian, T. Plants and rhizospheric environment: Affected by zinc oxide nanoparticles (ZnO NPs). A review. Plant Physiol. Biochem. 2022, 185, 91–100. [Google Scholar] [CrossRef]
- Verma, Y.; Singh, S.K.; Jatav, H.S.; Rajput, V.D.; Minkina, T. Interaction of zinc oxide nanoparticles with soil: Insights into the chemical and biological properties. Environ. Geochem. Health 2021, 44, 221–234. [Google Scholar] [CrossRef]
- Singh, K.; Madhusudanan, M.; Verma, A.K.; Kumar, C.; Ramawat, N. Engineered zinc oxide nanoparticles: An alternative to conventional zinc sulphate in neutral and alkaline soils for sustainable wheat production. 3 Biotech 2021, 11, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Prakash, V.; Rai, P.; Sharma, N.C.; Singh, V.P.; Tripathi, D.K.; Sharma, S.; Sahi, S. Application of zinc oxide nanoparticles as fertilizer boosts growth in rice plant and alleviates chromium stress by regulating genes involved in oxidative stress. Chemosphere 2022, 303, 134554. [Google Scholar] [CrossRef] [PubMed]
- Mishra, Y.K.; Milani, N.; Hettiarachchi, G.M.; Kirby, J.K.; Beak, D.G.; Stacey, S.P.; McLaughlin, M.J. Fate of Zinc Oxide Nanoparticles Coated onto Macronutrient Fertilizers in an Alkaline Calcareous Soil. PLoS ONE 2015, 10, e0126275. [Google Scholar] [CrossRef]
- Lee, C.W.; Mahendra, S.; Zodrow, K.; Li, D.; Tsai, Y.C.; Braam, J.; Alvarez, P.J.J. Developmental phytotoxicity of metal oxide nanoparticles to Arabidopsis thaliana. Environ. Toxicol. Chem. 2009, 29, 669–675. [Google Scholar] [CrossRef] [PubMed]
- López-Moreno, M.L.; de la Rosa, G.; Hernández-Viezcas, J.Á.; Castillo-Michel, H.; Botez, C.E.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Evidence of the Differential Biotransformation and Genotoxicity of ZnO and CeO2 Nanoparticles on Soybean (Glycine max) Plants. Environ. Sci. Technol. 2010, 44, 7315–7320. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.-M.; Xu, H.-H.; Liu, W.-C.; Lu, Y.-T. Copper Regulates Primary Root Elongation Through PIN1-Mediated Auxin Redistribution. Plant Cell Physiol. 2013, 54, 766–778. [Google Scholar] [CrossRef] [PubMed]
- Raven, J.A.; Evans, M.C.; Korb, R.E. The role of trace metals in photosynthetic electron transport in O2-evolving organisms. Photosynth. Res. 1999, 60, 111–150. [Google Scholar] [CrossRef]
- Droppa, M.; Terry, N.; Horvath, G. Effects of Cu deficiency on photosynthetic electron transport. Proc. Natl. Acad. Sci. USA 1984, 81, 2369–2373. [Google Scholar] [CrossRef]
- Uchida, R. Essential nutrients for plant growth: Nutrient functions and deficiency symptoms. In Plant Nutrient Management in Hawaii’s Soils; University of Hawaii at Manoa, College of Agriculture & Tropical Resources: Honolulu, HI, USA, 2000; pp. 31–55. [Google Scholar]
- Zhen, Y.; Ge, L.; Chen, Q.; Xu, J.; Duan, Z.; Loor, J.J.; Wang, M. Latent Benefits and Toxicity Risks Transmission Chain of High Dietary Copper along the Livestock–Environment–Plant–Human Health Axis and Microbial Homeostasis: A Review. J. Agric. Food Chem. 2022, 70, 6943–6962. [Google Scholar] [CrossRef]
- Feigl, G.; Kumar, D.; Lehotai, N.; Tugyi, N.; Molnár, Á.; Ördög, A.; Szepesi, Á.; Gémes, K.; Laskay, G.; Erdei, L.; et al. Physiological and morphological responses of the root system of Indian mustard (Brassica juncea L. Czern.) and rapeseed (Brassica napus L.) to copper stress. Ecotoxicol. Environ. Saf. 2013, 94, 179–189. [Google Scholar] [CrossRef]
- Brown, P.H.; Bellaloui, N.; Wimmer, M.A.; Bassil, E.S.; Ruiz, J.; Hu, H.; Pfeffer, H.; Dannel, F.; Römheld, V. Boron in Plant Biology. Plant Biol. 2008, 4, 205–223. [Google Scholar] [CrossRef]
- Ryden, P.; Sugimoto-Shirasu, K.; Smith, A.C.; Findlay, K.; Reiter, W.-D.; McCann, M.C. Tensile Properties of Arabidopsis Cell Walls Depend on Both a Xyloglucan Cross-Linked Microfibrillar Network and Rhamnogalacturonan II-Borate Complexes. Plant Physiol. 2003, 132, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Robertson, G.A.; Loughman, B.C. Rubidium Uptake and Boron Deficiency in Vicia faba L. J. Exp. Bot. 1973, 24, 1046–1052. [Google Scholar] [CrossRef]
- Dell, B.; Huang, L. Physiological response of plants to low boron. Plant Soil 1997, 193, 103–120. [Google Scholar] [CrossRef]
- Shen, Z.; Liang, Y.; Shen, K. Effect of boron on the nitrate reductase activity in oilseed rape plants. J. Plant Nutr. 1993, 16, 1229–1239. [Google Scholar] [CrossRef]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants; Academic Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Miwa, K.; Takano, J.; Fujiwara, T. Improvement of seed yields under boron-limiting conditions through overexpression of BOR1, a boron transporter for xylem loading, in Arabidopsis thaliana. Plant J. 2006, 46, 1084–1091. [Google Scholar] [CrossRef] [PubMed]
- Shireen, F.; Nawaz, M.; Chen, C.; Zhang, Q.; Zheng, Z.; Sohail, H.; Sun, J.; Cao, H.; Huang, Y.; Bie, Z. Boron: Functions and Approaches to Enhance Its Availability in Plants for Sustainable Agriculture. Int. J. Mol. Sci. 2018, 19, 1856. [Google Scholar] [CrossRef]
- Rajput, V.D.; Singh, A.; Minkina, T.; Rawat, S.; Mandzhieva, S.; Sushkova, S.; Shuvaeva, V.; Nazarenko, O.; Rajput, P.; Komariah; et al. Nano-Enabled Products: Challenges and Opportunities for Sustainable Agriculture. Plants 2021, 10, 2727. [Google Scholar] [CrossRef]
- Sekhon, B. Nanotechnology in agri-food production: An overview. Nanotechnol. Sci. Appl. 2014, 7, 31–53. [Google Scholar] [CrossRef]

| Fertilizer Composition | Method | Particle Size, nm | Object/Plant Culture | Experimental Conditions | Optimal Concentration | Experiment Results | Refs. |
|---|---|---|---|---|---|---|---|
| U-modified HA NPs | Wet chemical method with encapsulation of urea-modified HA nanoparticles into micro/nanoporous cavities of Glyricidia sepium (Jacg.) Kunth Walp. under pressure | 100 × 150 | Nitrogen release | Three types of soils with different pH (4.2, 5.2, and 7.0) | - | The nanoparticles showed slow release of nitrogen even at day 60 compared to the control. The control released nitrogen earlier and in large quantities until about day 30, but this was followed by the release of nitrogen in low and patchy quantities. | [41] |
| Chitosan NPs | Biosynthesis from shrimp waste by Penicillium oxalicum Currie and Thom | 10–20 | Triticum aestivum L. var. Misr-1 and Gemaiza-11 | Foliar spraying in a field experiment | 14 L∙ha−1 | ↑ chlorophyll content (+13–19% depending on the variety) ↑ number of shoots (+5–11%) ↑ spike length (+8–28%) ↑ number of spikelets (+7–8%) ↑ spike weight (+5–13%) ↑ 1000-grain weight (+7–16%) ↑ grain yield (+27–31%) Plant height did not change | [32] |
| U-doped amorphous Ca3(PO4)2-NPs | Batch method | 13.5 | Triticum durum Desf. | Cultivation in a growth chamber in a substrate consisting of a 1:1 mixture of soil and sand under simulated sunlight | 15 kg∙ha−1 | ↑ number of shoots per plant (+11%) ↑ FW (+30%) ↑ number of spikelets (+30%) ↑ spike weight (+36%) ↑ 1000-grain weight (+23%) ↑ Grain quantity (+27%) ↑ protein content (+13%) | [33] |
| Cucumis sativus L. | Cultivation in an aerated hydroponic solution followed by provocation of nitrogen starvation for 7 days | 3.7 mM | ↑ total root length (2.5 times), number (2.4 times), and surface area (2.3 times) ↑ Ca, P, and S ↑ N uptake (1.7 times) ↑ gene expression CsDUR3 (5 times) SPAD level did not change | [25] | |||
| Vitis vinifera L. | Field experiment | 0.4 kg∙ha−1 | ↑ N content in berries (+28.6%) ↑ amino acid concentrations in plants ↑ arginine concentrations in the musts (+21%) ↓ proline concentration compared to commercial urea treatment (6 kg∙ha−1) | [44] | |||
| Nitrogen-doped CDs | Hydrothermal method | 2.6 | Lactuca sativa L. | Hydroponic culture | 100 mg∙L−1 | ↑ FW (+42%) ↑ nutrient content ↑ chlorophyll content (+12.7%) ↑ ETR (+39%) ↑ light energy conversion efficiency (Y (II)) (+31%) ↑ photosynthesis rates—Rubisco activity (+61%) | [36] |
| HA-U-NPs | Chemical method with centrifugation | 38.7 | Triticum aestivum L. Pusa HD 3086 | Cultivation in pots under controlled conditions | 75 kg∙ha−1 | ↑ plant height (+13%) ↑ spike length (+24%) ↑ spike weight (+79%) ↑ number of spikelets (3.6 times) ↑ Grain quantity (+2.8) ↑ Mg (2.4 times), N (2.4 times), P (2.6 times), K (1.8 times), Ca (5.5 times), and Fe (3 times) in grains ↑ protein content (2 times) ↑ phospholipid concentration (+51%) ↑ proline concentration (+12%) | [26] |
| Mg-doped HA-U-NPs | Chemical method with centrifugation | 28.3 | Triticum aestivum L. Pusa HD 3086 | Cultivation in pots under controlled conditions | 75 kg∙ha−1 | ↑ plant height (+13%) ↑ spike length (+24%) ↑ spike weight (+79%) ↑ stem weight (+56%) ↑ number of spikelets (3.2 times) ↑ Grain quantity (+2.5) ↑ Mg (3 times), N (2.7 times), P (3.8 times), K (1.9 times), Ca (5.4 times), and Fe (3.7 times) in grains ↑ protein content (2 times) ↑ phospholipid concentration (+28%) ↑ proline concentration (+20%) | [26] |
| Zn-doped HA-U-NPs | Chemical method with centrifugation | 20.8 | Triticum aestivum L. Pusa HD 3086 | Cultivation in pots under controlled conditions | 37.5 kg∙ha−1 | ↑ plant height (+10%) ↑ spike length (+18%) ↑ spike weight (+61.5%) ↑ stem weight (+40%) ↑ number of spikelets (2.8 times) ↑ Grain quantity (+2.5) ↑ N (2.2 times), P (2.3 times), Mg (2.1 times), K (1.8 times), Fe (2.8 times), and Ca (4.2 times) in grains ↑ protein content (+50%) | [26] |
| HA-NPs | One-step wet chemical method | 8–22 | Glycine max L. | Greenhouse conditions | 21.8 mg∙L−1 P | ↑ growth rate (+32.6%) ↑ seed yield (+20.4%) ↑ shoot DW (+18.2%) ↑ root DW (+41.2%) | [37] |
| HA-NPs | Chemical method with centrifugation | 100 | Zea mays L. yellow cultivar | Foliar application in field conditions | 50 mg∙L−1 | ↑ height (+12.6%) ↑ leaf surface area (+27.7%) ↑ FW (+53%) ↑ green cob yield, t/ha (+29.4%) ↑ chlorophyll concentration (+52%) ↑ protein content (+22.1%) ↑ TPC (+12.9%) ↑ total flavonoids (+33.3%) ↑ total indoles (+32.4%) | [38,78] |
| Zea mays L. white cultivar | 100 mg∙L−1 | ↑ height (+17.0%) ↑ leaf surface area (+18.6%) ↑ FW (+58.8%) ↑ green cob yield, t/ha (+45.2%) ↑ chlorophyll concentration (+53%) ↑ TPC (+28.0%) ↑ total flavonoids (+10.0%) ↑ total indoles (+25.5%) | |||||
| HA-NPs with different surface charges | Wet chemical deposition and subsequent surface functionalization with glycine or dibasic ammonium citrate to obtain positively or negatively surface charged-nano-apatite | 25.7 | Helianthus annuus L. | Cultivation in pots for 35 days using two phosphorus-deficient soils (Ultisol and Vertisol) | 150 mg∙kg−1 P | In Ultisol (pH 4.7): ↑ FW (6.4–11.6 times) ↑ P in shoots (1.5 times) and Ca (2 times) ↓ Fe (8 times) and Zn (2 times) In Vertisol (pH 8.2): NPS did not significantly affect biomass ↓ Ca (3 times) | [79,80] |
| K-NPs | Green synthesis using Morus alba L. extract | 21–30 | Triticum aestivum L. (var. HD2967) | Field conditions, sandy loam soil, and foliar treatment | 20, 40, and 60 mg∙L−1 | All K-NP concentrations ↑ number of spikes (1.5–2 times) ↑ yield per hectare (2–3 times) ↑ protein content (30–50%) ↑ photosynthetic pigments (+50–60%) compared to the bulk analog (K2SO4) and control (without added potassium) | [81] |
| DAP with KFeO2-NP nano-coating | Chemical (sol–gel) method | 7–18 | Clayey and clayey-loamy soils; assessment of the dynamics of the release of N, P, K, and Fe | Incubation studies on clay and loamy soils | 10% nano-coating | Provides controlled release of P and N over a longer period compared to traditional DAP. ↑ release pattern of K (+8–12.5%), P (7–10 times), N (3 times), and Fe (2.5–3 times) in 60 days depending on soil type | [42] |
| CaCO3-NPs | Heterogeneous phase precipitation of Ca(OH)2 after hydration of CaO and subsequent calcination | 20–80 | Arachis hypogaea L. var. Luhua No. 4 | Greenhouse study, sand substrate, 80 days of cultivation | 160 mg∙L−1 Ca | ↑ Ca (+0.4%), N (+0.9%), P (+0.04%), and K (0.5%) ↑ total branches (+10%) ↑ leaf surface area (+21%) ↑ DW (+19%) ↑ soluble sugar content (+33%) ↑ protein content (+90%) compared to negative control and results are equivalent to Ca(NO3)2 treatments | [82] |
| Arachis hypogaea L. | Two-year experiment in field conditions, foliar spraying | 200 mg∙L−1 | ↑ plant height (+10%) ↑ branching number (+9.5%) ↑ growth rate (+16%) ↑ seed yield (1.6 times) ↑ 100-seed weight (1.6 times) ↑ protein content (+10.5%) ↑ oil (+6%) ↑ P (+12%) and K (2.5 times) | [39] | |||
| Khazra nano-chelated calcium (Khazra Sodour Ahrar Shargh Co., Tehran, Iran) | - | - | Malus domestica Borkh. cv. Red Delicious | Spraying 70 days after full flowering, a month before harvest, and during storage | 25 mg∙L−1 | ↑ fruit firmness, TA, TSS, TPC, TAA, and fiber content in fruits ↓ internal browning ↓ FW, total soluble solids compared to control ↓ polygalacturonase activity, pectin methylesterase, and β-galactosidase in fruits that were treated with both CaCO3-NPs and CaCl2 With increasing shelf life, the quality of fruits treated with CaCO3-NPs was higher than that of fruits treated with CaCl2 | [83] |
| Sr0.96Mg0.02Ca0.02Fe12O19, SrMgCa nano-HF | Sol–gel autoignition method | 42.4 | Hordeum vulgare L. | Cultivation in the field, treatment at the stages of germination and growth separately | 125–250 mg∙L−1 | ↑ germination rate (+20%) ↑ plant height (+38%) ↑ FW (+20%) ↑ protein soluble content (+41%) ↑ chlorophyll concentration (+33–42%) compared to untreated control Higher doses ↓ growth parameters | [27] |
| 500 mg∙L−1 | ↑ Fe (20 times), Ca (18 times), Mg (3 times), and Sr (60 times) in leaves | ||||||
| Mg-NPs+ FeSO4 | Chemical method with centrifugation | 20 | Vigna unguiculata ssp. unguiculata | 85 days of cultivation in field, application of Mg and/or Fe 56 and 72 days after sowing | 0.5 + 0.5 g∙L−1 | ↑ yield (+14%) ↑ Fe (+27%) and Mg (+7%) in leaves ↑ plasma membrane stability | [84] |
| Mg-NPs | Chemical method with centrifugation | 100 | 0.5 g∙L−1 | ↑ Fe (+17%) and Mg (+9%) in leaves ↑ SPAD (+5%) ↓ yield (2.7 times) | |||
| Mg-NPs | - | - | Green bean Phaseolus vulgaris L. cv. ‘Stike’ | 60 days of cultivation after planting in a greenhouse; foliar plant treatments every 10 days (5 in total); a mixture of vermiculite and perlite in a 2:1 ratio was used as a substrate | 0.05–0.1 g∙L−1 | ↑ stem FW (+23%) ↑ root FW (+20%) ↑ pod production (+18%) ↑ total polyphenols (+21%) ↑ flavonoid content (970–1703 mg catechin/100 g FW, not determined without treatment) ↑ DPPH (+35%) when using 50 mg∙L−1 Mg-NPs compared to MgSO4 at the same concentration | [85] |
| S-NPs | Chemical method | 20–40 | Triticum aestivum L. | Research in the field and greenhouse, seed treatment time 15 min | 3.4 g∙L−1 | ↑ seed germination (3 times) ↑ early ripeness (3 weeks) ↑ number of productive shoots ↑ Grain quantity ↑ grain weight and yield (+30–100% depending on the variety) ↑ pathogen resistance | [86] |
| S-NPs | Chemical method | Solanum lycopersicum L. | Application to soil as a multifunctional fertilizer | 200 mg∙L−1 | ↑ root FW (+73%) ↑ shoot FW (+35%) ↑ linear electron flow ↑ quantum yield of photosystem II ↑ relative chlorophyll content | [87] | |
| S-NPs modified with stearic acid (cS) | 200 mg∙L−1 | ↑ root FW (+81%) ↑ shoot FW (+50%) ↑ linear electron flow ↑ quantum yield of photosystem II ↑ relative chlorophyll content ↑ content of tryptophan, tomatidine, and scopoletin in leaves | |||||
| S-NPs | Aqueous precipitation method | 35–45 | Helianthus annuus L. var. KBSH 42 | Field experiment | 40 kg∙ha−1 S | ↑ content of available S in the soil ↑ DW (+11–12%) ↑ gain yield (+15%) ↑ oil content (+14,7%), compared to plants fertilized with gypsum ↑ sulfur release (+7 days compared with gypsum) | [40] |
| Fertilizer Composition | Method | Particle Size, nm | Optimal Concentration | Object/Plant Culture | Experimental Conditions | Experiment Results | Ref. |
|---|---|---|---|---|---|---|---|
| Fe3O4-NPs | Chemical method | 20 | 0.5 g∙L−1 | Vigna unguiculata ssp. Unguiculata | Foliar treatment 56 and 72 days after sowing, testing a week after the last treatment | ↑ yield (+7%) ↑ chlorophyll content (+9%) ↑ Fe in leaves (+25%) | [84] |
| Fe3O4 + Mg-NPs | Chemical method | 20 + 100 | 0.5 + 0.5 g∙L−1 | ↑ yield (+9%) ↑ Fe in leaves (+25%) ↑ chlorophyll content (+10%) | |||
| SPION | Chemical method | 18.9–20.3 | 45 mg∙L−1 | Glycine max (L.) Merr. | Adding nanoparticles to a nutrient solution with aeration at the stage of 2 paired leaves in a greenhouse | ↑ chlorophyll content | [134] |
| Humic acid-coated Fe3O4-NPs | Chemical co-precipitation method | 60–72 | 40 mg∙L−1 | Sorghum bicolor L. Moench | Foliar treatment in the field a week after inoculation with Acremonium striticum | ↑ plant height (+2–5% depending on the variety) ↑ yield (+5%) ↑ gibberellic acid (2 times), ↓ fungal infection | [34] |
| Fe3O4-NPs | Synthesis by simple photochemical polymerization in situ | 6.3–6.6 | 100 mg∙g−1 | Vicia faba L. var. major Harz | Field experiment; spraying was carried out 60, 90, and 120 days after sowing and harvest took place after 98 days | ↑ plant height (+54%) ↑ FW (+27%) ↑ DW (+22%) ↑ leaf surface area (+63%) ↑ number of branches (2 times) ↑ number of pods (2 times) ↑ number of seeds (+50%) ↑ harvest index (2 times) ↑ 100-seed mass (+26%) ↑ total chlorophyll (+17%) ↑ carotenoids (+22%) ↑ photosynthesis rate (2.4 times) ↑ stomatal conductance (+67%) ↑ water use efficiency (2 times) ↑ gibberellic acid (+29%) ↑ indole-3-acetic acid (+37%) ↑ N (2 times), P (4 times), K (+35%), Ca (4 times), Fe (2.5 times), Zn (2 times), and Mn (+30%) ↑ total carbohydrate (+26%) ↑ crude protein (+34%) ↑ fat content (+29%) ↑ arginine (+33%) and leucine (+20%) ↓ abscisic acid (−44%) ↓ alanine (−5%) | [135] |
| SPION | Chemical method | 20 | 100 mg∙L−1 | Cucumis melo L. | Growing in pots for 5 weeks, NPs added to 1/2 Hoagland solution | ↑ FW (+9%) ↑ plant height (+17%) ↑ chlorophyll content at 3 weeks (+35%) ↑ vitamin C (+47%) | [136] |
| Fe2O3-NPs | Chemical co-precipitation method | 9 | 20 mg∙L−1 | Citrullus lanatus (Thunb.) Matsum. and Nakai | Germinating seeds in Petri dishes, spraying with 1/2 Hoagland’s solution | ↑ root activity (+23%) ↑ CAT (+24%) ↑ POD (2.3 times) ↑ SOD (+8%) ↑ MDA (11%) compared to Fe2+ treatment ↑ Fe content in root crop apoplasts | [29] |
| 18 | 20 mg∙L−1 | ↑ root activity (+31%) ↑ CAT (+23%) ↑ POD (+87%) ↑ SOD (+7%) ↑ MDA (9%) ↑ iron reductase activity (2.5 times) compared to Fe2+ treatment ↓ chlorophyll content (−8%) | |||||
| Fe2O3-NPs | Chemical method | 80–110 | 500 mg∙L−1 | Triticum aestivum L. | Seed treatment followed by growing in pots | ↑ FW (+17%) ↑ photosynthesis and transpiration ↑ content of photosynthetic pigments in leaves (+20–30%) ↑ Fe (+25%), P (+27%), and K (+7%) ↑ ascorbate peroxidase activity ↓ MDA (−20%) | [30] |
| 20–40 | 500 mg∙L−1 | Treatment of seedlings in a hydroponic installation with Hoagland’s solution in greenhouse conditions | ↑ root length (+30%) ↑ plant height ↑ FW (4 times) ↑ DW (3 times) ↑ chlorophyll content (+50%) ↑ carotenoids (+42%) | [137] | |||
| Yttrium doping-stabilized γ-Fe2O3 NPs | Sol–gel method | 1–10 | 2000 mg∙L−1 (200 mL per 1 plant) | Brassica napus L. | Cultivation in pots in a climate chamber after treatment drought conditions were created (4 days), after which, measurements were taken | ↑ leaf growth rate (+52%) ↑ FW (+67%) ↑ chlorophyll content (+11%) ↓ H2O2 (−45%) ↓ MDA (−28%) | [138] |
| γ-Fe2O3 NPs | Chemical method | 17–23 | 50 mg∙L−1 | Citrus maxima (Burm.) Merr. | The experiment was carried out in a climate chamber (30 days) equipped with a hydroponic system (1/2 Hoagland’s nutrient solution without iron); plants were sprayed with a solution of nanoparticles | ↑ Fe (2 times) ↓ FW (−18%), compared with Fe(II)-EDTA Chlorophyll content did not change | [139] |
| 17–23 | The experiment was carried out in a climate chamber (30 days) equipped with a hydroponic system (1/2 Hoagland’s nutrient solution without iron); plants were sprayed with a solution of nanoparticles Nanoparticles were added to the nutrient solution | NPs penetrated into plant roots but did not move from roots to shoots (root barrier) ↑ chlorophyll content (+23%) ↑ root activity (+24%) ↑ soluble protein (+78%) compared to control (without Fe) ↑ Fe absorption ↑ gene expression level FRO2 ↓ gene expression level NRAMP3 | [140] | ||||
| 17–23 | Citrullus lanatus (Thunb.) Matsum. and Nakai | ↑ soluble sugar content (+74%) ↑ protein content (+18%) ↑ chlorophyll content (+5%) ↑ SOD (+36%) ↑ POD (+17%) | [141] | ||||
| 20 | 200 mg∙L−1 | Cucumis melo L. | Growing in pots for 5 weeks, nanoparticles added to 1/2 Hoagland solution | ↑ FW (+9%) ↑ plant height (+13%) ↑ chlorophyll content at 3 weeks (+37%) ↑ soluble protein content (+35%) ↑ vitamin C (+35%) ↓ Fe in leaves (−15%) ↓ Fe in fruits (−35%) | [136] | ||
| 6 | 1000 mg∙L−1 | Glycine max (L.) Merr. | Cultivation in Petri dishes; root and foliar treatments of plants in a greenhouse (in pots) | ↑ root length on day 5 (+34.5%) | [142] | ||
| Citrate-coated Fe2O3-NPs | 500 mg∙L−1 | Glycine max (L.) Merr. | ↑ photosynthetic parameters during foliar spraying at the eight-membered leaf stage SPAD index (+7%) | ||||
| ZVI-NPs | Chemical method | 53–55 | 500 mg∙kg−1 of soil | Arabidopsis thaliana (L.) Heynh. | Cultivation of plants in a climate chamber for plant growth; a suspension of nanoparticles was added to the soil | ↑ DW (+38%) ↑ leaf surface area (+53%) ↑ CO2 assimilation rate (+27%) ↑ stomatal conductance (+40%) ↑ transpiration rate (+48%) ↑ plasma membrane H+-ATPase activity ↑ stomatal aperture ↑ P (+73%) ↑ Fe uptake by plant roots (+25%) ↑ accumulation of carbohydrates: glucose (+44%), sucrose (+27%), and starch (+52%) ↓ Mn (−25%) ↓ Zn (−25%) | [143,144] |
| 250 mg∙kg−1 of soil | Cucumis sativus L. | Growth chamber in Petri dishes/hydroponics/soil using 1/4 Hoagland solution | ↑ Fe in roots (5 times) after 3 weeks of growing ↓ Fe in shoots (5 times) after 3 weeks of growing compared to Fe-EDTA treatment | [145] | |||
| 20 | 50 mg∙L−1 | Oryza sativa L. | Cultivation in ½ Kimura solution in 2 variants: without Fe and with 0.05 mM Fe(II)-EDTA; measurements were carried out on day 14 after treatment | ↑ chlorophyll content (+31%) ↑ Fe in roots (7 times) ↑ Fe in leaves (7.5 times) compared to negative control ↓ oxidative stress and concentrations of stress-related phytohormones: gibberellins (−42%) and indole-3-acetic acid (−42%) | [146] | ||
| Fe3O4-NPs | Chemical method | 20 | 50 mg∙L−1 | ↑ chlorophyll content (+27%) ↑ Fe in roots (5 times) ↑ Fe in leaves (6 times) compared to negative control ↓ oxidative stress and concentrations of stress-related phytohormones: gibberellins (−46%) and indole-3-acetic acid (−39%) | |||
| Mn-NPs | Chemical method | 20 | 0.05 mg∙L−1 | Vigna radiata (L.) R. Wilczek | Growing plants in perlite medium for 15 days in a phito chamber | ↑ root length (+2%) ↑ stem length (+11%) ↑ number of nodules (+28%) ↑ DW (+50%) ↑ FW (+35%) ↑ absorption of nitrate nitrogen by roots (+48%) ↑ absorption of nitrate nitrogen by leaves (+22%) compared to MnSO4 treatment | [147,148] |
| MnO-NPs | Commercial product US Research Nanomaterials (Houston, TX) | 40 | 0.1 mg∙L−1 | Solanum melongena L. | Plants were grown in a greenhouse on a soilless medium infected with Fusarium wilt fungus for up to 6 weeks, then processed and planted in open ground a week later | ↑ yield (+31%) ↓ area under the disease progression curve (AUDPC) (−28%) | [35] |
| Mn0.5Zn0.5Fe2O4-NPs | Green microwave-assisted hydrothermal method using microwaves at 160 °C | 5–8 | 10 mg∙L−1 | Cucurbita pepo L. | Foliar treatment 20 days after sowing in the field | ↑ yield (+49–53%) compared to untreated plants | [31] |
| 20 mg∙L−1 | ↑ organic matter content (+76–77%) ↑ total energy (250–253 kcal∙g−1) in fruits | ||||||
| Green microwave-assisted hydrothermal method using microwaves at 180 °C | 10–11 | 30 mg∙L−1 | ↑ organic matter content (+73%) ↑ total energy (260 and 258 kcal∙g−1) in leaves | ||||
| Fe–Mn nanocomposites | Green technology using Azadirachta indica leaf extract as a reducing and ethylene glycol as a stabilizing agent | 10–12 | 200 mg∙L−1 | Triticum aestivum L. | Seedlings were grown on nutrient-free sand and treatment solutions were applied by solid matrix priming and foliar treatment Plants were exposed to NaCl salinity | ↑ percentage of germination (2 times) ↑ shoot length (+41%) ↑ shoot FW (+13%) ↑ root length (+12%) ↑ shoot DW (+30%) ↑ root DW (+21%) ↑ MDA (+54%) ↑ proline content (2.2 times) | [149] |
| Fe–Mn nanocomposite-doped GQDs | Synthesis of graphene quantum dots from natural polymer starch | 17 | 200 mg∙L−1 | ↑ shoot FW (2 times) ↑ root FW (+21%) ↑ root DW (+47.6%) ↑ shoot DW (+17%) ↑ MDA (+72%) ↑ proline content (2.1 times) | |||
| 500 mg∙L−1 | ↑ shoot length (+27%) ↑ CAT (+40.5%) ↑ POD (+103%) ↑ glutathione reductase (+130%) ↑ NADPH- oxidase (+141%) ↑ MDA (+43%) ↑ proline content (2.3 times) ↓ stress ↓ root length (−7%) ↓ number of roots (−9%) | ||||||
| ZnO-NPs | Biosynthesis from soil fungus Aspergillus fumigatus TFR-8 | 20–24 | 10 mg∙L−1 | Vigna radiata (L.) R.Wilczek | Plants were grown in pots in greenhouse conditions; NPs were sprayed after 2 weeks of cultivation and samples were taken 2 weeks after treatment | ↑ stem length (+32%) ↑ root length (3 times) ↑ root volume (+61%) ↑ number of nodules (+13%) ↑ enzyme activity: acid phosphatases (2 times), alkaline phosphatases (+53%), phytase (+83%), and dehydrogenase (2 times) ↑ P uptake (5.5 times) ↑ Zn content in leaves (+63%) ↑ Zn content in seeds (2 times) ↑ soil microbial population: bacteria (6 times) and fungi (3 times), incl. actinomycetes (up to 16%) ↑ biochemical parameters: total soluble protein (2 times) and chlorophyll content (4.4 times) compared to the bulk ZnO | [150] |
| ZnO-NPs | Chemical method | 20 | 20 mg∙L−1 | Vigna radiata (L.) R.Wilczek | Incubation in plant agar medium, 60 h | ↑ root length (+42%) ↑ shoot DW (+41%) ↑ shoot length (+98%) ↑ root DW (+76%) | [151] |
| 1 mg∙L−1 | Cicer arietinum L. | ↑ root length (+53%) ↑ root DW (+37%) ↑ shoot length (+6%) ↑ shoot DW (+27%) | |||||
| ZnO-NPs | Precipitation method (Meliorum Technologies, New York, USA) | 9–11 | 400 mg∙kg−1 | Cucumis sativus L. | Growing plants in pots in loamy-sandy soil for 53 days; NPs were applied to the soil | ↑ starch content (+57%) ↑ Mg (+18%) and Zn (+70%) in fruits ↑ root DW (+10%) ↓ Mo (−40%) and Cu (−25%) | [152,153] |
| 800 mg∙kg−1 | ↑ glutelin content (2 times) ↑ Mg (+7%) and Zn (2.5 times) ↑ root DW (+60%) ↑ fruit DW (+6%) ↓ Mo (−53%) and Cu (−19%) | ||||||
| ZnO-NPs | Nanostructured and Amorphous Materials Inc. (Houston, TX, USA) | 12–24 | 1 g∙L−1 | Capsicum chinense Jacq. | Growing in greenhouse conditions; foliar treatments were sprayed to cover the foliage twice at each of the following stages: vegetative growth (VG 45–89 days), flowering (FL 90–140 days), fruit development (FG 141–170 days), and maturity (M 171–205 days) to obtain total Zn amounts of 0.8 and 1.6 mg per plant | ↑ plant height (101%, 9%, and 13% during treatments at stages FL, FG, and M, respectively) ↑ stem diameter (2%, 7%, and 19%) ↑ chlorophyll content (+19%, 23%, and 16%) ↑ number of fruits (+9%) ↑ fruit weight (+3.6%) ↑ yield (+12%) ↑ FW (+2.2%) ↑ DW (+4.3%) compared to ZnSO4 treatments | [154] |
| 2 g∙L−1 | ↑ capsaicin content (+19%) ↑ dihydrocapsaicin content (+11%) ↑ maintenance of Scoville thermal units (+16%) ↑ content of total phenols (+14%) ↑ content of total flavonoids in fruits: soluble (+50%) and bound (+27%) ↑ antioxidant capacity (+15%): DPPH (+32%) and FRAP (antioxidant capacity restored by iron) (+20.5%) ↓ plant height (−10.5% and 11.6% in FG and M treatments) ↓ chlorophyll content (−8.5%, 4.3%, and 6.2% during treatments at stages FL, FG, and M) ↓ number of fruits (−7.3%) ↓ fruit weight (−3.8%) ↓ fruit yield (−11%) ↓ FW (−3%) ↓ DW (−10%) | ||||||
| ZnO-NPs | Chemical method | 20 | 2 g∙L−1 | Brassica napus L. Raphanus raphanistrum subsp. Sativus Lolium perenne L. Lactuca sativa L. Zea mays L. Cucumis sativus L. | Germination in Petri dishes for 5 days in dark conditions | ↑ radish root length (+56%) ↑ rapeseed root length (+38%) ↓ germination of corn seeds (−30%) | [155] |
| Zn-NPs | 35 | 2 g∙L−1 | ↓ seed germination of L. perenne (−41%) | ||||
| ZnO-NPs | Chemical method | 25 | 1 g∙L−1 | Arachis hypogaea L. | Field research | ↑ seed germination (+16%) ↑ shoot length (3 times) ↑ root length (2.4 times) ↑ seedling viability (2.5 times) at the germination stage compared to the ZnSO4 treatment ↑ plant growth (+88%) ↑ chlorophyll content (+42%) at the flowering stage ↑ number of pods per plant (+12%) ↑ yield (+29%) ↓ flowering time (−2 days) | [156] |
| ZnO-NPs | Chemical method | 20 | 0.5 g∙L−1 | Glycine max (L.) Merr. | Growing in laboratory conditions; 1 day of stressful conditions (drought) | ↑ percentage of germination (+89.5%) ↑ germination rate (+6.9%) of seeds in drought conditions | [157] |
| Sol–gel method | 38 | 200 mg∙kg−1 | 120 days of cultivation in open ground | ↑ DW (+15%) ↑ root length (+23%) ↑ root volume (+15%) ↑ root area (+19%) compared with ZnCl2 treatment | [158] | ||
| ZnO-NPs | Chemical method | 20–50 | 2 g∙L−1 | Triticum aestivum L. | 7 days of cultivation in cups with sand in a greenhouse | ↑ chlorophyll a content (+12%) ↑ chlorophyll b content (+12.5%) ↑ protein content (23%) | [159] |
| ZnO-NPs | Biosynthesis from soil fungus Rhizoctonia bataticola TFR-6 | 15–25 | First 2 weeks—10 mg∙L−1, then 4 weeks—16 L ∙ha−1 | Pennisetum glaucum (L.) R.Br. | 6 weeks of cultivation, field experiment, arid zone, foliar treatments | ↑ shoot length (+15%) ↑ root length (+4%) ↑ root volume (+24%) ↑ chlorophyll content (+24%) ↑ total amount of soluble protein (+38.7%) in leaves at the critical stage of growth (6 weeks) of the crop | [160] |
| ZnO-NPs | Sol–gel method | 27–29 | 100 mg∙L−1 (per 1 kg of soil) | Solanum lycopersicum L. | 66 days of cultivation, greenhouse conditions, application of NPs on day 14, measurements on day 28 (application onto soil and aerosol spraying) | ↑ leucopene content in fruits (3 times) with foliar treatment ↑ DW (+41%) when applied to the soil | [161] |
| 250 mg∙L−1 (per 1 kg of soil) | ↑ plant height (+25%) when applied to the soil ↑ root length (+50%) with foliar treatment | ||||||
| 1000 mg∙L−1 (per 1 kg of soil) | ↑ chlorophyll content (3 times) when applied to the soil ↑ yield (+81.9% with foliar treatment and +305.4% when applied to the soil) ↑ leucopene content in fruits (2.5 times) when applied to the soil ↓ leucopene content in fruits (3 times when foliar treatment was applied) ↓ DW (−11% when applied to the soil) | ||||||
| ZnO-NPs | Chemical method | 20 | 10 mg∙L−1 | Allium cepa L. | 10 days, cultivation in Petri dishes | ↑ FW (+11%) ↑ DW (+11%) | [162] |
| ZnO-NPs | Biological synthesis using extracellular secretions of Aspergillus fumigatus TFR-8 | 1–7 | 10 mg∙L−1 | Cyamopsis tetragonoloba L. | 6 weeks of cultivation, foliar treatment on day 14 | ↑ FW (+27%) ↑ shoot length (+32%) ↑ root length (+66%) ↑ root area (+74%) ↑ chlorophyll content (+276%) ↑ total soluble protein in leaves (+27%) ↑ rhizospheric microbial population (+11–14%) ↑ acid phosphatase (+74%) ↑ alkaline phosphatase (+49%) ↑ phytase (+72%) in clusterbean rhizosphere ↑ gum content in clusterbean seeds (+7.5%) | [163] |
| ZnO-NPs | Chemical method | 30 | 500 mg∙L−1 | Sorghum bicolor var M-35-1 | Potted experiment under controlled conditions | ↑ leaf area index (+7%) ↑ DW (+16%) ↑ grain yield (+9.5%) ↑ Zn content in grain (+5.6%) compared to bulk ZnSO4 | [164] |
| ZnO-NPs | Chemical method | 10 | 1.6 g∙L−1 | Medicago sativa L. | Germination in Petri dishes | ↓ germination percentage (−40%) ↓ root length (2 times) | [165] |
| 200 mg∙L−1 | Cucumis sativus L. | ↑ root length (2.7 times) ↓ FW (−13%) | |||||
| 1600 mg∙L−1 | ↑ germination percentage (+10%) | ||||||
| 800 g∙L−1 | Solanum lycopersicum L. | ↓ root length (−43%), ↑ FW (+35%) | |||||
| 1600 mg∙L−1 | ↓ germination percentage (−20%) ↓ root length (−42%) | ||||||
| ZnO-NPs | Green synthesis using leaf extract of Moringa oleifera Lam. | 15–30 | 10–250 mg∙L−1 | Amaranthus caudatus L. | Germination in Petri dishes | Did not have a significant effect on germination | [166] |
| 500 mg∙L−1 | ↓ germination percentage (−5–10%) | ||||||
| 10 mg∙L−1 | Growing in pots in greenhouse conditions, spraying with NPs was carried out in the 4-leaf phase, watering with Hoagland’s solution, sampling was carried out 30 days after treatment | ↑ plant height (+35%) ↑ FW (+67%) ↑ agronomic efficiency (3.7 times) ↑ physiological efficiency (3.9 times) Zn concentration in plants did not change compared to the bulk analog (ZnSO4) | |||||
| ZnO-NPs | Green synthesis using leaf litter of E. lanceolatus | 100 | 200 mg∙L−1 | Zea mays L. var. PG2458 | Seed primer | ↑ germination percentage (+13%) ↑ seed vigor index (+50%) ↑ shoot length (+27%) ↑ root length (+71%) ↑ FW (+12%) compared to bulk analog (ZnSO4) | [167] |
| Foliar treatments in greenhouse conditions 40 days before ripening | ↑ leaf surface area (+10%) ↑ stem diameter (+3%) ↑ number of leaves (+15%) ↑ chlorophyll a content (+20%) ↑ chlorophyll b content (+53%) ↑ carotenoid content (+14%) ↑ protein content (+23%) ↑ SOD (+20%) ↑ CAT (+50%) ↑ Zn in grains (+33%) ↑ total Zn content (+33%) compared to bulk analog (ZnSO4) | ||||||
| Urea coated by ZnO-NPs | Chemical method | 50–90 | 0.5% | Triticum aestivum L. | Growing in soil in a field | ↑ plant height ↑ root length ↑ root volume ↑ grain yield ↑ DW | [168] |
| ZnO-NPs | Green synthesis using leaf extract of Terminalia bellirica (Gaertn.) Roxb. | 22 | 200 mg∙L−1 | Brassica juncea L. | Growing in the field | ↑ length of roots and shoots ↑ number of seeds ↑ seed weight ↑ oil content ↓ disease damage (−70%) | [169,170] |
| ZnO-NPs | Chemical method | 20 | 10, 20, 50, 100, 200, and 1000 mg∙L−1 | Lolium perenne L. | 12 days of growing in hydroponics | ↓ FW Morphological abnormalities: root tips shriveled, epidermal and cortical root cells were severely vacuolated or destroyed | [171] |
| Cu-NPs | Chemical method | 50 | 200, 400, 600, 800, and 1000 mg∙L−1 | Vigna radiata (L.) R.Wilczek Triticum aestivum subsp. Aestivum | Growing on plant agar medium | NPs were toxic and bioavailable to both species, with mung being more sensitive to the trace element than prenica (toxicity concentrations of 335 and 570 mg∙L−1, respectively) | [172] |
| Cu-NPs | 100, 500 mg∙L−1 | Cucurbita pepo L. | 14 days of growing in hydroponics without humic acid and with its addition (50 mg∙L−1) | Cu-NPs and bulk Cu solution at all concentrations were found to be phytotoxic; humic acid decreased the ion content in bulk Cu solution but increased Cu2+ in Cu-NPs solutions | [173] | ||
| Cu-NPs | Chemical method | 40–60 | 13 and 66 g∙kg−1 | Lactuca sativa L. | 15 days of cultivation in soil; assessment of microbial colonies and ecotoxicity of NPs | NPs did not have a significant effect on soil microbiota and seed germination; Cu-NPs at higher concentrations and the combination of Au-NPs + Cu-NPs significantly affected plant growth after 15 days of incubation | [174] |
| Au-NPs + Cu-NPs | 100 + 50 | 13 + 13 mg∙kg−1 | ↑ ratio of shoots and roots compared to control | ||||
| 70% CuO, 30% Cu2O (Cu-NPs) | Chemical method | 30 | 0.025, 0.25, 0.5, 1, and 5 mg∙L−1 Cu | Elodea densa Planch | 3 days of growing in hydroponics | ↑ lipid peroxidation (up to 120 and 180%) ↑ CAT and SOD activity (1.5–2.0 times) phytotoxicity was observed at a concentration of 1.0 mg∙L−1 | [175] |
| Mo-NPs | Chemical method | 100–250 | 8 mg∙L−1 | Cicer arietinum L. | Soil rhizosphere study “Experience options:” Control/microbiological preparation/colloidal Mo-NPs/microbiological preparation + Mo-NPs | ↑ number of nodules (0.6/6.7/3.3/12.8 pcs per plant) ↑ mass of nodules (90/560/770/780 mg per plant) ↑ antioxidant enzyme activity ↑ activity of symbiotic bacteria | [176] |
| 100 | 100 mg∙L−1 | Nicotiana tabacum L. | Root and foliar treatments when grown in plastic containers in an artificial climate condition | ↑ lignification of root cells ↑ the number of vascular bundles in tissues, especially when applied using root irrigation ↑ photosynthetic rate (+131%) ↑ MDA Foliar treatment: ↑ chlorophyll concentration (+67%) ↑ protein content (+61%) ↑ stomatal conductance (5 times) ↑ Mo in roots (14 times), in roots (9 times) Root treatment: ↑ Mo in roots (11 times), in roots (8 times) ↑ soluble sugar content (+67%) ↑ protein content (+73%) ↑ chlorophyll content (3.6 times) ↑ plant height (+50%) ↑ FW (+67%) ↑ DW (+75%) | [177] | ||
| MoS2 NS | Chemical method | 20 × 106 | 10 mg∙kg−1 | Glycine max (L.) Merr. | Cultivation in a greenhouse for 115 days, watered with Hoagland’s solution | ↑ seed yield (+30%) compared to the application of traditional molybdenum fertilizers (Na2MoO4) ↑ nitrogenase activity (+122%) ↑ total nitrogen content in nodules (+27%) ↑ Mo (2 times) | [178] |
| Ca-NPs, B-NPs, and nanocomplexes | Chemical method | 60 | 200 mg∙L−1 | Arachis hypogea L. | Field experiment, foliar spraying at the vegetation stage (30 and 60 days after sowing) | ↑ plant height (+13%) ↑ branching number (+10%) ↑ DW (+11.2%) ↑ growth rate (+22%) ↑ SPAD (+13%) ↑ seed yield (1.8 times) ↑ 100-seed weight (+16%) ↑ protein content (+26%) ↑ oil (+17%) ↑ N (+27%), P (+10%), and K (2.5 times) | [39] |
| Ca3(BO3)2-NPs | Combination of co-precipitation and heat treatment methods | 80 | 30 mg∙L−1 | Lactuca sativa L. | Plants were grown in greenhouse conditions for 60 days on a modified Hoagland solution with and without boron; NPs were sprayed at intervals of 10 days | ↑ shoot height (2.7 times) ↑ root length (1.9 times) ↑ FW (+58%) ↓ DPPH activity (−32%) | [179] |
| Cucurbita pepo L. | ↑ shoot height (+18%) ↑ root length (+66%) ↑ phenolic compounds (+51%) compared to control (without B) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semenova, N.A.; Burmistrov, D.E.; Shumeyko, S.A.; Gudkov, S.V. Fertilizers Based on Nanoparticles as Sources of Macro- and Microelements for Plant Crop Growth: A Review. Agronomy 2024, 14, 1646. https://doi.org/10.3390/agronomy14081646
Semenova NA, Burmistrov DE, Shumeyko SA, Gudkov SV. Fertilizers Based on Nanoparticles as Sources of Macro- and Microelements for Plant Crop Growth: A Review. Agronomy. 2024; 14(8):1646. https://doi.org/10.3390/agronomy14081646
Chicago/Turabian StyleSemenova, Natalia A., Dmitriy E. Burmistrov, Sergey A. Shumeyko, and Sergey V. Gudkov. 2024. "Fertilizers Based on Nanoparticles as Sources of Macro- and Microelements for Plant Crop Growth: A Review" Agronomy 14, no. 8: 1646. https://doi.org/10.3390/agronomy14081646
APA StyleSemenova, N. A., Burmistrov, D. E., Shumeyko, S. A., & Gudkov, S. V. (2024). Fertilizers Based on Nanoparticles as Sources of Macro- and Microelements for Plant Crop Growth: A Review. Agronomy, 14(8), 1646. https://doi.org/10.3390/agronomy14081646









