Unveiling Nitrogen Fertilizer in Medicinal Plant Cultivation
Abstract
1. Introduction
2. Materials and Methods
2.1. Nitrogen Fertilization Database of Reported Medicinal Plants
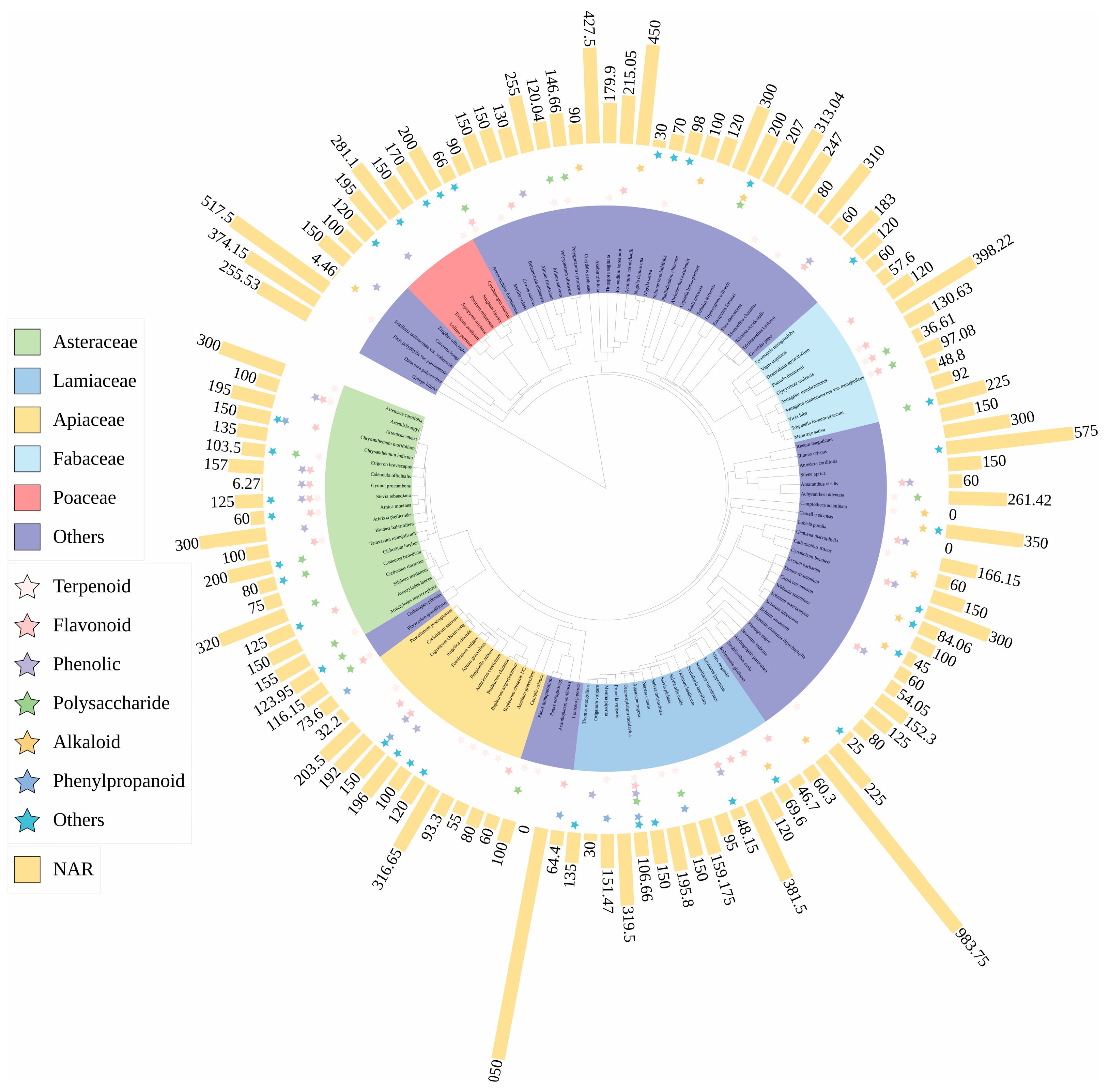
2.2. Phylogenetic Tree of Medicinal Species
2.3. Statistics and Calculation of Phylogenetic Distribution of N Fertilization
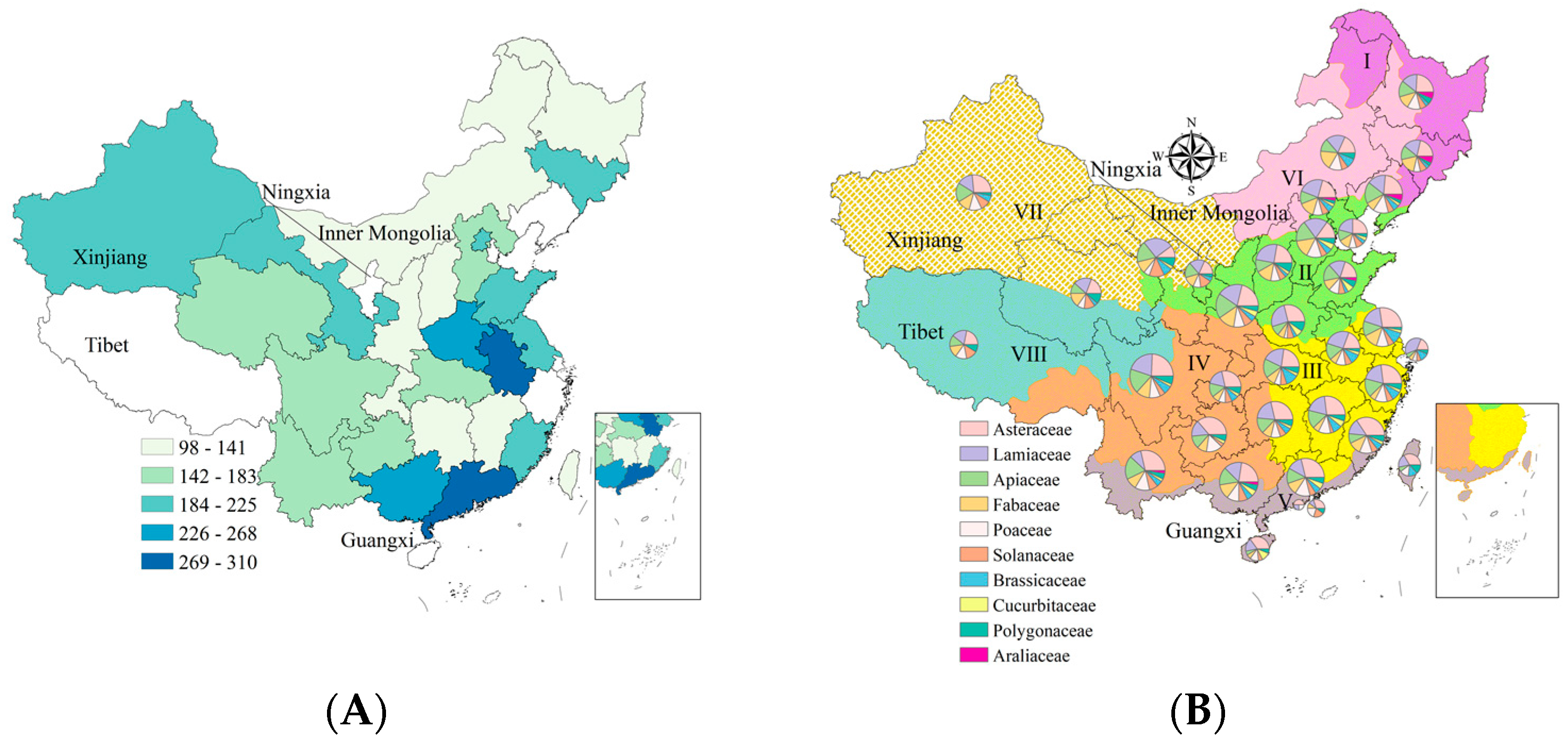
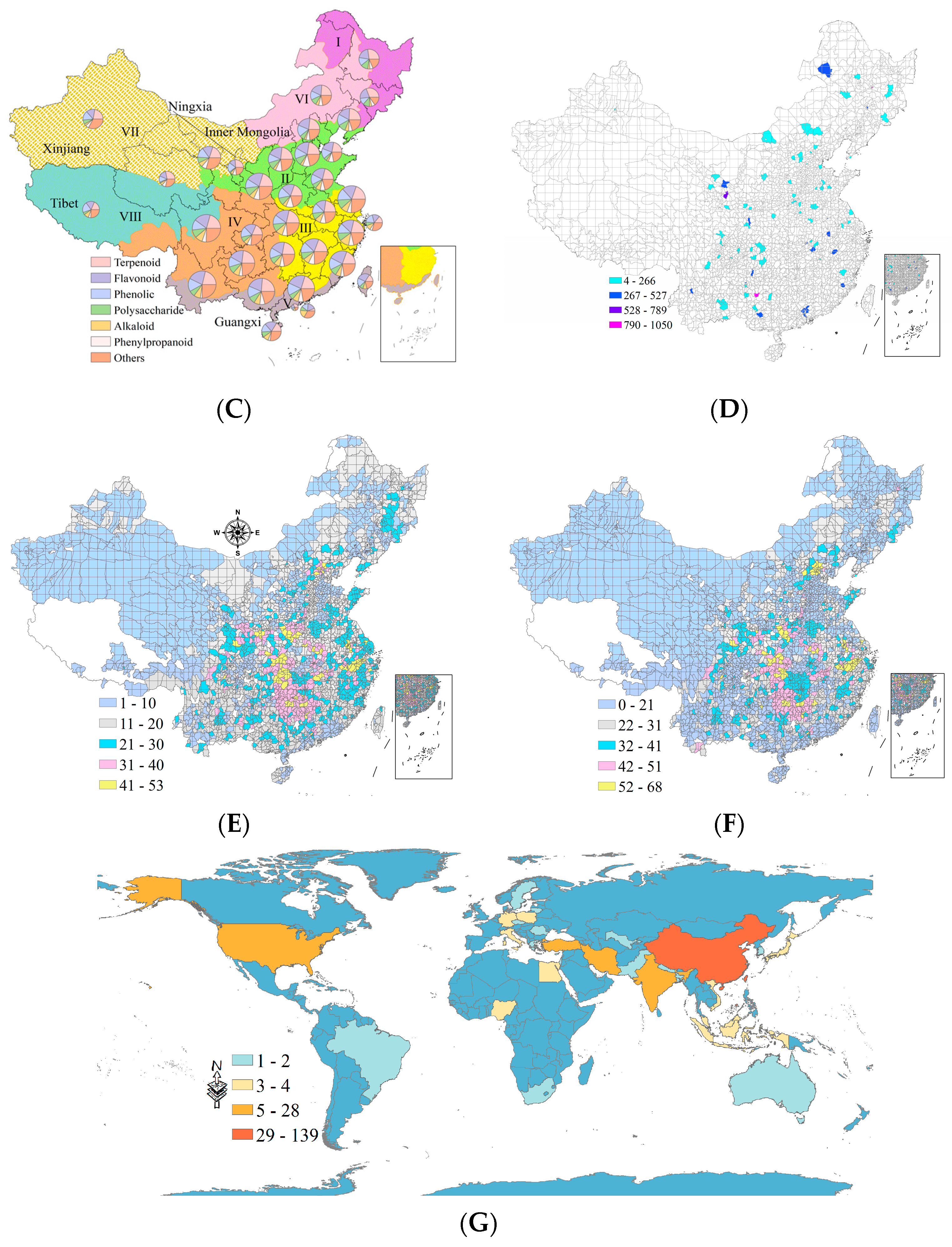
2.4. Geographical Distribution of Reported Medicinal Plants and N Fertilization
2.5. Statistical Analyses
3. Results and Discussion
3.1. Phylogenetic Distribution of Reported Medicinal Species with N Fertilization
3.2. Global-, Provincial-, and County-Level Distribution of Medicinal Plants with N Fertilization
3.3. Amount of Nitrogen Fertilizer Applied to Medicinal Crops
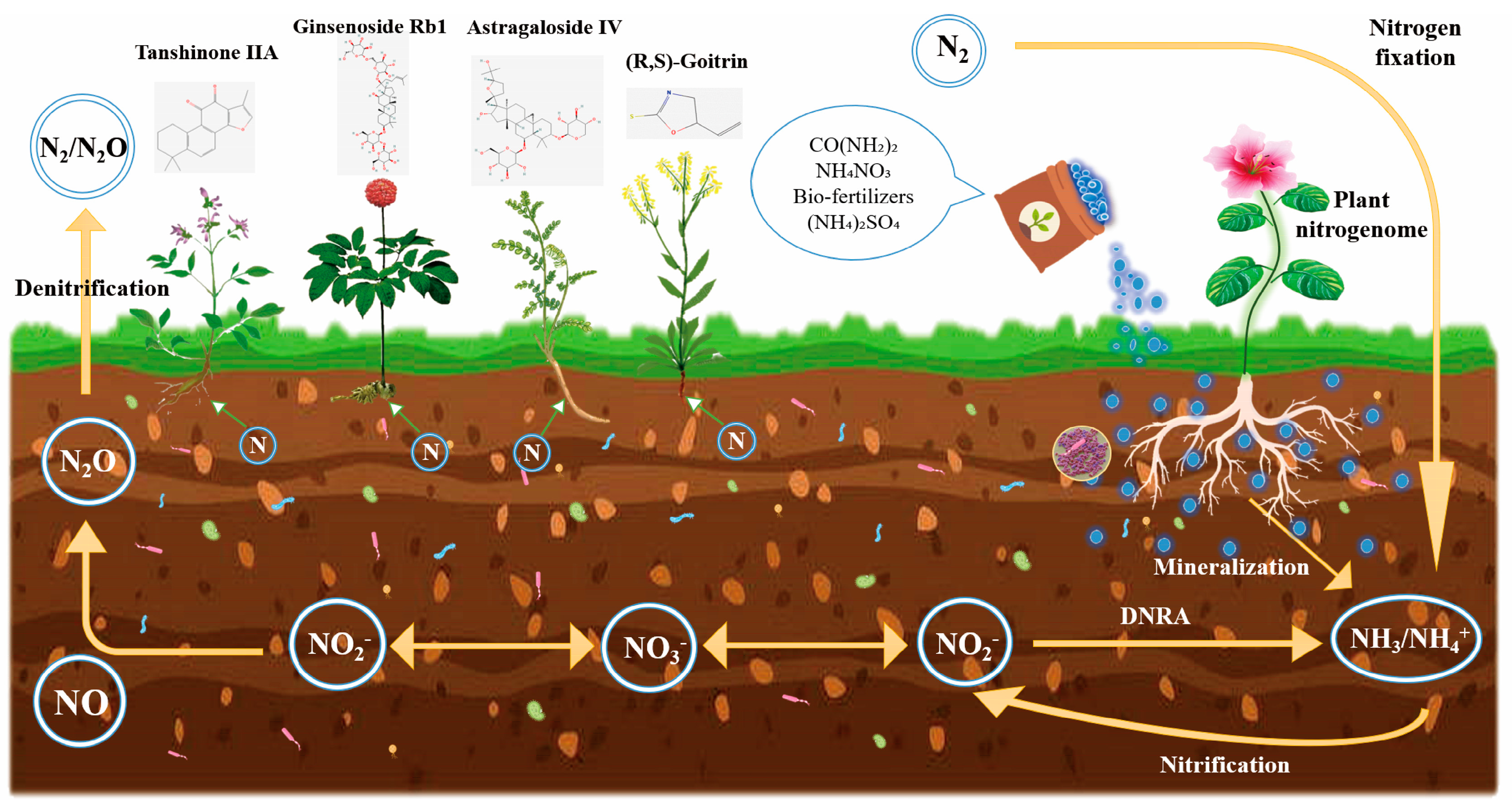
3.4. Effects of N Fertilizer on Medicinal Phytometabolites
3.5. Pot Experiments, Hydroponic Studies, and Others
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Swarbreck, S.M.; Wang, M.; Wang, Y.; Kindred, D.; Sylvester-Bradley, R.; Shi, W.; Varinderpal-Singh; Bentley, A.R.; Griffiths, H. A roadmap for lowering crop nitrogen requirement. Trends Plant Sci. 2019, 24, 892–904. [Google Scholar] [CrossRef] [PubMed]
- Oliva, M.; Guy, A.; Galili, G.; Dor, E.; Schweitzer, R.; Amir, R.; Hacham, Y. Enhanced production of aromatic amino acids in tobacco plants leads to increased phenylpropanoid metabolites and tolerance to stresses. Front Plant Sci. 2021, 11, 604349. [Google Scholar] [CrossRef]
- Bergman, M.E.; Davis, B.; Phillips, M.A. Medically useful plant terpenoids: Biosynthesis, occurrence, and mechanism of action. Molecules 2019, 24, 3961. [Google Scholar] [CrossRef] [PubMed]
- Hao, D.C.; Wang, L.; Gao, W.; Xie, H.T.; Bao, X.L.; Jia, Z.J.; Wang, L.F. Disentangling effects of moisture/gas regimes on microbial community, network configuration and nitrogen turnover of black soil. Eurasian Soil Sci. 2021, 54 (Supp. 1), 42–61. [Google Scholar] [CrossRef]
- Hao, D.C.; Su, X.Y.; Xie, H.T.; Bao, X.L.; Zhang, X.D.; Wang, L.F. Effects of tillage patterns and stover mulching on N2O production, nitrogen cycling genes and microbial dynamics in black soil. J. Environ. Manag. 2023, 345, 118458. [Google Scholar] [CrossRef]
- Wang, L.; Hao, D.C.; Fan, S.S.; Xie, H.T.; Bao, X.L.; Jia, Z.J.; Wang, L.F. N2O emission and nitrification/denitrification bacterial communities in upland black soil under combined effects of early and immediate moisture. Agriculture 2022, 12, 330. [Google Scholar] [CrossRef]
- Tegeder, M.; Masclaux-Daubresse, C. Source and sink mechanisms of nitrogen transport and use. New Phytol. 2018, 217, 35–53. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Kang, C.Z.; Wan, X.F.; Wang, S.; Lyu, C.G.; Zhang, W.J.; Yuan, Q.J.; Yan, B.B.; Guo, L.P. Current situation of nitrogen application and its effects on yield and quality of Chinese materia medica. China J. Chin. Mater. Med. 2021, 46, 1883–1892. [Google Scholar]
- Dilena, E.; Close, D.C.; Hunt, I.; Garland, S.M. Investigating how nitrogen nutrition and pruning impacts on CBD and THC concentration and plant biomass of Cannabis sativa. Sci. Rep. 2023, 13, 19533. [Google Scholar] [CrossRef]
- Huang, P. Effects of fertilization on the yield and root diameter of C. pilosula. J. Chin. Med. Mater. 1999, 22, 1–5. [Google Scholar]
- Sugier, D.; Sugier, P.; Jakubowicz-Gil, J.; Gawlik-Dziki, U.; Zając, A.; Król, B.; Chmiel, S.; Kończak, M.; Pięt, M.; Paduch, R. Nitrogen fertilization and solvents as factors modifying the antioxidant and anticancer potential of Arnica montana L. flower head extracts. Plants 2023, 12, 142. [Google Scholar] [CrossRef] [PubMed]
- Cun, Z.; Wu, H.M.; Zhang, J.Y.; Shuang, S.P.; Hong, J.; An, T.X.; Chen, J.W. High nitrogen inhibits biomass and saponins accumulation in a medicinal plant Panax notoginseng. PeerJ 2023, 11, e14933. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.H.; Jaafar, H.Z.; Rahmat, A.; Rahman, Z.A. Involvement of nitrogen on flavonoids, glutathione, anthocyanin, ascorbic acid and antioxidant activities of Malaysian medicinal plant Labisia pumila Blume (Kacip Fatimah). Int. J. Mol. Sci. 2012, 13, 393–408. [Google Scholar] [CrossRef] [PubMed]
- Xia, F.Q. The Study of Salvia Nutritional Characteristics and Fertilizer Law. Master’s Thesis, Henan University of Science and Technology, Luoyang, China, 2010. [Google Scholar]
- Zhang, M.; Sharma, A.; León, F.; Avery, B.; Kjelgren, R.; McCurdy, C.R.; Pearson, B.J. Effects of nutrient fertility on growth and alkaloidal content in Mitragyna speciosa (Kratom). Front. Plant Sci. 2020, 11, 597696. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Yu, L.; He, P.; Feng, H.; Wang, J.; Zhang, H.; Song, Y.; Liu, R.; Li, Y. Integrated transcriptome and metabolome analysis reveals the regulation of phlorizin synthesis in Lithocarpus polystachyus under nitrogen fertilization. BMC Plant Biol. 2024, 24, 366. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Xu, G.; Luo, M.; Tan, Q.S.; Luo, S.; Deng, C.F. Effect of nitrogen, phosphorus, and potassium fertilizer application on the yield and quality of Peucedanum praeruptorum. Chin. Trad. Pat. Med. 2024, 46, 329–333. [Google Scholar]
- Fan, Y.; Xu, E.; Wang, G.; He, D.; Ma, J.; Liu, Y.; Li, X.; Luo, A. Transcriptional and physiological analysis reveal new insights into the regulation of fertilization (N, P, K) on the growth and synthesis of medicinal components of Dendrobium denneanum. Int. J. Mol. Sci. 2023, 24, 1522. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Yang, Q.; Tang, X.; Cheng, X.; Zhang, C.; Pan, H. Effects of combined application of nitrogen, phosphorous and potassium on the yield and quality of Desmodium styracifolium. Guihaia 2014, 34, 426–430. [Google Scholar]
- Song, P.Z. Effect of Different Nitrogen Application Rates on the Yield and Quality of Codonopsis pilosula ‘Lu Dangshen’. Master’s Thesis, Shanxi Agricultural University, Jinzhong, China, 2022. [Google Scholar]
- Tseng, H.M.; Lu, T.M.; Ng, L.T. Responses of Cynanchum taiwanianum and its bioactive compound biosynthesis to levels of nitrogen and potassium fertilization. Agronomy 2022, 12, 180. [Google Scholar] [CrossRef]
- Liu, J.; Lu, F.; Zhu, Y.; Wu, H.; Ahmad, I.; Dong, G.; Zhou, G.; Wu, Y. The effects of planting density and nitrogen application on the growth quality of alfalfa forage in saline soils. Agriculture 2024, 14, 302. [Google Scholar] [CrossRef]
- Wang, Y.; He, X.; Li, F.; Deng, H.; Wang, Z.; Huang, C.; Han, Y.; Ba, Y.; Lei, L.; Zhang, C. Effects of water and nitrogen coupling on the photosynthetic characteristics, yield, and quality of Isatis indigotica. Sci. Rep. 2021, 11, 17356. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.W.; Liu, Y.; Li, J.; Yang, Y.; Miao, Y.; Liu, D.H. Effect of different nitrogen application rates on growth, yield and quality of two-year-old Belamcanda chinensis. Chin. J. Exp. Tradit. Med. Formulae 2022, 28, 135–141. [Google Scholar]
- Parrey, Z.A.; Shah, S.H.; Fayaz, M.; Casini, R.; Elansary, H.O.; Mohammad, F. Nitrogen supplementation modulates morphological, biochemical, yield and quality attributes of peppermint. Plants 2023, 12, 809. [Google Scholar] [CrossRef] [PubMed]
- Izadi, Z.; Biabani, A.; Sabouri, H.; Bahreininejad, B. The effect of different levels of urea and planting density on the phytochemical characteristics, alkaloids, and yield of the medicinal plant jimsonweed (Datura stramonium L.). Crop Sci. 2022, 62, 1264–1276. [Google Scholar] [CrossRef]
- Tavarini, S.; Sgherri, C.; Ranieri, A.M.; Angelini, L.G. Effect of nitrogen fertilization and harvest time on steviol glycosides, flavonoid composition, and antioxidant properties in Stevia rebaudiana Bertoni. J. Agric. Food Chem. 2015, 63, 7041–7050. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Nada, R.S.; Mady, E.; Ashmawi, A.E.; Gashash, E.A.; Elateeq, A.A.; Suliman, A.A.; Al-Harbi, N.A.; Al-Qahtani, S.M.; Zarad, M.M.; et al. Effect of organic and bio-fertilization on fruit yield, bioactive constituents, and estragole content in fennel fruits. Agronomy 2023, 13, 1189. [Google Scholar] [CrossRef]
- Gao, S.C. Effect of Different Nitrogen Levels and Forms on the Growth and Metabolism in Isodon rubescens. Master’s Thesis, Zhengzhou University, Guiyang, China, 2021. [Google Scholar]
- He, X.; Qiu, H.; Xie, K.; Wang, Y.C.; Hu, J.; Li, F.Q.; An, J. Effects of water saving and nitrogen reduction on the yield, quality, water and nitrogen use efficiency of Isatis indigotica in Hexi Oasis. Sci. Rep. 2022, 12, 550. [Google Scholar] [CrossRef]
- Hao, D.C.; Lyu, H.Y.; Wang, F.; Xiao, P.G. Evaluating potentials of species rich taxonomic groups in cosmetics and dermatology: Clustering and dispersion of skin efficacy of Asteraceae and Ranunculales plants on the species phylogenetic tree. Curr. Pharm. Biotechnol. 2023, 24, 279–298. [Google Scholar] [CrossRef]
- Shan, Z.J.; Ye, J.F.; Hao, D.C.; Xiao, P.G.; Chen, Z.D.; Lu, A.M. Distribution patterns and industry planning of commonly used traditional Chinese medicinal plants in China. Plant Divers. 2022, 44, 255–261. [Google Scholar] [CrossRef]
- Ou, X.; Cui, X.; Zhu, D.; Guo, L.; Liu, D.; Yang, Y. Lowering nitrogen and increasing potassium application level can improve the yield and quality of Panax notoginseng. Front. Plant Sci. 2020, 11, 595095. [Google Scholar] [CrossRef]
- Sun, J.; Li, W.; Zhang, Y.; Guo, Y.; Duan, Z.; Tang, Z.; Abozeid, A. Metabolomics analysis reveals potential mechanisms in Bupleurum L. (Apiaceae) induced by three levels of nitrogen fertilization. Agronomy 2021, 11, 2291. [Google Scholar] [CrossRef]
- Ye, S.; Peng, B.; Liu, T. Effects of organic fertilizers on growth characteristics and fruit quality in Pear-jujube in the Loess Plateau. Sci. Rep. 2022, 12, 13372. [Google Scholar] [CrossRef] [PubMed]
- Sugier, D.; Olesińska, K.; Sugier, P.; Wójcik, M. Chemical composition of essential oil from flower heads of Arnica Chamissonis Less. under a nitrogen impact. Molecules 2019, 24, 4454. [Google Scholar] [CrossRef] [PubMed]
- Ganjineh, E.; Babaii, F.; Mozafari, A.; Mirzaei Heydari, M.; Naseri, R. Effect of urea, compost, manure and bio-fertilizers on yield, percentage and composition of fatty acids of sesame seed oil (Sesamum indicum L.). Cell. Mol. Biol. 2019, 65, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Lakshmanan, P.; Wang, X.; Xiong, H.; Yang, L.; Liu, B.; Shi, X.; Chen, X.; Wang, J.; Zhang, Y.; et al. Global reactive nitrogen loss in orchard systems: A review. Sci. Total Environ. 2022, 821, 153462. [Google Scholar] [CrossRef]
- Cui, M.; Zeng, L.; Qin, W.; Feng, J. Measures for reducing nitrate leaching in orchards: A review. Environ. Pollut. 2020, 263 Pt B, 114553. [Google Scholar] [CrossRef]
- Jackson, R.D. Soil nitrate leaching under grazed cool-season grass pastures of the North Central US. J. Sci. Food Agric. 2020, 100, 5307–5312. [Google Scholar] [CrossRef]
- Ahmadi, F.; Samadi, A.; Rahimi, A. Improving growth properties and phytochemical compounds of Echinacea purpurea (L.) medicinal plant using novel nitrogen slow release fertilizer under greenhouse conditions. Sci. Rep. 2020, 10, 13842. [Google Scholar] [CrossRef]
- Elrys, A.S.; El-Maati, M.F.; Abdel-Hamed, E.M.; Arnaout, S.M.; El-Tarabily, K.A.; Desoky, E.M. Mitigate nitrate contamination in potato tubers and increase nitrogen recovery by combining dicyandiamide, moringa oil and zeolite with nitrogen fertilizer. Ecotoxicol. Environ. Saf. 2021, 209, 111839. [Google Scholar] [CrossRef]
- Askary, M.; Behdani, M.A.; Mollaei, H.; Fallahi, H.R.; Azarmi-Atajan, F.; Macinaei, H.M. Bioactive compounds and apoptotic effects of saffron (Crocus sativus L.) in different fertilizer conditions. Biochem. Syst. Ecol. 2024, 114, 104806. [Google Scholar] [CrossRef]
- Liava, V.; Karkanis, A.; Tsiropoulos, N. Yield and silymarin content in milk thistle (Silybum marianum (L.) Gaertn.) fruits affected by the nitrogen fertilizers. Ind. Crops Prod. 2021, 171, 113955. [Google Scholar] [CrossRef]
- Yaldiz, G.; Camlica, M.; Ozen, F. Biological value and chemical components of essential oils of sweet basil (Ocimum basilicum L.) grown with organic fertilization sources. J. Sci. Food Agric. 2019, 99, 2005–2013. [Google Scholar] [CrossRef]
- Wang, J.R.; Huo, A.L.; Zhang, X.L.; Gong, Y.H.; Han, S.; Liang, Z.S. Influences of nitrogen and phosphorus fertilizer on the yield and quality of chinese honeysuckle. Chin. J. Soil Sci. 2009, 40, 847–851. [Google Scholar]
- Yang, W.; Li, C.; Wang, S.; Zhou, B.; Mao, Y.; Rensing, C.; Xing, S. Influence of biochar and biochar-based fertilizer on yield, quality of tea and microbial community in an acid tea orchard soil. Appl. Soil Ecol. 2021, 166, 104005. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, J.; Ma, Y.; Yin, M.; Jia, Q.; Tian, R.; Kang, Y.; Qi, G.; Wang, C.; Jiang, Y.; et al. Appropriate water and nitrogen regulation improves the production of wolfberry (Lycium barbarum L.). Agronomy 2024, 14, 607. [Google Scholar] [CrossRef]
- Abkar, E.; Nabizadeh, E.; Roshdi, M.; Yezdanseta, S. Reaction of dragonhead medicinal plant to nitrogen and micronutrient nutrition. J. Plant Nutr. 2022, 45, 1906–1918. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, F.; He, X.; Dong, Y.; Sun, K.; Liu, S.; Wang, X.; Yang, H.; Zhang, W.; Lakshmanan, P.; et al. Comparative metabolomics reveals complex metabolic shifts associated with nitrogen-induced color development in mature pepper fruit. Front Plant Sci. 2024, 15, 1319680. [Google Scholar] [CrossRef] [PubMed]
- Hameed, M.K.; Umar, W.; Razzaq, A.; Aziz, T.; Maqsood, M.A.; Wei, S.; Niu, Q.; Huang, D.; Chang, L. Differential metabolic responses of lettuce grown in soil, substrate and hydroponic cultivation systems under NH4+/NO3− application. Metabolites. 2022, 12, 444. [Google Scholar] [CrossRef] [PubMed]
- Malaka, M.J.; Araya, N.A.; Soundy, P.; du Plooy, C.P.; Araya, H.T.; Jansen Van Rensburg, W.S.; Watkinson, E.; Levember, E.; Wadiwala, E.; Amoo, S.O. Biomass, essential oil yield, and composition of marjoram as influenced by interactions of different agronomic practices under controlled conditions. Plants. 2022, 12, 173. [Google Scholar] [CrossRef]
- Landi, S.; Berni, R.; Capasso, G.; Hausman, J.F.; Guerriero, G.; Esposito, S. Impact of nitrogen nutrition on Cannabis sativa: An update on the current knowledge and future prospects. Int. J. Mol. Sci. 2019, 20, 5803. [Google Scholar] [CrossRef]
- Bernstein, N.; Gorelick, J.; Zerahia, R.; Koch, S. Impact of N, P, K, and humic acid supplementation on the chemical profile of medical cannabis (Cannabis sativa L). Front Plant Sci. 2019, 10, 736. [Google Scholar] [CrossRef] [PubMed]
- Massuela, D.C.; Munz, S.; Hartung, J.; Nkebiwe, P.M.; Graeff-Hönninger, S. Cannabis Hunger Games: Nutrient stress induction in flowering stage—Impact of organic and mineral fertilizer levels on biomass, cannabidiol (CBD) yield and nutrient use efficiency. Front. Plant Sci. 2023, 14, 1233232. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.L., II; Pearson, B.; Kjelgren, R.; Brym, Z. Response of essential oil hemp (Cannabis sativa L.) growth, biomass, and cannabinoid profiles to varying fertigation rates. PLoS ONE 2021, 16, e0252985. [Google Scholar] [CrossRef] [PubMed]
- Farnisa, M.M.; Miller, G.C.; Solomon, J.K.Q.; Barrios-Masias, F.H. Floral hemp (Cannabis sativa L.) responses to nitrogen fertilization under field conditions in the high desert. PLoS ONE 2023, 18, e0284537. [Google Scholar] [CrossRef] [PubMed]
- Song, J.S.; Jung, S.; Jee, S.; Yoon, J.W.; Byeon, Y.S.; Park, S.; Kim, S.B. Growth and bioactive phytochemicals of Panax ginseng sprouts grown in an aeroponic system using plasma-treated water as the nitrogen source. Sci. Rep. 2021, 11, 2924. [Google Scholar] [CrossRef]
- Cun, Z.; Zhang, J.Y.; Hong, J.; Yang, J.; Gao, L.L.; Hao, B.; Chen, J.W. Integrated metabolome and transcriptome analysis reveals the regulatory mechanism of low nitrogen-driven biosynthesis of saponins and flavonoids in Panax notoginseng. Gene 2024, 901, 148163. [Google Scholar] [CrossRef]
- Cun, Z.; Li, X.; Zhang, J.Y.; Hong, J.; Gao, L.L.; Yang, J.; Ma, S.Y.; Chen, J.W. Identification of candidate genes and residues for improving nitrogen use efficiency in the N-sensitive medicinal plant Panax notoginseng. BMC Plant Biol. 2024, 24, 105. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jiang, H.; Yan, H.; Jiang, X.; Ma, Y.; Qin, Y. Carbon and nitrogen metabolism under nitrogen variation affects flavonoid accumulation in the leaves of Coreopsis tinctoria. PeerJ 2021, 9, e12152. [Google Scholar] [CrossRef]
- Gou, Y.; Jing, Y.; Song, J.; Nagdy, M.M.; Peng, C.; Zeng, L.; Chen, M.; Lan, X.; Htun, Z.L.L.; Liao, Z.; et al. A novel bHLH gene responsive to low nitrogen positively regulates the biosynthesis of medicinal tropane alkaloids in Atropa belladonna. Int. J. Biol. Macromol. 2024, 266 Pt 1, 131012. [Google Scholar] [CrossRef]
- Igarashi, M.; Fuchino, H.; Sakurai, M.; Matsuba, T.; Hishida, A. Efficient fertilization in the cultivation of Angelica acutiloba (Siebold & Zucc.) Kitag. in Hokkaido: Effect of amount of supplied nitrogen on growth, yield, and quality of A. acutiloba. J. Nat. Med. 2022, 76, 298–305. [Google Scholar]
- Lasheen, F.F.; Negm, A.H.; Hassan, S.E.; Azab, E.; Gobouri, A.A.; Hewidy, M. Nitrogen, phosphorous, and potassium application rate on the young seedling growth of Salvadora persica. Agriculture 2021, 11, 291. [Google Scholar] [CrossRef]
- Mao, X.; Zhang, G.; Liu, Z.; Huang, F.; Yu, F. Combined Application of Nitrogen and Phosphorus Promotes the Growth and Nutrient Accumulations of Cinnamomum camphora Container Seedlings. Agriculture 2024, 14, 280. [Google Scholar] [CrossRef]
- Guo, L.L.; Guo, S.; Dong, L.L.; Shen, L.; Li, X.W.; Xu, J.; Chen, S.L. Key techniques for precision cultivation of nitrogenous fertilizer of pollution-free ginseng. China J. Chin. Mater. Med. 2018, 43, 1427–1433. [Google Scholar]
- Karlińska, E.; Kaczorowska, O.; Romanowska, B.; Kosmala, M. Nutritional and polyphenolic composition of Agrimonia procera Wallr. from experimental cultivation with different levels of nitrogen fertilization. Molecules 2022, 27, 7597. [Google Scholar] [CrossRef] [PubMed]
- Hao, D.L.; Zhou, J.Y.; Li, L.; Qu, J.; Li, X.H.; Chen, R.R.; Kong, W.Y.; Li, D.D.; Li, J.J.; Guo, H.L.; et al. An appropriate ammonium: Nitrate ratio promotes the growth of centipedegrass: Insight from physiological and micromorphological analyses. Front. Plant Sci. 2023, 14, 1324820. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, F.; Samadi, A.; Sepehr, E.; Rahimi, A.; Shabala, S. Optimizing hydroponic culture media and NO3-/NH4+ ratio for improving essential oil compositions of purple coneflower (Echinacea purpurea L.). Sci. Rep. 2021, 11, 8009. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, F.; Samadi, A.; Sepehr, E.; Rahimi, A.; Shabala, S. Increasing medicinal and phytochemical compounds of coneflower (Echinacea purpurea L.) as affected by NO3−/NH4+ ratio and perlite particle size in hydroponics. Sci. Rep. 2021, 11, 15202. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Dixon, M.; Saxena, P.K. Growing environment and nutrient availability affect the content of some phenolic compounds in echinacea purpurea and echinacea angustifolia. Planta Med. 2006, 72, 1407–1414. [Google Scholar] [CrossRef]
- Geem, K.R.; Kim, J.; Bae, W.; Jee, M.G.; Yu, J.; Jang, I.; Lee, D.Y.; Hong, C.P.; Shim, D.; Ryu, H. Nitrate enhances the secondary growth of storage roots in Panax ginseng. J. Ginseng Res. 2023, 47, 469–478. [Google Scholar] [CrossRef]
- Salehi, A.; Tasdighi, H.; Gholamhoseini, M. Evaluation of proline, chlorophyll, soluble sugar content and uptake of nutrients in the German chamomile (Matricaria chamomilla L.) under drought stress and organic fertilizer treatments. Asian Pac. J. Trop. Biomed. 2016, 6, 886–891. [Google Scholar] [CrossRef]
- Chaneva, G.; Tomov, A.; Paunov, M.; Hristova, V.; Ganeva, V.; Mihaylova, N.; Anev, S.; Krumov, N.; Yordanova, Z.; Tsenov, B.; et al. Jewel orchid’s biology and physiological response to aquaponic water as a potential fertilizer. Plants 2022, 11, 3181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, M.; Li, Q.; Cao, J.; Zhang, C.; Zhang, F.; Song, Z.; Chen, X. Accumulation and distribution characteristics of biomass and nitrogen in bitter gourd (Momordica charantia L.) under different fertilization strategies. J. Sci. Food Agric. 2018, 98, 2681–2688. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.X.; Huang, G.J.; Guo, J.L.; Guo, C.X.; Wang, Y.L.; Zhao, J.N.; Yang, Z.P. Effects of different amounts of nitrogen fertilizer on the growth and quality of direct seeding Scutellaria baicalensis Georgi. J. Shanxi Agric. Univ. 2021, 49, 1331–1335. [Google Scholar]
- Zhou, Q.M.; Tian, W.; Wu, Z.M.; Wen, C.X.; Liu, M.; Xie, X.L. Effects of N fertilizer on Mongolia Skullcap growth and yeild. J. Hebei Agric. Sci. 2007, 11, 88–90. [Google Scholar]
- Cui, J.B.; Zhang, J.; Hu, L.; Yang, Q.S.; Zhang, J.; Qi, J.S. Effect of different nitrogen fertilizer and fertilizer amount on yield and alkaloid content of Corydalis corydalis. J. Hebei Agric. Univ. 2022, 50, 124–130. [Google Scholar]
- Luo, R. Effects of Nitrogen Application Rate and Intercropping Astragalus Sinicus on Growth, Yield and Quality of Atractylodes macrocephala Koidz. Master’s Thesis, Guizhou University, Guiyang, China, 2022. [Google Scholar]
- Zhang, L.; Ma, G.S.; Wang, J.; Zhang, Y.L.; Xin, L.F.; Sun, W.H. Effects of different nitrogen forms on growth and absorption of nitrogen, phosphorus and potassium of Atractylodes lancea. North. Hortic. 2022, 15, 106–112. [Google Scholar]
- Lv, X.L.; Li, M.; Li, Y.X.; Bai, H.B.; Hui, J.; Ma, H.J.; Li, S.H.; Guo, S.H.; Xu, X. Effects of different ratios of water and nitrogen on growth, water use efficiency and flavonoid content of Glycyrrhiza uralensis. Plant Physiol. J. 2023, 59, 421–431. [Google Scholar]
- Sheng, Y.Z. Effects of Water and Salt Stress and Fertilization on the Quality of Glycyrrhiza uralensis Fisch. and G. macrophylla Pall. Master’s Thesis, Northwest A&F University, Xianyang, China, 2022. [Google Scholar]
- Zhang, S. Effects of N, P and K on growth, photosynthetic characteristics and quality of Epimedium Koreanum Nakai. Master’s Thesis, Jilin Agricultural University, Changchun, China, 2020. [Google Scholar]
- Lu, Z.G.; Zhu, Y.Q.; Lu, Y.C.; Zhang, G.H.; Wang, Q.X.; Long, G.Q.; Yang, S.C. Effects of nitrogen, phosphorus, and potassium fertilizer application on yield and output of the main extracts of Erigeron breviscapus. Plant Sci. 2019, 37, 55–62. [Google Scholar]
- Liu, H.; Jin, S.Y.; Yang, L.X.; Wang, Q.B.; Guo, S.L.; Wang, Z.Y. Effect of nitrogen fertilizer on carbon metabolism and growth and development of Acanthopanax senticosus seedlings. Chin. J. Exp. Tradit. Med. Formulae 2020, 26, 152–156. [Google Scholar]
- Cao, X.X. Effects of Nitrogen Fertilizer on Growth and Nitrate Accumulation of American Ginseng. Master’s Thesis, Changchun Normal University, Changchun, China, 2019. [Google Scholar]
- Ou Yang, Z.; Mohammad, M.A.; Lv, M.J.; Cui, Y.H.; Zhang, Y.H.; Sang, Y.H.; Wang, X.K. Effects of combined application of nitrogen and potassium on dry weight and Gulumbin content of root tuber. J. Chin. Med. Mater. 2023, 46, 2945–2950. [Google Scholar]
- Deng, Y.W. The Effects of Reduced Application of Nitrogen, Phosphorus and Potassium on Polygonatum cyrtonema Hua and Fertilization Optimization. Master’s Thesis, Southwest University, El Paso, TX, USA, 2020. [Google Scholar]
- Li, S.Y. Effects of Nitrogen, Phosphorus and Potassium Combined Application on the Quality, Yield and Soil Microecology of Bupleurum chinense DC. Master’s Thesis, Jilin Agricultural University, Changchun, China, 2023. [Google Scholar]
- He, M.H. Effect of Fertilization Combination of Nitrogen, Phosphorus, and Potassium on Yield and Quality of Pueraria thomsonii. Master’s Thesis, Guangxi University, Nanning, China, 2020. [Google Scholar]
- Gong, Z.D. Effects of combined application of nitrogen, phosphorus, and potassium on the growth and main active component of Tripterygium wilfordii. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2021. [Google Scholar]
- Li, X.Y.; Xia, X.H.; Tao, J.K.; Huang, D.N.; Luo, J.Y.; Wu, X.G. Effect of different nitrogen application rate on growth and yield of wild chrysanthemum under intercropping system. Guangdong Agric. Sci. 2019, 46, 15–20. [Google Scholar]
- Yang, H.L.; Liao, Z.W.; Qiu, D.Y. Effect of nitrogen application on nitrogen metabolism of Angelica sinensis. Agric. Sci.-Technol. Inf. 2020, 18, 49–52. [Google Scholar]
- Cao, Y.W. Effect of Nitrogen Reduction on Growth, Physiology of Isatis indigotica Fortune and Metabolomics Analysis. Master’s Thesis, Nanjing Agricultural University, Nanjing, China, 2020. [Google Scholar]
- Feng, M.L. Effects of Biochar and Nitrogen Additions on Soil Nutrient, Biomass and Photosynthetic Physiological Characteristics of Bletilla striata. Master’s Thesis, Jiangxi Agricultural University, Nanchang, China, 2019. [Google Scholar]
- Chai, Y.F. Effects of Water and Nitrogen Interaction on the Growth, Yield and Quality of Safflower. Master’s Thesis, Henan Agricultural University, Zhengzhou, China, 2022. [Google Scholar]
- Liu, J.X. Effects of Water and Nitrogen Coupling on Yield and Quality of sabvia miltiorrhiza Bunge. Master’s Thesis, Shandong Agricultural University, Taian, China, 2021. [Google Scholar]
- Wang, Q.S.; Chen, Z.M.; Lin, X.Q.; Tan, X.H.; Huang, S.Q.; Zou, S.Q.; Zou, X.X. Effect of water and nitrogen coupling on growth and quality of Anoectochilus roxburghii. J. Northwest A&F Univ. 2024, 52, 1–12. [Google Scholar]
- Chen, X.L. Effects of Exogenous Nitrogen on Rhizosphere Microbial Community Structure and Accumulation of Medicinal Components in Phellodendron chinense Schneid. Master’s Thesis, Central South University of Forestry & Technology, Changsha, China, 2022. [Google Scholar]
- Li, D.D.; Qiu, H.; Cui, L.J.; Zhang, G.Y.; Wang, G.D.; Qiang, Y. Effects of different nitrogen, phosphorus and potassium ratio on the Aconitum carmichaeli Debx. growth characteristics and the processed Heishunpian quality. Soil Fertil. Sci. China 2023, 10, 177–183. [Google Scholar]
- Xu, Z.H. Effect of Nitrogen Fertilizer on Nutrient Uptake and Yield and Quality of Dandelion. Master’s Thesis, Xinjiang Agricultural University, Ürümqi, China, 2016. [Google Scholar]
- Ma, L.; Chen, C.J.; Miao, Y.H.; Guo, L.P.; Liu, D.H. The appropriate nitrogen fertilization rate to enhance Artemisia argyi yield and quality. J. Plant Nutr. Fertil. 2021, 27, 1665–1674. [Google Scholar]
- Lv, Z.G.; Lv, Y.C.; Zhang, G.H.; Long, G.Q.; Yang, S.C. Effects of combined application of N, P, K fertilizers on yield and quality of Erigeron breviscapus and its fertilizer interaction. J. Nucl. Agric. Sci. 2019, 33, 616–622. [Google Scholar]
- Zhang, W.J.; Luo, X.; Guo, Y.H.; Dong, X.H.; Zhang, Z.M.; Zhai, Z.X. Effects of nitrogen phosphorus and potassium fertilization on yield and essential oil content of Schizonepetate nuifolia. North. Hortuculture 2010, 16, 194–196. [Google Scholar]
- Fan, F. Studies on the Nutrition Characteristics and the Effect of Application Ratio Nitrogen, Phosphorus and Potassium Fertilizer on Rehmannia glutinosa Libosch. f h. Master’s Thesis, Henan Agricultural University, Zhengzhou, China, 2008. [Google Scholar]
- Zhu, Z.B. Study on Prescription Fertilization and the Law of Water Requirement of Bupleurum chinense DC. Master’s Thesis, Northwest A&F University, Xianyang, China, 2005. [Google Scholar]
- Gao, X. Integrated Agronomic Measures of Astragalus membranaceus (Fisch.) Bge. var. mongholicus (Bge.) Hisao Normative Cultivation in Sand Area. Master’s Thesis, Northwest A&F University, Xianyang, China, 2017. [Google Scholar]
- Cheng, M.M. The Influence of Nitrogen, Phosphorus and Potassium on Growth and Secondary Metabolism of Astragalus mong. Master’s Thesis, Northwest A&F University, Xianyang, China, 2016. [Google Scholar]
- Gao, Q.G. The Influence Different Harvest Time and Fertilization on Root Growth Index and the Main Secondary Metabolites Content of Mongolian astragalus. Master’s Thesis, Northwest A&F University, Xianyang, China, 2015. [Google Scholar]
- Song, Q.Y. Effects of Combined Application of Nitrogen, Phosphorus and Potassium on Yield and Quality of Astragalus membranaceus. Master’s Thesis, Beijing University of Chinese Medicine, Beijing, China, 2017. [Google Scholar]
- Yang, X.Z.; Zhuang, Y.; Li, Q.S.; Jin, X.M. Effect of different proportion fertilizing on the yield and nutritional components of Agropyron michnoi. Hubei Agric. Sci. 2016, 55, 692–694. [Google Scholar]
- Wu, C.; Chen, X.F.; Yang, W.Y.; Meng, J.; Zeng, Y.; Cheng, T. Fertilizer effect on yield and quality of Gentiana crassicaulis. J. Chin. Med. Mater. 2015, 38, 1798–1803. [Google Scholar]
- Wang, J. Effect of Mineral Nutrition on Growth and Effective Ingredients of Platycodon grandiflorum. Master’s Thesis, Northwest A&F University, Xianyang, China, 2012. [Google Scholar]
- Ma, X.C. Effects of Density and Fertilizer on Yield and Quality and Mineral Element Absorption of Bupleurum falcatum L. Master’s Thesis, Shandong Agricultural University, Taian, China, 2010. [Google Scholar]
- Wu, M.Z.; Wei, Y.C.; Du, L.E.; Wang, R.L.; Yu, X.P. Effects of application of fertilizer on yield and quality of Bupleurun falcatum. J. Hebei Agric. Sci. 1999, 3, 13–16. [Google Scholar]
- Zuo, X.Y. Study on Nutrition Characteristics of Nitrogen, Phosphorus and Potassium of Achyranthes bidentata and Effect of Fertilization on Yield and Quality. Master’s Thesis, Henan Agricultural University, Zhengzhou, China, 2008. [Google Scholar]
- Wei, S.Y. Effect of N P and K fertilizers application on the yield and indirubin contents of Baphicanthus cusia Bremek. Soil Fertil. Sci. China 2007, 3, 43–46. [Google Scholar]
- Wang, K.C.; Tang, X.Q.; Wu, J.; Sun, L.P. Effects of formula fertilization to yield and content on polysaccharide of Isatis indigotica. China J. Chin. Mater. Med. 2007, 32, 2588–2591. [Google Scholar]
- Li, J.L.; Xiong, J.F.; Zhang, H.T.; Wei, S.F.; Yang, S.C.; He, Z.J.; Zhang, X.F. Effects of NPK fertilizer on yield and total saponin contents in Paris polyphilla var. yunnanensis. J. Yunnan Agric. Uni. 2016, 31, 895–901. [Google Scholar]
- Ji, Y.; Zhang, J.J.; Wang, L.Q.; Lin, H.M.; Huang, J.J.; Chen, Z.X.; Wang, X.Z.; Tao, Y.F. Effects of nitrogen phosphorus and potassium mixed fertilizer application on growth dynamics and yield of Glycyrrhiza uralensis. Pratacultural Sci. 2014, 31, 717–723. [Google Scholar]
- Liu, J.L. Establishment of Quality Evaluation Method and Standardized Production of Operation Procedure of Ligusticum chuanxiong. Master’s Thesis, Sichuan Agricultural University, Ya’an, China, 2014. [Google Scholar]
- Xu, N.F.; Liu, S.J.; Lan, C.Y.; Jiao, J.; Shu, Y.; Fu, Z.Q. Effects of N, P and K formula fertilization on yield of yam. Shandong Agric. Sci. 2014, 46, 79–82. [Google Scholar]
- Zhao, H.G. Effect of Different Soil Moisture and Fertilizer on Panax notoginseng (Burk) F. H. Chen. Master’s Thesis, Northwest A&F University, Xianyang, China, 2013. [Google Scholar]
- Wei, M.L.; Sun, Y.Q.; Huang, T.W.; Wang, B.Y.; Chen, Z.J.; Wang, C.L. Effects on the growth and the contents of saponin with different level of nitrogen applied in Panax notginseng. Res. Pract. Chin. Med. 2008, 22, 17–20. [Google Scholar]
- Wang, Z.H. Studie on the Nutrient Absorption Characteristics and Formula Fertilization Technology of Polygonatum, sibiricum Red. Master’s Thesis, Northwest A&F University, Xianyang, China, 2012. [Google Scholar]
- Zhai, C.X.; Wen, C.X.; Wang, K.H.; Zhang, Y.C.; Wang, L.Y.; Li, Q.Y.; Chen, L.L. Effects of N, P and K fertilizer on roots growth and nutrition content of Salvia miltiorrhiza. Acta Agric. Boreali-Sin. 2008, 23 (Suppl. 1), 220–223. [Google Scholar]
- Xue, Y.F. Effects of Different Nitrogen and Phosphate Rates on Yield and Quality of Salvia miltiorrhiza Bge. Master’s Thesis, Shandong Agricultural University, Taian, China, 2008. [Google Scholar]
- Li, J.H. Studies on the Nutrition Characteristic and Fertilization Effect in Bletilla striata. Master’s Thesis, Guizhou University, Guiyang, China, 2007. [Google Scholar]
- Dou, M.M.; Shi, F.; Ma, L.H.; Feng, Y.; Zhang, Y.Q.; Li, Y.X.; Chen, X.F. Effects of applying micro-element fertilizer on the yield of Alisma orientalis. J. Chin. Med. Mater. 2017, 40, 7–11. [Google Scholar]
- Zhang, Q.F.; Liu, B.; Shi, H.; Cai, X.M.; Lv, Z.Q.; Feng, X.Q. Effects of N, P and K nutrition on growth and quality of Chinese traditional herb Alisma orientale. J. Plant Resour. Environ. 2006, 15, 39–42. [Google Scholar]
- Deng, Q.L.; Yang, Z.M.; Chen, Y.; Zhang, Y.Q.; Lei, F.Y.; Li, S.J.; Liu, Z.D.; Chen, X.F. Effects of combined application of nitrogen, phosphorus and potassium on the yield and total alkaloid content of Fr. wabu. Acta Agric. Boreali-Occident. Sinica 2019, 28, 1138–1146. [Google Scholar]
- Huang, P.; Chen, M.; An, Z.; Xue, S. Effect of combined N and K application on leaf yield and qualities of Boussingaultia var. pseudobaselloides. Chin. Agric. Sci. Bull. 2009, 25, 240–243. [Google Scholar]
- Zhang, X.; Zhu, H.X.; Sun, C.H. Study on nitrogen, phosphorus and potassium nutrient absorption and balanced fertilization of garlic. Soils Fertil. Sci. China 1998, 2, 10–13. [Google Scholar]
- Jiang, Z.P.; Wei, G.P.; Meng, Y.C.; Zhou, G.Y.; Su, T.M.; Wei, H.Y. Effect of optimum fertilization model on yield and growth of chinese traditional medicinal materials Euonyus fortunei in karst area. Southwest China J. Agric. Sci. 2009, 22, 1649–1653. [Google Scholar]
- Li, X.; Ma, J.Q.; Chen, T.X.; Qiu, S. Effects of different proportion fertilizer of N, P and K on yield and quality of Leonurus japonicus. Mod. Agric. Sci. Technol. 2019, 6, 32–33. [Google Scholar]
- Zhang, Y. Study on Nutritional Conditions and Mechanism of Control of Leonurus alkaloids. Master’s Thesis, Beijing University of Chinese Medicine, Beijing, China, 2007. [Google Scholar]
- Xue, Q.; Wang, K.C.; Sui, L.; Liang, Y.F.; Su, Y.Y.; Yang, J.W. Effects of combined application of nitrogen, phosphorus and potassium on the growth and yield of patchouli. J. Chin. Med. Mater. 2018, 41, 784–789. [Google Scholar]
- Yang, Q.; Tang, X.M.; Cheng, X.X.; Zhang, C.R.; Chen, L.X.; Lu, T. Effect of nitrogen, phosphorous and potassium fertilizers on the growth and schaftoside content of Desmodium styracifolium. Chin. Agric. Sci. Bull. 2013, 29, 206–209. [Google Scholar]
- Wu, Y.K.; Li, L.Y.; Hu, Y. Optmal measure for cultivation of Artemisia annua with high seeds yield. China J. Chin. Mater. Med. 2009, 34, 2144–2148. [Google Scholar]
- Yang, S.P.; Yang, X.; Huang, J.G.; Li, L.Y. Effects of application of N, P and K and plant density on growth of Artemisia annua and yield of artemisinin. China J. Chin. Mater. Med. 2009, 34, 2290–2295. [Google Scholar]
- Zhang, C.Y.; Tan, Z.J.; Tao, C.L.; Zhang, Z.X.; Hu, N.B. Genetic diversity analysis of germplasm resources of Psammosilene tunicoides. Chin. Tradit. Herb. Drugs 2007, 38, 1067–1070. [Google Scholar]
- Gu, C.; Wang, L.H.; Zhao, Z.; Liu, H.C.; Luo, C.L.; Li, J.L.; Luo, F.L.; Huang, M.J. Effects of combined application of nitrogen, phosphorus and potassium on the growth, yield and quality of Alsinia alsinense. Jiangsu Agric. Sci. 2018, 46, 146–151. [Google Scholar]
- Bian, L.M.; Zhang, X.J.; Sheng, J.H. Effect of nitrogen on growth, yield and contents of essential oil of Dracocephalum moldavica L. J. Inner Mongolia Agric. Univ. 2009, 30, 45–47. [Google Scholar]
- Zhang, Z.M. Effects of nitrogen fertilizer on seedling growth of Fraxinus rhynchophlla seedlings. For. Investig. Des. 2017, 46, 38–40. [Google Scholar]
- Zhu, L.X.; Wang, J.H.; Bi, J.J.; Guan, X.; Li, J.L.; Jia, S.J. Effect of N application rates on nutrients accumulation, transformation and yield of Chrysanthemum morifolium. Plant Nutr. Fertil. Sci. 2010, 16, 992–997. [Google Scholar]
- Zhu, Y.; Zhao, Y.P.; Zhou, Y.F.; Liu, Q.; Yang, X.Z.; He, Y.P.; He, Y. Effects of different N, P, K combined application on yield of marigold. North. Hortic. 2010, 19, 101–103. [Google Scholar]
- Zhang, R.C.; Xin, J.; Guo, Q.M. Effects of “3414” fertilizer experiment on yield of Trichosanthes kirilowii Maxim. Crops 2016, 32, 150–155. [Google Scholar]
- Guo, Q.S.; Chen, Y.H.; Liu, L.; Wang, C.Y.; Zhang, X.X. Effect of optimized fertilization on spicas biomass and yield of Prunella vulgaris. China J. Chin. Mater. Med. 2011, 36, 2932–2936. [Google Scholar]
- Han, M.; Yang, L.M.; Han, D.Y.; Yang, L. Effect of fertilization on medicinal material output of Tribulus terrestris. J. Jilin Agric. Univ. 2009, 31, 178–180. [Google Scholar]
- Cao, L.; Zhang, S.W.; Shao, A.J.; Huang, L.Q. Study on effect of fertilizers on spring shoots growth of Akebia trifoliate. China J. Chin. Mater. Med. 2008, 33, 1540–1542. [Google Scholar]
- Li, N.; Yang, T.X. Effects of different nitrogen treatments on nitrogen uptake and utilization and yield of Semen vaccariae. China J. Chin. Mater. Med. 2018, 41, 2044–2047. [Google Scholar]
- Yu, W.W.; Cao, F.L.; Xie, Y.C. Effects of N, P, K combinations fertilization on yield and quality of Ginkgo nuts. J. Central South Univ. Forest. Technol. 2013, 33, 9–15. [Google Scholar]
- Alaei, F.; Farahani, S.M.; Habibi, H.; Fotokian, M.H.; Khodadadi, M. Coriander response to nitrogen fertilizer sources in different competing levels of weeds. Heliyon 2024, 10, e26816. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; Jaafar, H.Z.E. Involvement of carbohydrate, protein and phenylanine ammonia lyase in up-regulation of secondary metabolites in Labisia pumila under various CO2 and N2 level. Molecules 2011, 16, 4172–4190. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, Z.; Cui, R.; Su, L.; Sun, X.; Borras-Hidalgo, O.; Li, K.; Wei, J.; Yue, Q.; Zhao, L. Effects of nitrogen application on phytochemical component levels and anticancer and antioxidant activities of Allium fistulosum. PeerJ 2021, 9, e11706. [Google Scholar] [CrossRef]
- Wang, W.L.; Wang, Z.; Xu, F.L. Effects of nitrogen, phosphorus and potassium application on growth and active ingredient of Astragalus membranaceus. China J. Chin. Mater. Med. 2008, 33, 1802–1806. [Google Scholar]
- Moscatello, S.; Battistelli, A.; Mattioni, M.; Proietti, S. Yield, fructans accumulation, and nutritional quality of young chicory plants as related to genotype and nitrogen fertilization. Agronomy 2023, 13, 1752. [Google Scholar] [CrossRef]
- Olarewaju, O.; Alashi, A.; Taiwo, K.; Oyedele, D.; Adebooye, C.; Aluko, R. Influence of nitrogen fertilizer micro-dosing on phenolic content, antioxidant, and anticholinesterase properties of aqueous extracts of three tropical leafy vegetables. J. Food Biochem. 2018, 42, 12566. [Google Scholar] [CrossRef]
- Tan, P.; Wang, S.; Ameen, A.; Xie, J.; Jiang, J.Q.; Han, L.P. Fertilizer application to balance nitrogen and phosphorus nutrients and improve agronomic traits of okra (Abelmoschus esculentus L.) in coastal saline soil under subsurface pipe salt drainage. J. Soil Sci. Plant Nutr. 2023, 23, 5454–5467. [Google Scholar] [CrossRef]
- Ranjbar, F.; Pessarakli, M.; Rezvani Moghaddam, P.; Koocheki, A. Responses of anise medicinal plant species in terms of essential oil contents and concentrations to different planting times and various nitrogen fertilizer sources under semi-arid climatic conditions. Commun. Soil Sci. Plant Anal. 2017, 48, 801–807. [Google Scholar] [CrossRef]
- Liu, Z.; Xing, Y.; Jin, D.; Liu, Y.; Lu, Y.; Chen, Y.; Chen, D.; Zhang, X. Improved nitrogen utilization of Faba Bean (Vicia faba L.) roots and plant physiological characteristics under the combined application of organic and inorganic fertilizers. Agriculture 2022, 12, 1999. [Google Scholar] [CrossRef]
- Jiang, K.; Peng, S.; Yin, Z.; Li, X.; Xie, L.; Shen, M.; Li, D.; Gao, J. Effects of N, P, K nutrition levels on the growth, flowering attributes and functional components in Chrysanthemum morifolium. Horticulturae 2024, 10, 226. [Google Scholar] [CrossRef]
- Renata, B.; Petras, R.V.; Pranas, V.; Edita, D. Influence of nitrogen fertilizers on the yield and composition of Thyme (Thymus vulgaris). J. Agric. Food Chem. 2003, 51, 7751–7758. [Google Scholar]
- El Gendy, A.G.; El Gohary, A.E.; Omer, E.A.; Hendawy, S.F.; Hussein, M.S.; Petrova, V.; Stancheva, I. Effect of nitrogen and potassium fertilizer on herbage and oil yield of chervil plant (Anthriscus cerefolium L.). Ind. Crops Prod. 2015, 69, 167–174. [Google Scholar] [CrossRef]
- Xiong, F.; Nie, X.; Zhao, X.; Yang, L.; Zhou, G. Effects of different nitrogen fertilizer levels on growth and active compounds of rhubarb from Qinghai plateau. J. Sci. Food Agric. 2019, 99, 2874–2882. [Google Scholar] [CrossRef] [PubMed]
- Palchetti, E.; Moretta, M.; Calamai, A.; Mancini, M.; Dell’Acqua, M.; Brilli, L.; Armanasco, P.; Masoni, A. Effects of nitrogen fertilization and plant density on proso millet (Panicum miliaceum L.) growth and yield under mediterranean pedoclimatic conditions. Agriculture 2023, 13, 1657. [Google Scholar] [CrossRef]
- Śniegowska, J.; Biesiada, A.; Gasiński, A. Influence of the nitrogen fertilization on the yield, biometric characteristics and chemical composition of Stevia rebaudiana bertoni grown in Poland. Molecules 2024, 29, 1865. [Google Scholar] [CrossRef]
- Bartzialis, D.; Giannoulis, K.D.; Gintsioudis, I.; Danalatos, N.G. Assessing the efficiency of different nitrogen fertilization levels on sorghum yield and quality characteristics. Agriculture 2023, 13, 1253. [Google Scholar] [CrossRef]
- Majid, W.H.D.W.; Yusoff, M.M.; Misran, A.; Arisah, F.M. Effects of fertilizer rate on growth performance and phytochemical compounds of Gynura procumbens. Asian J. Plant Sci. 2024, 23, 132–145. [Google Scholar] [CrossRef]
- Stumpf, B.; Yan, F.; Honermeier, B. Influence of nitrogen fertilization on yield and phenolic compounds in wheat grains (Triticum aestivum L. ssp. aestivum). J. Plant Nutr. Soil Sci. 2019, 182, 111–118. [Google Scholar] [CrossRef]
- Shrestha, B.; Stringam, B.L.; Darapuneni, M.K.; Lombard, K.A.; Sanogo, S.; Higgins, C.; Djaman, K. Effect of irrigation and nitrogen management on potato growth, yield, and water and nitrogen use efficiencies. Agronomy 2024, 14, 560. [Google Scholar] [CrossRef]
- Mahanti, N.K.; Upendar, K.; Chakraborty, S.K. Comparison of artificial neural network and linear regression model for the leaf morphology of fenugreek (Trigonella foenum graecum) grown under different nitrogen fertilizer doses. Smart Agric. Tech. 2022, 2, 100058. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, X.Z.; Ma, L.N.; Lin, Y.S.; Huang, X.G. Chinese yam yield is affected by soil nutrient levels and interactions among N, P, and K fertilizers. Chin. Herb. Med. 2023, 15, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Jabborova, D.; Sayyed, R.Z.; Azimov, A.; Jabbarov, Z.; Matchanov, A.; Enakiev, Y.; Baazeem, A.; Sabagh, A.E.; Danish, S.; Datta, R. Impact of mineral fertilizers on mineral nutrients in the ginger rhizome and on soil enzymes activities and soil properties. Saudi J. Biol. Sci. 2021, 28, 5268–5274. [Google Scholar] [CrossRef]
- Subedi, P.; Bhattarai, P.; Lamichhane, B.; Khanal, A.; Shrestha, J. Effect of different levels of nitrogen and charcoal on growth and yield traits of chili (Capsicum annuum L.). Heliyon 2023, 9, e13353. [Google Scholar] [CrossRef]
- Sęczyk, Ł.; Król, B.; Kołodziej, B. In vitro bioaccessibility and activity of Greek oregano (Origanum vulgare L. ssp. hirtum (link) Ietswaart) compounds as affected by nitrogen fertilization. J. Sci. Food Agric. 2020, 100, 2410–2417. [Google Scholar]
- Ashraf, M.; Ali, Q.; Iqbal, Z. Effect of nitrogen application rate on the content and composition of oil, essential oil and minerals in black cumin (Nigella sativa L.) seeds. J. Sci. Food Agric. 2006, 86, 871–876. [Google Scholar] [CrossRef]
- Amiri, M.B.; Jahan, M.; Moghaddam, P.R. An exploratory method to determine the plant characteristics affecting the final yield of Echium amoenum Fisch. & C.A. Mey. under fertilizers application and plant densities. Sci. Rep. 2022, 12, 1881. [Google Scholar]
- Yin, Z.C.; Guo, W.Y.; Liang, J.; Xiao, H.Y.; Hao, X.Y.; Hou, A.F.; Zong, X.X.; Leng, T.R.; Wang, Y.J.; Wang, Q.Y.; et al. Effects of multiple N, P, and K fertilizer combinations on adzuki bean (Vigna angularis) yield in a semi-arid region of northeastern China. Sci. Rep. 2019, 9, 19408. [Google Scholar] [CrossRef] [PubMed]
- Aboyeji, C.M.; Dahunsi, S.O.; Olaniyan, D.O.; Dunsin, O.; Adekiya, A.O.; Olayanju, A. Performance and quality attributes of okra (Abelmoschus esculentus (L.) Moench) fruits grown under soil applied Zn-fertilizer, green biomass and poultry manure. Sci. Rep. 2021, 11, 8291. [Google Scholar] [CrossRef]
- Peng, L.C.; Ng, L.T. Impacts of nitrogen and phosphorus fertilization on biomass, polyphenol contents, and essential oil yield and composition of Vitex negundo Linn. Agriculture 2022, 12, 859. [Google Scholar] [CrossRef]
- Mudau, F.N.; Soundy, P.; Du Toit, E.S.; Olivier, J. Variation in polyphenolic content of Athrixia phylicoides (L.) (bush tea) leaves with season and nitrogen application. S. Afr. J. Bot. 2006, 72, 398–402. [Google Scholar] [CrossRef]
- Akamine, H.; Hossain, A.; Ishimine, Y.; Yogi, K.; Hokama, K.; Iraha, Y.; Aniya, Y. Effects of application of N, P And K alone or in combination on growth, yield and curcumin content of Turmeric (Curcuma Longa L.). Plant Prod. Sci. 2007, 10, 151–154. [Google Scholar] [CrossRef]
- Pham, T.L.; Nguyen, V.H.; Hoang, T.L.T.; Ha, T.T.T.; Phan, C.N.; Vu, X.D.; Tran, T.T. Improvement of the field productivity in Rehmannia glutinosa Libosch. Crops by application of N, P and K fertilizers and plant spacing. Asian J. Plant Sci. 2020, 19, 214–222. [Google Scholar] [CrossRef]
- Mota, J.H.; Melo, E.P.D.; Soares, T.S.; Vieira, M.D.C. Phosphatic and nitrogenous fertilization effects on tansagem medicinal plant (Plantago major L.) growth. Ciencia Agrotecnol. 2008, 32, 1748–1753. [Google Scholar] [CrossRef]
- Zahra, T.; Ahmad, A.; Gholamreza, A.; Seyed, S.; Mohammad, S. The effect of planting arrangement, phosphorus and nitrogen on physiological traits and guar yield (Cyamopsis tetragonoloba L.). Legume Res. 2023, 46, 1205–1210. [Google Scholar]
- Rezaei, R.; Valadabadi, S.A.; Shirani Rad, A.H.; Sayfzadeh, S.; Hadidi Masouleh, E. The effects of application of biological fertilizers and different amounts of urea fertilizer sources under low water stress conditions on physiological traits of medicinal plant (Calendula officinalis L.). Appl. Ecol. Environ. Res. 2018, 16, 4813–4827. [Google Scholar] [CrossRef]
- Hoang, H.-L.; Tran, H.T.T.; Do, T.D.; Rehman, H.; Kawarazuka, N.; Tran, B.K.; Truong, D.H.T. Ammonium-nitrogen supply induces growth, physiological and secondary metabolites changes in Centella asiatica L. Urban. Legume Res. 2022, 45, 1005–1009. [Google Scholar] [CrossRef]
- Lv, R.; Elsabagh, M.; Obitsu, T.; Sugino, T.; Kurokawa, Y.; Kawamura, K. Changes of photosynthetic pigments and phytol content at different levels of nitrogen fertilizer in Italian ryegrass fresh herbage and hay. Grassland Sci. 2022, 68, 53–59. [Google Scholar] [CrossRef]
- Hejcman, M.; Křišťálová, V.; Cervená, K.; Hrdličková, J.; Pavlů, V. Effect of nitrogen, phosphorus and potassium availability on mother plant size, seed production and germination ability of Rumex crispus. Weed Res. 2012, 52, 260–268. [Google Scholar] [CrossRef]
- Hoang, C.K.; Pham, N.H.; Le, C.H.; Tran, H.T.N.; Dang, H.T.C.; Chu, H.H.; Brouwer, B.; Le, H.M. Impact of nitrogen fertilizer on the mycorrhizal inoculating potential and fungal community structure in rhizosphere of medicinal plant Curcuma longa L. Geomicrobiol. J. 2019, 36, 385–395. [Google Scholar] [CrossRef]
- Karimi, B.; Rokhzadi, A.; Rahimi, A.R. RSM modeling of nitrogen use efficiency, biomass and essential oil of Salvia officinalis L. as affected by fertilization and plant density. J. Plant Nutri. 2021, 44, 1067–1084. [Google Scholar] [CrossRef]
- Behzadi, M.; Javanmard, A.S.; Khakdan, F.; Mohsenzadeh, S. Effects of urea supplementation and different substrates on the production of indole alkaloid reserpine in Catharanthus roseus plants. Plant Biosyst. 2022, 156, 1011–1018. [Google Scholar] [CrossRef]
- Singh, M. Effect of nitrogen and potassium fertilizer on growth, herbage and oil yield of irrigated palmarosa (Cymbopogon martinii [Roxb.] wats. var. motia burk) in a semi-arid tropical climate. Arch. Agron. Soil Sci. 2008, 54, 395–400. [Google Scholar] [CrossRef]
- Liu, Z.; Adams, J.C.; Viator, H.P.; Constantin, R.J.; Carpenter, S.B. Influence of soil fertilization, plant spacing, and cokppicing on growth, stomatal conductance, abscisic acid, and camptothecin levels in Camptotheca acuminata seedlings. Physiol. Plant. 1999, 105, 402–408. [Google Scholar] [CrossRef]
- Telci, I.; Izgi, M.N.; Ozek, T.; Yasak, S.; Yur, S.; Ozek, G. Effects of different nitrogen doses on thymoquinone and fatty acid composition in seed oil of black cumin (Nigella sativa L.). J. Am. Oil Chem. Soc. 2022, 99, 229–237. [Google Scholar] [CrossRef]
- Keshavarz-Mirzamohammadi, H.; Tohidi-Moghadam, H.R.; Hosseini, S.J. Is there any relationship between agronomic traits, soil properties and essential oil profile of peppermint (Mentha piperita L.) treated by fertilizer treatments and irrigation regimes? Ann. Appl. Biol. 2021, 179, 331–344. [Google Scholar] [CrossRef]
- Ghiasy-Oskoee, M.; AghaAlikhani, M.; Sefidkon, F.; Mokhtassi-Bidgoli, A.; Ayyari, M. Blessed thistle growth, essential oil content, yield and composition as influenced by plant density and nitrogen fertilizer. J. Essent. Oil Bear. Plants 2020, 23, 276–291. [Google Scholar] [CrossRef]
- Kiymaz, S.; Altun, B.; Ertek, A. Effect of different water regimes and nitrogen applications on the growth, yield, essential oil content, and quality parameters of the oil rose (Rosa damascena Mill.). J. Plant Nutri. 2022, 45, 2108–2122. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, S.; Sood, S.; Kaundal, M.; Agnihotri, V.K. Effect of manures and inorganic fertilizers on growth, yield, and essential oil of damask rose (Rosa damascena Mill.) and chemical properties of soil in western Himalayas. J. Plant Nutri. 2017, 40, 1604–1615. [Google Scholar] [CrossRef]
- Salmasi, S.Z.; Bonab, S.M.; Ghassemi-Golezani, K. Changes in essential oil composition of dill (Anethum graveolens L.) grains in response to water limitation and nitrogen fertilizer. J. Essent. Oil Bear. Plants 2016, 19, 374–378. [Google Scholar] [CrossRef]
- Liu, W.; Yin, D.X.; Zhang, T.; Hou, X.G.; Qiao, Q.; Song, P. Major fatty acid compositions and antioxidant activity of cultivated Paeonia ostii under different nitrogen fertilizer application. Chem. Biodivers. 2020, 17, e20006. [Google Scholar] [CrossRef]
- Naderi, M.R.; Bannayan, M.; Goldani, M.; Alizadeh, A. Effect of nitrogen application on growth and yield of pumpkin. J. Plant Nutri. 2017, 40, 890–907. [Google Scholar] [CrossRef]
- Daneshvar, H.; Babalar, M.; Díaz-Pérez, J.C.; Nambeesan, S.; Delshad, M.; Tabrizi, L. Evaluation of organic and mineral fertilizers on plant growth, minerals, and postharvest quality of celery (Apium graveolens L.). J. Plant Nutri. 2023, 46, 1712–1729. [Google Scholar] [CrossRef]
- Ünlükara, A.; Varol, İ.S.; Güneş, A. Effects of various fertilizers and different nitrogen doses on pumpkin seed and plant water consumption. Commun. Soil Sci. Plant Anal. 2022, 53, 590–601. [Google Scholar] [CrossRef]
- Yang, W. Effect of nitrogen, phosphorus and potassium fertilizer on growth and seed germination of Capsella bursa-pastoris (L.) Medikus. J. Plant Nutri. 2018, 41, 636–644. [Google Scholar] [CrossRef]
- Shiwakoti, S.; Shannon, D.A.; Wood, C.W.; Joshee, N.; Rimando, A.; Lawrence, K.S.; Kemppainen, B. Nitrogen, phosphorus, and potassium effects on biomass yield and flavonoid content of American skullcap (Scutellaria lateriflora). J. Plant Nutr. 2016, 39, 1240–1249. [Google Scholar] [CrossRef]
- Shakeri, E.; Modarres-Sanavy, S.A.M.; Amini Dehaghi, M.; Tabatabaei, S.A.; Moradi-Ghahderijani, M. Improvement of yield, yield components and oil quality in sesame (Sesamum indicum L.) by N-fixing bacteria fertilizers and urea. Arch. Agron. Soil Sci. 2016, 62, 547–560. [Google Scholar] [CrossRef]
- Nasir, S.; Khan, M.M.A. Critical dose of nitrogen and phosphorus enhanced growth, yield and alkaloid content in Withania somnifera L. J. Plant Nutr. 2012, 35, 1705–1724. [Google Scholar] [CrossRef]
- Pinto, J.V.D.C.; Vieira, M.D.C.; Zárate, N.A.; Formagio, A.S.; Cardoso, C.A.; Carnevali, T.O.; Souza, P.H.D. Effect of soil nitrogen and phosphorus on early development and essential oil composition of Schinus terebinthifolius Raddi. J. Essent. Oil Bear. Plants 2016, 19, 247–257. [Google Scholar] [CrossRef]
- Fu, L.Z.; Zhao, L.M.; Lv, H.Q.; Yan, M.Q.; Zheng, Y.Q.; Liu, Q.; Wang, L.Y. Effects of nitrogen forms on main phytochemical content and antioxidant activity in root tubers of Tetrastigma hemsleyanum. China J. Chin. Mater. Med. 2019, 4, 696–702. [Google Scholar]
- Ma, X.D.; Guo, Y.H.; Li, M.Y.; Yu, X.X.; Xu, Y.J.; Song, W.J.; Feng, J. Response of drought stress of Lycium ruthenicum Murr. under different nitrogen applications. J. Agric. Sci. Technol. 2022, 24, 421–431. [Google Scholar]
- Shi, Y.C.; Jiang, L.Q.; Zou, R.; Tang, J.M.; Jiang, Y.S.; Xiong, Z.C. Effects of nitrogen fertilizer and paclobutrazol on second flower characters of Sophora japonica. J. Shanxi Agric. Sci. 2018, 46, 1871–1874. [Google Scholar]
- Liu, G.Y.; Cui, X.A.; Wang, Q.; Wang, W.; Li, Y.; Zhou, J.H. Effect of combined application of nitrogen, phosphorus and potassium fertilizers on Brugmansia arborea with yellow flower growth and scopolamine content. J. Gansu Agric. Univ. 2023, 58, 75–82. [Google Scholar]
- Ji, L.L. Physiological Response of Coix Lacrima-Jobi to Increased CO2 Concentration and Nitrogen Application. Master’s Thesis, Guizhou University, Guiyang, China, 2021. [Google Scholar]
- Chen, P.F. Effects of Nitrogen Supply on Growth, Nutritional Quality and Effective Constituents of Dracocephalum moldavica (L.). Master’s Thesis, Hebei University, Baodong, China, 2023. [Google Scholar]
- Zhang, X.Y.; Li, Y.F.; Liu, G.H.; Xu, Z.S.; Deng, B. Effects of nitrogen application on growth and triterpenoids accumulation of 1-year-old Cyclocarya paliurus. J. Beijing Forest. Univ. 2020, 42, 60–68. [Google Scholar]
- Hu, J.T.; Li, J.L.; Wang, H.L.; Luo, C.L.; Liu, H.C.; Zhao, Z. Effects of fertilization and water treatment on the growth of annual Polygonum multiflorum Thunb. Tillage Cultiv. 2023, 43, 41–46. [Google Scholar]
- Li, L.; Cai, C.T.; Liu, G.Z. Effects of light and nitrogen level on leaf growth and photosynthesis of Rauvolfia vomitoria. J. Wuhan Bot. Res. 2010, 28, 206–212. [Google Scholar] [CrossRef]
- Hu, J.T.; Zhao, Z.; Wang, H.L.; Li, J.L.; Luo, C.L.; Liu, H.C. The effect of several cultivated physiological indexes of Polygonum multiflorum Thunb under different treatments with fertilizer and water. Lishizhen Med. Mater. Med. Res. 2012, 23, 2863–2866. [Google Scholar]
- Hu, X.L. Magnolia offcinalis Nutrition Diagnosis and Quality Evaluation Research. Master’s Thesis, Fujian Agriculture and Forestry University, Fuzhou, China, 2014. [Google Scholar]
- Zhang, J.H.; Feng, B.B.; Xu, X.Y.; Yao, Y.H. Optimum effect model of fertilizer impact on yield and quality of Lonicerae Flos. Southwest China J. Agric. Sci. 2013, 26, 1546–1552. [Google Scholar]
- Wang, Z.; Zhao, T.; Ma, L.; Chen, C.J.; Miao, Y.H.; Guo, L.P.; Liu, D.H. Mechanisms governing the impact of nitrogen stress on the formation of secondary metabolites in Artemisia argyi leaves. Sci. Rep. 2023, 13, 12866. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.M.; Xu, X.Y.; Zhang, T.; Yang, Y.H.; Tong, H.Y.; Yuan, H.Y. Comparative transcriptome analysis provides insights into steviol glycoside synthesis in stevia (Stevia rebaudiana Bertoni) leaves under nitrogen deficiency. Plant Cell Rep. 2021, 40, 1709–1722. [Google Scholar] [CrossRef]
- Xing, Z.; Bi, G.; Li, T.; Zhang, Q.; Knight, P.R. Nitrogen fertilization improves growth and bioactive compound content for Salvia miltiorrhiza Bunge. Horticulturae 2023, 9, 254. [Google Scholar] [CrossRef]
- Ma, D.; Teng, W.; Mo, Y.T.; Yi, B.; Chen, W.L.; Pang, Y.P.; Wang, L. Effects of nitrogen, phosphorus, and potassium fertilization on plant growth, element levels in plants and soil, and the relationships among nutrient concentrations, plant yield, and nutrient status in Erythropalum scandens (Blume). J. Plant Nutr. 2024, 47, 82–96. [Google Scholar] [CrossRef]
- Ye, Z.; Zhang, H.; Lin, X.; Huang, S.; Zou, S.; Zou, X. Effect of biochar using N, P, and K fertilizers on growth and quality of Lithocarpus litseifolius. Agronomy 2024, 14, 728. [Google Scholar] [CrossRef]
- Susanto, D.; Amirta, R. The application of NPK fertilizer boosts the nutrient uptake status and biomass production of Vernonia amygdalina. Nusant. Biosci. 2020, 12. [Google Scholar] [CrossRef]
- Lyu, Y.; Porat, R.; Yermiyahu, U.; Heler, Y.; Holland, D.; Dag, A. Effects of nitrogen fertilization on pomegranate fruit, aril and juice quality. J. Sci. Food Agric. 2020, 100, 1678–1686. [Google Scholar] [CrossRef]
- Szalai, G.; Dai, N.; Danin, A.; Dudai, N.; Barazani, O. Effect of nitrogen source in the fertilizing solution on nutritional quality of three members of the Portulaca oleracea aggregate. J. Sci. Food Agric. 2010, 90, 2039–2045. [Google Scholar] [CrossRef]
- Liu, W.; Zhu, D.W.; Liu, D.H.; Geng, M.J.; Zhou, W.B.; Mi, W.J.; Yang, T.W.; Hamilton, D. Influence of nitrogen on the primary and secondary metabolism and synthesis of flavonoids in Chrysanthemum morifolium Ramat. J. Plant Nutr. Soil Sci. 2010, 33, 240–254. [Google Scholar]
- Liu, D.H.; Liu, W.; Zhu, D.W.; Geng, M.J.; Zhou, W.B.; Yang, T.W. Nitrogen effects on total flavonoids, chlorogenic acid, and antioxidant activity of the medicinal plant Chrysanthemum morifolium. J. Plant Nutr. Soil Sci. 2010, 173, 268–274. [Google Scholar] [CrossRef]
- Zou, S.; Huang, C.; Chen, Y.; Bai, X.; Li, W.; He, B. Patterns of nitrogen and phosphorus along a chronosequence of tea plantations in subtropical China. Agriculture 2024, 14, 110. [Google Scholar] [CrossRef]
- Wu, C.S.; Gao, Q.H.; Kjelgren, R.K.; Guo, X.D.; Wang, M. Yields, phenolic profiles and antioxidant activities of Ziziphus jujube Mill. in response to different fertilization treatments. Molecules 2013, 18, 12029–12040. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.M. Effects of Combined Application of Nitrogen and Potassium on Growth and Quality of Trichosanthes rosthornii Harms. and Its Production Area Prediction. Master’s Thesis, Nanjing Agricultural University, Nanjing, China, 2020. [Google Scholar]
- Liu, Y.; Ou, X.H.; Hu, Y.P.; Xu, J.; He, H.; Zhou, T. Effect of nitrate on the growth and heterophyllin B of Pseudostellaria heterophylla. North. Hortic. 2024, 7, 104–111. [Google Scholar]
- Wang, Y.; Bu, H.; Wang, H.; Zhang, P.; Jin, L. Effects of applying nitrogen and potassium on Lilium lancifolium growth and accumulation of secondary metabolites in bulbs. Horticulturae 2023, 9, 396. [Google Scholar] [CrossRef]
- Cebani, S.; Jimoh, M.O.; Sogoni, A.; Wilmot, C.M.; Laubscher, C.P. Nutrients and phytochemical density in Mesembryanthemum crystallinum L. cultivated in growing media supplemented with dosages of nitrogen fertilizer. Saudi J. Biol. Sci. 2024, 31, 103876. [Google Scholar] [CrossRef] [PubMed]
- Chrysargyris, A.; Hajisolomou, E.; Xylia, P.; Tzortzakis, N. Ammonium to total nitrogen ratio affects the purslane (Portulaca oleracea L.) growth, nutritional, and antioxidant status. Heliyon 2023, 9, e21644. [Google Scholar] [CrossRef]
- Zhu, Z.; Yuan, H.; Wei, Y.; Li, P.; Zhang, P.; Xie, D. Effects of ammonia nitrogen and sediment nutrient on growth of the submerged plant Vallisneria natans. Clean Soil Air Water 2015, 43, 1653–1659. [Google Scholar] [CrossRef]
- Fontana, E.; Hoeberechts, J.; Nicola, S.; Cros, V.; Battista Palmegiano, G.; Giorgio Peiretti, P. Nitrogen concentration and nitrate/ammonium ratio affect yield and change the oxalic acid concentration and fatty acid profile of purslane (Portulaca oleracea L.) grown in a soilless culture system. J. Sci. Food Agric. 2006, 86, 2417–2424. [Google Scholar] [CrossRef]
- Luo, J.; Mu, M.J.; Wang, Q.; Wen, M.; Zhu, F.R.; Wu, X.H.; Zhou, N. Effect of different nitrogen forms and concentrations on yield and quality of Fritillaria thunbergii. Chin. J. Exp. Tradit. Med. Formulae 2020, 26, 168–174. [Google Scholar]
- Pompeiano, A.; Patton, A.J. Growth and root architecture responses of zoysia grass to changes in fertilizer nitrate: Urea ratio. J. Plant Nutr. Eurasian Soil Sci. 2017, 180, 528–534. [Google Scholar] [CrossRef]
- Naseri, A.; Alirezalu, A.; Noruzi, P.; Alirezalu, K. The effect of different ammonium to nitrate ratios on antioxidant activity, morpho-physiological and phytochemical traits of Moldavian balm (Dracocephalum moldavica). Sci. Rep. 2022, 12, 16841. [Google Scholar] [CrossRef]
- Noh, K.; Jeong, B.R. Silicon supplementation alleviates adverse effects of ammonium on Ssamchoo grown in home cultivation system. Plants 2022, 11, 2882. [Google Scholar] [CrossRef]
- Liao, Y.; Zhao, S.; Zhang, W.; Zhao, P.; Lu, B.; Moody, M.L.; Tan, N.; Chen, L. Chromosome-level genome and high nitrogen stress response of the widespread and ecologically important wetland plant Typha angustifolia. Front. Plant Sci. 2023, 14, 1138498. [Google Scholar] [CrossRef]
- Loem, E.; Haneklaus, S.; Schnug, E. Comparative effects of sulfur and nitrogen fertilization and post-harvest processing parameters on the glucotropaeolin content of Tropaeolum majus L. J. Sci. Food Agric. 2007, 87, 1576–1585. [Google Scholar]
- Nurfalah, R.; Ridwan, T.; Aziz, S.A.; Rafi, M.; Takemori, H.; Batubara, I. Growth performance and secondary metabolite production of Adenostemma madurense using different fertilizers. J. Saudi Soc. Agric. Sci. 2024, 23, 177–183. [Google Scholar] [CrossRef]
- Özbucak, T.; Ertürk, Ö.; Yıldız, O.; Bayrak, A.; Kara, M.; Sahin, H.; Kıralan, M. The effect of different manures and synthetic fertilizer on biochemical and antimicrobial properties of Mentha piperita L. J. Food Biochem. 2014, 38, 424–432. [Google Scholar] [CrossRef]
- Mahmoud, N.; Abdou, M.A.H.; Salaheldin, S.; Soliman, W.S.; Abbas, A.M. The Impact of irrigation intervals and NPK/yeast on the vegetative growth characteristics and essential oil content of lemongrass. Horticulturae 2023, 9, 365. [Google Scholar] [CrossRef]
- Ma, J.H.; Faqir, Y.; Chai, Y.L.; Wu, S.M.; Luo, T.; Liao, S.Y.; Kaleri, A.R.; Tan, C.J.; Qing, Y.X.; Kalhoro, M.T.; et al. Chitosan microspheres-based controlled release nitrogen fertilizers enhance the growth, antioxidant, and metabolite contents of Chinese cabbage. Sci. Horticulturae 2023, 308, 111542. [Google Scholar] [CrossRef]
- Mogren, L.M.; Olsson, M.E.; Gertsson, U.E. Effects of cultivar, lifting time and nitrogen fertilizer level on quercetin content in onion (Allium cepa L.) at lifting. J. Sci. Food Agric. 2007, 87, 470–476. [Google Scholar] [CrossRef]
- Cojocaru, A.; Vlase, L.; Munteanu, N.; Stan, T.; Teliban, G.C.; Burducea, M.; Stoleru, V. Dynamic of phenolic compounds, antioxidant activity, and yield of rhubarb under chemical, organic and biological fertilization. Plants 2020, 9, 355. [Google Scholar] [CrossRef] [PubMed]
- Endo, A. Dune soil nitrogen leaching for Chinese-yam cultivation: Impact of microbe-decomposable slow-release fertilizer. Heliyon 2024, 10, e30545. [Google Scholar] [CrossRef] [PubMed]
- Haghaninia, M.; Javanmard, A.; Kahrizi, D.; Bahadori, M.B.; Machiani, M.A. Optimizing oil quantity and quality of camelina (Camelina sativa L.) with integrative application of chemical, nano and bio-fertilizers under supplementary irrigation and rainfed condition. Plant Stress 2024, 11, 100374. [Google Scholar] [CrossRef]
- Chowdhury, T.; Chowdhury, M.A.H.; Qingyue, W.; Enyoh, C.E.; Wang, W.; Khan, M.S.I. Nutrient uptake and pharmaceutical compounds of Aloe vera as influenced by integration of inorganic fertilizer and poultry manure in soil. Heliyon 2021, 7, e07464. [Google Scholar] [CrossRef]
- Sarwar, M.; Patra, J.K.; Ali, A.; Maqbool, M.; Arshad, M.I. Effect of compost and NPK fertilizer on improving biochemical and antioxidant properties of Moringa oleifera. S. Afr. J. Bot. 2020, 129, 62–66. [Google Scholar] [CrossRef]
- Chotimah, H.; Jaya, A.; Suparto, H.; Saraswati, D.; Nawansyah, W. Utilizing organic fertilizers on two types of soil to improve growth and yield of bawang dayak (Eleutherine americana Merr). Afr. J. Agric. Sci. 2021, 43, 164–173. [Google Scholar] [CrossRef]
- Vasca-Zamfir, D.; Balan, D.; Luta, G.; Gherghina, E.; Tudor, V. Effect of fertilization regime on Murraya exotica plants growth and bioactive compounds. Rom. Biotechnol. Lett. 2019, 24, 245–253. [Google Scholar] [CrossRef]
- Fotohi Chiyaneh, S.; Rezaei-Chiyaneh, E.; Amirnia, R.; Keshavarz Afshar, R.; Siddique, K.H.M. Intercropping medicinal plants is a new idea for forage production: A case study with ajowan and fenugreek. Food Energy Secur. 2024, 13, e501. [Google Scholar] [CrossRef]
- Mohammadi, S.M.; Sefidkon, F.; Asadi-Sanam, S.; Kalatejari, S. The changes of carvacrol content and essential oil yield of Satureja khuzestanica Jamzad in response to different fertilizer sources. Flavour Fragr. J. 2023, 38, 37–48. [Google Scholar] [CrossRef]
- Faridvand, S.; Rezaei-Chiyaneh, E.; Battaglia, M.L.; Gitari, H.I.; Raza, M.A.; Siddique, K.H.M. Application of bio and chemical fertilizers improves yield, and essential oil quantity and quality of Moldavian balm (Dracocephalum moldavica L.) intercropped with mung bean (Vigna radiata L.). Food Energy Secur. 2022, 11, e319. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Wang, J.; Xing, W.H.; Liu, Y.; Wan, Y.P.; Li, N.; Wang, J.Y.; Liu, G.Y.; Wang, R.; et al. Characterization of the volatile compounds in white radishes under different organic fertilizer treatments by HS-GC-IMS with PCA. Flavour Fragr. J. 2023, 38, 83–94. [Google Scholar] [CrossRef]
| MLR | Root | Stem | Leaf | Flower | Fruit | Five Most Reported Species a |
|---|---|---|---|---|---|---|
| Total | ||||||
| p value | 0.00 *** | 0.00 *** | 0.00 *** | 0.00 *** | 0.0019 ** | 0.0105 * |
| Effect | AP(+), TN(+), AN(+) | AP(+), TN(+), AK(+) | AP(+), AK(-), TN(+) | AP(+) | AP(+), TN(-), AN(+) | TN(+), AN(+), AK(-) |
| China | ||||||
| p value | 0.0253 * | 0.0005 *** | 0.0109 * | 0.0172 * | 0.5434 | 0.0002 *** |
| Effect | AN(+) | AN(+) | AK(+), AP(-), TN(+) | AP(+) | ||
| Other countries | ||||||
| p value | 0.6319 | 0.0001 *** | 0.0017 ** | 0.0007 *** | 0.002 ** | 0.00 *** |
| Effect | AK(+), TN(+) | -- | AK(+) | -- | AK(+), AN(+) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, D.; Luan, Y.; Wang, Y.; Xiao, P. Unveiling Nitrogen Fertilizer in Medicinal Plant Cultivation. Agronomy 2024, 14, 1647. https://doi.org/10.3390/agronomy14081647
Hao D, Luan Y, Wang Y, Xiao P. Unveiling Nitrogen Fertilizer in Medicinal Plant Cultivation. Agronomy. 2024; 14(8):1647. https://doi.org/10.3390/agronomy14081647
Chicago/Turabian StyleHao, Dacheng, Yuanyuan Luan, Yaoxuan Wang, and Peigen Xiao. 2024. "Unveiling Nitrogen Fertilizer in Medicinal Plant Cultivation" Agronomy 14, no. 8: 1647. https://doi.org/10.3390/agronomy14081647
APA StyleHao, D., Luan, Y., Wang, Y., & Xiao, P. (2024). Unveiling Nitrogen Fertilizer in Medicinal Plant Cultivation. Agronomy, 14(8), 1647. https://doi.org/10.3390/agronomy14081647







