Response of the Edamame Germplasm to Early-Season Diseases in the United States
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Field Resistance Screening for Bacterial Pustule
2.3. Identification of the Causative Agent of Edamame Bacteria Pustule
2.3.1. Collection of Samples in the Field and Bacteria Isolation
2.3.2. DNA Extraction, Sequencing, and Phylogenetic Analyses
2.4. Growth Chamber Screening for Seedling Diseases
2.4.1. Inoculum Preparation and Resistance Evaluation for Rhizoctonia Root and Stem Rot
2.4.2. Southern Blight
2.4.3. Pythium Root Rot
2.5. Statistics Analysis
3. Results
3.1. Evaluation of Edamame Bacterial Pustule
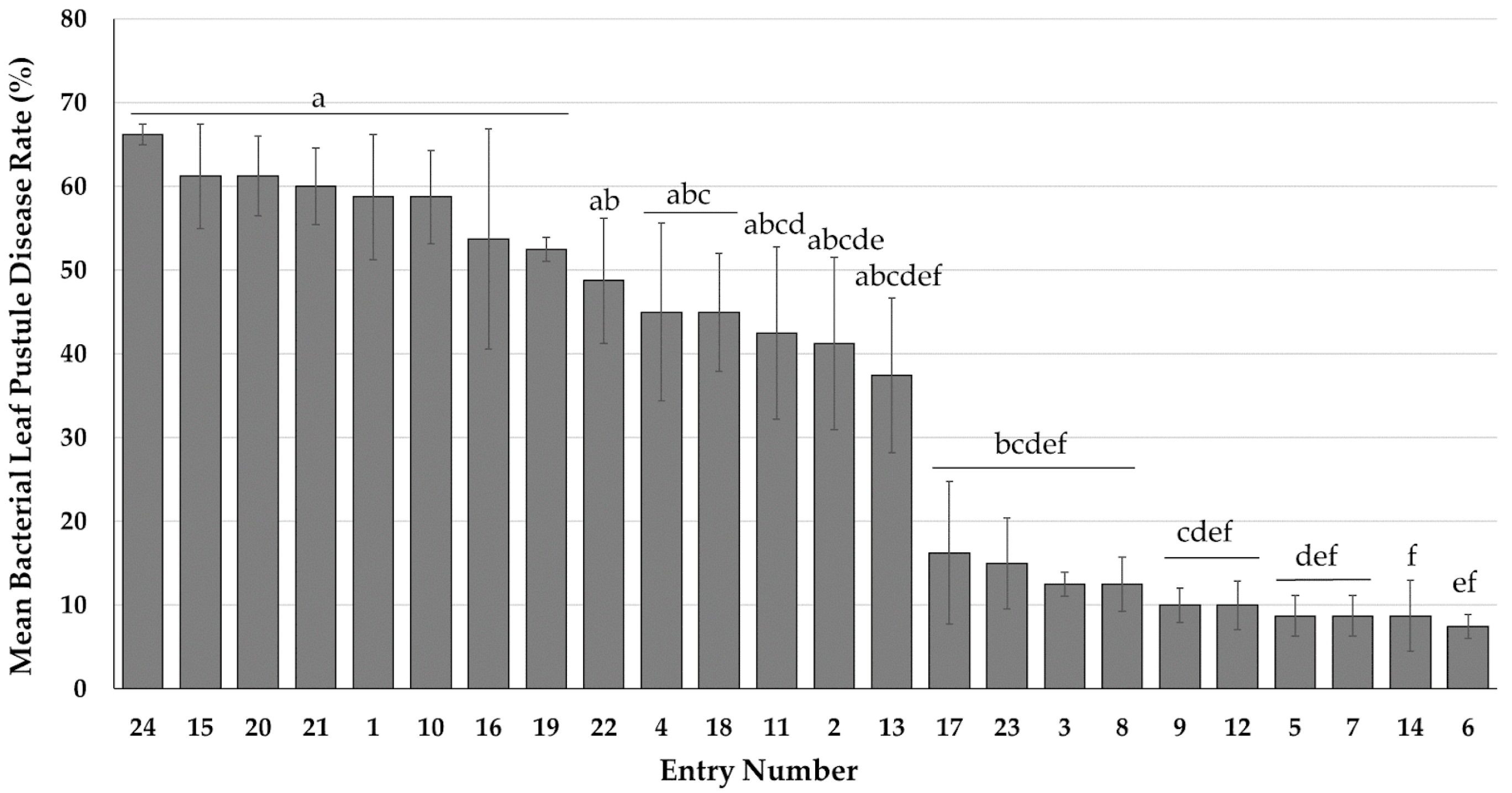
3.1.1. Disease Incidence and Severity of Bacterial Pustule in Virginia
3.1.2. Variations in Disease Development across Various Edamame Reaction Groups
| Entry No. | Variety Name | Type | Source | Group a |
|---|---|---|---|---|
| 1 | UA Kirksey | CV | UA, USA | 1 |
| 2 | R15-10280 | BL | UA, USA | 2 |
| 3 | R17-2750 | BL | UA, USA | 3 |
| 4 | R17-2776 | BL | UA, USA | 2 |
| 5 | R17-2965 | BL | UA, USA | 3 |
| 6 | R18-9725 | BL | UA, USA | 3 |
| 7 | R18-9770 | BL | UA, USA | 3 |
| 8 | R18-9782 | BL | UA, USA | 3 |
| 9 | R18-9794 | BL | UA, USA | 3 |
| 10 | R18-9808 | BL | UA, USA | 1 |
| 11 | VT Sweet | CV | VT, USA | 2 |
| 12 | V16-0293 | BL | VT, USA | 3 |
| 13 | V16-0521 | BL | VT, USA | 2 |
| 14 | V16-0523HP | BL | VT, USA | 3 |
| 15 | V16-0527 | BL | VT, USA | 1 |
| 16 | V16-0546 | BL | VT, USA | 1 |
| 17 | V16-0688HP | BL | VT, USA | 3 |
| 18 | V18-0785 | BL | VT, USA | 2 |
| 19 | V18-1025 | BL | VT, USA | 1 |
| 20 | V19-0196 | BL | VT, USA | 1 |
| 21 | V19-0288 | BL | VT, USA | 1 |
| 22 | V19-0290 | BL | VT, USA | 2 |
| 23 | V19-0322 | BL | VT, USA | 3 |
| 24 | V19-0368 | BL | VT, USA | 1 |
| CK1 | Agate | CV | Japan | NA |
| CK2 | Gardensoy 21 | CV | UI, USA | NA |
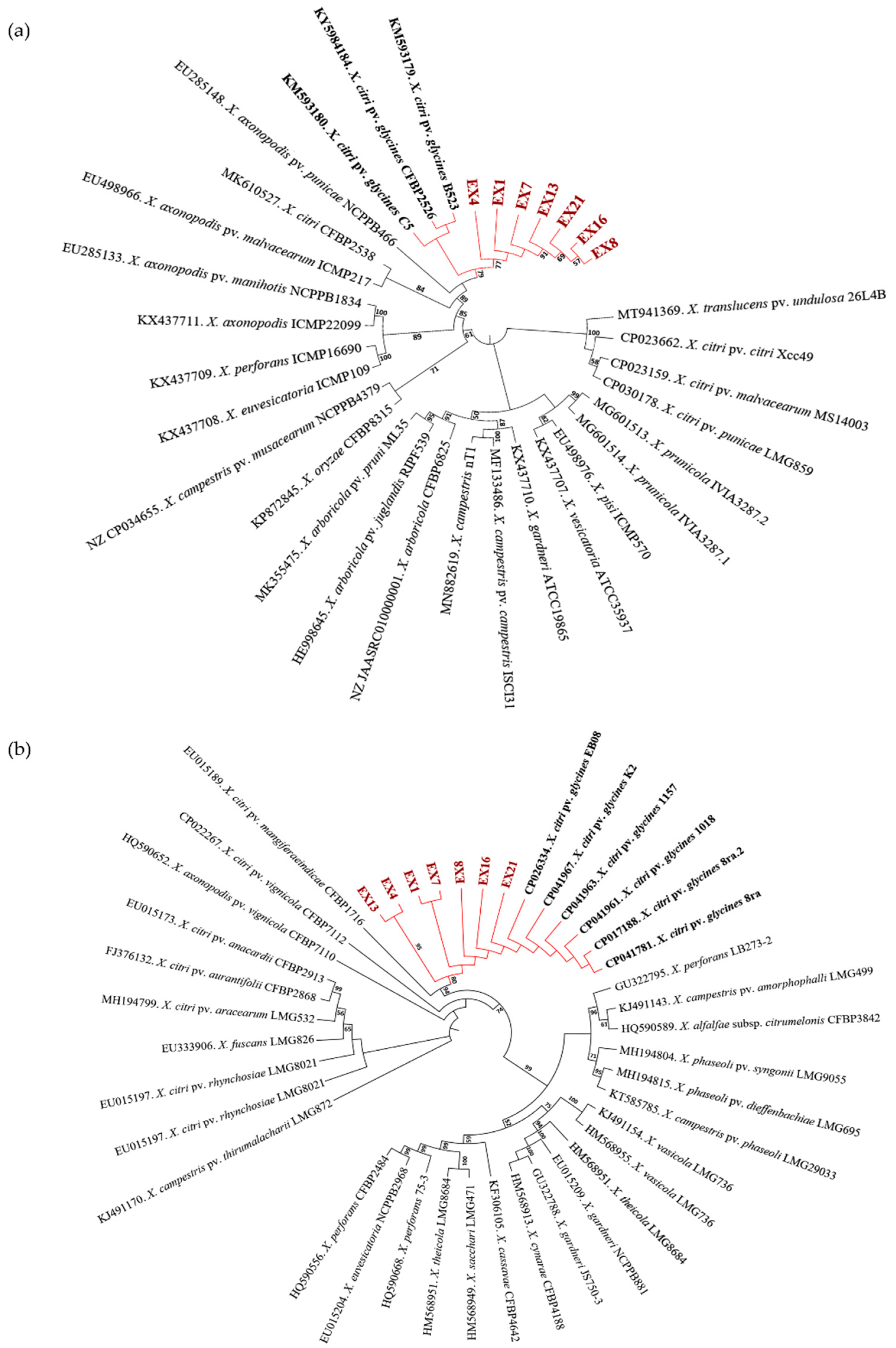
3.2. Identification of the Causative Agent of Bacterial Pustule
3.2.1. Isolation of Xanthomonas and Pathogenicity Determination
3.2.2. PCR Amplification and Phylogenetic Analysis
3.3. Seedling Resistance Screening to Soilborne Diseases
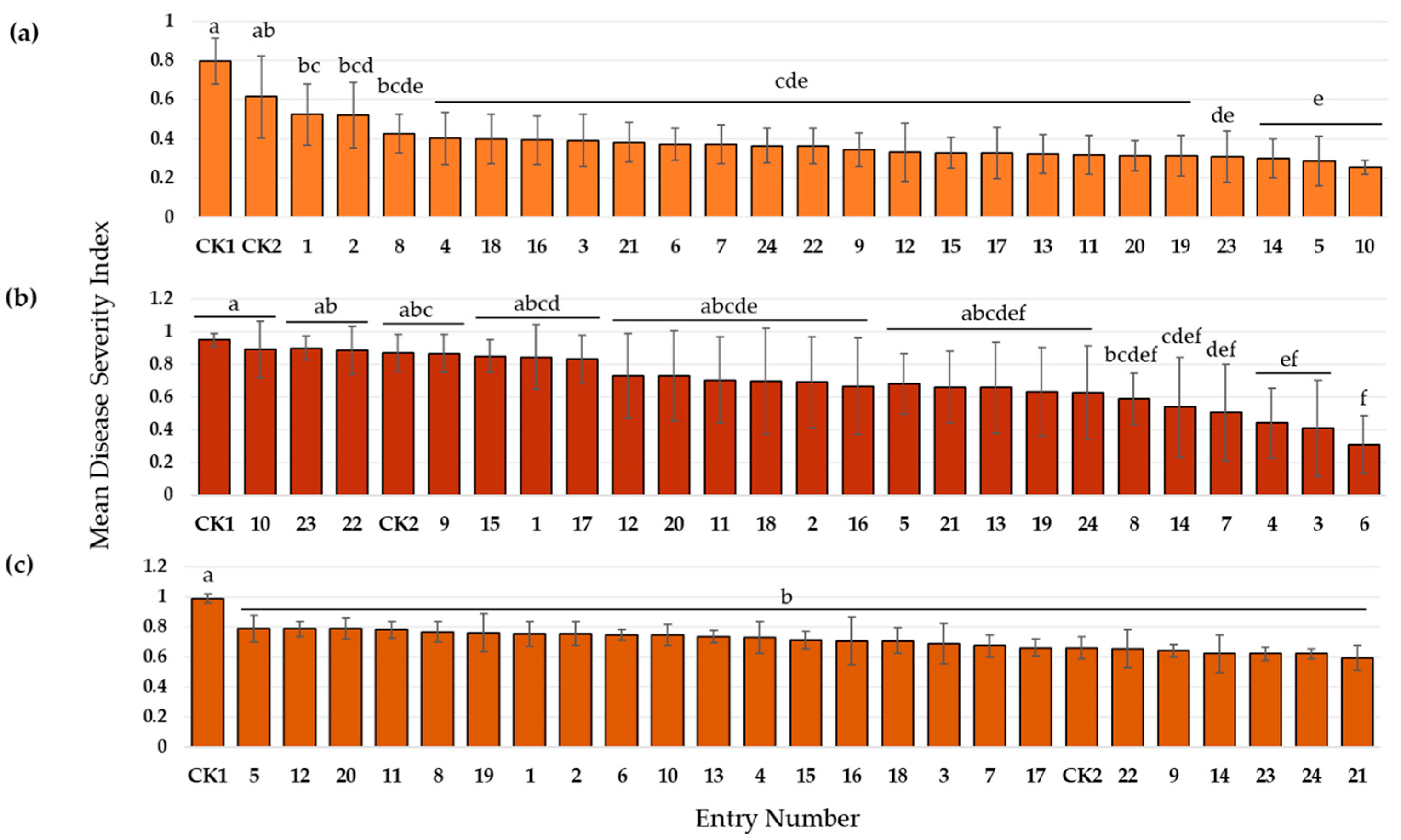
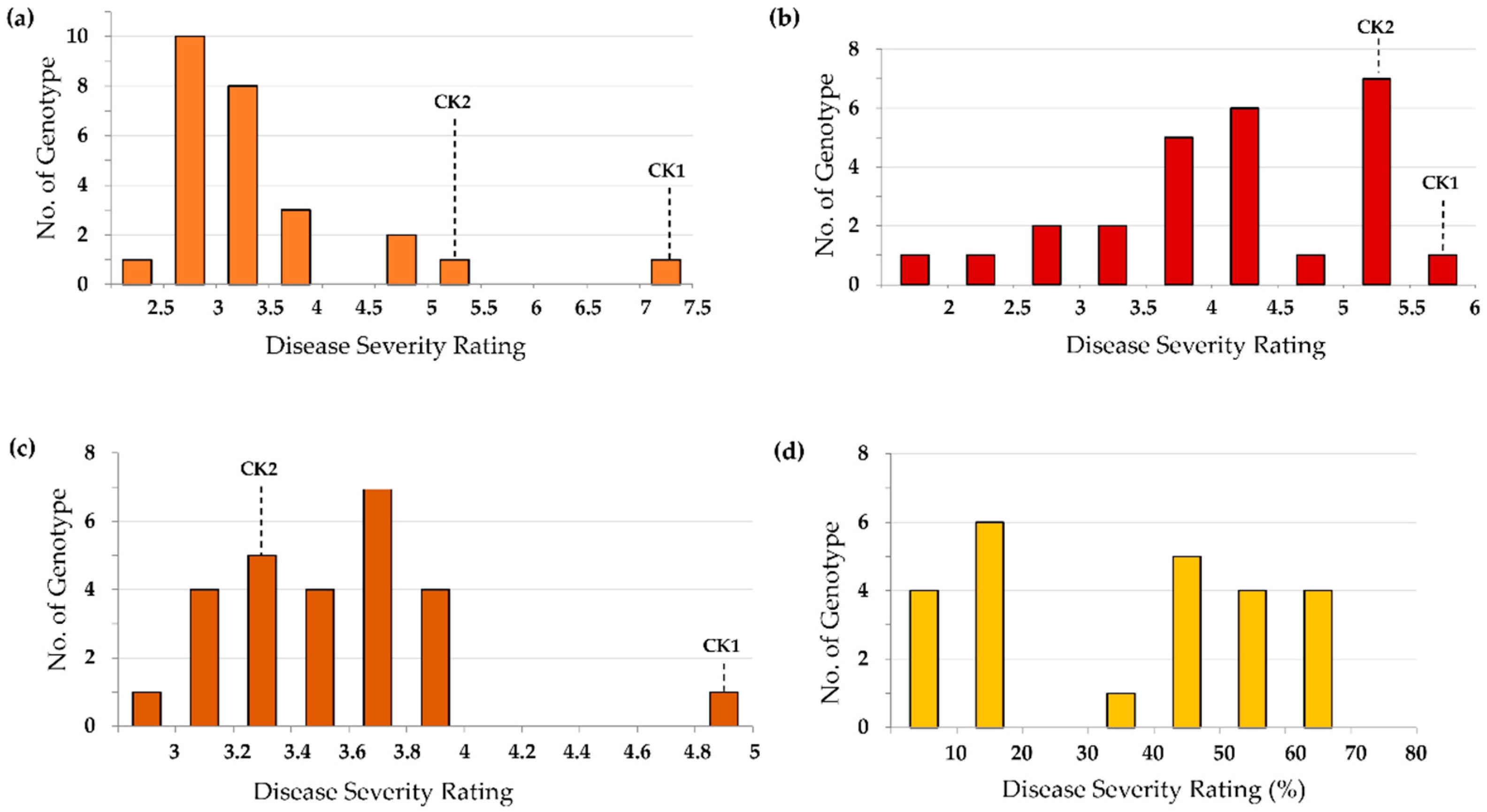
3.3.1. Resistance to Rhizoctonia Root and Stem Rot
3.3.2. Resistance to Southern Blight
3.3.3. Resistance to Pythium Root Rot
3.3.4. Overall Performance of Newly Developed Genotypes against Four Edamame Diseases
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zeipiņa, S.; Alsiņa, I.; Lepse, L. Insight in Edamame Yield and Quality Parameters: A Review. Res. Rural. Dev. 2017, 2, 40–45. [Google Scholar]
- Kaiser, C.; Ernst, M. Edamame. University of Kentucky, College of Agriculture, Food and Enviroment, Cooperative Extension Service. 2020. CCD-CP-94. Available online: https://www.uky.edu/ccd/sites/www.uky.edu.ccd/files/edamame.pdf (accessed on 25 July 2024).
- Williams, M.M.; Bradley, C.A. Fludioxonil + Mefenoxam Seed Treatment Improves Edamame Seedling Emergence. Horttechnology 2017, 27, 846–851. [Google Scholar] [CrossRef]
- Allen, T.W.; Bradley, C.A.; Sisson, A.J.; Byamukama, E.; Chilvers, M.I.; Coker, C.M.; Collins, A.A.; Damicone, J.P.; Dorrance, A.E.; Dufault, N.S.; et al. Soybean Yield Loss Estimates Due to Diseases in the United States and Ontario, Canada, from 2010 to 2014. Plant Health Prog. 2017, 18, 19–27. [Google Scholar] [CrossRef]
- Reiter, M.; Samtani, J.; Quezada, E.Q.; Singh, V.; Doughty, H.; Kuhar, T. 2022–2023 Mid-Atlantic Commercial Vegetable Production Recommendations. Available online: https://www.pubs.ext.vt.edu/456/456-420/456-420.html (accessed on 19 December 2022).
- Panth, M.; Hassler, S.C.; Baysal-Gurel, F. Methods for Management of Soilborne Diseases in Crop Production. Agriculture 2020, 10, 16. [Google Scholar] [CrossRef]
- Campo, R.J.; Araujo, R.S.; Hungria, M. Nitrogen Fixation with the Soybean Crop in Brazil: Compatibility between Seed Treatment with Fungicides and Bradyrhizobial Inoculants. Symbiosis 2009, 48, 154–163. [Google Scholar] [CrossRef]
- Zilli, J.É.; Ribeiro, K.G.; Campo, R.J.; Hungria, M. Influence of Fungicide Seed Treatment on Soybean Nodulation and Grain Yield. Rev. Bras. Ciência Solo 2009, 33, 917–923. [Google Scholar] [CrossRef]
- Lin, F.; Li, W.; McCoy, A.G.; Wang, K.; Jacobs, J.; Zhang, N.; Huo, X.; Wani, S.H.; Gu, C.; Chilvers, M.I.; et al. Identification and Characterization of Pleiotropic and Epistatic QDRL Conferring Partial Resistance to Pythium Irregulare and P. Sylvaticum in Soybean. Theor. Appl. Genet. 2022, 135, 3571–3582. [Google Scholar] [CrossRef] [PubMed]
- Kang, I.-J.; Kim, K.S.; Beattie, G.A.; Chung, H.; Heu, S.; Hwang, I. Characterization of Xanthomonas citri Pv. Glycines Population Genetics and Virulence in a National Survey of Bacterial Pustule Disease in Korea. Plant Pathol. J. 2021, 37, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Scaboo, A.M.; Dombek, D.G.; Rupe, J.C.; Robbins, R.T.; Chen, P. Soybean Cultivar UA Kirksey. U.S. Patent US8,766,051, 23 January 2014. [Google Scholar]
- Zhang, B.; Lord, N.; Kuhar, T.; Duncan, S.; Huang, H.; Ross, J.; Rideout, S.; Arancibia, R.; Reiter, M.; Li, S.; et al. ‘VT Sweet’: A Vegetable Soybean Cultivar for Commercial Edamame Production in the Mid-Atlantic USA. J. Plant Regist. 2022, 16, 29–33. [Google Scholar] [CrossRef]
- Capobiango da Fonseca, P.; Maria Barbosa, R.; de Oliveira Ferreira, D.; Badel, J.L.; Schuster, I.; Vieira, R.F.; Lopes da Silva, F. Genome-wide Association Study Reveals Molecular Markers and Genes Potentially Associated with Soybean (Glycine max) Resistance to Xanthomonas citri Pv. Glycines. Plant Breed. 2022, 141, 37–48. [Google Scholar] [CrossRef]
- Galkiewicz, J.P.; Kellogg, C.A. Cross-Kingdom Amplification Using Bacteria -Specific Primers: Complications for Studies of Coral Microbial Ecology. Appl. Environ. Microbiol. 2008, 74, 7828–7831. [Google Scholar] [CrossRef] [PubMed]
- Mondal, K.K.; Verma, G.; Mani, C. Phylogenetic Relatedness of Xanthomonas Axonopodis Pv. Punicae, the Causal Agent of Bacterial Blight of Pomegranate Based on Two Loci, 16S RRNA and GyrB. Ann. Microbiol. 2013, 63, 801–804. [Google Scholar] [CrossRef]
- Martins, L.; Fernandes, C.; Blom, J.; Dia, N.C.; Pothier, J.F.; Tavares, F. Xanthomonas Euroxanthea Sp. Nov., a New Xanthomonad Species Including Pathogenic and Non-Pathogenic Strains of Walnut. Int. J. Syst. Evol. Microbiol. 2020, 70, 6024–6031. [Google Scholar] [CrossRef] [PubMed]
- Gagnevin, L.; Leach, J.E.; Pruvost, O. Genomic Variability of the Xanthomonas Pathovar Mangiferaeindicae, Agent of Mango Bacterial Black Spot. Appl. Environ. Microbiol. 1997, 63, 246–253. [Google Scholar] [CrossRef] [PubMed]
- El-Esawi, M.A.; Alaraidh, I.A.; Alsahli, A.A.; Alamri, S.A.; Ali, H.M.; Alayafi, A.A. Bacillus Firmus (SW5) Augments Salt Tolerance in Soybean (Glycine max L.) by Modulating Root System Architecture, Antioxidant Defense Systems and Stress-Responsive Genes Expression. Plant Physiol. Biochem. 2018, 132, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Bradley, C.A.; Hartman, G.L.; Nelson, R.L.; Mueller, D.S.; Pederson, W.L. Response of Ancestral Soybean Lines and Commercial Cultivars to Rhizoctonia Root and Hypocotyl Rot. Plant Dis. 2001, 85, 1091–1095. [Google Scholar] [CrossRef] [PubMed]
- Peña, P.A.; Steadman, J.R.; Eskridge, K.M.; Urrea, C.A. Identification of Sources of Resistance to Damping-off and Early Root/Hypocotyl Damage from Rhizoctonia Solani in Common Bean (Phaseolus vulgaris L.). Crop. Prot. 2013, 54, 92–99. [Google Scholar] [CrossRef]
- Singh, B.; Technol, R.U.-J.A. Undefined Management of Southern Stem Blight of Soybean by PCNB-Resistant Mutants of Trichoderma Harzianum 4572 Incited by Sclerotium Rolfsii. Ijat-Aatsea. Com. 2009, 5, 85–98. [Google Scholar]
- Paparu, P.; Acur, A.; Kato, F.; Acam, C.; Nakibuule, J.; Nkuboye, A.; Musoke, S.; Mukankusi, C. Morphological and Pathogenic Characterization of Sclerotium Rolfsii, the Causal Agent of Southern Blight Disease on Common Bean in Uganda. Plant Dis. 2020, 104, 2130–2137. [Google Scholar] [CrossRef]
- Klepadlo, M.; Balk, C.S.; Vuong, T.D.; Dorrance, A.E.; Nguyen, H.T. Molecular Characterization of Genomic Regions for Resistance to Pythium Ultimum Var. Ultimum in the Soybean Cultivar Magellan. Theor. Appl. Genet. 2019, 132, 405–417. [Google Scholar] [CrossRef]
- Chiang, K.S.; Liu, H.I.; Bock, C.H. A Discussion on Disease Severity Index Values. Part I: Warning on Inherent Errors and Suggestions to Maximise Accuracy. Ann. Appl. Biol. 2017, 171, 139–154. [Google Scholar] [CrossRef]
- Segre, J.A. What Does It Take to Satisfy Koch’s Postulates Two Centuries Later?: Microbial Genomics and Propionibacteria Acnes. J. Investig. Dermatol. 2013, 133, 2141–2142. [Google Scholar] [CrossRef] [PubMed]
- Darrasse, A.; Bolot, S.; Serres-Giardi, L.; Charbit, E.; Boureau, T.; Fisher-Le Saux, M.; Briand, M.; Arlat, M.; Gagnevin, L.; Koebnik, R.; et al. High-Quality Draft Genome Sequences of Xanthomonas axonopodis pv. glycines Strains CFBP 2526 and CFBP 7119. Genome Announc. 2013, 1, 6. [Google Scholar] [CrossRef] [PubMed]
- Schoch, C.L.; Ciufo, S.; Domrachev, M.; Hotton, C.L.; Kannan, S.; Khovanskaya, R.; Leipe, D.; Mcveigh, R.; O’Neill, K.; Robbertse, B.; et al. NCBI Taxonomy: A Comprehensive Update on Curation, Resources and Tools. Database 2020, 2020, baaa062. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Guerra, C.A.; Cano-Díaz, C.; Egidi, E.; Wang, J.-T.; Eisenhauer, N.; Singh, B.K.; Maestre, F.T. The Proportion of Soil-Borne Pathogens Increases with Warming at the Global Scale. Nat. Clim. Chang. 2020, 10, 550–554. [Google Scholar] [CrossRef]
- Zhao, F.; Cheng, W.; Wang, Y.; Gao, X.; Huang, D.; Kong, J.; Antwi-Boasiako, A.; Zheng, L.; Yan, W.; Chang, F.; et al. Identification of Novel Genomic Regions for Bacterial Leaf Pustule (BLP) Resistance in Soybean (Glycine max L.) via Integrating Linkage Mapping and Association Analysis. Int. J. Mol. Sci. 2022, 23, 2113. [Google Scholar] [CrossRef]
- Weber, C.R.; Dunleavy, J.M.; Fehr, W.R. Effect of Bacterial Pustule on Closely Related Soybean Lines 1. Agron. J. 1966, 58, 544–545. [Google Scholar] [CrossRef]
- Prathuangwong, S.; Amnuaykit, K. Studies on Tolerance and Rate-Reducing Bacterial Pustule of Soybean Cultivars/Lines. Agric. Nat. Resour. 1987, 21, 408–426. [Google Scholar]
- Duppong, L.M.; Hatterman-Valenti, H. Yield and Quality of Vegetable Soybean Cultivars for Production in North Dakota. Horttechnology 2005, 15, 896–900. [Google Scholar] [CrossRef]
- Li, X.; Welbaum, G.E.; Rideout, S.L.; Singer, W.; Zhang, B. Vegetable Soybean and Its Seedling Emergence in the United States. In Legumes Research; IntechOpen: London, UK, 2022; Volume 1. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zaia, R.; Liu, K.; Xu, X.; Silva, M.D.; Rojas, A.; Welbaum, G.E.; Zhang, B.; Rideout, S. Response of the Edamame Germplasm to Early-Season Diseases in the United States. Agronomy 2024, 14, 1660. https://doi.org/10.3390/agronomy14081660
Li X, Zaia R, Liu K, Xu X, Silva MD, Rojas A, Welbaum GE, Zhang B, Rideout S. Response of the Edamame Germplasm to Early-Season Diseases in the United States. Agronomy. 2024; 14(8):1660. https://doi.org/10.3390/agronomy14081660
Chicago/Turabian StyleLi, Xiaoying, Rafael Zaia, Kathryn Liu, Xueming Xu, Marcos Da Silva, Alejandro Rojas, Gregory E. Welbaum, Bo Zhang, and Steven Rideout. 2024. "Response of the Edamame Germplasm to Early-Season Diseases in the United States" Agronomy 14, no. 8: 1660. https://doi.org/10.3390/agronomy14081660






