Lipidomics in Plants Under Abiotic Stress Conditions: An Overview
Abstract
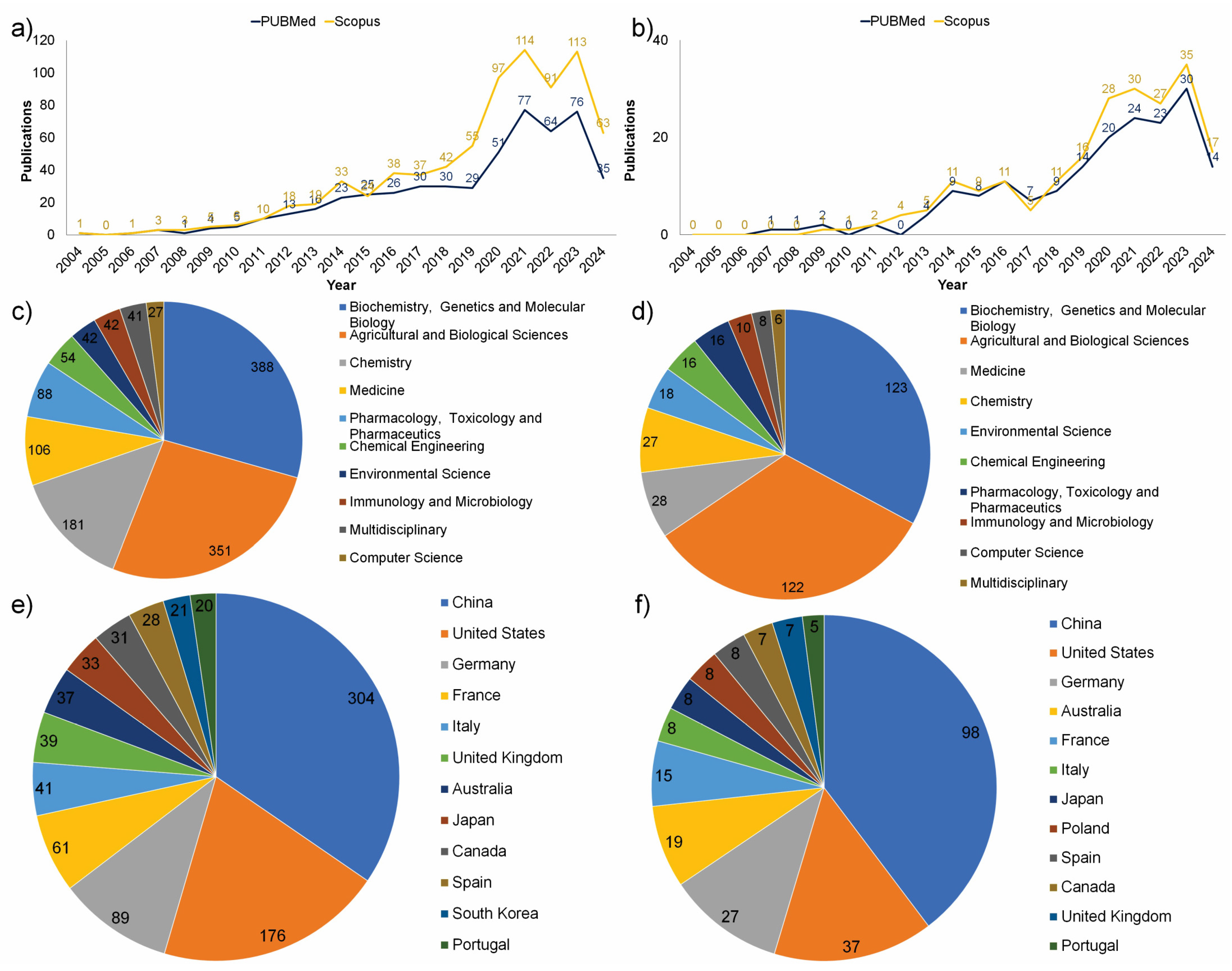
:1. Introduction
2. Overview of Current Research on Plant Lipidomics
3. Abiotic Stress Responses
3.1. Temperature Stress
3.2. Water Stress
3.3. Salt and Alkali Stress
3.4. Heavy Metals
3.5. Nutrient Deficiency
3.6. Light
4. Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cassim, A.M.; Gouguet, P.; Gronnier, J.; Laurent, N.; Germain, V.; Grison, M.; Boutté, Y.; Gerbeau-Pissot, P.; Simon-Plas, F.; Mongrand, S. Plant Lipids: Key Players of Plasma Membrane Organization and Function. Prog. Lipid Res. 2019, 73, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Reszczyńska, E.; Hanaka, A. Lipids Composition in Plant Membranes. Cell Biochem. Biophys. 2020, 78, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Zhang, L.; Duan, Z.; Lin, J. Coordination of Phospholipid-Based Signaling and Membrane Trafficking in Plant Immunity. Trends Plant Sci. 2021, 26, 407–420. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, G.E.; Lada, R.R.; Caldwell, C.D.; Udenigwe, C.; MacDonald, M.T. Potential Roles of Fatty Acids and Lipids in Postharvest Needle Abscission Physiology. Am. J. Plant Sci. 2019, 10, 1069–1089. [Google Scholar] [CrossRef]
- Nakamura, Y. Plant Phospholipid Diversity: Emerging Functions in Metabolism and Protein–Lipid Interactions. Trends Plant Sci. 2017, 22, 1027–1040. [Google Scholar] [CrossRef]
- Colin, L.A.; Jaillais, Y. Phospholipids across Scales: Lipid Patterns and Plant Development. Curr. Opin. Plant Biol. 2020, 53, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Qu, Y.; Zhang, Q. Phospholipids: Molecules Regulating Cytoskeletal Organization in Plant Abiotic Stress Tolerance. Plant Signal. Behav. 2014, 9, e28337. [Google Scholar] [CrossRef] [PubMed]
- Hölzl, G.; Dörmann, P. Chloroplast Lipids and Their Biosynthesis. Annu. Rev. Plant Biol. 2019, 70, 51–81. [Google Scholar] [CrossRef] [PubMed]
- Kalisch, B.; Dörmann, P.; Hölzl, G. DGDG and Glycolipids in Plants and Algae. Subcell. Biochem. 2016, 86, 51–83. [Google Scholar] [CrossRef] [PubMed]
- Basnet, R.; Zhang, J.; Hussain, N.; Shu, Q. Characterization and Mutational Analysis of a Monogalactosyldiacylglycerol Synthase Gene OsMGD2 in Rice. Front. Plant Sci. 2019, 10, 992. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Nagano, M.; Jin, S.; Miyagi, A.; Yamaguchi, M.; Kawai-Yamada, M.; Ishikawa, T. Plant-Unique Cis/Trans Isomerism of Long-Chain Base Unsaturation Is Selectively Required for Aluminum Tolerance Resulting from Glucosylceramide-Dependent Plasma Membrane Fluidity. Plants 2020, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Dunn, T.; Lynch, D.; Michaelson, L.; Napier, J. A Post-genomic Approach to Understanding Sphingolipid Metabolism in Arabidopsis thaliana. Ann. Bot. 2004, 93, 483–497. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.J.; Hou, L.P.; Bao, J.J.; Wang, L.J.; Chen, X.Y. Sphingolipid Metabolism, Transport, and Functions in Plants: Recent Progress and Future Perspectives. Plant Commun. 2021, 2, 100214. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chen, M.; Li, Y.; Huang, X.; Niu, D.; Rashid, A.; Xu, C.; Wang, K. Adjustments of Both Phospholipids and Sphingolipids Contribute to Cold Tolerance in Stony Hard Peach Fruit by Continuous Ethylene. Postharvest Biol. Technol. 2021, 171, 111332. [Google Scholar] [CrossRef]
- Valitova, J.N.; Sulkarnayeva, A.G.; Minibayeva, F.V. Plant Sterols: Diversity, Biosynthesis, and Physiological Functions. Biochemistry 2016, 81, 819–834. [Google Scholar] [CrossRef] [PubMed]
- Tarkowská, D.; Krampolová, E.; Strnad, M. Plant Triterpenoid Crosstalk: The Interaction of Brassinosteroids and Phytoecdysteroids in Lepidium sativum. Plants 2020, 9, 1325. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Nemoto, K.; Shimizu, M.; Abe, A.; Asai, S.; Ishihama, N.; Matsuoka, S.; Daimon, T.; Ojika, M.; Kawakita, K.; et al. Recognition of Pathogen-Derived Sphingolipids in Arabidopsis. Science 2022, 376, 857–860. [Google Scholar] [CrossRef] [PubMed]
- Bates, P.D. Understanding the Control of Acyl Flux through the Lipid Metabolic Network of Plant Oil Biosynthesis. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2016, 1861, 1214–1225. [Google Scholar] [CrossRef]
- Lu, J.; Xu, Y.; Wang, J.; Singer, S.D.; Chen, G. The Role of Triacylglycerol in Plant Stress Response. Plants 2020, 9, 472. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Shanklin, J. Triacylglycerol Metabolism, Function, and Accumulation in Plant Vegetative Tissues. Annu. Rev. Plant Biol. 2016, 67, 179–206. [Google Scholar] [CrossRef] [PubMed]
- Chapman, K.D.; Aziz, M.; Dyer, J.M.; Mullen, R.T. Mechanisms of Lipid Droplet Biogenesis. Biochem. J. 2019, 476, 1929–1942. [Google Scholar] [CrossRef] [PubMed]
- Barros, J.A.S.; Siqueira, J.A.B.; Cavalcanti, J.H.F.; Araújo, W.L.; Avin-Wittenberg, T. Multifaceted Roles of Plant Autophagy in Lipid and Energy Metabolism. Trends Plant Sci. 2020, 25, 1141–1153. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Benning, C. Functions of Triacylglycerols during Plant Development and Stress. Curr. Opin. Biotechnol. 2018, 49, 191–198. [Google Scholar] [CrossRef]
- Wang, L.; Qian, B.; Zhao, L.; Liang, M.H.; Zhan, X.; Zhu, J. Two Triacylglycerol Lipases Are Negative Regulators of Chilling Stress Tolerance in Arabidopsis. Int. J. Mol. Sci. 2022, 23, 3380. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Zhou, C.; Fan, J.; Shanklin, J.; Xu, C. Mechanisms and Functions of Membrane Lipid Remodeling in Plants. Plant J. 2021, 107, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Ischebeck, T.; Krawczyk, H.E.; Mullen, R.T.; Dyer, J.M.; Chapman, K.D. Lipid Droplets in Plants and Algae: Distribution, Formation, Turnover and Function. Semin. Cell Dev. Biol. 2020, 108, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, T.; Han, J.; Li, M.; Zhao, Y.; Su, T.; Ma, C. Plant Autophagy: An Intricate Process Controlled by Various Signaling Pathways. Front. Plant Sci. 2021, 12, 754982. [Google Scholar] [CrossRef] [PubMed]
- Farquharson, K.L. Autophagy Contributes to Plant Lipid Homeostasis. Plant Cell 2019, 31, 1427–1428. [Google Scholar] [CrossRef] [PubMed]
- Barros, J.A.S.; Magen, S.; Lapidot-Cohen, T.; Rosental, L.; Brotman, Y.; Araújo, W.L.; Avin-Wittenberg, T. Autophagy Is Required for Lipid Homeostasis during Dark-Induced Senescence. Plant Physiol. 2021, 185, 1547–1558. [Google Scholar] [CrossRef] [PubMed]
- Cahoon, E.B.; Li-Beisson, Y. Plant Unusual Fatty Acids: Learning from the Less Common. Curr. Opin. Plant Biol. 2020, 55, 66–73. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Ding, N.Z. Plant Unsaturated Fatty Acids: Multiple Roles in Stress Response. Front. Plant Sci. 2020, 11, 562785. [Google Scholar] [CrossRef] [PubMed]
- Philippe, G.; Sørensen, I.; Jiao, C.; Sun, X.; Fei, Z.; Domozych, D.S.; Rose, J.K. Cutin and Suberin: Assembly and Origins of Specialized Lipidic Cell Wall Scaffolds. Curr. Opin. Plant Biol. 2020, 55, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Macabuhay, A.; Arsova, B.; Walker, R.; Johnson, A.; Watt, M.; Roessner, U. Modulators or Facilitators? Roles of Lipids in Plant Root–Microbe Interactions. Trends Plant Sci. 2022, 27, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Cavaco, A.R.; Matos, A.R.; Figueiredo, A. Speaking the Language of Lipids: The Cross-Talk between Plants and Pathogens in Defence and Disease. Cell. Mol. Life Sci. 2021, 78, 4399–4415. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, Y.; Saito, K. Roles of Lipids as Signaling Molecules and Mitigators during Stress Response in Plants. Plant J. 2014, 79, 584–596. [Google Scholar] [CrossRef] [PubMed]
- Huby, E.; Napier, J.A.; Baillieul, F.; Michaelson, L.V.; Dhondt-Cordelier, S. Sphingolipids: Towards an Integrated View of Metabolism during the Plant Stress Response. New Phytol. 2020, 225, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, Y.L.; Li, Y.K.; Chen, D.K.; He, J.F.; Yao, N. Functions of Sphingolipids in Pathogenesis During Host–Pathogen Interactions. Front. Microbiol. 2021, 12, 701041. [Google Scholar] [CrossRef] [PubMed]
- Koelmel, J.P.; Napolitano, M.P.; Ulmer, C.Z.; Vasiliou, V.; Garrett, T.J.; Yost, R.A.; Prasad, M.N.V.; Godri Pollitt, K.J.; Bowden, J.A. Environmental Lipidomics: Understanding the Response of Organisms and Ecosystems to a Changing World. Metabolomics 2020, 16, 56. [Google Scholar] [CrossRef] [PubMed]
- Waadt, R.; Seller, C.A.; Hsu, P.-K.; Takahashi, Y.; Munemasa, S.; Schroeder, J.I. Plant Hormone Regulation of Abiotic Stress Responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 680–694. [Google Scholar] [CrossRef] [PubMed]
- Higashi, Y.; Saito, K. Lipidomic Studies of Membrane Glycerolipids in Plant Leaves under Heat Stress. Prog. Lipid Res. 2019, 75, 100990. [Google Scholar] [CrossRef] [PubMed]
- Tenenboim, H.; Burgos, A.; Willmitzer, L.; Brotman, Y. Using Lipidomics for Expanding the Knowledge on Lipid Metabolism in Plants. Biochimie 2016, 130, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.A.; Niazi, A.K.; Akhtar, J.; Saifullah; Farooq, M.; Souri, Z.; Karimi, N.; Rengel, Z. Acquiring Control: The Evolution of ROS-Induced Oxidative Stress and Redox Signaling Pathways in Plant Stress Responses. Plant Physiol. Biochem. 2019, 141, 353–369. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Joshi, S.; Patil, S.; Khare, T.; Kumar, V. Reactive Oxygen, Nitrogen, Carbonyl and Sulfur Species and Their Roles in Plant Abiotic Stress Responses and Tolerance. J. Plant Growth Regul. 2022, 41, 119–142. [Google Scholar] [CrossRef]
- Signorelli, S.; Tarkowski, Ł.P.; Van den Ende, W.; Bassham, D.C. Linking Autophagy to Abiotic and Biotic Stress Responses. Trends Plant Sci. 2019, 24, 413–430. [Google Scholar] [CrossRef] [PubMed]
- Gross, R.W. The Evolution of Lipidomics through Space and Time. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Gross, R.W. The Foundations and Development of Lipidomics. J. Lipid Res. 2022, 63, 100164. [Google Scholar] [CrossRef] [PubMed]
- Kehelpannala, C.; Rupasinghe, T.; Hennessy, T.; Bradley, D.; Ebert, B.; Roessner, U. The State of the Art in Plant Lipidomics. Mol. Omics 2021, 17, 894–910. [Google Scholar] [CrossRef]
- Narayanan, S.; Zoong-Lwe, Z.S.; Gandhi, N.; Welti, R.; Fallen, B.; Smith, J.R.; Rustgi, S. Comparative Lipidomic Analysis Reveals Heat Stress Responses of Two Soybean Genotypes Differing in Temperature Sensitivity. Plants 2020, 9, 457. [Google Scholar] [CrossRef]
- Zhang, X.; Ni, Y.; Xu, D.; Busta, L.; Xiao, Y.; Jetter, R.; Guo, Y. Integrative Analysis of the Cuticular Lipidome and Transcriptome of Sorghum Bicolor Reveals Cultivar Differences in Drought Tolerance. Plant Physiol. Biochem. 2021, 163, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.J.; Qi, H.; Cheng, B.; Hussain, S.; Peng, Y.; Liu, W.; Feng, G.; Zhao, J.; Li, Z. Enhanced Adaptability to Limited Water Supply Regulated by Diethyl Aminoethyl Hexanoate (DA-6) Associated With Lipidomic Reprogramming in Two White Clover Genotypes. Front. Plant Sci. 2022, 13, 879331. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Cui, H.; Ma, C.; Li, Y.; Yang, C.; Wang, K.; Sun, Y. Lipidomic Metabolism Associated with Acetic Acid Priming-Induced Salt Tolerance in Carex rigescens. Plant Physiol. Biochem. 2021, 167, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Buffagni, V.; Zhang, L.; Senizza, B.; Rocchetti, G.; Ferrarini, A.; Miras-Moreno, B.; Lucini, L. Metabolomics and Lipidomics Insight into the Effect of Different Polyamines on Tomato Plants under Non-Stress and Salinity Conditions. Plant Sci. 2022, 322, 111346. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Cheng, B.; Zhao, Y.; Luo, L.; Zhang, Y.; Feng, G.; Han, L.; Peng, Y.; Zhang, X. Metabolic Regulation and Lipidomic Remodeling in Relation to Spermidine-Induced Stress Tolerance to High Temperature in Plants. Int. J. Mol. Sci. 2022, 23, 12247. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gao, Y.; Zhuang, L.; Huang, B. Phosphatidic Acid Priming-Enhanced Heat Tolerance in Tall Fescue (Festuca arundinacea) Involves Lipidomic Reprogramming of Lipids for Membrane Stability and Stress Signaling. Plant Growth Regul. 2023, 99, 527–538. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, J.; Yan, B.; Wei, Y.; Ge, S.; Li, J.; Han, Y.; Li, Z.; Zhao, C.; Xu, J. The Adjustment of Membrane Lipid Metabolism Pathways in Maize Roots Under Saline–Alkaline Stress. Front Plant Sci. 2021, 12, 635327. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Wang, Z.; Xu, C.; Li, L.; Yang, C. Multiomics Analysis Provides Insights into Alkali Stress Tolerance of Sunflower (Helianthus annuus L.). Plant Physiol. Biochem. 2021, 166, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Tarazona, P.; Feussner, K.; Feussner, I. An Enhanced Plant Lipidomics Method Based on Multiplexed Liquid Chromatography-Mass Spectrometry Reveals Additional Insights into Cold- and Drought-Induced Membrane Remodeling. Plant J. 2015, 84, 621–633. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Thelen, J.J. Acyl-Lipid Desaturase 1 Primes Cold Acclimation Response in Arabidopsis. Physiol. Plant 2016, 158, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, Q.; Chen, J.; Jiang, Y. Revealing Further Insights on Chilling Injury of Postharvest Bananas by Untargeted Lipidomics. Foods 2020, 9, 894. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, S.; Wu, J.; Liu, H.; Wang, P.; Xu, L. Insights into Membrane Lipids Modification in Barley Leaves as an Adaptation Mechanism to Cold Stress. Plant Growth Regul. 2024, 103, 369–388. [Google Scholar] [CrossRef]
- Kong, X.; Wei, B.; Gao, Z.; Zhou, Y.; Shi, F.; Zhou, X.; Zhou, Q.; Ji, S. Changes in Membrane Lipid Composition and Function Accompanying Chilling Injury in Bell Peppers. Plant Cell Physiol. 2018, 59, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Lam, S.M.; Zuo, J.; Yuan, S.; Lv, J.; Shi, J.; Gao, L.; Chen, B.; Sui, Y.; Shui, G.; et al. Lipidomics Reveals the Difference of Membrane Lipid Catabolism between Chilling Injury Sensitive and Non-Sensitive Green Bell Pepper in Response to Chilling. Postharvest Biol. Technol. 2021, 182, 111714. [Google Scholar] [CrossRef]
- Wang, Y.; Ji, S.; Dai, H.; Kong, X.; Hao, J.; Wang, S.; Zhou, X.; Zhao, Y.; Wei, B.; Cheng, S.; et al. Changes in Membrane Lipid Metabolism Accompany Pitting in Blueberry during Refrigeration and Subsequent Storage at Room Temperature. Front. Plant Sci. 2019, 10, 829. [Google Scholar] [CrossRef] [PubMed]
- Zainal, P.W.; Syukri, D.; Fahmy, K.; Imaizumi, T.; Thammawong, M.; Tsuta, M.; Nagata, M.; Nakano, K. Lipidomic Profiling to Assess the Freshness of Stored Cabbage. Food Anal. Methods 2023, 16, 304–317. [Google Scholar] [CrossRef]
- Dhaliwal, L.K.; Shim, J.; Auld, D.; Angeles-Shim, R.B. Fatty Acid Unsaturation Improves Germination of Upland Cotton (Gossypium Hirsutum) under Cold Stress. Front. Plant Sci. 2024, 15, 1286908. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Qian, Y.; Mao, B. Dendrobium Multi-Omics Reveal Lipid Remodeling in Response to Freezing. Metabolites 2022, 12, 1216. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wei, Y.; Zhang, J.; Yang, L.; Liu, X.; Zhang, H.; Shao, W.; He, L.; Li, Z.; Zhang, Y.; et al. Membrane Lipids’ Metabolism and Transcriptional Regulation in Maize Roots Under Cold Stress. Front. Plant Sci. 2021, 12, 639132. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Jiang, H.; Li, M.; Wang, D.; Xiang, H.; Zeng, R.; Chen, L.; Zhang, X.; Zuo, J.; Yang, S.; et al. Genetic and Lipidomic Analyses Reveal the Key Role of Lipid Metabolism for Cold Tolerance in Maize. J. Genet. Genom. 2024, 51, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Tian, B.; Li, W. Membrane Lipid Remodelling of Meconopsis Racemosa after Its Introduction into Lowlands from an Alpine Environment. PLoS ONE 2014, 9, e106614. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Eiriksson, F.F.; Thorsteinsdóttir, M.; Simonsen, H.T. Lipidomic Analysis of Moss Species Bryum Pseudotriquetrum and Physcomitrium Patens under Cold Stress. Plant Environ. Interac. 2022, 3, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Chen, J.; Xu, F.; He, X.; Yang, S.; Zhu, Y.; Li, W.; Zheng, G. Analysis of Changes in the Panax Notoginseng Glycerolipidome in Response to Long-Term Chilling and Heat. Plant Divers. 2020, 42, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, K.; Wu, C.; Zhao, Y.; Yin, X.; Zhang, B.; Grierson, D.; Chen, K.; Xu, C. Effect of Ethylene on Cell Wall and Lipid Metabolism during Alleviation of Postharvest Chilling Injury in Peach. Cells 2019, 8, 1612. [Google Scholar] [CrossRef] [PubMed]
- Cheong, B.E.; Ho, W.W.H.; Biddulph, B.; Wallace, X.; Rathjen, T.; Rupasinghe, T.W.T.; Roessner, U.; Dolferus, R. Phenotyping Reproductive Stage Chilling and Frost Tolerance in Wheat Using Targeted Metabolome and Lipidome Profiling. Metabolomics 2019, 15, 144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhao, X.; Bi, M.; Zhang, H.; He, Y.; Cui, Y.; Qi, M. Membrane Lipid Metabolism and Scavenging of Reactive Oxygen Species Are Essential for Cold Resistance of Solanum habrochaites (LA1777): Lipidomics and Transcriptomes Analysis. Environ. Exp. Bot. 2023, 209, 105290. [Google Scholar] [CrossRef]
- Rabeh, K.; Sbabou, L.; Rachidi, F.; Ferradouss, A.; Laghmari, G.; Aasfar, A.; El Arroussi, H.; Ouajdi, M.; El Antry, S.; Belkadi, B.; et al. Lipidomic Profiling of Argania spinosa L. (Skeels) Following Drought Stress. Appl. Biochem. Biotechnol. 2023, 195, 1781–1799. [Google Scholar] [CrossRef] [PubMed]
- Sarabia, L.D.; Boughton, B.A.; Rupasinghe, T.; Callahan, D.L.; Hill, C.B.; Roessner, U. Comparative Spatial Lipidomics Analysis Reveals Cellular Lipid Remodelling in Different Developmental Zones of Barley Roots in Response to Salinity. Plant Cell Environ. 2020, 43, 327–343. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Wang, S.; Sun, L.; Wang, Y.; Fan, K.; Li, C.; Wang, H.; Bi, C.; Zhang, F.; Ding, Z. Dynamic Changes in Metabolic and Lipidomic Profiles of Tea Plants during Drought Stress and Re-Watering. Front. Plant Sci. 2022, 13, 978531. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wang, X.; Li, K.; Cai, Z. Spatially Resolved Metabolomics and Lipidomics Reveal Salinity and Drought-Tolerant Mechanisms of Cottonseeds. J. Agric. Food Chem. 2021, 69, 8028–8037. [Google Scholar] [CrossRef] [PubMed]
- Perlikowski, D.; Kierszniowska, S.; Sawikowska, A.; Krajewski, P.; Rapacz, M.; Eckhardt, Ä.; Kosmala, A. Remodeling of Leaf Cellular Glycerolipid Composition under Drought and Re-Hydration Conditions in Grasses from the Lolium-Festuca Complex. Front. Plant Sci. 2016, 7, 1027. [Google Scholar] [CrossRef] [PubMed]
- Kränzlein, M.; Schmöckel, S.M.; Geilfus, C.M.; Schulze, W.X.; Altenbuchinger, M.; Hrenn, H.; Roessner, U.; Zörb, C. Lipid Remodeling of Contrasting Maize (Zea mays L.) Hybrids under Repeated Drought. Front. Plant Sci. 2023, 14, 1050079. [Google Scholar] [CrossRef] [PubMed]
- Moradi, P.; Mahdavi, A.; Khoshkam, M.; Iriti, M. Lipidomics Unravels the Role of Leaf Lipids in Thyme Plant Response to Drought Stress. Int. J. Mol. Sci. 2017, 18, 2067. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Shen, Y.; Tao, F.; Yang, S.; Li, W. Submergence Induced Changes of Molecular Species in Membrane Lipids in Arabidopsis thaliana. Plant Divers. 2016, 38, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Shao, D.; Yu, C.; Chen, Y.; Qiu, X.; Chen, J.; Zhao, H.; Chen, K.; Wang, X.; Chen, P.; Gao, G.; et al. Lipids Signaling and Unsaturation of Fatty Acids Participate in Ramie Response to Submergence Stress and Hypoxia-Responsive Gene Regulation. Int. J. Biol. Macromol. 2024, 263, 130104. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Weng, X.; Tang, S.; Zhang, W.; Zhu, K.; Zhu, G.; Zhang, H.; Wang, Z.; Yang, J. Untargeted Lipidomic Analysis of Milled Rice under Different Alternate Wetting and Soil Drying Irrigation Regimes. J. Integr. Agric. 2024, in press. [CrossRef]
- Basu, S.; Kumari, S.; Kumar, P.; Kumar, G.; Rajwanshi, R. Redox Imbalance Impedes Photosynthetic Activity in Rice by Disrupting Cellular Membrane Integrity and Induces Programmed Cell Death under Submergence. Physiol. Plant. 2021, 172, 1764–1778. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Pan, R.; Zhou, M.; Xu, Y.; Zhang, W. Lipid Remodelling Plays an Important Role in Wheat (Triticum aestivum) Hypoxia Stress. Funct. Plant Biol. 2019, 47, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Higashi, Y.; Okazaki, Y.; Takano, K.; Myouga, F.; Shinozaki, K.; Knoch, E.; Fukushima, A.; Saito, K. HEAT INDUCIBLE LIPASE1 Remodels Chloroplastic Monogalactosyldiacylglycerol by Liberating α-Linolenic Acid in Arabidopsis Leaves under Heat Stress. Plant Cell 2018, 30, 1887–1905. [Google Scholar] [CrossRef] [PubMed]
- Higashi, Y.; Okazaki, Y.; Myouga, F.; Shinozaki, K.; Saito, K. Landscape of the Lipidome and Transcriptome under Heat Stress in Arabidopsis thaliana. Sci. Rep. 2015, 5, 10533. [Google Scholar] [CrossRef]
- Shiva, S.; Samarakoon, T.; Lowe, K.A.; Roach, C.; Vu, H.S.; Colter, M.; Porras, H.; Hwang, C.; Roth, M.R.; Tamura, P.; et al. Leaf Lipid Alterations in Response to Heat Stress of Arabidopsis thaliana. Plants 2020, 9, 845. [Google Scholar] [CrossRef]
- Korte, P.; Unzner, A.; Damm, T.; Berger, S.; Krischke, M.; Mueller, M.J. High Triacylglycerol Turnover Is Required for Efficient Opening of Stomata during Heat Stress in Arabidopsis. Plant J. 2023, 115, 81–96. [Google Scholar] [CrossRef]
- Qian, W.; Zhu, Y.; Chen, Q.; Wang, S.; Chen, L.; Liu, T.; Tang, H.; Yao, H. Comprehensive Metabolomic and Lipidomic Alterations in Response to Heat Stress during Seed Germination and Seedling Growth of Arabidopsis. Front. Plant Sci. 2023, 14, 1132881. [Google Scholar] [CrossRef] [PubMed]
- Sun, A.Z.; Chen, L.S.; Tang, M.; Chen, J.H.; Li, H.; Jin, X.Q.; Yi, Y.; Guo, F.Q. Lipidomic Remodeling in Begonia Grandis Under Heat Stress. Front. Plant Sci. 2022, 13, 843942. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.; Han, B.; Liu, A.; Xu, W. Integrated Lipidomic and Transcriptomic Analysis Reveals Triacylglycerol Accumulation in Castor Bean Seedlings under Heat Stress. Ind. Crops Prod. 2022, 180, 114702. [Google Scholar] [CrossRef]
- Zoong Lwe, Z.S.; Welti, R.; Anco, D.; Naveed, S.; Rustgi, S.; Narayanan, S. Heat Stress Elicits Remodeling in the Anther Lipidome of Peanut. Sci. Rep. 2020, 10, 22163. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, Y.; Huang, B. Lipidomic Reprogramming Associated with Drought Stress Priming-Enhanced Heat Tolerance in Tall Fescue (Festuca arundinacea). Plant Cell Environ. 2019, 42, 947–958. [Google Scholar] [CrossRef]
- Hu, H.; Jia, Y.; Hao, Z.; Ma, G.; Xie, Y.; Wang, C.; Ma, D. Lipidomics-Based Insights into the Physiological Mechanism of Wheat in Response to Heat Stress. Plant Physiol. Biochem. 2023, 205, 108190. [Google Scholar] [CrossRef] [PubMed]
- Figueira, E.; Matos, D.; Cardoso, P.; Pires, A.; Fernandes, C.; Tauler, R.; Bedia, C. A Biochemical and Lipidomic Approach to Perceive Halimione portulacoides (L.) Response to Mercury: An Environmental Perspective. Mar. Pollut. Bull. 2023, 186, 114393. [Google Scholar] [CrossRef] [PubMed]
- Andresen, E.; Lyubenova, L.; Hubáček, T.; Bokhari, S.N.H.; Matoušková, Š.; Mijovilovich, A.; Rohovec, J.; Küpper, H. Chronic Exposure of Soybean Plants to Nanomolar Cadmium Reveals Specific Additional High-Affinity Targets of Cadmium Toxicity. J. Exp. Bot. 2020, 71, 1628–1644. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Hu, C.; Wang, X.; Wang, Y.; Yu, M.; Xiao, H.; Shabala, S.; Wu, K.; Tan, Q.; Xu, S.; et al. Cadmium-Induced Changes in Composition and Co-Metabolism of Glycerolipids Species in Wheat Root: Glycerolipidomic and Transcriptomic Approach. J. Hazard. Mater. 2022, 423, 127115. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, A.; Yadav, D.K.; Szymańska, R.; Kruk, J.; Sedlářová, M.; Pospíšil, P. Singlet Oxygen Scavenging Activity of Tocopherol and Plastochromanol in Arabidopsis thaliana: Relevance to Photooxidative Stress. Plant Cell Environ. 2014, 37, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Burgos, A.; Szymanski, J.; Seiwert, B.; Degenkolbe, T.; Hannah, M.A.; Giavalisco, P.; Willmitzer, L. Analysis of Short-Term Changes in the Arabidopsis Thaliana Glycerolipidome in Response to Temperature and Light. Plant J. 2011, 66, 656–668. [Google Scholar] [CrossRef] [PubMed]
- Kargiotidou, A.; Deli, D.; Galanopoulou, D.; Tsaftaris, A.; Farmaki, T. Low Temperature and Light Regulate Delta 12 Fatty Acid Desaturases (FAD2) at a Transcriptional Level in Cotton (Gossypium hirsutum). J. Exp. Bot. 2008, 59, 2043–2056. [Google Scholar] [CrossRef] [PubMed]
- Callahan, G.; Fillier, T.; Pham, T.H.; Zhu, X.; Thomas, R. The Effects of Clearcut Harvesting on Moss Chloroplast Lipidome and Adaptation to Light Stress during Boreal Forest Regeneration. J. Environ. Manag. 2022, 315, 115126. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Chen, S.; Gu, M.; Chen, X.; Chen, X.; Yang, J.; Zhao, F.; Ye, N. Exploration of the Effects of Different Blue Led Light Intensities on Flavonoid and Lipid Metabolism in Tea Plants via Transcriptomics and Metabolomics. Int. J. Mol. Sci. 2020, 21, 4606. [Google Scholar] [CrossRef] [PubMed]
- Spicher, L.; Almeida, J.; Gutbrod, K.; Pipitone, R.; Dörmann, P.; Glauser, G.; Rossi, M.; Kessler, F. Essential Role for Phytol Kinase and Tocopherol in Tolerance to Combined Light and Temperature Stress in Tomato. J. Exp. Bot. 2017, 68, 5845–5856. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, Y.; Otsuki, H.; Narisawa, T.; Kobayashi, M.; Sawai, S.; Kamide, Y.; Kusano, M.; Aoki, T.; Hirai, M.Y.; Saito, K. A New Class of Plant Lipid Is Essential for Protection against Phosphorus Depletion. Nat. Commun. 2013, 4, 1510. [Google Scholar] [CrossRef] [PubMed]
- Matich, E.K.; Ghafari, M.; Camgoz, E.; Caliskan, E.; Pfeifer, B.A.; Haznedaroglu, B.Z.; Atilla-Gokcumen, G.E. Time-Series Lipidomic Analysis of the Oleaginous Green Microalga Species Ettlia oleoabundans under Nutrient Stress. Biotechnol. Biofuels 2018, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Custódio, M.; Maciel, E.; Domingues, M.R.; Lillebø, A.I.; Calado, R. Nutrient Availability Affects the Polar Lipidome of Halimione Portulacoides Leaves Cultured in Hydroponics. Sci. Rep. 2020, 10, 6583. [Google Scholar] [CrossRef] [PubMed]
- Lowenstein, D.P.; Mayers, K.; Fredricks, H.F.; Van Mooy, B.A.S. Targeted and Untargeted Lipidomic Analysis of Haptophyte Cultures Reveals Novel and Divergent Nutrient-Stress Adaptations. Org. Geochem. 2021, 161, 104315. [Google Scholar] [CrossRef]
- Kokabi, K.; Gorelova, O.; Ismagulova, T.; Itkin, M.; Malitsky, S.; Boussiba, S.; Solovchenko, A.; Khozin-Goldberg, I. Metabolomic Foundation for Differential Responses of Lipid Metabolism to Nitrogen and Phosphorus Deprivation in an Arachidonic Acid-Producing Green Microalga. Plant Sci. 2019, 283, 95–115. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Lou, H.; Tan, Z.; Ouyang, Z.; Zhang, Y.; Lu, S.; Guo, L.; Yang, B. Lipidomic and Metabolomic Analyses Reveal Changes of Lipid and Metabolite Profiles in Rapeseed during Nitrogen Deficiency. Plant Cell Physiol. 2023, 65, 904–915. [Google Scholar] [CrossRef] [PubMed]
- Honda, S.; Yamazaki, Y.; Mukada, T.; Cheng, W.; Chuba, M.; Okazaki, Y.; Saito, K.; Oikawa, A.; Maruyama, H.; Wasaki, J.; et al. Lipidome Profiling of Phosphorus Deficiency-Tolerant Rice Cultivars Reveals Remodeling of Membrane Lipids as a Mechanism of Low P Tolerance. Plants 2023, 12, 1365. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, R.; Wang, G.; Li, M.; Roth, M.; Welti, R.; Wang, X. Differential Changes in Galactolipid and Phospholipid Species in Soybean Leaves and Roots under Nitrogen Deficiency and after Nodulation. Phytochemistry 2013, 96, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.Y.; Burgos, A.; Ma, L.; Zhang, Q.; Tang, D.; Ruan, J. Lipidomics Analysis Unravels the Effect of Nitrogen Fertilization on Lipid Metabolism in Tea Plant (Camellia sinensis L.). BMC Plant Biol. 2017, 17, 165. [Google Scholar] [CrossRef] [PubMed]
- Pfaff, J.; Denton, A.K.; Usadel, B.; Pfaff, C. Phosphate Starvation Causes Different Stress Responses in the Lipid Metabolism of Tomato Leaves and Roots. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158763. [Google Scholar] [CrossRef]
- Li, S.; Liu, X.; Yin, L.; Wang, S.; Deng, X. Alteration in Lipid Metabolism Is Involved in Nitrogen Deficiency Response in Wheat Seedlings. Plant Physiol. Biochem. 2024, 214, 108883. [Google Scholar] [CrossRef] [PubMed]
- Natera, S.H.A.; Hill, C.B.; Rupasinghe, T.W.T.; Roessner, U. Salt-Stress Induced Alterations in the Root Lipidome of Two Barley Genotypes with Contrasting Responses to Salinity. Funct. Plant Biol. 2016, 43, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.W.H.; Hill, C.B.; Doblin, M.S.; Shelden, M.C.; van de Meene, A.; Rupasinghe, T.; Bacic, A.; Roessner, U. Integrative Multi-Omics Analyses of Barley Rootzones under Salinity Stress Reveal Two Distinctive Salt Tolerance Mechanisms. Plant Commun. 2020, 1, 100031. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Boughton, B.A.; Hill, C.B.; Feussner, I.; Roessner, U.; Rupasinghe, T.W.T. Insights Into Oxidized Lipid Modification in Barley Roots as an Adaptation Mechanism to Salinity Stress. Front. Plant Sci. 2020, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, L.; Li, X.; Luo, M. Detailed Sphingolipid Profile Responded to Salt Stress in Cotton Root and the GhIPCS1 Is Involved in the Regulation of Plant Salt Tolerance. Plant Sci. 2022, 316, 111174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Lyu, W.; Gao, Y.; Zhang, X.; Sun, Y.; Huang, B. Choline-Mediated Lipid Reprogramming as a Dominant Salt Tolerance Mechanism in Grass Species Lacking Glycine Betaine. Plant Cell Physiol. 2020, 61, 2018–2030. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Liu, L.; Rupasinghe, T.W.T.; Roessner, U.; Barkla, B.J. Salt Stress Alters Membrane Lipid Content and Lipid Biosynthesis Pathways in the Plasma Membrane and Tonoplast. Plant Physiol. 2022, 189, 805–826. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Kou, M.; Gao, Z.; Liu, Y.; Xuan, Y.; Liu, Y.; Tang, Z.; Cao, Q.; Li, Z.; Sun, J. Involvement of Phosphatidylserine and Triacylglycerol in the Response of Sweet Potato Leaves to Salt Stress. Front. Plant Sci. 2019, 10, 1086. [Google Scholar] [CrossRef] [PubMed]
- Pretorius, C.J.; Zeiss, D.R.; Dubery, I.A. The Presence of Oxygenated Lipids in Plant Defense in Response to Biotic Stress: A Metabolomics Appraisal. Plant Signal. Behav. 2021, 16, 1989215. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.; Li, Q.; Zhang, X.; Hao, T.; Liu, N.; Yang, Z.; Yu, J. Lipid Composition Remodeling Plays a Critical Role during the Differential Responses of Leaves and Roots to Heat Stress in Bermudagrass. Environ. Exp. Bot. 2023, 213, 105423. [Google Scholar] [CrossRef]
- Han, X.; Yang, Y. Phospholipids in Salt Stress Response. Plants 2021, 10, 2204. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Huang, Y.; Liu, C.; Chen, K.; Li, M. Functions and Interaction of Plant Lipid Signalling under Abiotic Stresses. Plant Biol. 2023, 25, 361–378. [Google Scholar] [CrossRef] [PubMed]
- Li, H.M.; Yu, C.W. Chloroplast Galactolipids: The Link between Photosynthesis, Chloroplast Shape, Jasmonates, Phosphate Starvation and Freezing Tolerance. Plant Cell Physiol. 2018, 59, 1128–1134. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Yang, Z.; Xiao, X. Membrane Lipid Metabolism Is Involved in Regulating γ-Aminobutyric Acid-Mediated Cold Tolerance in Peach Fruit. Food Front. 2023, 4, 297–307. [Google Scholar] [CrossRef]
- Lopes, A.S.; Dias, T.J.; Henschel, J.M.; da Silva, J.H.B.; de Oliveira Sousa, V.F.; Targino, V.A.; da Silva Leal, M.P.; da Silva Gomes, D.; de Albuquerque, M.B.; Batista, D.S. Methyl Jasmonate Mitigates Drought Stress in Purple Basil by Enhancing Photosynthesis and Secondary Metabolism. J. Plant Growth Regul. 2024. [Google Scholar] [CrossRef]
- Sato, H.; Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Complex Plant Responses to Drought and Heat Stress under Climate Change. Plant J. 2024, 117, 1873–1892. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kidokoro, S.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Regulatory Networks in Plant Responses to Drought and Cold Stress. Plant Physiol. 2024, 195, 170–189. [Google Scholar] [CrossRef] [PubMed]
- Bidabadi, S.S.; VanderWeide, J.; Sabbatini, P. Exogenous Melatonin Improves Glutathione Content, Redox State and Increases Essential Oil Production in Two Salvia Species under Drought Stress. Sci. Rep. 2020, 10, 6883. [Google Scholar] [CrossRef] [PubMed]
- Kulak, M. Recurrent Drought Stress Effects on Essential Oil Profile of Lamiaceae Plants: An Approach Regarding Stress Memory. Ind. Crops Prod. 2020, 154, 112695. [Google Scholar] [CrossRef]
- Sharma, P.; Lakra, N.; Goyal, A.; Ahlawat, Y.K.; Zaid, A.; Siddique, K.H.M. Drought and Heat Stress Mediated Activation of Lipid Signaling in Plants: A Critical Review. Front. Plant Sci. 2023, 14, 1216835. [Google Scholar] [CrossRef] [PubMed]
- Cameron, K.D.; Teece, M.A.; Smart, L.B. Increased Accumulation of Cuticular Wax and Expression of Lipid Transfer Protein in Response to Periodic Drying Events in Leaves of Tree Tobacco. Plant Physiol. 2006, 140, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Zhao, P.; Tan, Z.; Peng, Y.; Xu, L.; Jin, Y.; Wei, F.; Guo, L.; Yao, X. Combining Physio-Biochemical Characterization and Transcriptome Analysis Reveal the Responses to Varying Degrees of Drought Stress in Brassica Napus L. Int. J. Mol. Sci. 2022, 23, 8555. [Google Scholar] [CrossRef] [PubMed]
- Riyazuddin, R.; Nisha, N.; Singh, K.; Verma, R.; Gupta, R. Involvement of Dehydrin Proteins in Mitigating the Negative Effects of Drought Stress in Plants. Plant Cell Rep. 2022, 41, 519–533. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Peng, F.; Xiao, Y.; Yu, W.; Zhang, Y.; Gao, H. Exogenous Phosphatidylcholine Treatment Alleviates Drought Stress and Maintains the Integrity of Root Cell Membranes in Peach. Sci. Hortic. 2020, 259, 108821. [Google Scholar] [CrossRef]
- Xu, L.; Pan, R.; Zhang, W. Membrane Lipids Are Involved in Plant Response to Oxygen Deprivation. Plant Signal. Behav. 2020, 15, 1771938. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.J.; Zhou, Y.; Chen, Q.F.; Xiao, S. New Insights into the Role of Lipids in Plant Hypoxia Responses. Prog. Lipid Res. 2021, 81, 101072. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, B.; Mishegyan, A.; Nagalingam, S.; Guenther, A.; Joshee, N.; Sherman, S.H.; Basu, C. Physiological and Genetic Responses of Lentil (Lens culinaris) under Flood Stress. Plant Stress 2023, 7, 100130. [Google Scholar] [CrossRef]
- Ibrahimova, U.; Kumari, P.; Yadav, S.; Rastogi, A.; Antala, M.; Suleymanova, Z.; Zivcak, M.; Tahjib-Ul-Arif, M.; Hussain, S.; Abdelhamid, M.; et al. Progress in Understanding Salt Stress Response in Plants Using Biotechnological Tools. J. Biotechnol. 2021, 329, 180–191. [Google Scholar] [CrossRef]
- Henschel, J.M.; Dias, T.J.; de Moura, V.S.; de Oliveira Silva, A.M.; Lopes, A.S.; da Silva Gomes, D.; Araujo, D.J.; Silva, J.B.M.; da Cruz, O.N.; Batista, D.S. Hydrogen Peroxide and Salt Stress in Radish: Effects on Growth, Physiology, and Root Quality. Physiol. Mol. Biol. Plants 2024. [Google Scholar] [CrossRef]
- Lopes, A.S.; Dias, T.J.; Henschel, J.M.; da Silva, T.I.; de Moura, V.S.; Silva, A.M.O.; da Silva Ribeiro, J.E.; Diniz Neto, M.A.; de Oliveira, A.B.; Batista, D.S. Methyl Jasmonate Mitigates Salt Stress and Increases Quality of Purple Basil (Ocimum basilicum L.). S. Afr. J. Bot. 2024, 171, 710–718. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, Q.; Liu, M.; Zhou, H.; Ma, C.; Wang, P. Regulation of Plant Responses to Salt Stress. Int. J. Mol. Sci. 2021, 22, 4609. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.; Peng, T.; Xue, S. Mechanisms of Plant Saline-Alkaline Tolerance. J. Plant Physiol. 2023, 281, 153916. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Hou, X.; Liang, X. Response Mechanisms of Plants Under Saline-Alkali Stress. Front. Plant Sci. 2021, 12, 667458. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Passioura, J.B.; Colmer, T.D.; Byrt, C.S. Osmotic Adjustment and Energy Limitations to Plant Growth in Saline Soil. New Phytol. 2020, 225, 1091–1096. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Liu, L.; Barkla, B.J. Membrane Lipid Remodeling in Response to Salinity. Int. J. Mol. Sci. 2019, 20, 4264. [Google Scholar] [CrossRef] [PubMed]
- Rawat, N.; Singla-Pareek, S.L.; Pareek, A. Membrane Dynamics during Individual and Combined Abiotic Stresses in Plants and Tools to Study the Same. Physiol. Plant. 2021, 171, 653–676. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yu, A.; Sun, Y.; Hu, Q.; Kang, J.; Chen, L.; Zhu, X.; Yang, Q.; Long, R. Lipid Composition Remodeling and Storage Lipid Conversion Play a Critical Role in Salt Tolerance in Alfalfa (Medicago sativa L.) Leaves. Environ. Exp. Bot. 2023, 205, 105144. [Google Scholar] [CrossRef]
- Omoto, E.; Iwasaki, Y.; Miyake, H.; Taniguchi, M. Salinity Induces Membrane Structure and Lipid Changes in Maize Mesophyll and Bundle Sheath Chloroplasts. Physiol. Plant 2016, 157, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Helm, L.T.; Shi, H.; Lerdau, M.T.; Yang, X. Solar-Induced Chlorophyll Fluorescence and Short-Term Photosynthetic Response to Drought. Ecol. Appl. 2020, 30, e02101. [Google Scholar] [CrossRef]
- Morales-Cedillo, F.; González-Solís, A.; Gutiérrez-Angoa, L.; Cano-Ramírez, D.L.; Gavilanes-Ruiz, M. Plant Lipid Environment and Membrane Enzymes: The Case of the Plasma Membrane H+-ATPase. Plant Cell Rep. 2015, 34, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Bejaoui, F.; Salas, J.J.; Nouairi, I.; Smaoui, A.; Abdelly, C.; Martínez-Force, E.; Youssef, N. Ben Changes in Chloroplast Lipid Contents and Chloroplast Ultrastructure in Sulla Carnosa and Sulla Coronaria Leaves under Salt Stress. J. Plant Physiol. 2016, 198, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Barkla, B.J.; Garibay-Hernández, A.; Melzer, M.; Rupasinghe, T.W.T.; Roessner, U. Single Cell-Type Analysis of Cellular Lipid Remodelling in Response to Salinity in the Epidermal Bladder Cells of the Model Halophyte Mesembryanthemum crystallinum. Plant Cell Environ. 2018, 41, 2390–2403. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.F.; Li, Y.; Mi, X.F.; Li, Y.L.; Zhai, C.Y.; Yang, G.F.; Wang, Z.Y.; Zhang, K. Physiological and Lipidomic Response of Exogenous Choline Chloride Alleviating Salt Stress Injury in Kentucky Bluegrass (Poa pratensis). Front. Plant Sci. 2023, 14, 1269286. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Xiao, Z.; Wang, Z.; Lam, H.M.; Chye, M.L. Galactolipid and Phospholipid Profile and Proteome Alterations in Soybean Leaves at the Onset of Salt Stress. Front. Plant Sci. 2021, 12, 644408. [Google Scholar] [CrossRef]
- Yu, W.; Yu, Y.; Wang, C.; Zhang, Z.; Xue, Z. Mechanism by Which Salt Stress Induces Physiological Responses and Regulates Tanshinone Synthesis. Plant Physiol. Biochem. 2021, 164, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Sui, N.; Han, G. Salt-Induced Photoinhibition of PSII Is Alleviated in Halophyte Thellungiella Halophila by Increases of Unsaturated Fatty Acids in Membrane Lipids. Acta Physiol. Plant 2014, 36, 983–992. [Google Scholar] [CrossRef]
- Salama, K.H.A.; Mansour, M.M.F. Choline Priming-Induced Plasma Membrane Lipid Alterations Contributed to Improved Wheat Salt Tolerance. Acta Physiol. Plant 2015, 37, 170. [Google Scholar] [CrossRef]
- Salama, K.H.A.; Mansour, M.M.F.; Ali, F.Z.M.; Abou-Hadid, A.F. NaCl-Induced Changes in Plasma Membrane Lipids and Proteins of Zea mays L. Cultivars Differing in Their Response to Salinity. Acta Physiol. Plant 2007, 29, 351–359. [Google Scholar] [CrossRef]
- You, Z.; Zhang, Q.; Peng, Z.; Miao, X. Lipid Droplets Mediate Salt Stress Tolerance in Parachlorella kessleri. Plant Physiol. 2019, 181, 510–526. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, S.; Qi, Z.; Yang, C.; Ding, D.; Xiao, B.; Wang, S.; Yang, C. Regulation of Root Exudation in Wheat Plants in Response to Alkali Stress. Plants 2024, 13, 1227. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, S.; Sun, B.; Osman, F.M.; Qi, Z.; Ding, D.; Liu, X.; Ding, J.; Zhang, Z. Carboxylic Acid Accumulation and Secretion Contribute to the Alkali-Stress Tolerance of Halophyte Leymus Chinensis. Front. Plant Sci. 2024, 15, 1366108. [Google Scholar] [CrossRef] [PubMed]
- Skorzynska-Polit, E. Lipid Peroxidation in Plant Cells, Its Physiological Role and Changes under Heavy Metal Stress. Acta Soc. Bot. Pol. 2007, 76, 49–54. [Google Scholar] [CrossRef]
- Zhang, F.Q.; Wang, Y.S.; Lou, Z.P.; Dong, J. De Effect of Heavy Metal Stress on Antioxidative Enzymes and Lipid Peroxidation in Leaves and Roots of Two Mangrove Plant Seedlings (Kandelia candel and Bruguiera gymnorrhiza). Chemosphere 2007, 67, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Devi, S.R.; Prasad, M.N.V. Membrane Lipid Alterations in Heavy Metal Exposed Plants. In Heavy Metal Stress in Plants; Springer: Berlin/Heidelberg, Germany, 1999. [Google Scholar] [CrossRef]
- de Bang, T.C.; Husted, S.; Laursen, K.H.; Persson, D.P.; Schjoerring, J.K. The Molecular–Physiological Functions of Mineral Macronutrients and Their Consequences for Deficiency Symptoms in Plants. New Phytol. 2021, 229, 2446–2469. [Google Scholar] [CrossRef] [PubMed]
- Francis, B.; Aravindakumar, C.T.; Brewer, P.B.; Simon, S. Plant Nutrient Stress Adaptation: A Prospect for Fertilizer Limited Agriculture. Environ. Exp. Bot. 2023, 213, 105431. [Google Scholar] [CrossRef]
- Terrer, C.; Jackson, R.B.; Prentice, I.C.; Keenan, T.F.; Kaiser, C.; Vicca, S.; Fisher, J.B.; Reich, P.B.; Stocker, B.D.; Hungate, B.A.; et al. Nitrogen and Phosphorus Constrain the CO2 Fertilization of Global Plant Biomass. Nat. Clim. Chang. 2019, 9, 684–689. [Google Scholar] [CrossRef]
- Tränkner, M.; Tavakol, E.; Jákli, B. Functioning of Potassium and Magnesium in Photosynthesis, Photosynthate Translocation and Photoprotection. Physiol. Plant 2018, 163, 414–431. [Google Scholar] [CrossRef] [PubMed]
- Lan, P.; Li, W.; Schmidt, W. “Omics” Approaches Towards Understanding Plant Phosphorus Acquisition and Use. In Phosphorus Metabolism in Plants; Wiley Blackwell: New York, NY, USA, 2015; Volume 48, pp. 65–98. ISBN 9781118958841. [Google Scholar]
- Singer, S.D.; Zou, J.; Weselake, R.J. Abiotic Factors Influence Plant Storage Lipid Accumulation and Composition. Plant Sci. 2016, 243, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yavari, N.; Tripathi, R.; Wu, B.-S.; MacPherson, S.; Singh, J.; Lefsrud, M. The Effect of Light Quality on Plant Physiology, Photosynthetic, and Stress Response in Arabidopsis thaliana Leaves. PLoS ONE 2021, 16, e0247380. [Google Scholar] [CrossRef] [PubMed]
- Lakshmanan, M.; Lim, S.H.; Mohanty, B.; Kim, J.K.; Ha, S.H.; Lee, D.Y. Unraveling the Light-Specific Metabolic and Regulatory Signatures of Rice through Combined in Silico Modeling and Multiomics Analysis. Plant Physiol. 2015, 169, 3002–3020. [Google Scholar] [CrossRef] [PubMed]
| Stress Condition | Plant Species | Tissue | Analytical Technique | Reference |
|---|---|---|---|---|
| Alkali | Maize | Roots | ESI-MS/MS | Xu et al. [55] |
| Sunflower | Seeds | LC-MS | Lu et al. [56] | |
| Cold | Arabidopsis thaliana | Leaves | LC-MS | Tarazona et al. [57] |
| Arabidopsis thaliana | Leaves | GC-MS | Chen et al. [58] | |
| Banana | Fruits | LC-MS | Liu et al. [59] | |
| Barley | Leaves | LC-MS | Zhao et al. [60] | |
| Bell pepper | Fruits | ESI-MS/MS | Kong et al. [61] | |
| Bell pepper | Fruits | LC-MS | Xu et al. [62] | |
| Blueberry | Fruits | GC-MS | Wang et al. [63] | |
| Cabbage | Leaves | LC-MS | Zainal et al. [64] | |
| Cotton | Seeds | LC-MS | Dhaliwal et al. [65] | |
| Dendrobium | Leaves | UPLC-QTOF-MS | Zhan et al. [66] | |
| Maize | Roots | ESI-MS/MS | Zhao et al. [67] | |
| Maize | Leaves | LC-MS/MS | Gao et al. [68] | |
| Meconopsis racemosa | Leaves | ESI-MS/MS | Zheng et al. [69] | |
| Moss species | Leaves | UPLC-QTOF-MS | Lu et al. [70] | |
| Panax notoginseng | Leaves | ESI-MS/MS | Liu et al. [71] | |
| Peach | Fruits | GC-MS | Zhu et al. [72] | |
| Peach | Fruits | ESI-MS/MS | Chen et al. [14] | |
| Wheat | Leaves | LC-QqQ-MS | Cheong et al. [73] | |
| Wild tomato | Leaves | UPLC-QTOF-MS | Zhang et al. [74] | |
| Drought | Arabidopsis thaliana | Leaves | LC-MS/MS | Tarazona et al. [57] |
| Argan tree | Leaves | GC-MS | Rabeh et al. [75] | |
| Barley | Roots | LC-MS | Sarabia et al. [76] | |
| Camellia sinensis | Leaves | GC-MS and LC-MS | Shen et al. [77] | |
| Cotton | Seeds | LC-MS/MS | Liu et al. [78] | |
| Italian ryegrass × tall fescue | Leaves | UPLC-MS | Perlikowski et al. [79] | |
| Maize | Leaves | LC-MS | Kränzlein et al. [80] | |
| Sorghum | Leaf cuticle | GC-MS | Zhang et al. [49] | |
| Thyme | Leaves | DIFT-ICR-MS | Moradi et al. [81] | |
| Waterlogging | Arabidopsis thaliana | Leaves | ESI-MS/MS | Wang et al. [82] |
| Boehmeria nivea | Leaves | UPLC-QTOF/MS | Shao et al. [83] | |
| Rice | Grains | LC-MS | Xu et al. [84] | |
| Rice | Leaves | LC-MS | Basu et al. [85] | |
| Wheat | Leaves | ESI-MS/MS | Xu et al. [86] | |
| Heat | Arabidopsis thaliana | Leaves | LC-MS | Higashi et al. [87] |
| Arabidopsis thaliana | Leaves | LC-MS | Higashi et al. [88] | |
| Arabidopsis thaliana | Leaves | LC-MS | Shiva et al. [89] | |
| Arabidopsis thaliana | Leaves | LC-MS | Korte et al. [90] | |
| Arabidopsis thaliana | Seeds and seedlings | ESI/MS | Qian et al. [91] | |
| Begonia | Leaves | LC-MS | Sun et al. [92] | |
| Castor bean | Leaves | UPLC-QTOF/MS | Zhang et al. [93] | |
| Panax notoginseng | Leaves | ESI-MS/MS | Liu et al. [71] | |
| Peanut | Anthers | ESI-MS/MS | Lwe et al. [94] | |
| Soybean | Leaves | ESI-MS/MS | Narayanan et al. [48] | |
| Tall fescue | Leaves | ESI-MS/MS | Zhang et al. [95] | |
| Tall fescue | Leaves | ESI-MS/MS | Zhang et al. [54] | |
| Wheat | Leaves | LC-MS/MS | Hu et al. [96] | |
| Heavy metals | Halimione portulacoides | Leaves, stems, and roots | LC-MS | Figueira et al. [97] |
| Soybean | Leaves | LC-MS | Andresen et al. [98] | |
| Wheat | Roots | ESI-MS/MS | Wu et al. [99] | |
| Light | Arabidopsis thaliana | Leaves | HPLC | Rastogi et al. [100] |
| Arabidopsis thaliana | Leaves | UPLC-MS | Burgos et al. [101] | |
| Cotton | Leaves and roots | GC-MS | Kargiotidou et al. [102] | |
| Moss | Leaves | LC-MS | Callahan et al. [103] | |
| Tea plants | Leaves | UPLC-TOF-MS | Wang et al. [104] | |
| Tomato | Leaves | UHPLC-APCI-QTOF-MS | Spicher et al. [105] | |
| Nutrient deficiency | Arabidopsis thaliana | Leaves | LC-MS | Okazaki et al. [106] |
| Ettlia oleoabundans | Cells | LC–QTOF-MS | Matich et al. [107] | |
| Halimione portulacoides | Leaves | LC-MS | Custódio et al. [108] | |
| Haptophytes | Cells | HPLC–HRAM–MS | Lowenstein et al. [109] | |
| Lobosphaera incisa | Cells | LC-MS | Kokabi et al. [110] | |
| Rapeseed | Leaves | LC-MS/MS | Peng et al., 2023 [111] | |
| Rice | Leaves and roots | UPLC-QTOF-MS | Honda et al. [112] | |
| Soybean | Leaves and roots | ESI-MS/MS | Narasimhan et al. [113] | |
| Tea plant | Leaves | UPLC-MS | Liu et al. [114] | |
| Tomato | Leaves and roots | GC-MS | Pfaff et al. [115] | |
| Wheat | Leaves | GC-MS | Li et al. [116] | |
| Salt | Barley | Roots | HPLC-ESI-QTOF-MS | Natera et al. [117] |
| Barley | Roots | HPLC-ESI-QTOF-MS | Ho et al. [118] | |
| Barley | Roots | HPLC-ESI-QTOF-MS | Yu et al. [119] | |
| Carex rigescens | Leaves | ESI-MS/MS | Hu et al. [51] | |
| Cotton | Seeds | LC-MS/MS | Liu et al. [78] | |
| Cotton | Roots | LC-MS | Liu et al. [120] | |
| Kentucky bluegrass | Leaves | ESI-MS/MS | Zhang et al. [121] | |
| Mesembryanthemum crystallinum | Leaves | HPLC–ESI–MS/MS | Guo et al. [122] | |
| Sweet Potato | Leaves | UPLC-QTOF/MS | Yu et al. [123] | |
| Tomato | Leaves | UHPLC/QTOF-MS | Buffagni et al. [52] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henschel, J.M.; Andrade, A.N.d.; dos Santos, J.B.L.; da Silva, R.R.; da Mata, D.A.; Souza, T.; Batista, D.S. Lipidomics in Plants Under Abiotic Stress Conditions: An Overview. Agronomy 2024, 14, 1670. https://doi.org/10.3390/agronomy14081670
Henschel JM, Andrade ANd, dos Santos JBL, da Silva RR, da Mata DA, Souza T, Batista DS. Lipidomics in Plants Under Abiotic Stress Conditions: An Overview. Agronomy. 2024; 14(8):1670. https://doi.org/10.3390/agronomy14081670
Chicago/Turabian StyleHenschel, Juliane Maciel, Antônio Nunes de Andrade, Josefa Bruna Lima dos Santos, Rodrigo Ribeiro da Silva, Djair Alves da Mata, Tancredo Souza, and Diego Silva Batista. 2024. "Lipidomics in Plants Under Abiotic Stress Conditions: An Overview" Agronomy 14, no. 8: 1670. https://doi.org/10.3390/agronomy14081670






