Effect of γ-Irradiation on the Growth and Yield Response of Three Varieties of Pea (Pisum spp.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mutagenic Treatment and Main Characters Observed
2.2. Plant Material
2.3. Field Experiment Growth Conditions
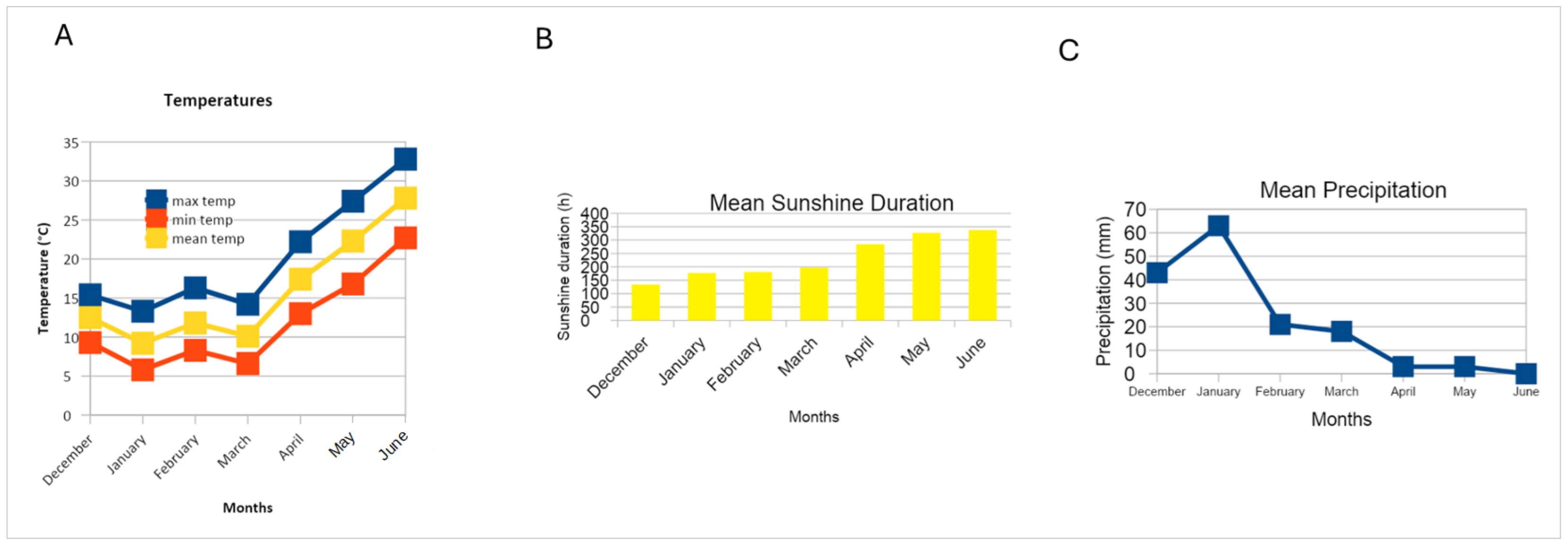
2.4. Weather Conditions
2.5. Data Collection
2.6. Statistical Analysis
3. Results and Discussion
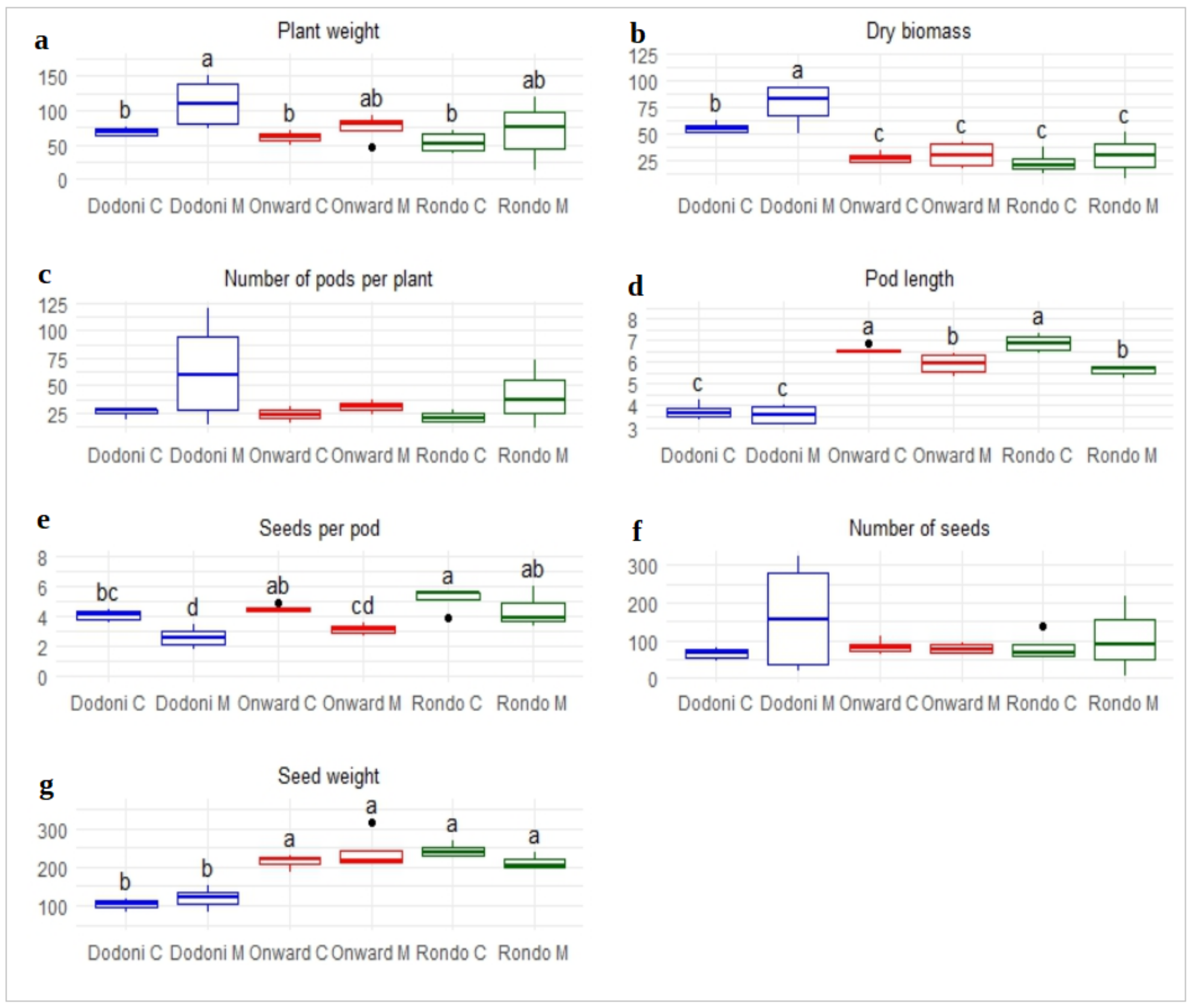
3.1. Agro-Morphological Traits
3.2. Post-Harvest Characteristics
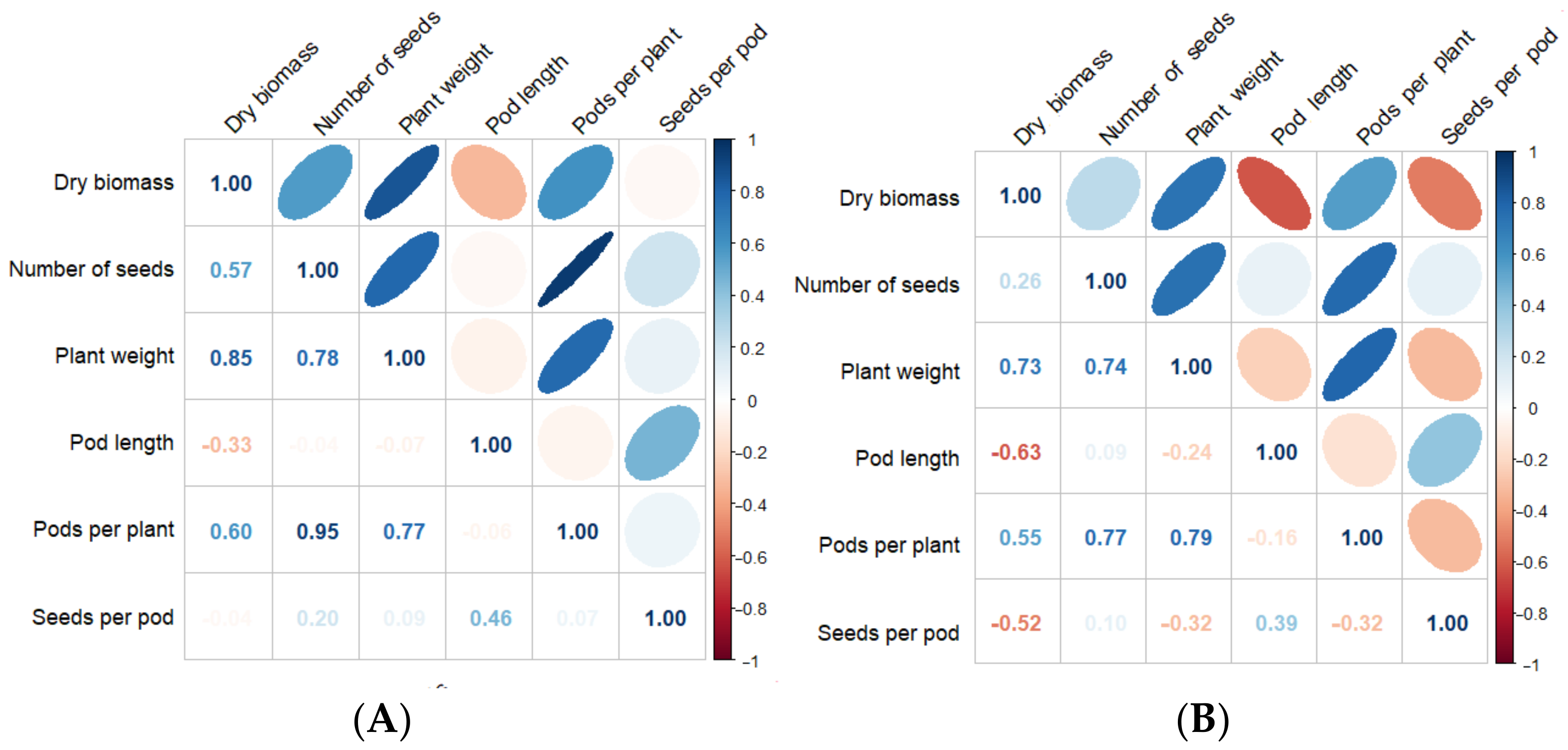
3.3. Correlation Coefficients
4. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Acquaah, G. Principles of Plant Genetics and Breeding; John Wiley&Sons, Ltd.: Chichester, UK, 2012; ISBN 978-1-118-31371-8. [Google Scholar]
- Kang, B.-H.; Busse, J.S.; Bednarek, S.Y. Members of the Arabidopsis Dynamin-Like Gene Family, ADL1, are Essential for Plant Cytokinesis and Polarized Cell Growth. Plant Cell 2003, 15, 899–913. [Google Scholar] [CrossRef]
- Desai, S.; Jadhav, A.; Ramteke, A.; Dhole, V.; Bapat, V.; Gaikwad, N. Genetic Improvement of Two Indian Non-Basmati Aromatic Rice Landraces through Physical and Chemical Mutagenesis. Int. J. Radiat. Biol. 2022, 98, 82–89. [Google Scholar] [CrossRef]
- Barve, S.S.; Straley, S.C. lcrR, a Low-Ca2(+)-Response Locus with Dual Ca2(+)-Dependent Functions in Yersinia Pestis. J. Bacteriol. 1990, 172, 4661–4671. [Google Scholar] [CrossRef]
- Pandey, P.K.; Bhowmik, P.; Kagale, S. Optimized Methods for Random and Targeted Mutagenesis in Field Pea (Pisum sativum L.). Front. Plant Sci. 2022, 13, 995542. [Google Scholar] [CrossRef]
- Acuna-Hidalgo, R.; Veltman, J.A.; Hoischen, A. New Insights into the Generation and Role of De Novo Mutations in Health and Disease. Genome Biol. 2016, 17, 241. [Google Scholar] [CrossRef]
- Powers, S.; Mirsky, E.; Bandaranayake, A.; Thavarajah, P.; Shipe, E.; Bridges, W.; Thavarajah, D. Field Pea (Pisum sativum L.) Shows Genetic Variation in Phosphorus Use Efficiency in Different P Environments. Sci. Rep. 2020, 10, 18940. [Google Scholar] [CrossRef]
- Vanmathi, S.; Arulbalachandran, D.; Soundarya, V. Effects of Gamma Radiation on Quantitative Traits and Genetic Variation of Three Successive Generations of Cowpea (Vigna unguiculata (L.) Walp.). Plant Sci. Today 2021, 8, 578–589. [Google Scholar] [CrossRef]
- Baadu, R.; Chong, K.P.; Gansau, J.A.; Mohamed Zin, M.R.; Dayou, J. A Systematic Review on Physical Mutagens in Rice Breeding in Southeast Asia. PeerJ 2023, 11, e15682. [Google Scholar] [CrossRef]
- Beyaz, R.; Yildiz, M.; Beyaz, R.; Yildiz, M. The Use of Gamma Irradiation in Plant Mutation Breeding. In Plant Engineering; IntechOpen: London, UK, 2017; ISBN 978-953-51-3608-8. [Google Scholar]
- Biermans, G.; Horemans, N.; Vanhoudt, N.; Vandenhove, H.; Saenen, E.; Van Hees, M.; Wannijn, J.; Vangronsveld, J.; Cuypers, A. Biological Effects of α-Radiation Exposure by (241)Am in Arabidopsis thaliana Seedlings are Determined Both by Dose Rate and (241)Am Distribution. J. Environ. Radioact. 2015, 149, 51–63. [Google Scholar] [CrossRef]
- International Atomic Energy Agency (IAEA). Book of Abstracts (IAEA-CN-263). In Proceedings of the FAO/IAEA International Symposium on Plant Mutation Breeding and Biotechnology, Vienna, Austria, 27–31 August 2018. [Google Scholar]
- Marcu, D.; Damian, G.; Cosma, C.; Cristea, V. Gamma Radiation Effects on seed Germination, Growth and Pigment Content, and ESR Study of Induced Free Radicals in Maize (Zea mays). J. Biol. Phys. 2013, 39, 625–634. [Google Scholar] [CrossRef]
- Pramanik, B.; Debnath, S.; Rahimi, M.; Helal, M.M.U.; Hasan, R. Morphometric frequency and spectrum of gamma-ray-induced chlorophyll mutants identified by phenotype and development of novel variants in lentil (Lens culinaris Medik.). PLoS ONE 2023, 18, e0286975. [Google Scholar] [CrossRef]
- Feng, Z.; Du, Y.; Chen, J.; Chen, X.; Ren, W.; Wang, L.; Zhou, L. Comparison and Characterization of Phenotypic and Genomic Mutations Induced by a Carbon-Ion Beam and Gamma-ray Irradiation in Soybean (Glycine max (L.) Merr.). Int. J. Mol. Sci. 2023, 24, 8825. [Google Scholar] [CrossRef]
- Rana, A.; Rana, V.; Kumar Sood, V.; Bakshi, S. Priyanka Mutagenic Sensitivity, Effectiveness and Efficiency of Gamma Rays and Ethyl Methane Sulfonate on Soft and Semi-Hard Bread Wheat (Triticum aestivum L.) Varieties in the north-Western Himalayan Climate. Int. J. Radiat. Biol. 2024, 100, 296–315. [Google Scholar] [CrossRef]
- Wi, S.G.; Chung, B.Y.; Kim, J.-S.; Kim, J.-H.; Baek, M.-H.; Lee, J.-W.; Kim, Y.S. Effects of Gamma Irradiation on Morphological Changes and Biological Responses in Plants. Micron. Oxf. Engl. 2007, 38, 553–564. [Google Scholar] [CrossRef]
- Majeed, A.; Muhammad, Z.; Ullah, R. Growth and Yield Response of Field Pea (Pisum sativum L.) To Gamma Irradiation Stress. Plant Breed. Seed Sci. 2016, 74, 27–35. [Google Scholar] [CrossRef]
- Majeed, T.; Wani, I.A.; Hamdani, A.M.; Bhat, N.A. Effect of Sonication and γ-Irradiation on the Properties of Pea (Pisum sativum) and Vetch (Vicia villosa) Starches: A Comparative Study. Int. J. Biol. Macromol. 2018, 114, 1144–1150. [Google Scholar] [CrossRef]
- Reese, I.; Schäfer, C.; Ballmer-Weber, B.; Beyer, K.; Dölle-Bierke, S.; van Dullemen, S.; Jappe, U.; Müller, S.; Schnadt, S.; Treudler, R.; et al. Vegan Diets from an Allergy Point of View—Position Paper of the DGAKI Working Group on Food Allergy. Allergol Sel. 2023, 31, 57–83. [Google Scholar] [CrossRef]
- Shanthakumar, P.; Klepacka, J.; Bains, A.; Chawla, P.; Dhull, S.B.; Najda, A. The Current Situation of Pea Protein and Its Application in the Food Industry. Molecules 2022, 27, 5354. [Google Scholar] [CrossRef]
- Luthra, S.; Singh, B.P.; Singh, R.; Nagar, B.L.; Singh, S.K. Genetic Variability in Garden Pea (Pisum sativum L.): A Review. Int. J. Environ. Clim. Change 2023, 13, 3859–3865. [Google Scholar] [CrossRef]
- Patel, D.; Davda, B.K.; Modha, K.G. Induction of Genetic Variability through Mutagenesis Insorghum [Sorghum bicolor (L.) Moench]. TPI 2023, 12, 1520–1523. [Google Scholar]
- Mba, C. Induced Mutations Unleash the Potentials of Plant Genetic Resources for Food and Agriculture. Agronomy 2013, 3, 200–231. [Google Scholar] [CrossRef]
- Bagi, G.; Bornemisza-Pauspertl, P.; Hidvégi, E.J. Inverse Correlation between Growth and Degrading Enzyme Activity of Seedlings after Gamma and Neutron Irradiation of Pea Seeds. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1988, 53, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Khan, W.M.; Muhammad, Z.; Akhtar, N.; Burni, T.; Younas, A.; Umar, N. 48. Gamma Radiation Induced Mutation in M2 Generation of Pea (Pisum sativum L.). Pure Appl. Biol. PAB 2018, 7, 832–839. [Google Scholar]
- Malumpong, C.; Youngkom, P.; Vanavichit, A.; Siang-Liw, M. Variation in Tolerance to Salinity Stress at the Reproductive Stage in a Large Fast Neutron Mutant Rice (Oryza sativa L.) Population. Int. J. Agric. Technol. 2019, 15, 445–464. [Google Scholar]
- Gatsiou, M. Production of Propagating Material of Forage Pea under Organic Conditions. Bachelor’s Thesis, Technological Educational Institute of Kalamata, School of Agricultural Technology, Antikalamos, Greece, 2012. [Google Scholar]
- Dalianis, K. Legumes for Grain and Hay; A. Stamoulis Publications: Athens, Greece, 1993. [Google Scholar]
- Girija, M.; Dhanavel, D.; Gnanamurthy, S. Gamma Rays and EMS Induced Flower Color and Seed Mutants in Cowpea (Vigna unguiculata L. Walp). Adv. Appl. Sci. Res. 2013, 4, 134–139. [Google Scholar]
- Sharma, S.; Singh, P.; Choudhary, O.P. Neemisha Nitrogen and Rice Straw Incorporation Impact Nitrogen Use Efficiency, Soil Nitrogen Pools and Enzyme Activity in Rice-Wheat System in North-Western India. Field Crops Res. 2021, 266, 108131. [Google Scholar] [CrossRef]
- El Ghareeb, I.M. Gamma Rays Mutagenic Influence in Development Pea Agronomic and Biochemical Characters. Ann. Agric. Sci. Moshtohor 2006, 44, 637–652. [Google Scholar]
- Deepalakshmi, A.J.; Kumar, C.R. Creation of Genetic Variability for Different Polygenic Traits in Blackgram {Vigna mungo (L.) Hepper} through Induced Mutagenesis. Legume Res. 2004, 27, 188–192. [Google Scholar]
- Masry, A.I.; Fayad, A.M.; Taher, D.I. Genetic Improvements in Pea (Pisum sativum L.) Through Irradiation by Gama Rays. J. Plant Prod. 2019, 10, 1089–1093. [Google Scholar] [CrossRef]
- Khan, K.; Iqbal, M.; Azim, A.; Ahmad, B.; Karim, F.; Sher, H. Effect of Gamma Irradiation on Yield and Yield Components of Barley (Hordeum vulgare L.). Pak. J. Biol. Sci. 2003, 6, 1695–1697. [Google Scholar] [CrossRef]
- Verma, R.; Purbiya, R. Effects of Gamma Radiations on Seed Germination and Morphological Characteristics of Pea (Pisum sativum L.). Indian J. Plant Sci. 2017, 6, 21–25. [Google Scholar]
- Thenuja, M.; Sutharsan, S.; Rifnas, L.M. Effects of Different Levels of Gamma Radiation on Growth and Yield Characteristics of Groundnut. Asian J. Res. Agric. For. 2024, 10, 1–10. [Google Scholar] [CrossRef]
- Esan, V.I.; Oke, G.O.; Ogunbode, T.O. Genetic Variation and Characterization of Bambara groundnut [Vigna subterranea (L.) verdc.] Accessions under Multi-Environments Considering Yield and Yield Components Performance. Sci. Rep. 2023, 13, 1498. [Google Scholar] [CrossRef] [PubMed]
- Elsherbiny, H.A.; Gaballah, M.M.; Hamad, H.S.; Sakr, S.M.; Elbadawy, O.A.; Alwutayd, K.M.; Boudiar, R.; Mansour, E.; Bleih, E.M. Inducing Potential Mutants in Rice Using Different Doses of Gamma Rays for Improving Agronomic Traits. Chil. J. Agric. Res. 2024, 84, 380–390. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarri, E.; Samolada, S.-M.; Katsileros, A.; Tomlekova, N.; Abraham, E.M.; Tani, E. Effect of γ-Irradiation on the Growth and Yield Response of Three Varieties of Pea (Pisum spp.). Agronomy 2024, 14, 1695. https://doi.org/10.3390/agronomy14081695
Sarri E, Samolada S-M, Katsileros A, Tomlekova N, Abraham EM, Tani E. Effect of γ-Irradiation on the Growth and Yield Response of Three Varieties of Pea (Pisum spp.). Agronomy. 2024; 14(8):1695. https://doi.org/10.3390/agronomy14081695
Chicago/Turabian StyleSarri, Efi, Styliani-Maria Samolada, Anastasios Katsileros, Nasya Tomlekova, Eleni M. Abraham, and Eleni Tani. 2024. "Effect of γ-Irradiation on the Growth and Yield Response of Three Varieties of Pea (Pisum spp.)" Agronomy 14, no. 8: 1695. https://doi.org/10.3390/agronomy14081695





