Effects of Phosphorus and Potassium Supply on Photosynthetic Nitrogen Metabolism, Nitrogen Absorption, and Nitrogen Utilization of Hydroponic Rice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Experimental Design
2.3. Sampling and Measurements
2.3.1. Determination of the Chlorophyll Components of the Leaves
2.3.2. Determination of N-Metabolism-Related Enzymes in the Leaves and Roots
2.3.3. Determination of the Antioxidant Enzyme Activity and MDA Content of the Leaves and Roots
2.3.4. Determination of the Leaf Area Index and Photosynthetic Indicators
2.3.5. Leaf Ultra-Microstructure
2.3.6. Determination of the N Contents of Different Organs of the Plant and Calculation of N Efficiency
2.3.7. Calculation of the Grain Yield and N Efficiency
2.3.8. Measurement of P and K Uptake Rate (NUR) in Nutrient Solution
2.4. Data Analysis
3. Results
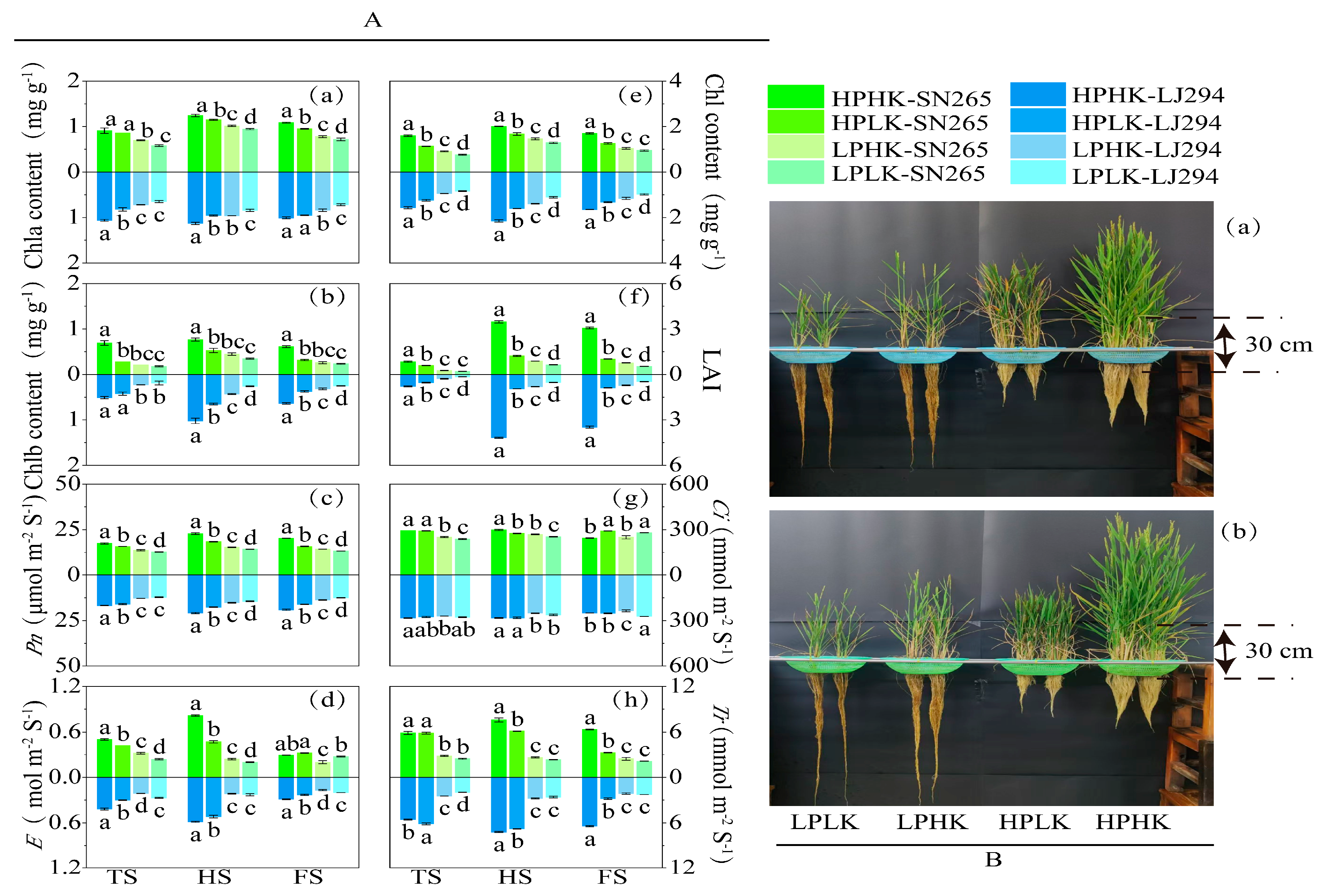
3.1. Leaf Chlorophyll Component, LAI, Photosynthesis, and Plant Morphological Characteristics
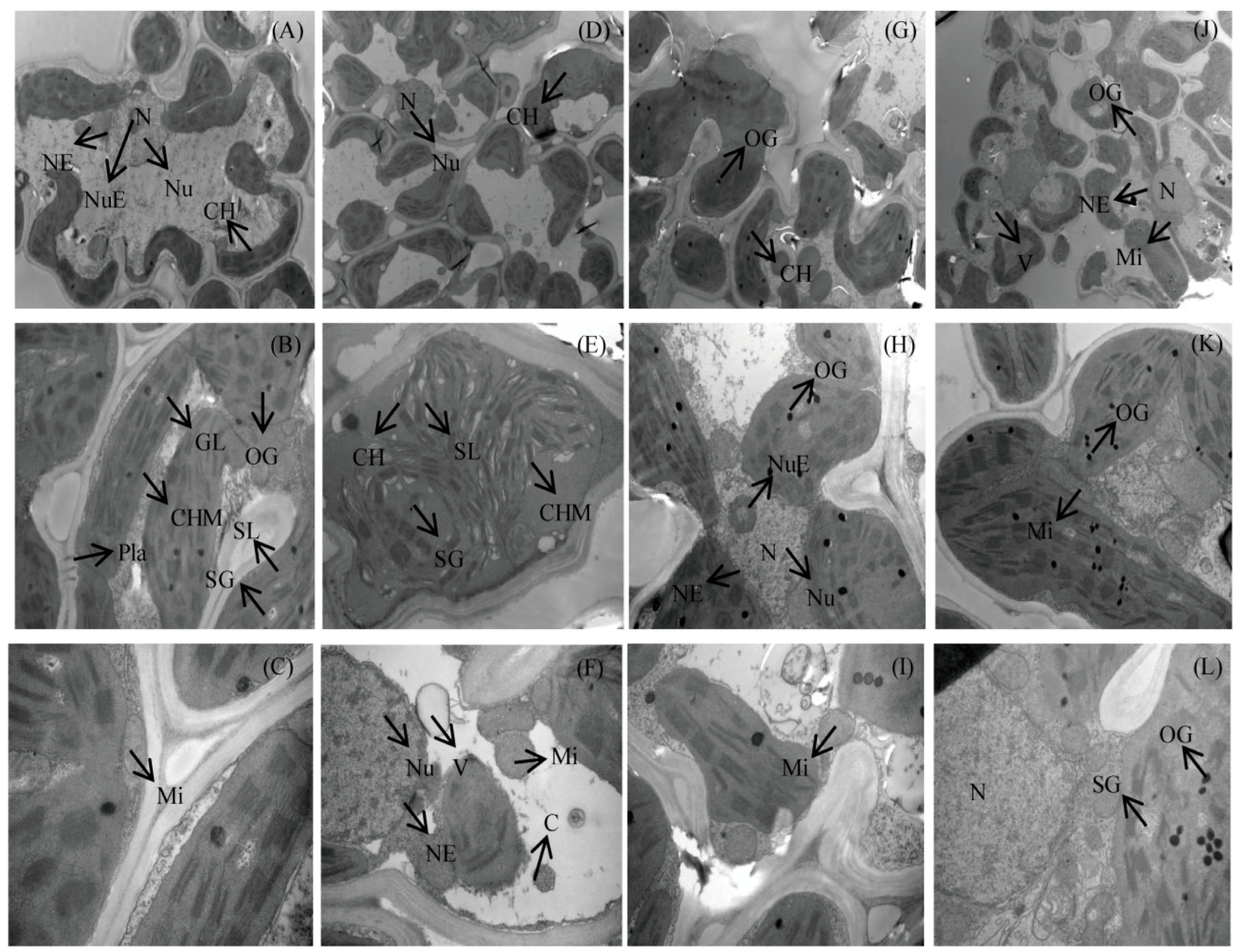
3.2. Ultrastructure of Mesophyll Cells at the Full-Heading Stage
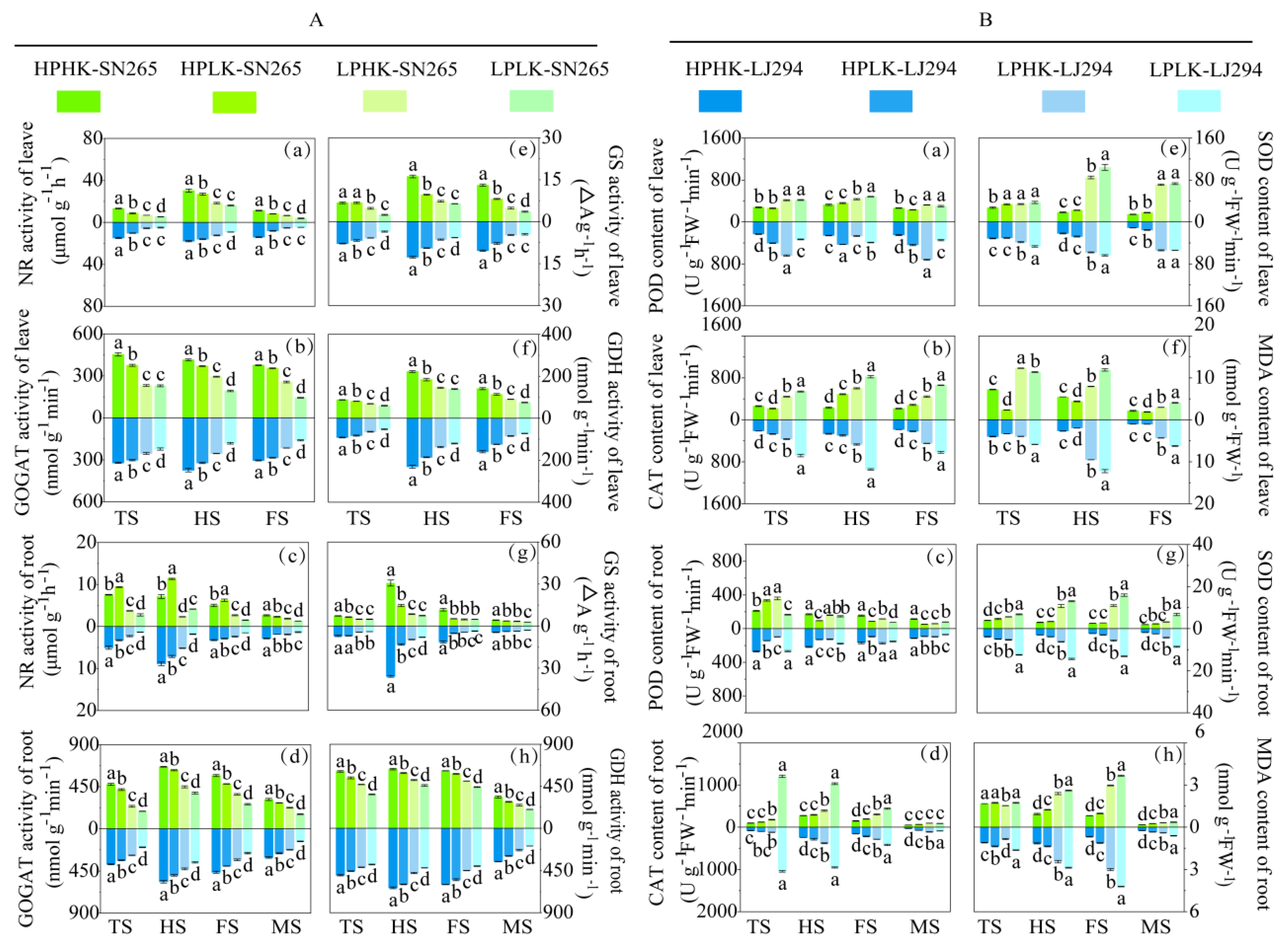
3.3. Leaf and Root Antioxidant Enzyme Activity, MDA Content, and N-Metabolizing Enzyme Activity
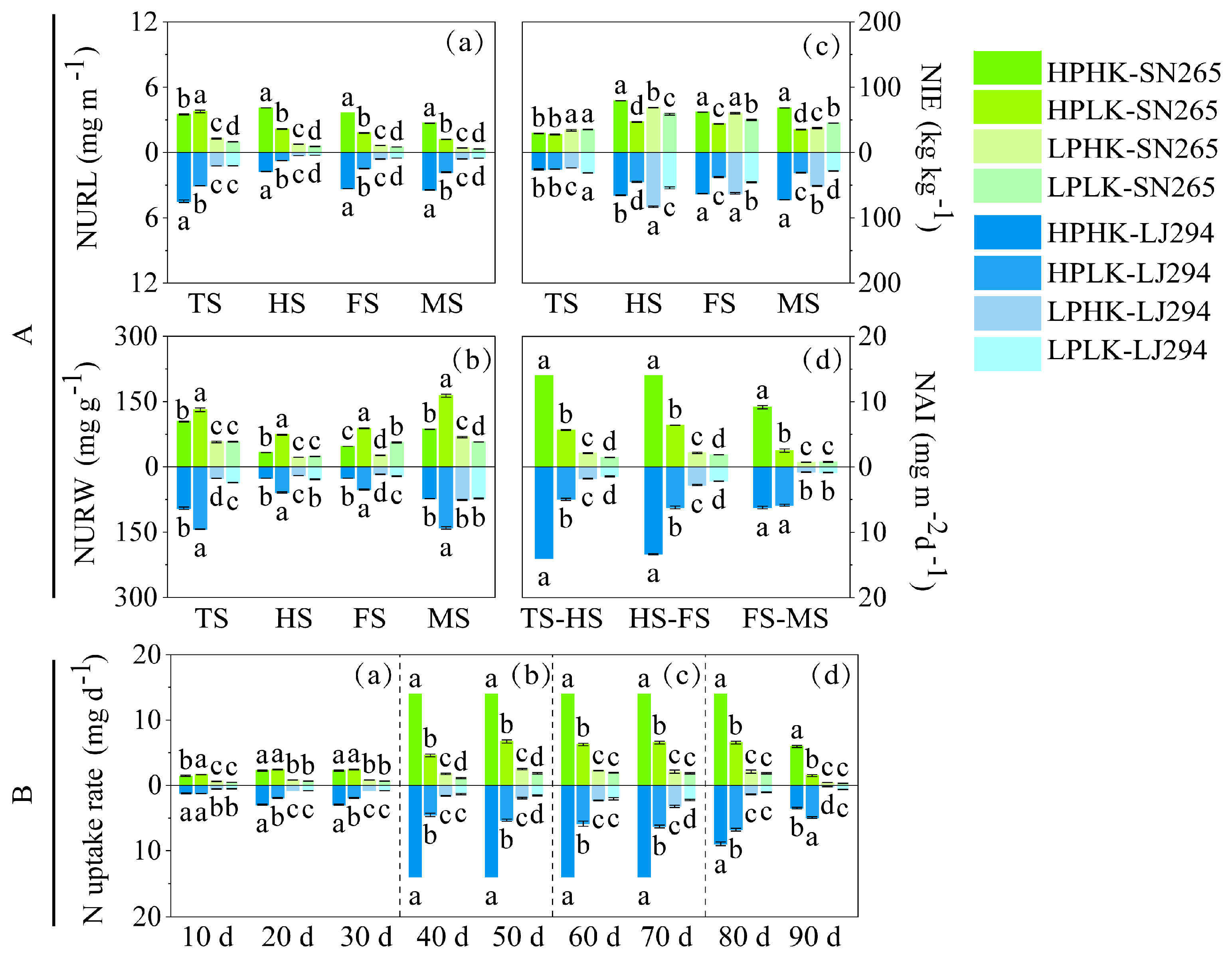
3.4. Content and Accumulation of N in Various Organs
3.5. Root- and Grain-Yield-Related N Efficiency
3.6. Nitrogen Uptake Rate (NUR) in Water Samples
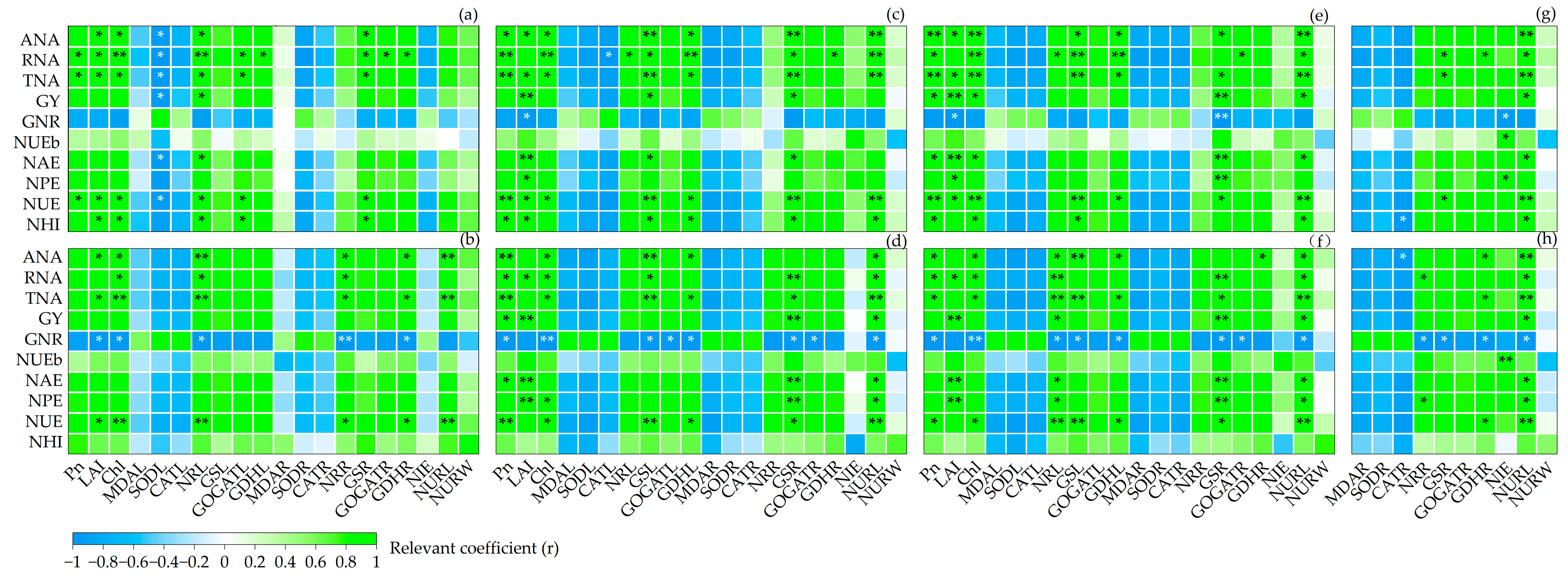
3.7. Correlations between the Indicators
4. Discussion
4.1. The Response Characteristics of Plant Growth and Stress Adaptation to the P and K Supply (Sufficient or Lacking)
4.2. The Characteristics of Plant Photosynthetic N Metabolism in Response to the P and K Supply (Sufficient or Lacking)
4.3. The Response Characteristics of Related N Uptake and N Efficiency in the Root–Grain–Nutrient Solution in Response to the P and K Supply (Sufficient or Lacking)
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ye, T.; Li, Y.; Zhang, J.; Hou, W.; Zhou, W.; Lu, J.; Xing, Y.; Li, X. Nitrogen, phosphorus, and potassium fertilization affects the flowering time of rice (Oryza sativa L.). Glob. Ecol. Conserv. 2019, 20, e00753. [Google Scholar] [CrossRef]
- Yi, J.; Gao, J.; Zhang, W.; Zhao, C.; Wang, Y.; Zhen, X. Differential uptake and utilization of two forms of nitrogen in japonica rice cultivars from north-eastern China. Front. Plant Sci. 2019, 10, 1061. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, J.; Zhang, H.; Lian, A.; Gao, F.; Zhang, Z.; Guo, Z. The Impact of Fertilizer Type on Dry Matter, Nitrogen Partitioning, and Yield of Spring Maize with Film-Side Sowing. Agronomy 2023, 13, 2999. [Google Scholar] [CrossRef]
- Jing, B.; Shi, W.; Diao, M. Nitrogen application levels based on critical nitrogen absorption regulate processing tomatoes productivity, nitrogen uptake, nitrate distributions, and root growth in Xinjiang, China. J. Arid. Land 2023, 15, 1231–1244. [Google Scholar] [CrossRef]
- Du, J.Y.; Ru, M.; Ni, W.Z. Effects of fertilization with reducing nitrogen, controlling phosphorus and stabilizing potassium on rice yield and nutrient accumulation. J. Plant Nutr. Fertil. 2013, 19, 523–533. [Google Scholar]
- Bernardi, A.C.D.C.; Carmello, Q.A.D.C.; Carvalho, S.A.D.; Machado, E.C.; Medina, C.L.; Gomes, M.D.M.D.A.; Lima, D.M. Nitrogen, phosphorus and potassium fertilization interactions on the photosynthesis of containerized citrus nursery trees. J. Plant Nutr. 2015, 38, 1902–1912. [Google Scholar] [CrossRef]
- Meike, S.; Peter, D.; Hölzl, G. Membrane Remodelling in Phosphorus-Deficient Plants. Annu. Plant Rev. Online 2018, 48, 237–263. [Google Scholar]
- Cochrane, T.T.; Cochrane, T.A. The vital role of potassium in the osmotic mechanism of stomata aperture modulation and its link with potassium deficiency. Plant Signal. Behav. 2009, 4, 240–243. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The critical role of potassium in plant stress response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef]
- Wang, S.; Liang, D.; Li, C.; Hao, Y.; Ma, F.; Shu, H. Influence of drought stress on the cellular ultrastructure and antioxidant system in leaves of drought-tolerant and drought-sensitive apple rootstocks. Plant Physiol. Biochem. 2012, 51, 81–89. [Google Scholar] [CrossRef]
- Ali, E.; Hussain, S.; Jalal, F.; Khan, M.A.; Imtiaz, M.; Said, F.; Ismail, M.; Khan, S.; Ali, H.; Shah, F.; et al. Salicylic acid-mitigates abiotic stress tolerance via altering defense mechanisms in Brassica napus (L.). Front. Plant Sci. 2023, 14, 1187260. [Google Scholar] [CrossRef]
- Zambrosi, F.C.; Mesquita, G.L.; Tanaka, F.A. Assessment of leaf ultrastructure offers insights into mechanisms regulating sugarcane performance under low-phosphorus stress. Acta Physiol. Plant. 2020, 42, 54. [Google Scholar] [CrossRef]
- Li, Q.; Sun, J. Microstructure and Ultrastructure in Plant Leaves of Cucumber as Influenced by Phosphorus. In Proceedings of the 2016 ASABE Annual International Meeting, American Society of Agricultural and Biological Engineers, Orlando, FL, USA, 17–20 June 2016; p. 1. [Google Scholar]
- Jiang, H.M.; Yang, J.C.; Zhang, J.F. Effects of external phosphorus on the cell ultrastructure and the chlorophyll content of maize under cadmium and zinc stress. Environ. Pollut. 2007, 147, 750–756. [Google Scholar] [CrossRef]
- Tang, Z.H.; Zhang, A.J.; Wei, M.; Chen, X.G.; Liu, Z.H.; Li, H.M.; Ding, Y.F. Physiological response to potassium deficiency in three sweet potato (Ipomoea batatas [L.] Lam.) genotypes differing in potassium utilization efficiency. Acta Physiol. Plant. 2015, 37, 184. [Google Scholar] [CrossRef]
- Zhao, D.; Oosterhuis, D.M.; Bednarz, C.W. Influence of potassium deficiency on photosynthesis, chlorophyll content, and chloroplast ultrastructure of cotton plants. Photosynthetica 2001, 39, 103–109. [Google Scholar] [CrossRef]
- Iqbal, A.; Qiang, D.; Xiangru, W.; Huiping, G.; Hengheng, Z.; Xiling, Z.; Meizhen, S. Phosphorus and carbohydrate metabolism contributes to low phosphorus tolerance in cotton. BMC Plant Biol. 2023, 23, 97. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, K.K.; Wang, L.T.; Zhao, L.L.; Wang, P.C. Transcriptome analysis provides insights into the response of Lotus corniculatus roots to low-phosphorus stress. Front. Plant Sci. 2023, 14, 1089380. [Google Scholar] [CrossRef]
- Meng, X.; Chen, W.W.; Wang, Y.Y.; Huang, Z.R.; Ye, X.; Chen, L.S.; Yang, L.T. Effects of phosphorus deficiency on the absorption of mineral nutrients, photosynthetic system performance and antioxidant metabolism in Citrus grandis. PLoS ONE 2021, 16, e0246944. [Google Scholar] [CrossRef]
- Zahoor, R.; Zhao, W.; Dong, H.; Snider, J.L.; Abid, M.; Iqbal, B.; Zhou, Z. Potassium improves photosynthetic tolerance to and recovery from episodic drought stress in functional leaves of cotton (Gossypium hirsutum L.). Plant Physiol. Biochem. 2017, 119, 21–32. [Google Scholar] [CrossRef]
- Qi, D.U.; Zhao, X.H.; Le, X.I.A.; Jiang, C.J.; Wang, X.G.; Yi, H.A.N.; Yu, H.Q. Effects of potassium deficiency on photosynthesis, chloroplast ultrastructure, ROS, and antioxidant activities in maize (Zea mays L.). J. Integr. Agric. 2019, 18, 395–406. [Google Scholar]
- Hu, W.; Coomer, T.D.; Loka, D.A.; Oosterhuis, D.M.; Zhou, Z. Potassium deficiency affects the carbon-nitrogen balance in cotton leaves. Plant Physiol. Biochem. 2017, 115, 408–417. [Google Scholar] [CrossRef]
- Qu, C.; Liu, C.; Ze, Y.; Gong, X.; Hong, M.; Wang, L.; Hong, F. Inhibition of nitrogen and photosynthetic carbon assimilation of maize seedlings by exposure to a combination of salt stress and potassium-deficient stress. Biol. Trace Elem. Res. 2011, 144, 1159–1174. [Google Scholar] [CrossRef]
- Hou, W.; Xue, X.; Li, X.; Khan, M.R.; Yan, J.; Ren, T.; Cong, J.; Lu, J. Interactive effects of nitrogen and potassium on: Grain yield, nitrogen uptake and nitrogen use efficiency of rice in low potassium fertility soil in China. Field Crops Res. 2019, 236, 14–23. [Google Scholar] [CrossRef]
- Zhou, Q.; Tang, J.; Liu, C.; Huang, K.; Huang, X. Effects of phosphate fertilizer application on the growth and yield of tartary buckwheat under low-nitrogen condition. Agronomy 2023, 13, 1886. [Google Scholar] [CrossRef]
- Rao, D.-Y.; Zhou, F.; Zhou, S.-Y.; Liu, K.; Zi, X.-J.; Yang, Y.-Q.; Wu, B.-Z. Effects of nitrogen application rate and phosphorus fertilizer management on silage maize yield and nutrient utilization. J. South. Agric. 2023, 5, 90–101. [Google Scholar]
- Chen, X.-Y.; Liu, P.; Cheng, Y.; Dong, S.-T.; Zhang, J.-W.; Zhao, B.; Ren, B.-Z.; Han, K. The root-layer regulation based on the depth of phosphate fertilizer application of summer maize improves soil nitrogen absorption and utilization. Acta Agron. Sin. 2020, 46, 238–248. [Google Scholar]
- Chen, Y.; Zhou, T.; Wang, K.; Cui, Y.; Zhang, C.; Xu, K. Effect of phosphorus on nitrogen use efficiency and soil nitrate content of the wheat/maize/soybean relay-intercropping system. J. Soil Water Conserv. 2014, 28, 191–196+208. [Google Scholar]
- Yu, T.-Y.; Li, X.-L.; Lu, Y.; Sun, X.-W.; Zheng, Y.-M.; Wu, Z.-F.; Shen, P.; Wang, C.-B. Effect of phosphorus (P) on nitrogen (N) uptake and utilization in peanut. Acta Agron. Sin. 2019, 45, 912–921. [Google Scholar]
- Li, Q.; Chen, X.; Li, Y.; Zhang, X. Effect of different N/P fertilizer application on rice yield and N use efficiency in irrigation area of Ningxia. Chin. J. Agrometeorol. 2010, 31, 379. [Google Scholar]
- Kundu, A.; Raha, P.; Dubey, A.N. Impact of source and method of potassium application on dry matter accumulation and partitioning of potassium in rice (Oryza sativa L.). J. Soil Sci. Plant Nutr. 2021, 21, 2252–2263. [Google Scholar] [CrossRef]
- Reis, A.F.D.B.; Nascente, A.S.; Almeida, R.E.M.D.; Chagas, A.F. Growth and nutrient contents in lowland rice due to phosphorus and potassium fertilization. Pesqui. Agropecuária Trop. 2018, 48, 98–108. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, Y.; Xu, H.; Wang, C.; Yang, Z.; Li, N.; Yan, F.; Li, Y.; Wang, H.; Ma, J. Effects of fertilizer levels on the absorption, translocation, and distribution of phosphorus and potassium in rice cultivars with different nitrogen-use efficiencies. J. Agric. Sci. 2016, 8, 38. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, Y.; Liu, H.; Yang, X.; Yuan, P.; Wang, C.; Shi, L. Optimal phosphorus management strategies to enhance crop productivity and soil phosphorus fertility in rapeseed–rice rotation. Chemosphere 2023, 337, 139392. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, J. Split application of potassium improves yield and potassium uptake of rice under deficient soils. J. Soil Water Conserv. 2021, 20, 213–220. [Google Scholar] [CrossRef]
- Zhang, S.Z.; Hua, B.Z.; Zhang, F. Induction of the activities of antioxidative enzymes and the levels of malondialdehyde in cucumber seedlings as a consequence of Bemisia tabaci (Hemiptera: Aleyrodidae) infestation. Arthropod-Plant Interact. 2008, 2, 209–213. [Google Scholar] [CrossRef]
- Zhou, Q.; Jiang, Z.; Zhang, X.; Zhang, T.; Zhu, H.; Cui, B.; Li, Y.; Zhao, F.; Zhao, Z. Leaf anatomy and ultrastructure in senescing ancient tree, Platycladus orientalis L. (Cupressaceae). PeerJ 2019, 7, e6766. [Google Scholar] [CrossRef]
- Wang, N.; Fu, F.; Wang, H.; Wang, P.; He, S.; Shao, H.; Ni, Z.; Zhang, X. Effects of irrigation and nitrogen on chlorophyll content, dry matter and nitrogen accumulation in sugar beet (Beta vulgaris L.). Sci. Rep. 2021, 11, 16651. [Google Scholar] [CrossRef]
- Teng, Y.; Han, H.; Hao, Z.; Yang, H.; Li, J. Effect of vegetable cropping system on total nitrogen, phosphorus and COD in farmland leachate. Chin. J. Eco-Agric. 2017, 25, 759–768. [Google Scholar]
- Lv, X.; Han, J.; Liao, Y.; Liu, Y. Effect of phosphorus and potassium foliage application post-anthesis on grain filling and hormonal changes of wheat. Field Crops Res. 2017, 214, 83–93. [Google Scholar] [CrossRef]
- Li, L.; Yang, H.; Ren, W.; Liu, B.; Cheng, D.; Wu, X.; Gong, J.; Peng, L.; Huang, F. Physiological and biochemical characterization of sheepgrass (Leymus chinensis) reveals insights into photosynthetic apparatus coping with low-phosphate stress conditions. J. Plant Biol. 2016, 59, 336–346. [Google Scholar] [CrossRef]
- Hafsi, C.; Romero-Puertas, M.C.; del Río, L.A.; Abdelly, C.; Sandalio, L.M. Antioxidative response of Hordeum maritimum L. to potassium deficiency. Acta Physiol. Plant. 2011, 33, 193–202. [Google Scholar] [CrossRef]
- Simkin, A.J.; Kapoor, L.; Doss, C.G.P.; Hofmann, T.A.; Lawson, T.; Ramamoorthy, S. The role of photosynthesis related pigments in light harvesting, photoprotection and enhancement of photosynthetic yield in planta. Photosynth. Res. 2022, 152, 23–42. [Google Scholar] [CrossRef]
- El-Mejjaouy, Y.; Lahrir, M.; Naciri, R.; Zeroual, Y.; Mercatoris, B.; Dumont, B.; Oukarroum, A. How far can chlorophyll a fluorescence detect phosphorus status in wheat leaves (Triticum durum L.). Environ. Exp. Bot. 2022, 194, 104762. [Google Scholar] [CrossRef]
- Ren, B.; Cui, H.; Camberato, J.J.; Dong, S.; Liu, P.; Zhao, B.; Zhang, J. Effects of shading on the photosynthetic characteristics and mesophyll cell ultrastructure of summer maize. Sci. Nat. 2016, 103, 67. [Google Scholar] [CrossRef]
- Sutinen, S.; Wallin, G.; Karlsson, P.E.; Skärby, L.; Selldén, G. Cell ultrastructure of needles from saplings of Norway spruce, Picea abies (L) Karst., exposed to ozone and low phosphorus supply in open-top chambers. Chemosphere 1998, 36, 691–696. [Google Scholar]
- Henningsen, J.N.; Venturas, M.D.; Quintero, J.M.; Garrido, R.R.; Mühling, K.H.; Fernández, V. Leaf surface features of maize cultivars and response to foliar phosphorus application: Effect of leaf stage and plant phosphorus status. Physiol. Plant. 2023, 175, e14093. [Google Scholar] [CrossRef]
- Wang, P.; Yin, Y.P.; Fu, G.Z.; Guo, Y.; Cai, R.G.; Liang, T.B.; Wang, Z.L. Effect of phosphorus on activities of enzymes related to nitrogen metabolism in flag leaves and protein content of wheat grains. J. Plant Nutr. Fertil. 2009, 15, 24–31. [Google Scholar]
- Ruiz, J.M.; Romero, L. Nitrogen metabolism and yield response of cucumber (Cucumis sativus L cv Brunex) plants to phosphorus fertilisation. J. Sci. Food Agric. 2000, 80, 2069–2073. [Google Scholar] [CrossRef]
- Azcón, R.; Gomez, M.; Tobar, R. Effects of nitrogen source on growth, nutrition, photosynthetic rate and nitrogen metabolism of mycorrhizal and phosphorus-fertilized plants of Lactuca sativa L. New Phytol. 1992, 121, 227–234. [Google Scholar] [CrossRef]
- Hu, W.; Lv, X.; Yang, J.; Chen, B.; Zhao, W.; Meng, Y.; Wang, Y.; Zhou, Z.; Oosterhuis, D.M. Effects of potassium deficiency on antioxidant metabolism related to leaf senescence in cotton (Gossypium hirsutum L.). Field Crops Res. 2016, 191, 139–149. [Google Scholar] [CrossRef]
- Ju, F.; Pang, J.; Huo, Y.; Zhu, J.; Yu, K.; Sun, L.; Loka, D.; Hu, W.; Zhou, Z.; Wang, S.; et al. Potassium application alleviates the negative effects of salt stress on cotton (Gossypium hirsutum L.) yield by improving the ionic homeostasis, photosynthetic capacity and carbohydrate metabolism of the leaf subtending the cotton boll. Field Crops Res. 2021, 272, 108288. [Google Scholar] [CrossRef]
- Pan, Y.; Lu, Z.; Lu, J.; Li, X.; Cong, R.; Ren, T. Effects of low sink demand on leaf photosynthesis under potassium deficiency. Plant Physiol. Biochem. 2017, 113, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Zahoor, R.; Dong, H.; Abid, M.; Zhao, W.; Wang, Y.; Zhou, Z. Potassium fertilizer improves drought stress alleviation potential in cotton by enhancing photosynthesis and carbohydrate metabolism. Environ. Exp. Bot. 2017, 137, 73–83. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Tittal, M.; Mir, R.A.; Agarwal, R.M. Alleviation of water and osmotic stress-induced changes in nitrogen metabolizing enzymes in Triticum aestivum L. cultivars by potassium. Protoplasma 2017, 254, 1953–1963. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Guo, P.; Wang, Z.; Liang, J.; Li, G.; He, W.; Zhen, A. Grafting improves growth and nitrogen-use efficiency by enhancing NO3− uptake, photosynthesis, and gene expression of nitrate transporters and nitrogen metabolizing enzymes in watermelon under reduced nitrogen application. Plant Soil 2022, 480, 305–327. [Google Scholar] [CrossRef]
- Zhou, H.; Zhao, Q.; He, R.; Zhang, W.; Zhang, H.; Wang, H.; Ao, X.; Yao, X.; Xie, F. Rapid effect of enriched nitrogen on soybean nitrogen uptake, distribution, and assimilation during early flowering stage. J. Soil Sci. Plant Nutr. 2022, 22, 3798–3810. [Google Scholar] [CrossRef]
- Yu, T.F.; Liu, X.J.; Hao, F. Effect of phosphate fertilizer application on alfalfa yield, nutritive value and N and P use efficiency. Acta Prataculturae Sin. 2018, 27, 154–163. [Google Scholar]
- Chen, P.; Li, L.; Xia, S.; Zhang, R.; Zhang, R.; Zeng, X.M.; Shuai, D.; Liu, Y.; Li, Z.G. Enhancement patterns of potassium on nitrogen transport and functional genes in cotton vary with nitrogen levels. Plant Sci. 2023, 335, 111824. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Zhang, W.; Gao, J.; Liu, M.; Zhou, Y.; He, D.; Zhao, Y.; Liu, S. Improvement of root characteristics due to nitrogen, phosphorus, and potassium interactions increases rice (Oryza sativa L.) yield and nitrogen use efficiency. Agronomy 2021, 12, 23. [Google Scholar] [CrossRef]
- Li, Q.; Kong, F.; Wu, Y.; Feng, D.; Jichao, Y. Increasing nitrogen accumulation and reducing nitrogen loss with N-efficient maize cultivars. Plant Prod. Sci. 2020, 23, 260–269. [Google Scholar] [CrossRef]
- Fosu-Mensah, B.Y.; Mensah, M. The effect of phosphorus and nitrogen fertilizers on grain yield, nutrient uptake and use efficiency of two maize (Zea mays L.) varieties under rain fed condition on Haplic Lixisol in the forest-savannah transition zone of Ghana. Environ. Syst. Res. 2016, 5, 22. [Google Scholar] [CrossRef]
- Qian, C.; Chen, L.; Ma, Q.; Li, C.; Zhu, M.; Zhu, X.; Ding, J.; Guo, W. Effects of phosphorus and potassium fertilizer on grain yield, nutrient uptake and utilization in low-gluten wheat. J. Plant Nutr. Fertil. 2023, 29, 287–299. [Google Scholar]

| Indicator Abbreviations | Definition and Calculation Method | Units |
|---|---|---|
| LNC | Leaf nitrogen content. | % |
| SNC | Stem nitrogen content. | % |
| RNC | Root nitrogen content. | % |
| ANC | Aboveground N content. The ratio of aboveground N accumulation to dry weight. | % |
| PNC | Panicle nitrogen content. | % |
| LNA | Leaf N accumulation. The product of leaf N content and leaf dry weight. | mg/hill |
| SNA | Stem N accumulation. The product of stem N content and stem dry weight. | mg/hill |
| RNA | Root N accumulation. The product of root N content and root dry weight. | mg/hill |
| ANA | Aboveground N accumulation. The product of aboveground N content and aboveground dry weight. | mg/hill |
| TNA | Plant N accumulation. Sum of aboveground and root N accumulation. | mg/hill |
| NAI | N absorb intensity. The ratio of the difference in plant N accumulation between adjacent fertility periods to time. | mg/(m2·d) |
| NIE | N internal uptake efficiency. Ratio of aboveground dry weight to plant N accumulation. | kg/kg |
| NAE | N agronomic efficiency. Ratio of seed yield to N application. | kg/kg |
| NUE | N uptake efficiency. The ratio of plant N accumulation to N application. | % |
| NPE | N physiological efficiency. Ratio of grain yield to plant N accumulation. | kg/kg |
| GNR | N requirements for 100 kg grain; 100-fold ratio of plant N accumulation to grain yield. | kg·N/100 kg |
| NHI | N harvest index; 100-fold ratio of seed N to plant N accumulation. | % |
| NTE | N translocation rate. Ratio between the difference between the N accumulation of nutrient organs at full-heading and maturity stage and the N accumulation of nutrient organs at full-heading stage. | % |
| NUEb | N dry matter production efficiency. The ratio of plant dry weight to N accumulation. | kg/kg·N |
| NuRL | Ratio of plant N accumulation to total root length. | mg/m |
| NuRW | Ratio of plant N accumulation to root dry weight. | mg/g |
| Treatments | GNR (kg ·N/100 kg) | NUEb (kg/kg ·N) | NAE (kg/kg) | NPE (kg/kg) | NUE (%) | NTE (%) | NHI (%) | Grain Yield (kg/hm2) |
|---|---|---|---|---|---|---|---|---|
| HPHK | 385.4 ± 5.3 b | 79.8 ± 0.7 a | 16.6 ± 0.1 a | 26.0 ± 0.4 a | 64.0 ± 0.3 a | 22.8 ± 0.4 b | 62.7 ± 0.2 a | 4392.5 ± 37.6 a |
| HPLK | 1001.4 ± 52.1 a | 41.3 ± 0.2 d | 2.9 ± 0.2 d | 10.0 ± 0.5 b | 28.5 ± 0.6 b | 28.7 ± 2.3 a | 49.8 ± 0.9 b | 757.4 ± 54.8 b |
| LPHK | 967.4 ± 83.3 a | 52.1 ± 1.1 c | 1.0 ± 0.1 c | 10.5 ± 1.0 b | 9.8 ± 0.2 c | 19.4 ± 1.7 b | 39.8 ± 0.6 d | 273.0 ± 28.4 c |
| LPLK | 992.2 ± 63.8 a | 62.8 ± 0.9 b | 0.8 ± 0.1 c | 10.2 ± 0.7 b | 7.9 ± 0.0 d | 13.1 ± 1.3 c | 43.0 ± 0.2 c | 213.5 ± 13.4 c |
| HPHK | 404.8 ± 6.5 c | 85.9 ± 1.9 a | 14.7 ± 0.3 a | 24.7 ± 0.4 a | 59.6 ± 0.3 a | 18.9 ± 0.9 ab | 54.3 ± 0.4 b | 3899.7 ± 70.4 a |
| HPLK | 914.7 ± 18.7 b | 38.2 ± 0.4 c | 3.4 ± 0.1 c | 10.9 ± 0.2 b | 31.3 ± 0.6 b | 17.0 ± 0.1 b | 57.4 ± 1.3 a | 907.2 ± 30.4 b |
| LPHK | 1065.5 ± 38.4 b | 64.4 ± 0.8 b | 1.0 ± 0.0 c | 9.4 ± 0.3 c | 10.2 ± 0.2 c | 7.8 ± 0.8 c | 32.0 ± 0.1 d | 254.8 ± 9.4 c |
| LPLK | 1339.9 ± 105.5 a | 42.1 ± 1.3 c | 0.7 ± 0.0 c | 7.6 ± 0.6 d | 8.8 ± 0.2 d | 23.1 ± 3.2 a | 46.7 ± 1.1 c | 175.7 ± 11.0 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Gao, J.; Zhong, M.; Chen, L.; Zhang, W. Effects of Phosphorus and Potassium Supply on Photosynthetic Nitrogen Metabolism, Nitrogen Absorption, and Nitrogen Utilization of Hydroponic Rice. Agronomy 2024, 14, 1726. https://doi.org/10.3390/agronomy14081726
Liu Y, Gao J, Zhong M, Chen L, Zhang W. Effects of Phosphorus and Potassium Supply on Photosynthetic Nitrogen Metabolism, Nitrogen Absorption, and Nitrogen Utilization of Hydroponic Rice. Agronomy. 2024; 14(8):1726. https://doi.org/10.3390/agronomy14081726
Chicago/Turabian StyleLiu, Ya, Jiping Gao, Min Zhong, Liqiang Chen, and Wenzhong Zhang. 2024. "Effects of Phosphorus and Potassium Supply on Photosynthetic Nitrogen Metabolism, Nitrogen Absorption, and Nitrogen Utilization of Hydroponic Rice" Agronomy 14, no. 8: 1726. https://doi.org/10.3390/agronomy14081726





