Study on the Diversity of Bacterial Communities in the Rhizosphere Soils of Different Wild Celery Species in Jilin Province
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Materials
2.2. Overview of the Study Area
2.3. Test Method
2.3.1. Sampling
2.3.2. Determination of Soil Physical and Chemical Indicators
2.3.3. Determination of Soil Microbial Community
2.4. Sample Data Processing and Analysis
3. Results
3.1. Determination of Soil Physical Ochemical Properties
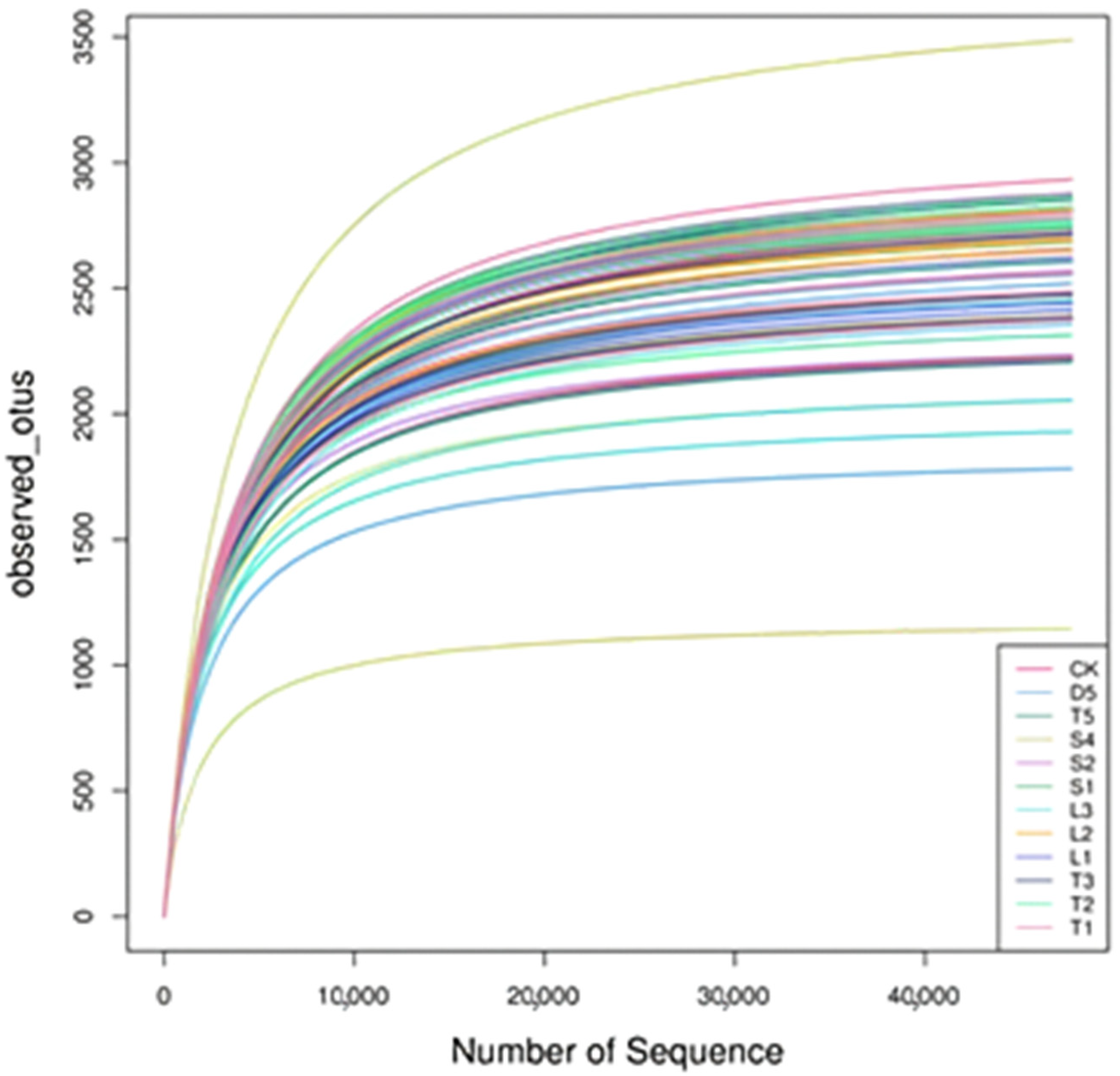
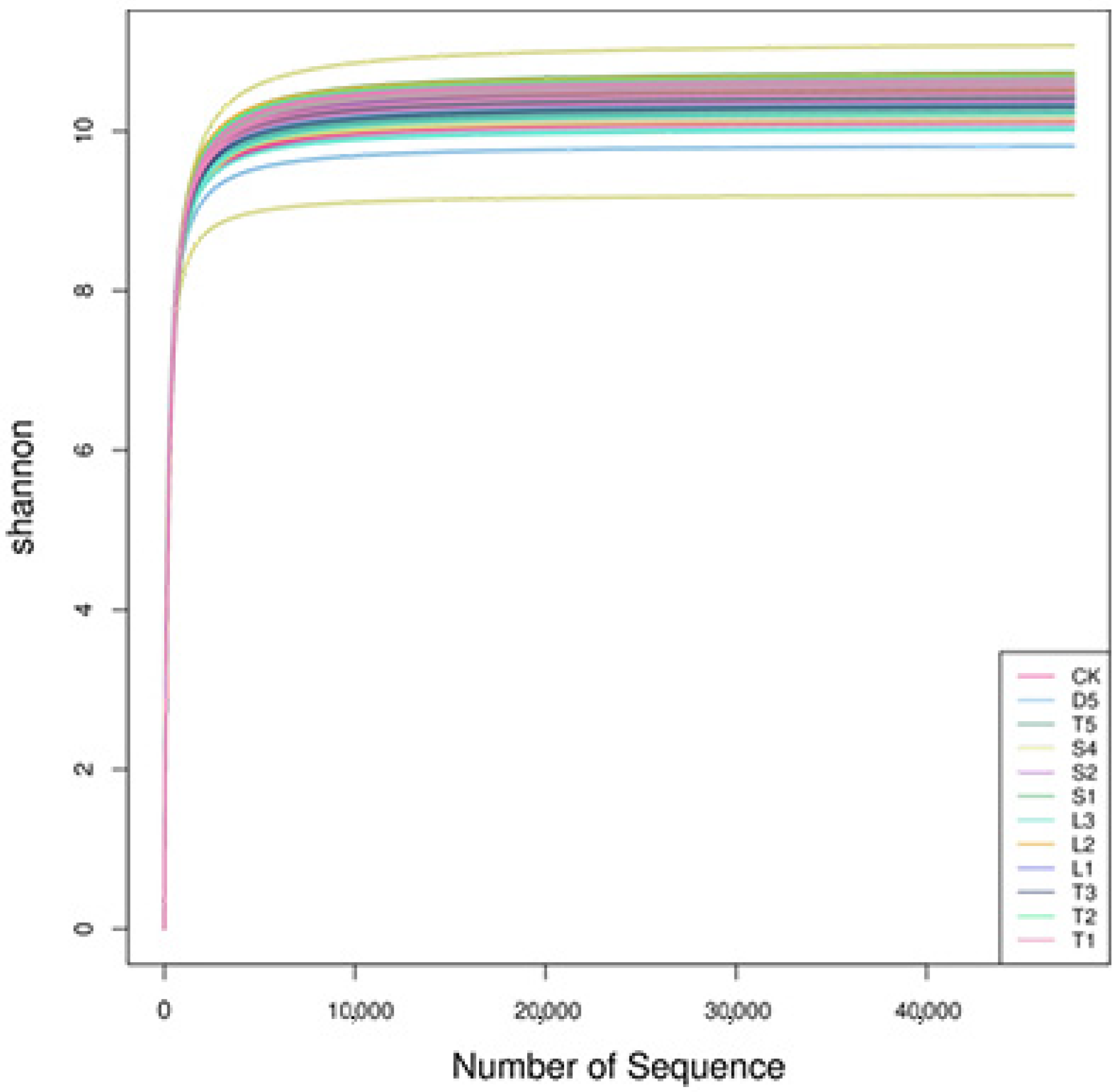
3.2. Analysis of Sequencing Data of Soil Samples
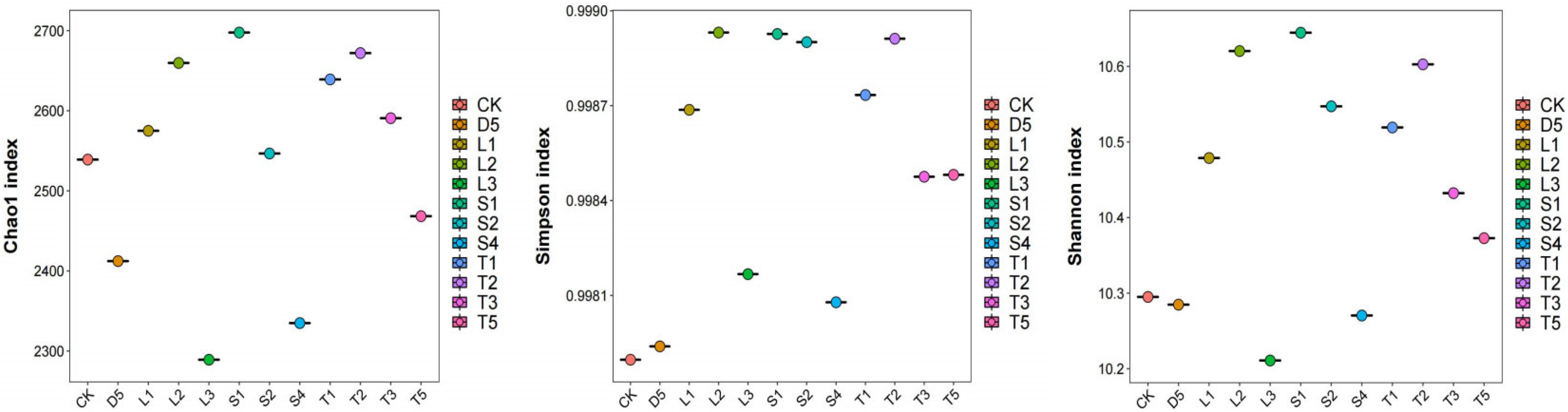
3.3. Alpha Diversity Analysis of Microbial Community
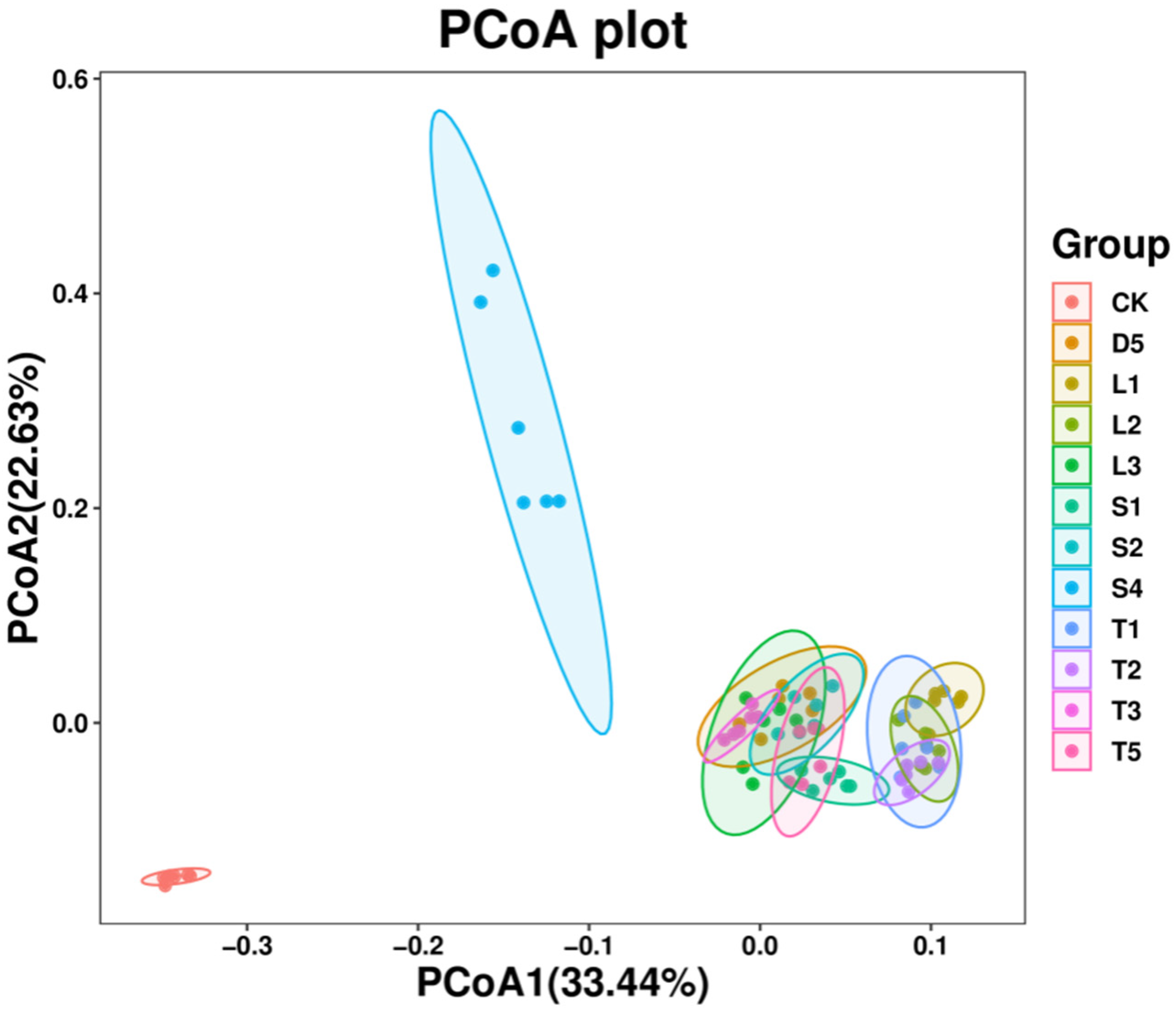
3.4. Beta Diversity Analysis of Microbial Community
Community Diversity Analysis
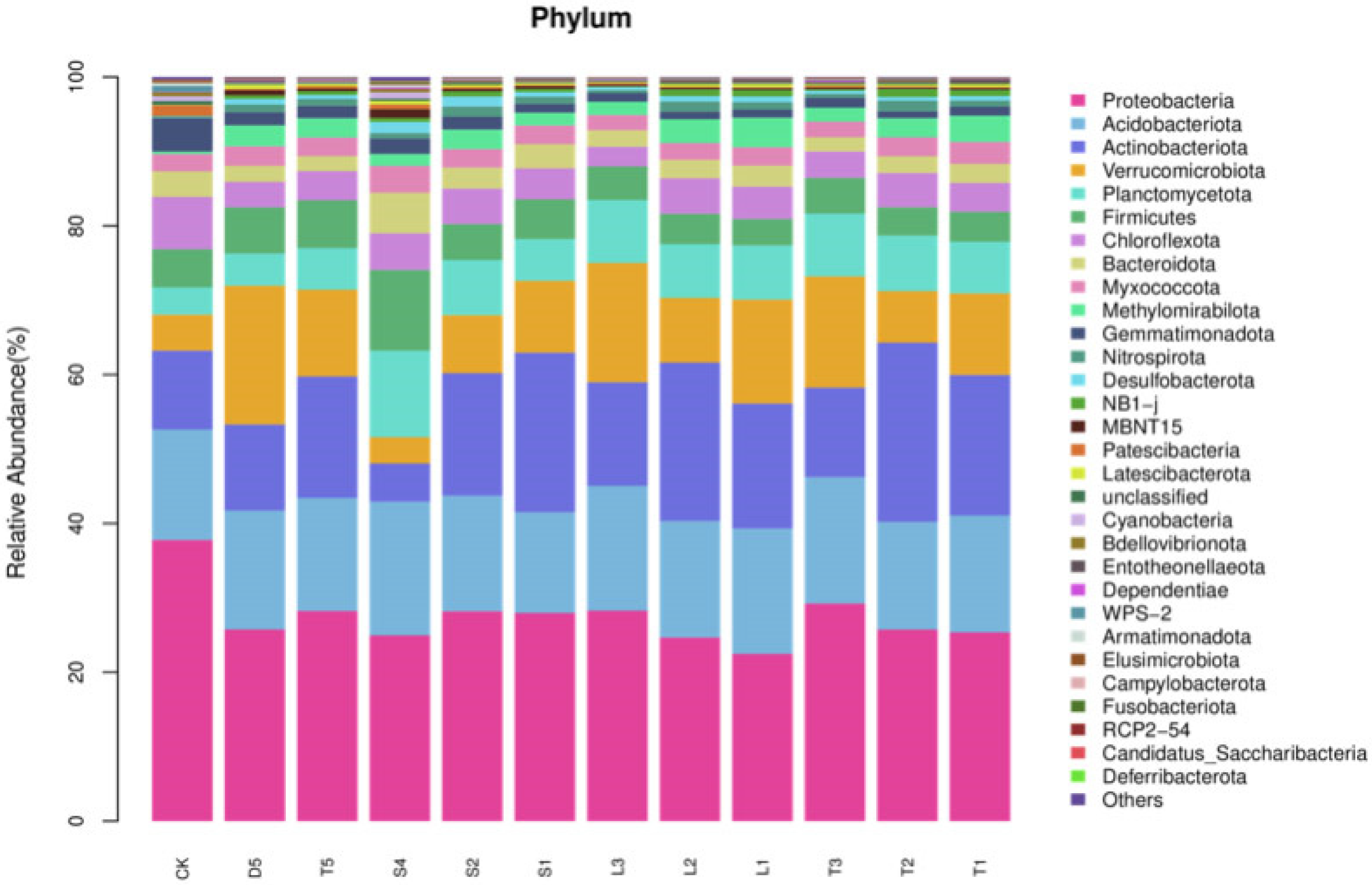
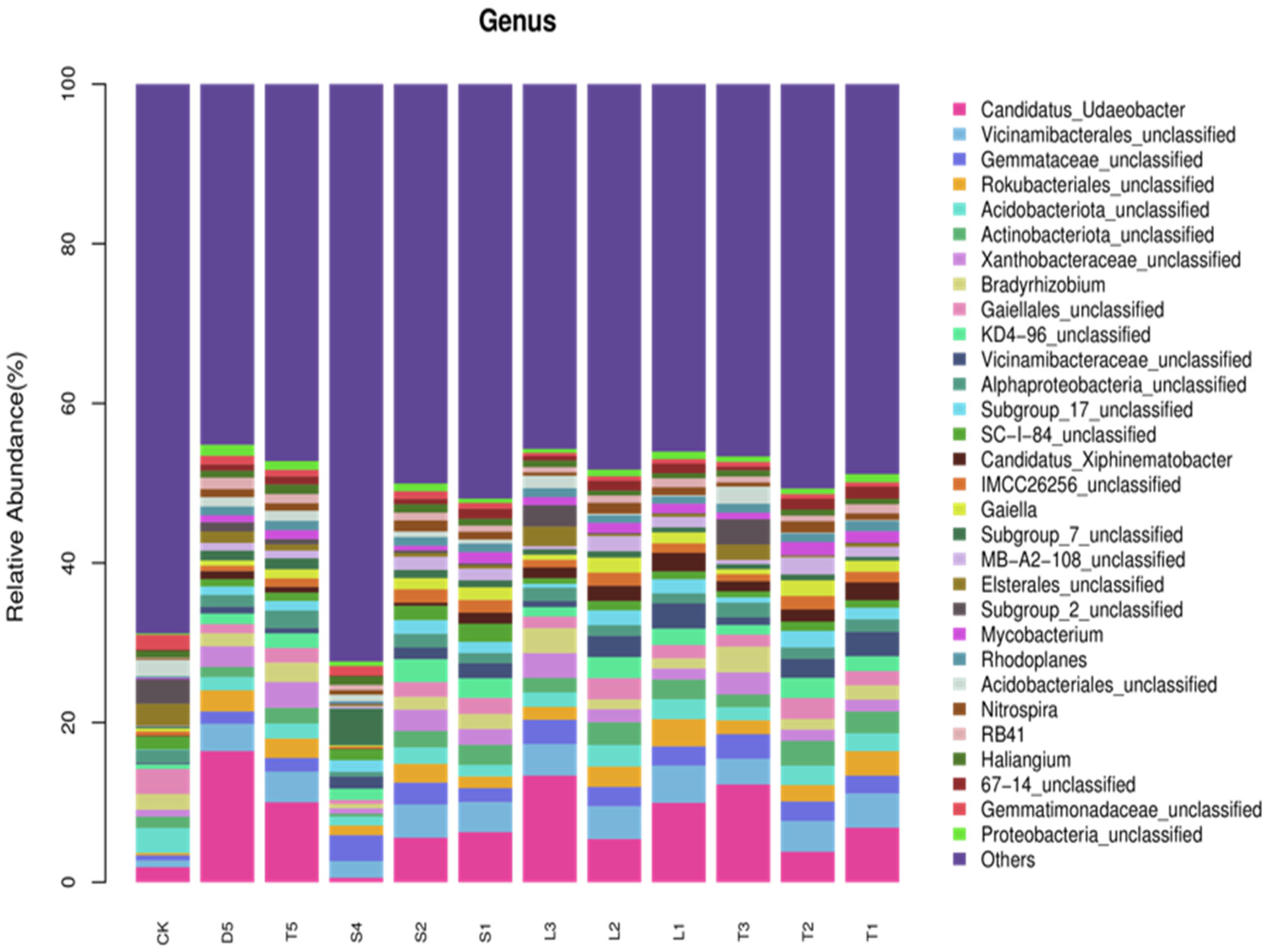
3.5. Analysis of Soil Bacterial Community Structure
3.6. Environmental Factor Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Pan, B.; Hui, Y.; Xv, Z.; Chi, X.; Li, M.; Yu, Y.; Sun, C.; Hou, L. Study and Prospect of Wild Vegetable Resources in Jilin Province. Chang. Veg. 2024, 22–25. [Google Scholar]
- Tan, S.; Chou, L.; Duan, O.; Jia, M.; Liu, Y.; Xiong, A. Effects of sodium hypochlorite treatment on soluble sugar content and related gene expression in celery seedlings. Acta Physiol. Sin. 2022, 58, 165–172. [Google Scholar]
- Ren, R.; Zhang, C.; Cao, X. Effects of microbial fertilizer on growth, yield and quality of celery. J. Jilin Agric. Univ. 2023, 1–9. [Google Scholar]
- Lu, X.; Feng, Z.; Jia, J.; Liang, J.; Zhao, W.; Bai, Y. Effects of continuous cropping on microbial community structure and diversity in celery rhizosphere soil. Acta Agri. Biotechnol. 2023, 31, 2466–2476. [Google Scholar]
- Xu, Y.; Chen, Z.; Li, X.; Tan, J.; Liu, F.; Wu, J. The mechanism of promoting rhizosphere nutrient turnover for arbuscular mycorrhizal fungi attributes to recruited functional bacterial assembly. Mol. Ecol. 2023, 32, 2335–2350. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Ji, Z.; Wu, A.; He, D.; Rensing, C.; Chen, Y.; Chen, C.; Wu, H.; Muneer, M.A.; Wu, L. Inconsistent responses of soil bacterial and fungal community’s diversity and network to magnesium fertilization in tea (Camellia sinensis) plantation soils. Appl. Soil Ecol. 2023, 191, 105055. [Google Scholar] [CrossRef]
- Bahram, M.; Hildebrand, F.; Forslund, S.K.; Anderson, J.L.; Soudzilovskaia, N.A.; Bodegom, P.M.; Bengtsson-Palme, J.; Anslan, S.; Coelho, L.P.; Harend, H.; et al. Structure and function of the global topsoil microbiome. Nature 2018, 560, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.; Zhang, H.; Chen, W.; Xu, W.; Guo, M. Detection of fungal community changes during wheat storage by high-throughput se-quencing technology. Food Sci. 2020, 41, 109–115. [Google Scholar]
- Nilsson, R.H.; Anslan, S.; Bahram, M.; Wurzbacher, C.; Baldrian, P.; Tedersoo, L. Mycobiome diversity: High-throughput sequencing and identification of fungi. Nat. Rev. Microbiol. 2019, 17, 95–109. [Google Scholar] [CrossRef]
- Xing, H.-Q.; Ma, J.-C.; Xu, B.-L.; Zhang, S.-W.; Wang, L.; Cao, L.; Yang, X.-M. Mycobiota of maize seeds revealed by rDNA-ITS sequence analysis of samples with varying storage times. Microbiologyopen 2018, 7, e00609. [Google Scholar] [CrossRef]
- Lv, J.; Li, C.; Yang, Z.; Liu, P.; Lu, M.; Ren, Y.; Tian, K.; Zhao, X.; Chen, Z. Response of soil microbial community to land use change in Napahai Plateau wetland. Soil Bull. 2023, 54, 682–694. [Google Scholar]
- Li, Q.; Wang, M.; Liu, H. Diversity and difference of microbial community structure in rapeseed rhizosphere soil at different stages. N. Hortic. 2023, 72–80. [Google Scholar]
- Bao, S. Agrochemical Analysis of Soil Mushrooms; China Agricultural Press: Beijing, China, 2005. [Google Scholar]
- Xu, Y.; Niu, J.; Chen, L.; Wang, S.; Dong, Z.; Wang, Z. Study on microbial changes in rhizosphere soil of cultivated Atractylodes lancea based on high-throughput sequencing technology. J. Crops 2022, 38, 221–228. [Google Scholar]
- Zhang, D.; Zhao, J.; Xie, S.; Hu, F.; Wu, Q.; Zhou, X. Analysis of microbial diversity in maize based on high-throughput sequencing. Acta Food Sin. 2023, 23, 305–314. [Google Scholar] [CrossRef]
- Ren, Y.; Han, C.; Yang, H.; Wei, B.; Cao, S.; Qian, Y.; Tang, Y. Study on soil microbial diversity of five urban landscape plants. Soil 2021, 53, 746–754. [Google Scholar]
- Zhang, J.; Xue, J.; Li, H.; Chai, X.; Zhao, S.; Li, L.; Zhang, S.; Jia, L.; Zhang, J.; Wang, G. Response of microbial community structure and diversity in wheat rhizosphere to water stress. J. Irrig. Drain. 2022, 41, 41–50. [Google Scholar]
- Deng, D.; Deng, T.; Zhou, Y.; Wang, J.; Yang, L. Soil microbial diversity and its response to soil physical and chemical properties in different banana varieties. J. Trop. Crops 2019, 40, 1858–1864. [Google Scholar]
- Xu, H.; Xue, Y.; Ding, H.; Qi, H.; Li, T.; Mao, W.; Cheng, S. Microbial Community Structure and Diversity of Peach Rhizosphere Soil in Dif-ferent Regions. China Fruit Veg. 2024, 44, 72–79. [Google Scholar]
- Wei, C.; Gu, W.; Tian, R.; Xu, F.; Han, Y.; Ji, Y.; Li, T.; Zhu, Y.; Lang, P.; Wu, W. Comparative analysis of the structure and function of rhizosphere microbiome of the Chinese medicinal herb Alisma in different regions. Arch. Microbiol. 2022, 204, 448. [Google Scholar] [CrossRef]
- Kui, L.; Chen, B.; Chen, J.; Sharifi, R.; Dong, Y.; Zhang, Z.; Miao, J. A Comparative Analysis on the Structure and Function of the Panax notoginseng Rhizosphere Microbiome. Front. Microbiol. 2021, 12, 673512. [Google Scholar] [CrossRef]
- Fischer, S.E.; Fischer, S.I.; Magris, S.; Mori, G.B. Isolation and characterization of bacteria from the rhizosphere of wheat. World J. Microbiol. Biotechnol. 2007, 23, 895–903. [Google Scholar] [CrossRef]
- Wang, G.; Liu, J.; Yu, Z. Research progress on ecology of soil acidobacter bacteria. Biotechnol. Bull. 2016, 32, 14–20. [Google Scholar]
- Jangid, K.; Williams, M.A.; Franzluebbers, A.J.; Sanderlin, J.S.; Reeves, J.H.; Jenkins, M.B.; Endale, D.M.; Coleman, D.C.; Whitman, W.B. Relative impacts of land-use, management intensity and fertilization upon soil microbial community structure in agricultural systems. Soil Biol. Biochem. 2008, 40, 2843–2853. [Google Scholar] [CrossRef]
- Liu, X.; Li, Z.; Liu, R.; Li, L.; Wang, W. Changes of bacterial community structure in rhizosphere soil of soybean at different growth stages. Guangxi Plant 2018, 38, 1363–1370. [Google Scholar]
- Bokulich, N.A.; Subramanian, S.; Faith, J.J.; Gevers, D.; Gordon, J.I.; Knight, R.; Mills, D.A.; Caporaso, J.G. Quality filtering greatly improves diversity estimates for Yili amplicon sequencing. Nat. Method 2013, 10, 57–59. [Google Scholar] [CrossRef]
- Li, Y.; Song, D.; Liang, S.; Dang, P.; Qin, X.; Liao, Y.; Siddique, K.H. Effects of No-tillage and Stubble on Soil Microbial Biomass Carbon, Nitrogen, and Enzyme Activities. Acta Ecol. Sin. 2009, 29, 5508–5515. [Google Scholar]
- Huang, W. Changes in Rhizosphere Microbial Flora and Screening of Key Microorganisms under Continuous Cropping of Wild Rehmannia Glutinosa. Master’s Thesis, Fujian Agriculture and Forestry University, Fuzhou, China, 2018. [Google Scholar]
- Zhang, Q.; Wang, C.; Sun, Z.; Li, S.; Liang, Y. Research Progress on Factors Influencing Soil Microbial Biomass and Diversity. North. Hortic. 2022, 116–121. [Google Scholar]
- Berendsen, R.L.; Vismans, G.; Yu, K.; Song, Y.; de Jonge, R.; Burgman, W.P.; Burmølle, M.; Herschend, J.; Bakker, P.A.H.M.; Pieterse, C.M.J. Disease-induced assemblage of a plant-beneficial bacterial consortium. ISME J. 2018, 12, 1496–1507. [Google Scholar] [CrossRef]
- Chen, C. The Effects of Different Crop Rotation Patterns on Soil Aggregate Distribution, Microbial Diversity, and Crop Yield. Master’s Thesis, Henan Agricultural University, Zhengzhou, China, 2023. [Google Scholar]
- Gong, Z. Study on Soil Fertility Status and Nutrient Distribution Characteristics of Panax Notoginseng Plants in Different Production Areas. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2014; pp. 26–60. [Google Scholar]
- Zhu, J. Study on the Mechanism of Accumulation of Active Ingredients in Different Developmental Stages of Gastrodia Elata. Master’s Thesis, Minzu University of China, Beijing, China, 2024. [Google Scholar]
- Qu, A. Characteristics of Soil Properties and Soil Quality Evaluation under Different Planted Forests in Stony Mountainous Areas of North China. Master’s Thesis, Henan Agricultural University, Zhengzhou, China, 2023. [Google Scholar]


| Survey Location | Survey Abbreviation | Latitude (Degree) | Longitude (Degree) | Elevation (M) | Sunshine Hour (h) | Average Annual Temperature (°C) | Average Annual Rainfall (mm) | Frost- Free Period (Days) | Canopy Density |
|---|---|---|---|---|---|---|---|---|---|
| Tonghua City | Tonghua | N125.75 | E41.68 | 320–670 | 7.61 | 5.5 °C | 870 | 145 | 0.7 |
| Linjiang City | Linjiang | N126.90 | E41.80 | 550–735 | 8.21 | 4 °C | 800 | 180 | 0.8 |
| Jiangyuan District | Jiangyuan | N126.59 | E42.06 | 630–840 | 8.11 | 4.5 °C | 880 | 140 | 0.7 |
| Dongfeng County | Dongfeng | N125.53 | E42.68 | 300–445 | 7.58 | 4.6 °C | 590 | 140 | 0.3 |
| Huadian City | Huadian | N126.75 | E42.98 | 320–780 | 7.81 | 3.7 °C | 748 | 125 | 0.6 |
| Sample | pH | Available Phosphorus (mg/kg) | Available Nitrogen (mg/kg) | Electric Conductivity (MS/cm) |
|---|---|---|---|---|
| Tonghua Large-leaf Celery | 6.27 ± 0.03 b | 16.47 ± 0.38 bc | 17.93 ± 0.26 d | 852.50 ± 17.50 bc |
| Linjiang Large-leaf Celery | 6.83 ± 0.14 a | 17.83 ± 0.09 b | 20.65 ± 0.48 c | 830.00 ± 13.23 c |
| Jiangyuan Large-leaf Celery | 6.12 ± 0.14 bc | 15.52 ± 0.20 c | 23.34 ± 0.83 b | 868.50 ± 5.29 b |
| Huadian Large-leaf Celery | 5.92 ± 0.03 c | 8.09 ± 0.04 d | 15.63 ± 0.37 e | 411.50 ± 5.77 d |
| Artificially Cultivated Large-leaf Celery | 5.64 ± 0.18 d | 179.13 ± 1.51 a | 186.70 ± 1.96 a | 1560.00 ± 11.46 a |
| Tonghua Water Celery | 6.37 ± 0.42 a | 13.37 ± 0.08 c | 11.96 ± 0.90 b | 269.33 ± 8.14 b |
| Linjiang Water Celery | 6.62 ± 0.27 a | 12.69 ± 0.13 c | 10.47 ± 0.27 b | 273.67 ± 13.65 b |
| Dongfeng Water Celery | 6.37 ± 0.17 a | 15.07 ± 0.05 b | 6.10 ± 1.30 c | 229.00 ± 3.77 c |
| Tonghua Old Mountain Celery | 6.88 ± 0.09 a | 15.7 ± 0.10 c | 18.12 ± 0.21 c | 785.17 ± 9.28 bc |
| Linjiang Old Mountain Celery | 6.78 ± 0.03 a | 17.37 ± 0.13 b | 20.37 ± 0.51 b | 770.00 ± 6.61 c |
| Jiangyuan Old Mountain Celery | 5.68 ± 0.04 b | 15.22 ± 0.12 c | 19.71 ± 0.34 b | 787.00 ± 7.55 b |
| Huadian Small-leaf Celery | 5.96 ± 0.02 | 7.96 ± 0.11 | 13.60 ± 1.19 | 426.67 ± 10.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Zou, Y.; Zhao, C.; Liu, S.; Yu, Y.; Jiang, J.; Zou, Y.; Qiao, J. Study on the Diversity of Bacterial Communities in the Rhizosphere Soils of Different Wild Celery Species in Jilin Province. Agronomy 2024, 14, 1735. https://doi.org/10.3390/agronomy14081735
Chen S, Zou Y, Zhao C, Liu S, Yu Y, Jiang J, Zou Y, Qiao J. Study on the Diversity of Bacterial Communities in the Rhizosphere Soils of Different Wild Celery Species in Jilin Province. Agronomy. 2024; 14(8):1735. https://doi.org/10.3390/agronomy14081735
Chicago/Turabian StyleChen, Shanshan, Yan Zou, Chunbo Zhao, Shuang Liu, Yue Yu, Junhai Jiang, Yue Zou, and Jianlei Qiao. 2024. "Study on the Diversity of Bacterial Communities in the Rhizosphere Soils of Different Wild Celery Species in Jilin Province" Agronomy 14, no. 8: 1735. https://doi.org/10.3390/agronomy14081735





