Selection and Characterization of Somaclonal Variants of Prata Banana (AAB) Resistant to Fusarium Wilt
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Preparation of the Foc Inoculum
2.3. Evaluation of Resistance in the Greenhouse
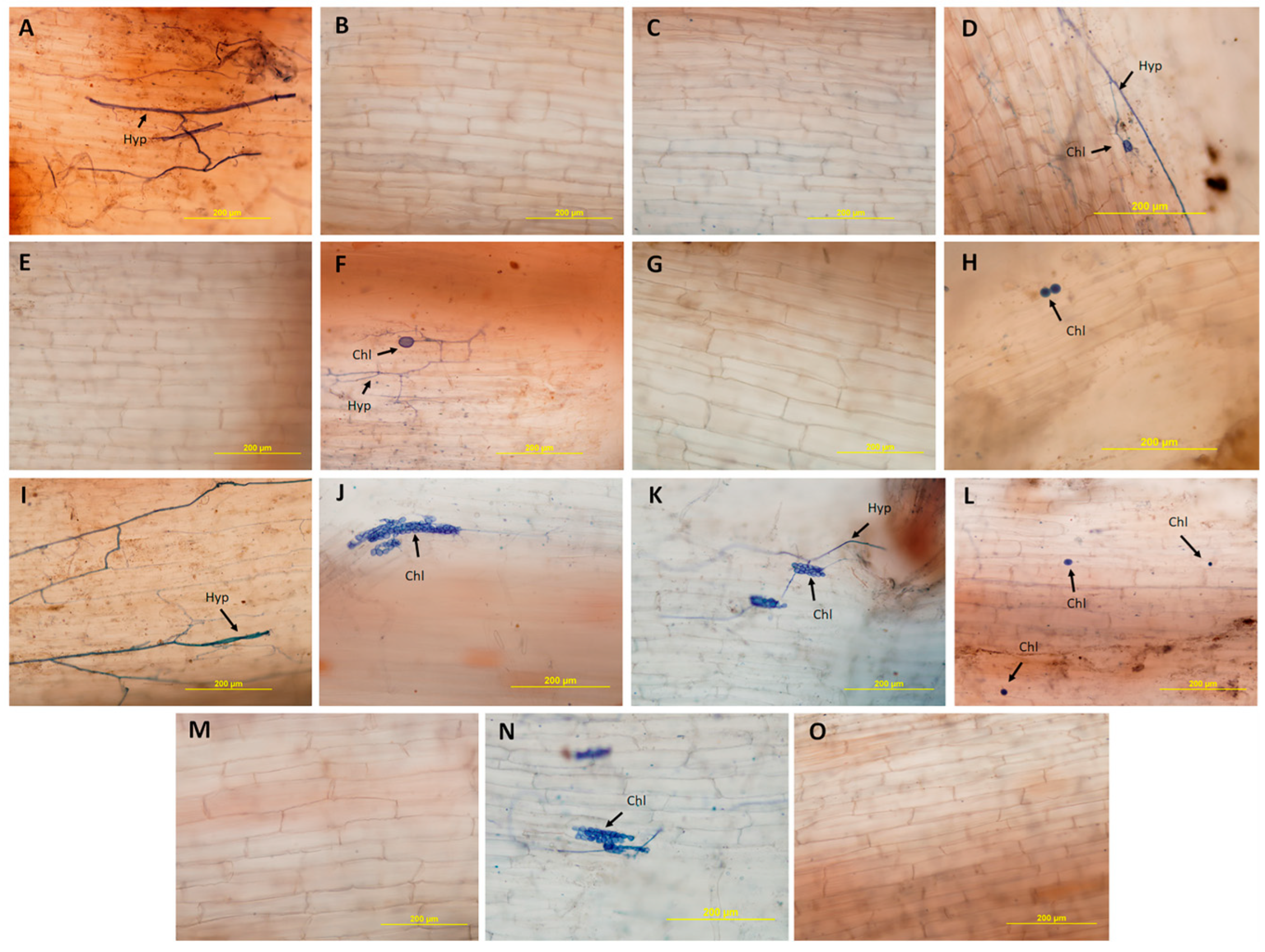
2.4. Histological and Histochemical Analysis
2.5. Molecular Analysis
Material Collection and DNA Extraction
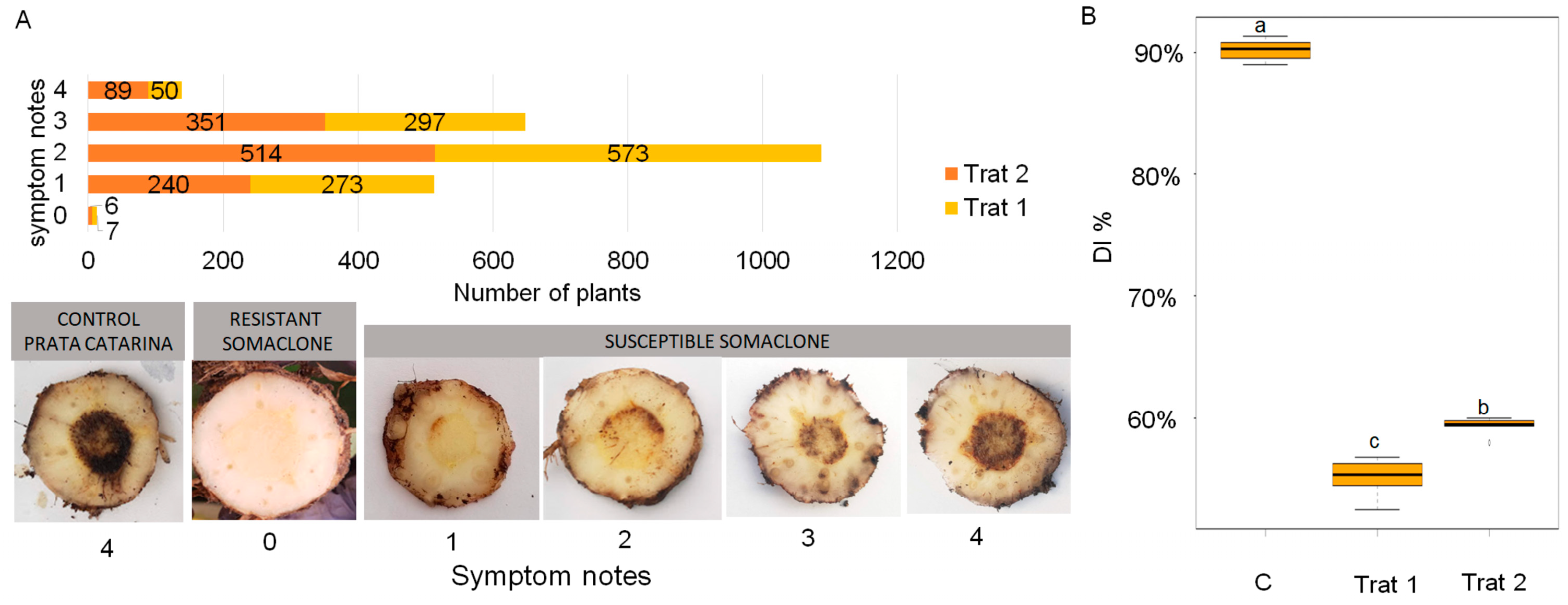
3. Results
3.1. Resistance Assessment in the Greenhouse
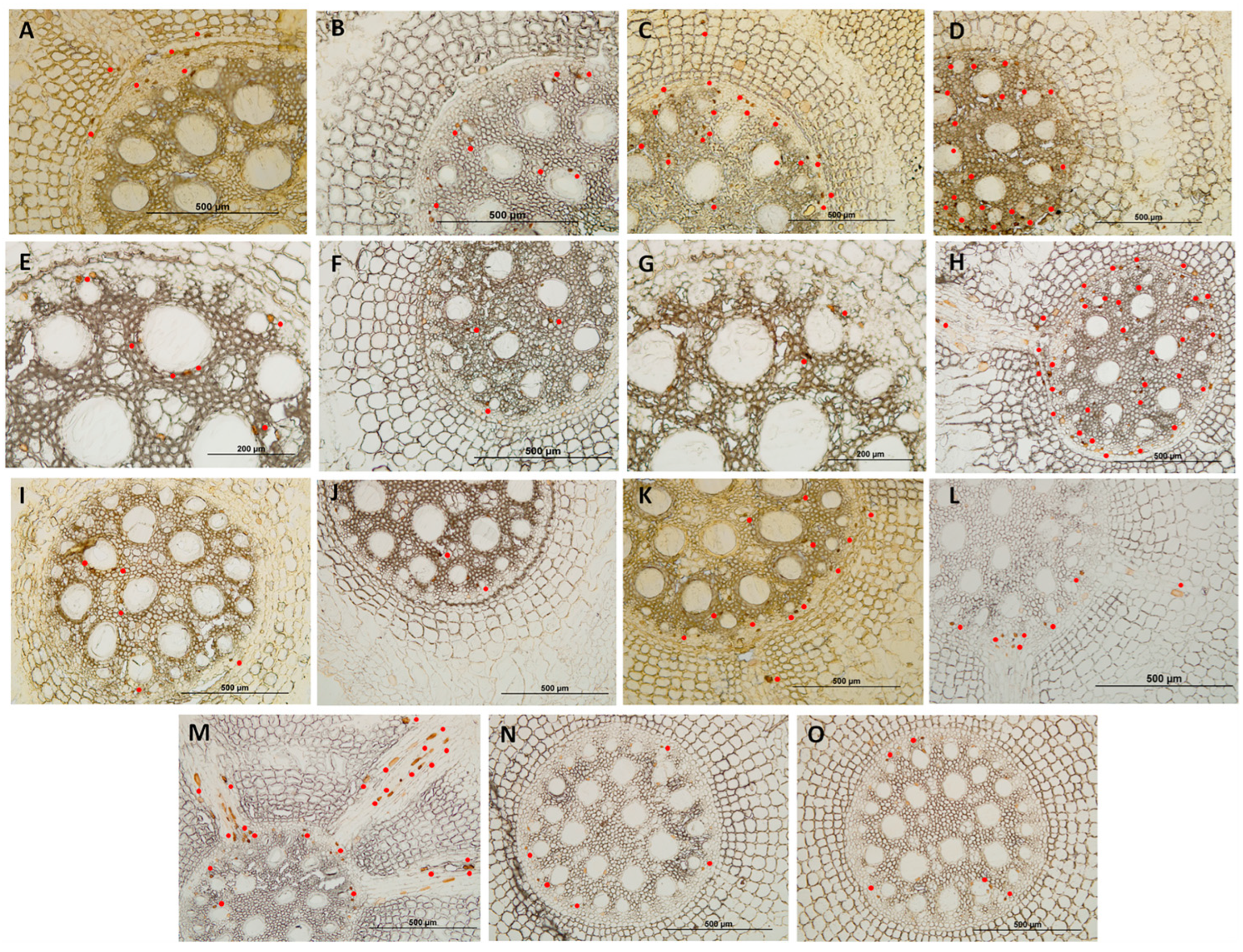
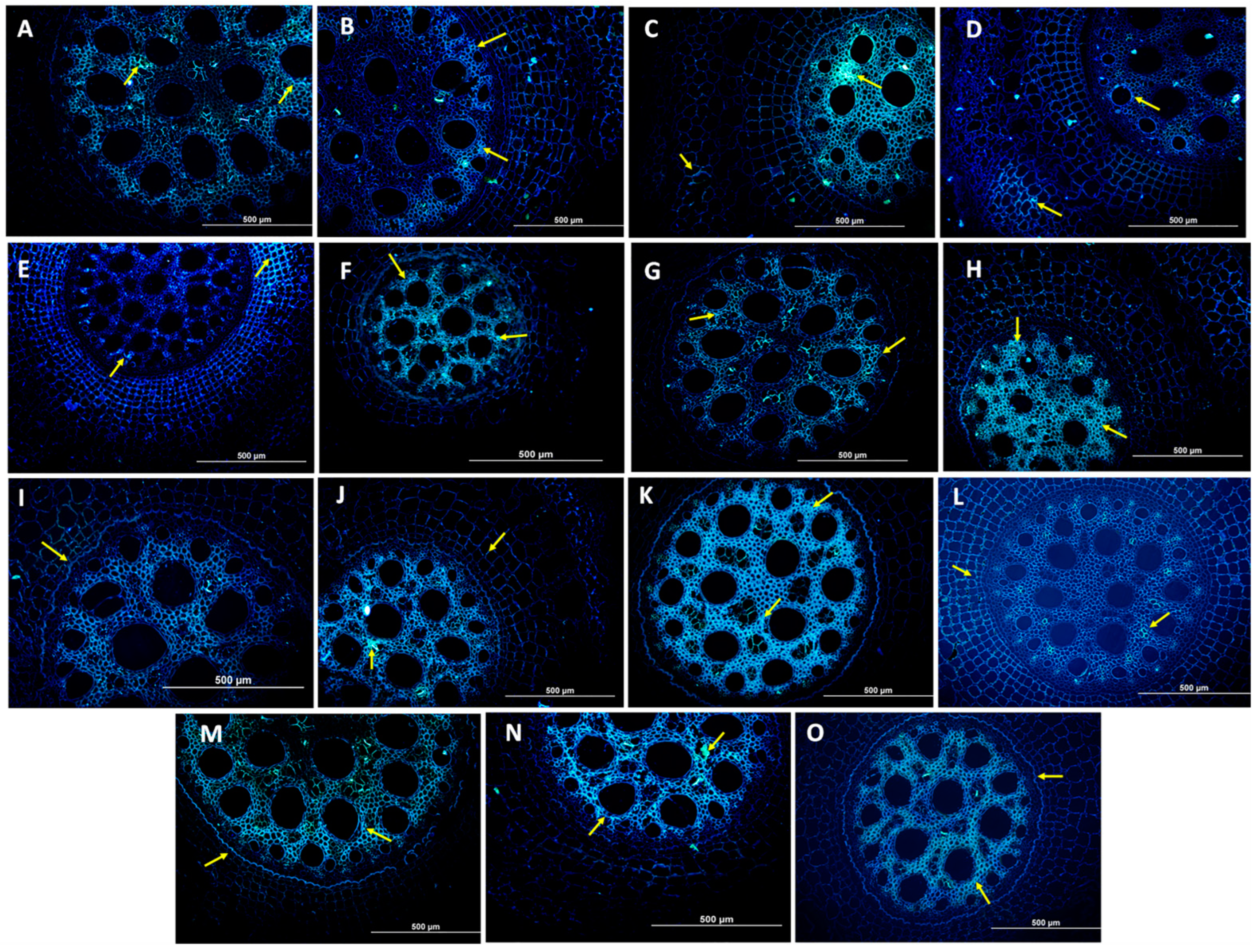
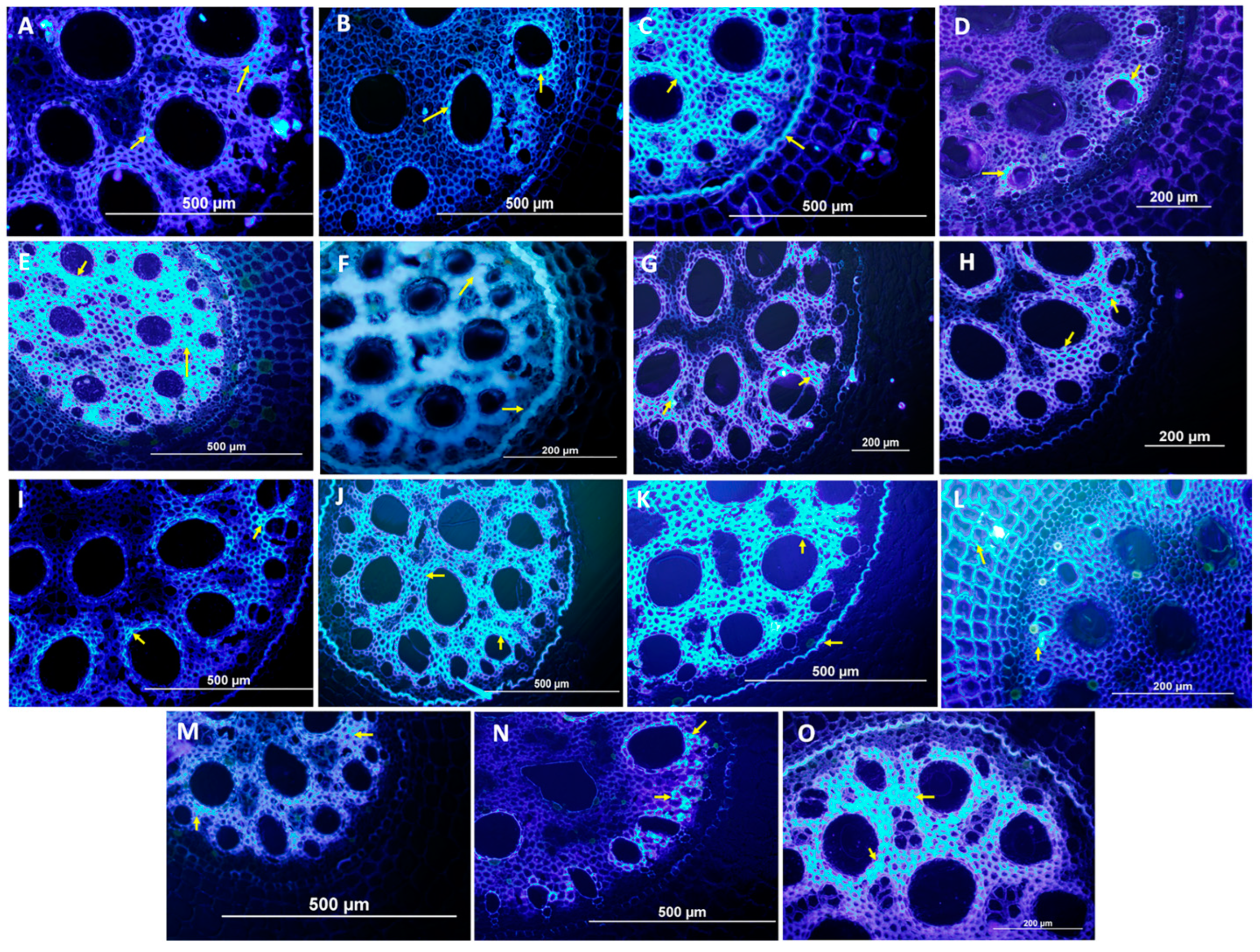
3.2. Histological and Histochemical Evaluation
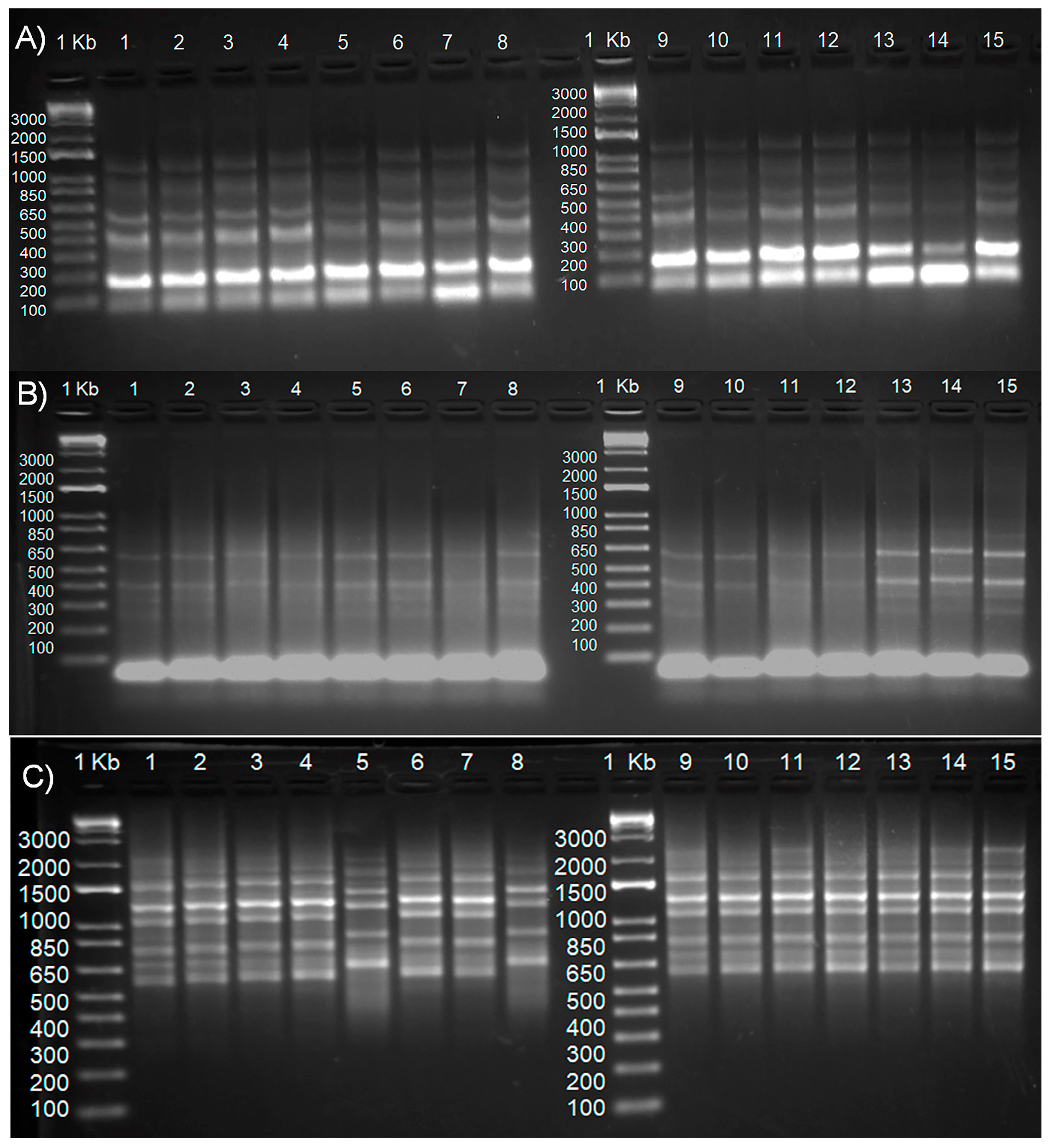
3.3. PCR Amplification and Marker Analysis
4. Discussion
4.1. Assessment of Resistance in the Greenhouse
4.2. Analysis of Resistance Mechanisms by Histological and Histochemical Evaluations
4.3. Analysis of the Extent of Genetic Diversity in Somaclones Using Molecular Markers IRAP, REMAP, and ISSR
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Available online: https://www.fao.org/world-banana-forum/zh/ (accessed on 8 June 2024).
- FAOSTAT. 2024. Available online: https://www.fao.org/faostat/en/#data (accessed on 10 May 2024).
- FAO. Banana Statistical Compendium 2021; Food and Agriculture Organization of the United Nations: Rome, Italy, 2022. [Google Scholar]
- Lichtemberg, L.A.; Hinz, R.H.; Malburg, J.L.; Peruch, L.A.M.; Sonego, M. Agriculture of Santa Catarina SCS451—Catarina—New Cultivar of Banana Plant of the Prata Subgroup; Epagri—Experimental Station of Itajaí: Santa Catarina, Brazil, 2011. [Google Scholar]
- Soni, K.B.; Jadhav, P.R.; Alex, S. Genetic Improvement of Banana. In Genetic Engineering of Crop Plants for Food and Health Security: Volume 1; Springer Nature: Singapore, 2024; pp. 305–329. [Google Scholar]
- Willadino, L.; Camara, T.R.; Ribeiro, M.B.; Amaral, D.O.J.; Suassuna, F.; Silva, M.V.D. Mechanisms of tolerance to salinity in banana: Physiological, biochemical, and molecular aspects. Rev. Bras. Frutic. 2017, 39, 1–8. [Google Scholar] [CrossRef]
- Nansamba, M.; Sibiya, J.; Tumuhimbise, R.; Karamura, D.; Kubiriba, J.; Karamura, E. Breeding banana (Musa spp.) for drought tolerance: A review. Plant Breed. 2020, 139, 685–696. [Google Scholar] [CrossRef]
- Monteiro, J.D.; Santos, M.; Santos, J.R.P.; Cares, J.E.; Marchão, R.L.; Amorim, E.P.; Costa, D.D.C. Identification of plant parasitic nematodes in triploid and tetraploid bananas in brazil. Rev. Caatinga 2020, 33, 865–877. [Google Scholar] [CrossRef]
- Sairam, S.; Selvarajan, R.; Handanahalli, S.S.; Venkataraman, S.; Sairam, S.; Selvarajan, R.; Handanahalli, S.S.; Venkataraman, S. Towards understanding the structure of the capsid of Banana Bunchy Top Virus. BioRxiv 2020. [Google Scholar] [CrossRef]
- Studholme, D.J.; Wicker, E.; Abrare, S.M.; Aspin, A.; Bogdanove, A.; Broders, K.; Dubrow, Z.; Grant, M.; Jones, J.B.; Karamura, G.; et al. Transfer of Xanthomonas campestris pv. arecae and X. campestris pv. musacearum to X. vasicola (Vauterin) as X. vasicola pv. arecae comb. nov. and X. vasicola pv. musacearum comb. nov. and description of X. vasicola pv. vasculorum pv. nov. Phytopathology 2020, 110, 1153–1160. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, F.D.S.; Sousa, Y.M.; Rocha, A.D.J.; Ferreira, C.F.; Haddad, F.; Amorim, E.P. Sources of black Sigatoka resistance in wild banana diploids. Rev. Bras. Frutic. 2020, 42, e-038. [Google Scholar] [CrossRef]
- Gonçalves, Z.S.; Haddad, F.; Amorim, V.B.O.; Ferreira, C.F.; Oliveira, S.A.S.; Amorim, E.P. Agronomic characterization and identification of banana genotypes resistant to Fusarium wilt race 1. Eur. J. Plant Pathol. 2019, 155, 1093–1103. [Google Scholar] [CrossRef]
- Ploetz, R.C. Fusarium wilt management in banana: A review with special reference to tropical race 4. Crop Prot. 2015, 73, 7–15. [Google Scholar] [CrossRef]
- Ploetz, R.C. Gone Bananas? Current and Future Impact of Fusarium Wilt on Production. In Plant Diseases and Food Security in the 21st Century; Scott, P., Strange, R., Korsten, L., Gullino, M.L., Eds.; Springer Nature: Cham, Switzerland, 2021; Volume 10, pp. 21–32. [Google Scholar] [CrossRef]
- García-Bastidas, F.A.; Quintero-Vargas, J.C.; Ayala-Vasquez, M.; Schermer, T.; Seidl, M.F.; Santos-Paiva, M.; Noguera, A.M.; Aguilera-Galvez, C.; Wittenberg, A.; Hofstede, R.; et al. First report of Fusarium wilt Tropical race 4 in Cavendish bananas caused by Fusarium odoratissimum in Colombia. Plant Dis. 2020, 104, 994. [Google Scholar] [CrossRef]
- Maryani, N.; Lombard, L.; Poerba, Y.S.; Subandiyah, S.; Crous, P.W.; Kema, G.H.J. Phylogeny and genetic diversity of the banana Fusarium wilt pathogen Fusarium oxysporum f. sp. cubense in the Indonesian centre of origin. Stud. Mycol. 2019, 92, 155–194. [Google Scholar] [CrossRef]
- Martínez, G.; Olivares, B.O.; Rey, J.C.; Rojas, J.; Cardenas, J.; Muentes, C.; Dawson, C. The Advance of FusariumWilt Tropical Race 4 in Musaceae of Latin America and the Caribbean: Current Situation. Pathogens 2023, 12, 277. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.N.; Bragança, C.A.D.; Ribeiro, L.R.; Amorim, E.P.; Silva, S.O.; Dita, M.; Laranjeira, F.F.; Haddad, F. Genetic structure of Fusarium oxysporum f. sp. cubense in diferent regions from Brazil. Plant Pathol. 2015, 64, 37–146. [Google Scholar] [CrossRef]
- Batista, I.C.A.; Heck, D.W.; Santos, A.; Alves, G.; Ferro, C.G.; Dita, M.; Haddad, F.; Michereff, S.J.; Correia, K.C.; da Silva, C.F.B.; et al. The Population of Fusarium oxysporum f. sp. cubense in Brazil Is Not Structured by Vegetative Compatibility Group or by Geographic Origin. Phytopathology 2022, 112, 2416–2425. [Google Scholar] [CrossRef] [PubMed]
- Dita, M.; Barquero, M.; Heck, D.; Mizubute, E.S.G.; Staver, C.P. Fusarium wilt of banana: Current knowledge on epidemiology and research needs toward sustainable disease management. Front. Plant Sci. 2018, 9, 1468–1488. [Google Scholar] [CrossRef]
- Pegg, K.G.; Coates, L.M.; O’Neill, W.T.; Turner, D.W. The epidemiology of fusarium wilt of Banana. Front. Plant Sci. 2019, 10, 1395. [Google Scholar] [CrossRef] [PubMed]
- Jamil, F.N.; Tang, C.N.; Saidi, N.B.; Lai, K.S.; Baharum, N.A. Fusarium wilt in banana: Epidemics and management strategies. In Horticultural Crops; Kossi Baimey, H., Hamamouch, N., Adjiguita Kolombia, Y., Eds.; IntechOpen: London, UK, 2019; pp. 229–331. [Google Scholar]
- Rocha, A.D.; Soares, J.M.; Nascimento, F.D.; Rocha, A.D.; Amorim, V.B.; Ramos, A.P.; Ferreira, C.F.; Haddad, F.; Amorim, E.P. Molecular, histological and histochemical responses of banana cultivars challenged with Fusarium oxysporum f. sp. cubense with different levels of virulence. Plants 2022, 11, 2339. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.D.; Rocha, A.D.; Nascimento, F.D.; Oliveira, W.D.; Soares, J.M.; Rebouças, T.A.; Morais Lino, L.S.; Haddad, F.; Ferreira, C.F.; Santos-Serejo, J.A.; et al. The role of somaclonal variation in plant genetic improvement: A systematic review. Agronomy 2023, 13, 730. [Google Scholar] [CrossRef]
- Davey, M.R.; Anthony, P. Plant Cell Culture: Essential Methods; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Bairu, M.W.; Aremu, A.O.; Van Staden, J. Somaclonal variation in plants: Causes and detection methods. Plant Growth Regul. 2011, 63, 147–173. [Google Scholar] [CrossRef]
- Jain, S.M.; Brar, D.S.; Ahloowalia, B.S. (Eds.) Somaclonal Variation and Induced Mutations in Crop Improvement; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Hou, B.H.; Tsai, Y.H.; Chiang, M.H.; Tsao, S.M.; Huang, S.H.; Chao, C.P.; Chen, H.M. Cultivar-specific markers, mutations, and chimerisim of Cavendish banana somaclonal variants resistant to Fusarium oxysporum f. sp. cubense tropical race 4. BMC Genom. 2022, 23, 470. [Google Scholar] [CrossRef]
- Rebouças, T.A.; Rocha, A.d.J.; Cerqueira, T.S.; Adorno, P.R.; Barreto, R.Q.; Ferreira, M.; Lino, L.S.M.; Amorim, V.B.D.; Santos-Serejo, J.A.D.; Haddad, F.; et al. Pre-selection of banana somaclones resistant to Fusarium oxysporum f. sp. cubense, subtropical race 4. Crop Prot. 2021, 147, 105692. [Google Scholar] [CrossRef]
- Hwang, S.C.; Ko, W.H. Cavendish banana cultivars resistant to Fusarium wilt acquired through somaclonal variation in Taiwan. Plant Dis. 2004, 88, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Munsamy, A.; Rutherford, R.S.; Snyman, S.J.; Watt, M.P. 5-Azacytidine as a tool to induce somaclonal variants with useful traits in sugarcane (Saccharum spp.). Plant Biotechnol. Rep. 2013, 7, 489–502. [Google Scholar] [CrossRef]
- Wang, K.; Wang, S.; Zhou, X.; Lin, Z.; Li, J.; Du, L.; Tang, Y.; Xu, H.; Yan, Y.; Ye, X. Development, identification, and genetic analysis of a quantitative dwarfing somatic variation line in wheat. Crop Sci. 2013, 53, 1032–1041. [Google Scholar] [CrossRef]
- Miyao, A.; Nakagome, M.; Ohnuma, T.; Yamagata, H.; Kanamori, H.; Katayose, Y.; Takahashi, A.; Matsumoto, T.; Hirochika, H. Molecular spectrum of somaclonal variation in regenerated rice revealed by whole-genome sequencing. Plant Cell Physiol. 2012, 53, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, T. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plantarum. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Velame, K.V.; Rocha, A.D.; Ferreira, M.D.; Haddad, F.; Amorim, V.B.; Pestana, K.N.; Ferreira, C.F.; Alves Santos de Oliveira, S.; Amorim, E.P. Evidence of Correlation between Pathogenicity, Avirulence Genes, and Aggressiveness of Fusarium oxysporum f. sp. cubense in Banana “Cavendish” and “Prata” Subgroups. Horticulturae 2024, 10, 228. [Google Scholar] [CrossRef]
- Madigan, M.T.; Martinko, J.M.; Bender, K.S.; Buckley, D.H.; Stahl, D.A. Microbiologia de Brock, 14th ed.; Artmed Editora: Porto Alegre, Brazil, 2016. [Google Scholar]
- Dita, M.A.; Pérez Vicente, L.; Martínez, E. Inoculation of Fusarium oxysporum f. sp. cubense causal agent of fusarium wilt in banana. In Technical Manual: Prevention and Diagnostic of Fusarium Wilt of Banana Caused by Fusarium oxysporum f. sp. cubense Tropical Race 4 (TR4); Pérez Vicente, L., Dita, M.A., de la Parte, E.M., Eds.; Food and Agriculture Organization of the United Nations: Rome, Italy, 2014; pp. 55–58. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; The R Foundation for Statistical Computing: Vienna, Austria, 2020; Volume 1. [Google Scholar]
- Karnovsky, M.J. A formaldehydeglutaraldehyde fixative of high osmolality for use in electron microscopy. J. Cell Biol. 1965, 27, 137. [Google Scholar]
- Johansen, D.A. Plant Microtechnique; Mc Graw Hill: New York, NY, USA, 1940; 523p. [Google Scholar]
- Hughes, J.; McCully, M.E. The use of an optical brightener in the study of plant structures. Stain Technol. 1975, 50, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. Isolation of plant DNA from fresh tissue. Focus Rockv. 1990, 12, 13–15. [Google Scholar]
- Ferreira, C.F.; Gutierrez, D.L.; Kreuze, J.F.; Iskra-Caruana, M.L.; Chabannes, M.; Barbosa, A.C.O.; Santos, T.A.; Silva, A.G.S.; Santos, R.M.F.; Amorim, E.P.; et al. Rapid plant dna and rna extraction protocol using a bench drill brief note. Genet. Mol. Res. 2019, 18, gmr18394. [Google Scholar] [CrossRef]
- Kalendar, R.; Grob, T.; Regina, M.; Suoniemi, A.; Schulman, A. IRAP and REMAP: Two new retrotransposon-based DNA fingerprinting techniques. Theor. Appl. Genet. 1999, 98, 704–711. [Google Scholar] [CrossRef]
- Sankar, A.A.; Moore, G.A. Evaluation of inter-simple sequence repeat analysis for mapping in Citrus and extension of the genetic linkage map. Theor. Appl. Genet. 2001, 102, 206–214. [Google Scholar] [CrossRef]
- Eeckhaut, T.; Van Houtven, W.; Bruznican, S.; Leus, L.; Van Huylenbroeck, J. Somaclonal variation in Chrysanthemum× morifolium protoplast regenerants. Front. Plant Sci. 2020, 11, 607171. [Google Scholar] [CrossRef] [PubMed]
- Erland, L.A.; Giebelhaus, R.T.; Victor, J.M.; Murch, S.J.; Saxena, P.K. The morphoregulatory role of thidiazuron: Metabolomics-guided hypothesis generation for mechanisms of activity. Biomolecules 2020, 10, 1253. [Google Scholar] [CrossRef] [PubMed]
- Famiani, F.; Proietti, P.; Pilli, M.; Battistelli, A.; Moscatello, S. Effects of application of thidiazuron (TDZ), gibberellic acid (GA 3), and 2,4-dichlorophenoxyacetic acid (2,4-D) on fruit size and quality of Actinidia deliciosa ‘Hayward’. N. Z. J. Crop Hortic. Sci. 2007, 35, 341–347. [Google Scholar] [CrossRef]
- Stern, R.; Shargal, A.; Flaishman, M. Thidiazuron increases fruit size of ‘Spadona’ and ‘Coscia’ pear (Pyrus communis L.). J. Hortic. Sci. Biotechnol. 2003, 78, 51–55. [Google Scholar] [CrossRef]
- Reynolds, A.; Wardle, D.; Zurowski, C.; Looney, N. Phenylureas CPPU and Thidiazuron Affect YieldComponents, Fruit Composition, and Storage Potential of Four Seedless Grape Selections. J. Am. Soc. Hortic. Sci. 1992, 1, 85–89. [Google Scholar] [CrossRef]
- Pasa, M.S.; Silva, C.P.D.; Carra, B.; Brighenti, A.F.; Souza, A.L.K.D.; Petri, J.L. Thidiazuron (TDZ) increases fruit set and yield of ‘Hosui’ and ‘Packham’s Triumph’ pear trees. An. Acad. Bras. Ciênc. 2017, 89, 3103–3110. [Google Scholar] [CrossRef]
- Yang, Y.-Z.; Lin, D.-C.; Guo, Z.-Y. Promotion of fruit development in cucumber (Cucumis sativus) by thidiazuron. Sci. Hortic. 1992, 50, 47–51. [Google Scholar] [CrossRef]
- Shen, X.; Kane, M.E.; Chen, J. Effects of genotype, explant source, and plant growth regulators on indirect shoot organogenesis in Dieffenbachia cultivars. In Vitro Cell Dev Biol Plant 2008, 44, 282–288. [Google Scholar] [CrossRef]
- Ali, H.M.; Khan, T.; Khan, M.A.; Ullah, N. The multipotent thidiazuron: A mechanistic overview of its roles in callogenesis and other plant cultures in vitro. Biotechnol. Appl. Biochem. 2022, 69, 2624–2640. [Google Scholar] [CrossRef] [PubMed]
- Tymoszuk, A.; Kulus, D. In Vitro Technology and Micropropagated Plants; MDPI: Basel, Switzerland, 2024. [Google Scholar] [CrossRef]
- Dewir, Y.H.; Murthy, H.N.; Ammar, M.H.; Alghamdi, S.S.; Al-Suhaibani, N.A.; Alsadon, A.A.; Paek, K.Y. In vitro rooting of leguminous plants: Difficulties, alternatives, and strategies for improvement. Hortic. Environ. Biotechnol. 2016, 57, 311–322. [Google Scholar] [CrossRef]
- Dewir, Y.H.; Nurmansyah Naidoo, Y.; Teixeira da Silva, J.A. Thidiazuron-induced abnormalities in plant tissue cultures. Plant Cell Rep. 2018, 37, 1451–1470. [Google Scholar] [CrossRef] [PubMed]
- Pop, R.; Pamfil, D.; Hârța, M.; Clapa, D.; Cordea, M.I. Assessment of somaclonal variation in micropropagated grapevine cultivars using molecular markers. Sci. Pap. Ser. B Hortic. 2023, LXVII, 1. [Google Scholar]
- Bidabadi, S.S.; Meon, S.; Wahab, Z.; Mahmood, M. Study of genetic and phenotypic variability among somaclones induced by BAP and TDZ in micropropagated shoot tips of banana (Musa spp.) using RAPD markers. J. Agric. Sci. 2010, 2, 49–60. [Google Scholar]
- Soares, J.M.; Rocha, A.D.; Nascimento, F.D.; Amorim, V.B.; Ramos, A.P.; Ferreira, C.F.; Haddad, F.; Amorim, E.P. Gene Expression, Histology and Histochemistry in the Interaction between Musa sp. and Pseudocercospora fijiensis. Plants 2022, 11, 1953. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Herrada, J.; Rico-Reséndiz, F.; Campos-Guillén, J.; Guevara-González, R.G.; Torres-Pacheco, I.; Cruz-Hernández, A. Establishment of in vitro regeneration system for Acaciella angustissima (Timbe) a shrubby plant endemic of México for the production of phenolic compounds. Ind. Crops Prod. 2016, 86, 49–57. [Google Scholar] [CrossRef]
- Dias, M.I.; Sousa, M.J.; Alves, R.C.; Ferreira, I.C. Exploring plant tissue culture to improve the production of phenolic compounds: A review. Ind. Crops Prod. 2016, 82, 9–22. [Google Scholar] [CrossRef]
- Wu, S.; Shan, L.; He, P. Microbial signature-triggered plant defense responses and early signaling mechanisms. Plant Sci. 2014, 228, 118–126. [Google Scholar] [CrossRef]
- Wang, B.; Andargie, M.; Fang, R. The function and biosynthesis of callose in high plants. Heliyon 2022, 8, e09248. [Google Scholar] [CrossRef] [PubMed]
- Ellinger, D.; Voigt, C.A. Callose biosynthesis in Arabidopsis with a focus on pathogen response: What we have learned within the last decade. Ann. Bot. 2014, 114, 1349–1358. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Lin, Z.; Yu, P.; Zeng, Y.; Du, S.; Huang, L.J. The multifarious role of callose and callose synthase in plant development and environment interactions. Front. Plant Sci. 2023, 14, 1183402. [Google Scholar] [CrossRef] [PubMed]
- De Quadros, F.M.; de Freitas, M.B.; Simioni, C.; Ferreira, C.; Stadnik, M.J. Redox status regulation and action of extra-and intravascular defense mechanisms are associated with bean resistance against Fusarium oxysporum f. sp. phaseoli. Protoplasma 2020, 257, 1457–1472. [Google Scholar] [CrossRef] [PubMed]
- Kikot, G.E.; Hours, R.A.; Alconada, T.M. Contribution of cell wall degrading enzymes to pathogenesis of Fusarium graminearum: A review. J. Basic Microbiol. 2009, 49, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Najjarzadeh, N.; Matsakas, L.; Rova, U.; Christakopoulos, P. How carbon source and degree of oligosaccharide polymerization affect production of cellulase-degrading enzymes by Fusarium oxysporum f. sp. lycopersici. Front. Microbiol. 2021, 12, 652655. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.F.; Zhu, Y.-J.; Li, Y.-D.; Liu, B. Studies on vascular infection of Fusarium oxysporum f. sp. cubense race 4 in banana by field survey and green fluorescent protein reporter. Int. J. Phytopathol. 2013, 2, 44–51. [Google Scholar] [CrossRef]
- Warman, N.M.; Aitken, E.A. The movement of Fusarium oxysporum f. sp. cubense (sub-tropical race 4) in susceptible cultivars of banana. Front. Plant Sci. 2018, 9, 420641. [Google Scholar] [CrossRef]
- Sheeja, T.E.; Kumar, I.P.V.; Giridhari, A.; Minoo, D.; Rajesh, M.K.; Babu, K.N. Amplified Fragment Length Polymorphism: Applications and recent developments. Mol. Plant Taxon. 2021, 2222, 187–218. [Google Scholar]
- Muhammad, A.J.; Othman, F.Y. Characterization of Fusarium wilt-resistant and Fusarium wilt-susceptible somaclones of banana cultivar Rastali (Musa AAB) by random amplified polymorphic DNA and retrotransposon markers. Plant Mol. Biol. Report. 2005, 23, 241–249. [Google Scholar] [CrossRef]
- Skarzyńska, A.; Pawełkowicz, M.; Pląder, W. Genome-wide discovery of DNA variants in cucumber somaclonal lines. Gene 2020, 736, 144412. [Google Scholar] [CrossRef] [PubMed]
| Initiator Identification | Nucleotide Sequence (5–3) | Annealing Temperature (°C) |
|---|---|---|
| REMAP * | ||
| LTR reverse 7286 |
GGAA11CATAGCATGGATAA TAAACGATTATC | 54 °C; 58 °C |
| 8081 | (GA)9C | 54 °C |
| 8082 | (CT)9G | 54 °C |
| 8385 | (CAC)7G | 58 °C |
| 8386 | (GTG)7C | 58 °C |
| 8387 | (CA)10G | 54 °C |
| 8564 | (CAC)7T | 58 °C |
| 8565 | GT(CAC)7 | 58 °C |
| IRAP ** | ||
| LTR6149 + TR6150 | CTCGCTCGCCCACTACATCAACCGCGTTTATT CTGGTTCGCCCCATCTCTATCTATCCACACATGTA | 42 °C |
| LTR6150 + 5′LTR2 | CTGGTTCGCCCCATCTCTATCTATCCACACATGTA ATCATTGCCTCTAGGGCATAATTC | 42 °C |
| 3′LTR + LTR6150 | TGTTTCCCATGCGACGTTCCCCAACA CTGGTTCGCCCCATCTCTATCTATCCACACATGTA | 42 °C |
| 5′LTR2 + Nikita | ATCATTGCCTCTAGGGCATAATTC CGCATTTGTTCAAGCCTAAACC | 42 °C |
| 3′LTR + Nikita | TGTTTCCCATGCGACGTTCCCCAACA CGCATTTGTTCAAGCCTAAACC | 46 °C |
| Nikita + LTR6149 | CGCATTTGTTCAAGCCTAAACC CTCGCTCGCCCACTACATCAACCGCGTTTATT | 46 °C |
| 5′LTR2 + LTR6150 | ATCATTGCCTCTAGGGCATAATTC CTGGTTCGCCCCATCTCTATCTATCCACACATGTA | 46 °C |
| Sukula + LTR6150 | GATAGGGTCGCATCTTGGGCGTGAC CTGGTTCGCCCCATCTCTATCTATCCACACATGTA | 46 °C |
| ISSR *** | ||
| ISSR-7 | (AG)9 | 48 °C |
| ISSR-23 | (AG)8AT | 45 °C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, M.d.S.; Rebouças, T.A.; Rocha, A.d.J.; Oliveira, W.D.d.S.; Santos, A.C.L.S.d.; Jesus, J.P.F.L.d.; Ramos, A.P.d.S.; Ferreira, C.F.; Santos-Serejo, J.A.d.; Haddad, F.; et al. Selection and Characterization of Somaclonal Variants of Prata Banana (AAB) Resistant to Fusarium Wilt. Agronomy 2024, 14, 1740. https://doi.org/10.3390/agronomy14081740
Ferreira MdS, Rebouças TA, Rocha AdJ, Oliveira WDdS, Santos ACLSd, Jesus JPFLd, Ramos APdS, Ferreira CF, Santos-Serejo JAd, Haddad F, et al. Selection and Characterization of Somaclonal Variants of Prata Banana (AAB) Resistant to Fusarium Wilt. Agronomy. 2024; 14(8):1740. https://doi.org/10.3390/agronomy14081740
Chicago/Turabian StyleFerreira, Mileide dos Santos, Tamyres Amorim Rebouças, Anelita de Jesus Rocha, Wanderley Diaciso dos Santos Oliveira, Ana Carolina Lima Santos dos Santos, João Pedro Falcón Lago de Jesus, Andresa Priscila de Souza Ramos, Claudia Fortes Ferreira, Janay Almeida dos Santos-Serejo, Fernando Haddad, and et al. 2024. "Selection and Characterization of Somaclonal Variants of Prata Banana (AAB) Resistant to Fusarium Wilt" Agronomy 14, no. 8: 1740. https://doi.org/10.3390/agronomy14081740






