Effects of Biogas Digestate on Winter Wheat Yield, Nitrogen Balance, and Nitrous Oxide Emissions under Organic Farming Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Site and Weather Conditions
2.2. Experimental Set Up
2.3. Fertilization with Biogas Digestate
2.4. Biomass and Soil Samples
2.5. N2O Measurement
2.6. Calculation of Cumulative N2O Emissions
2.7. Statistical Analysis
3. Results
3.1. Grain Yield and Protein Content
3.2. N Uptake, N Surplus, and N Use Efficiency
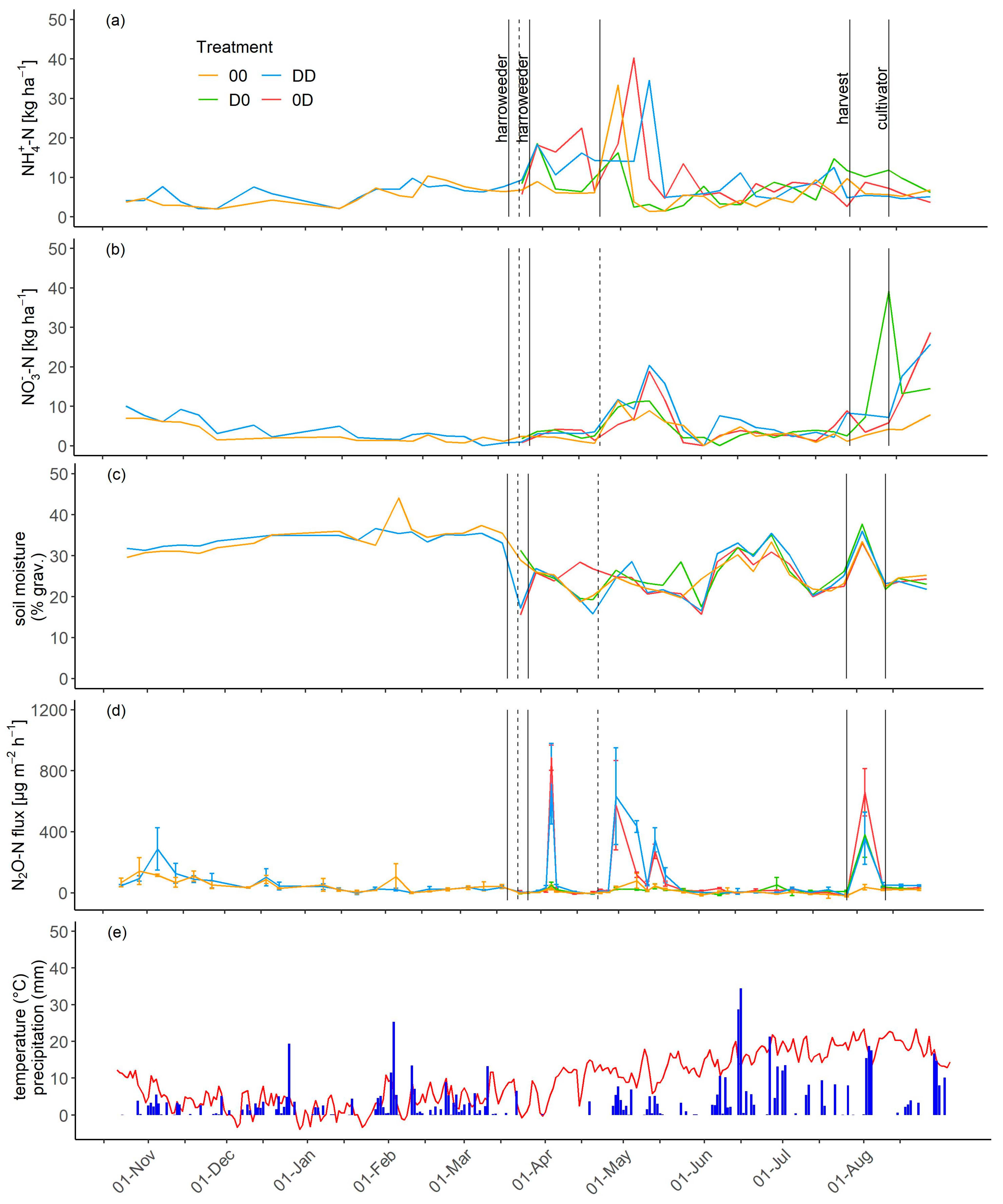
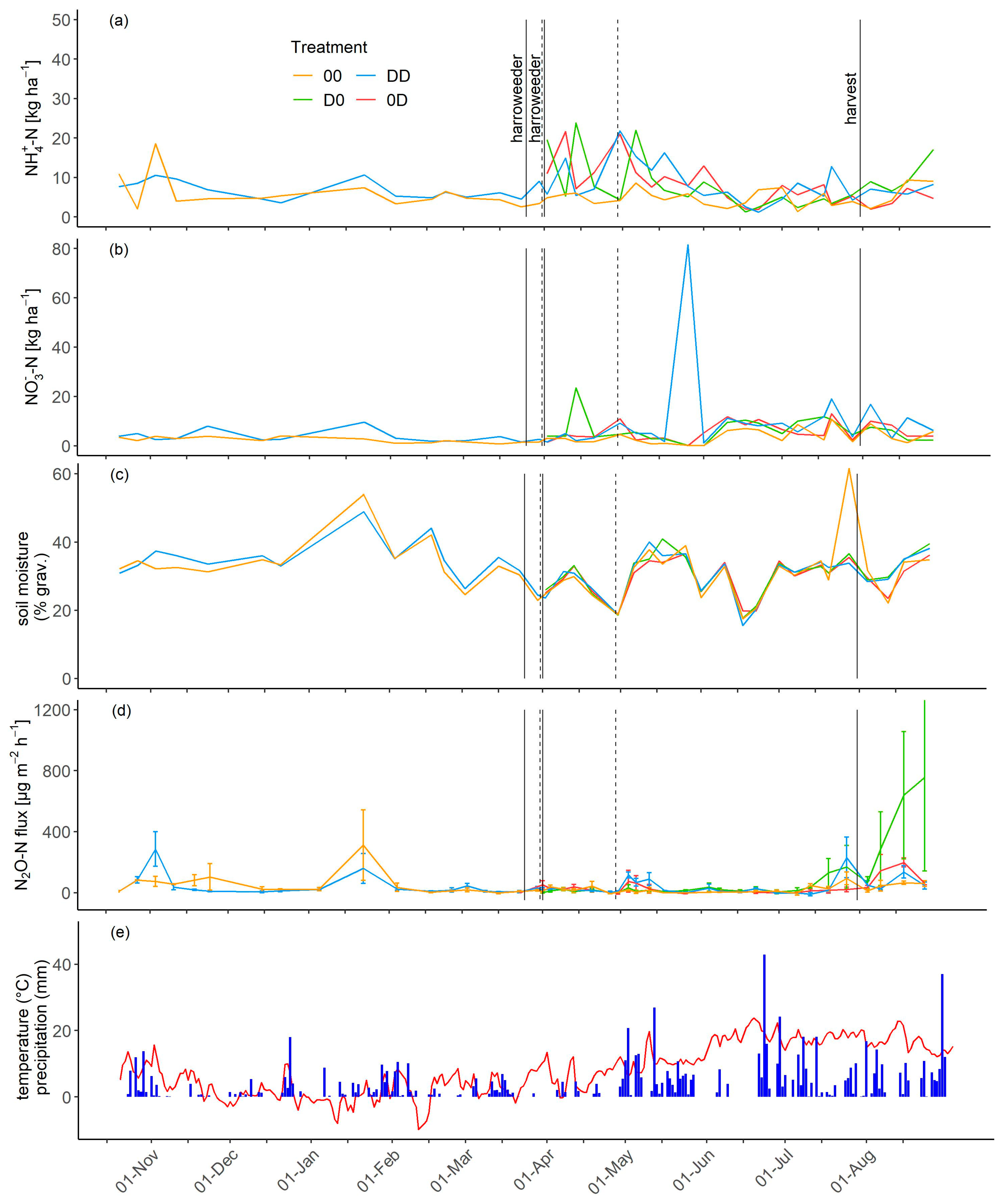
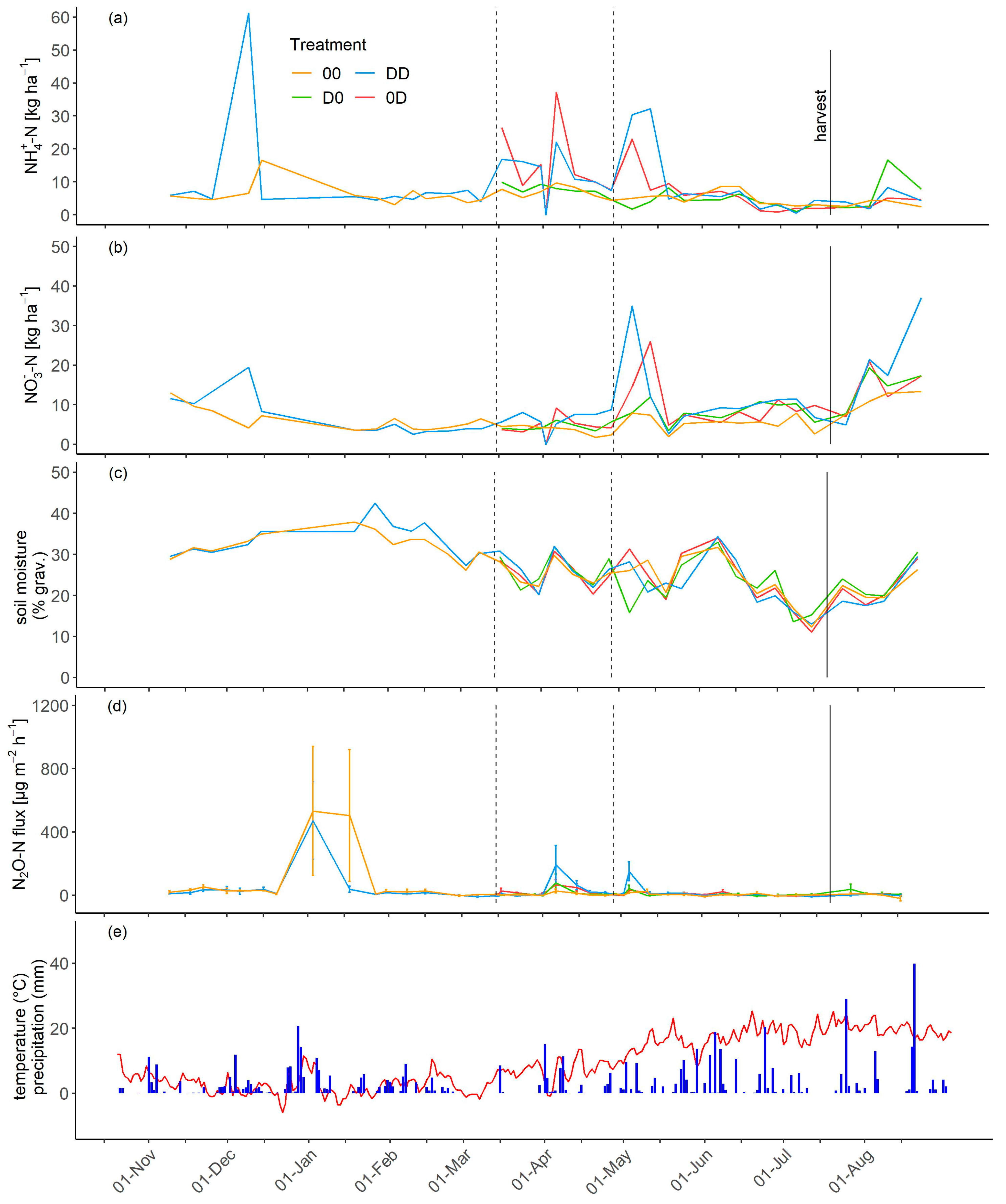
3.3. NH4+, NO3−, and Soil Moisture Dynamics
3.4. N2O Fluxes
3.5. Cumulative N2O-N Emissions and Emission Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Date | 26 March 2019 | 26 April 2019 | 23 March 2020 | 23 April 2020 | 31 March 2021 | 29 April 2021 | 14 March 2022 | 28 April 2022 |
|---|---|---|---|---|---|---|---|---|
| Treatment * | BBCH 20/21 | BBCH 30/31 | BBCH 20/21 | BBCH 30/31 | BBCH 20/21 | BBCH 30/31 | BBCH 20/21 | BBCH 30/31 |
| 00 | - | - | - | - | - | - | - | - |
| D0 | - | - | - | - | - | - | - | - |
| 0D [kg ha−1] | 116.7 | 116.7 | 132.3 | 132.3 | 114.4 | 114.4 | 111.6 | 111.6 |
| DD [kg ha−1] | 116.7 | 116.7 | 132.3 | 132.3 | 114.4 | 114.4 | 111.6 | 111.6 |
| Autumn and Winter | Spring and Summer | Fertilization until Harvest | Post-Harvest | Whole Year | |
|---|---|---|---|---|---|
| 2019 | 26 March 2019–03 September 2019 (161 days) | 26 March 2019–24 July 2019 (120 days) | 24 July 2019–03 September 2019 (41 days) | ||
| 2020 | 14 October 2019–24 March 2020 (162 days) | 24 March 2020–25 August 2020 (154 days) | 24 March 2020–28 July 2020 (126 days) | 28 July 2020– 25 August 2020 (28 days) | 14 October 2019–28 July 2020 (288 days) |
| 2021 | 20 October 2020–30 March 2021 (161 days) | 01 April 2021–26 August 2021 (147 days) | 01 April 2021–27 July 2021 (117 days) | 27 July 2021–26 August 2021 (30 days) | 20 October 2020–27 July 2021 (280 days) |
| 2022 | 09 November 2021–14 March 2022 (125 days) | 14 March 2022–16 August 2022 (155 days) | 14 March 2022–20 July 2022 (128 days) | 21 July 2022–16 August 2022 (26 days) | 09 November 2021–20 July 2022 (253 day) |
References
- Meyer, A.K.P.; Ehimen, E.A.; Holm-Nielsen, J.B. Future European biogas: Animal manure, straw and grass potentials for a sustainable European biogas production. Biomass Bioenergy 2018, 111, 154–164. [Google Scholar] [CrossRef]
- Theuerl, S.; Herrmann, C.; Heiermann, M.; Grundmann, P.; Landwehr, N.; Kreidenweis, U.; Prochnow, A. The Future Agricultural Biogas Plant in Germany: A Vision. Energies 2019, 12, 396. [Google Scholar] [CrossRef]
- Paolini, V.; Petracchini, F.; Segreto, M.; Tomassetti, L.; Naja, N.; Cecinato, A. Environmental impact of biogas: A short review of current knowledge. J. Environ. Sci. Health Part A 2018, 53, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Carter, M.S.; Hauggaard-Nielsen, H.; Heiske, S.; Jensen, M.; Thomsen, S.T.; Schmidt, J.E.; Johansen, A.; Ambus, P. Consequences of field N2O emissions for the environmental sustainability of plant-based biofuels produced within an organic farming system. GCB Bioenergy 2012, 4, 435–452. [Google Scholar] [CrossRef]
- Tilche, A.; Galatola, M. The potential of bio-methane as bio-fuel/bio-energy for reducing greenhouse gas emissions: A qualitative assessment for Europe in a life cycle perspective. Water Sci. Technol. 2008, 57, 1683–1692. [Google Scholar] [CrossRef] [PubMed]
- Cayuela, M.L.; Kuikman, P.J.; Oenema, O.; Bakker, R.R.; Groeningen, J.W. Bioenergy residues as soil amendments: Climate-relevant C and N dynamics during decomposition. In Proceedings of the 2010 19th World Congress of Soil Science, Soil Solutions for a Changing World, Brisbane, Australia, 1–6 August 2010. [Google Scholar]
- Dittert, K.; Senbayram, M.; Wienforth, B.; Kage, H.; Muehling, K.H. Greenhouse gas emissions in biogas production systems. In Proceedings of the International Plant Nutrition Colloquium XVI, Sacramento, CA, USA, 26–30 August 2009. [Google Scholar]
- Cayuela, M.L.; Oenema, O.; Kuikman, P.J.; Bakker, R.R.; van Groenigen, J.W. Bioenergy by-products as soil amendments? Implications for carbon sequestration and greenhouse gas emissions. GCB Bioenergy 2010, 84, 201–213. [Google Scholar] [CrossRef]
- Panuccio, M.R.; Papalia, T.; Attinà, E.; Giuffrè, A.; Muscolo, A. Use of digestate as an alternative to mineral fertilizer: Effects on growth and crop quality. Arch. Agron. Soil Sci. 2019, 65, 700–711. [Google Scholar] [CrossRef]
- Baral, K.R.; Labouriau, R.; Olesen, J.E.; Petersen, S.O. Nitrous oxide emissions and nitrogen use efficiency of manure and digestates applied to spring barley. Agric. Ecosyst. Environ. 2017, 239, 188–198. [Google Scholar] [CrossRef]
- NOAA. Trends in Atmospheric Nitrous Oxide (N2O): Global Monitoring Laboratory. Available online: https://gml.noaa.gov/ccgg/trends_n2o/ (accessed on 29 July 2024).
- Tian, H.; Xu, R.; Canadell, J.G.; Thompson, R.L.; Winiwarter, W.; Suntharalingam, P.; Davidson, E.A.; Ciais, P.; Jackson, R.B.; Janssens-Maenhout, G.; et al. A comprehensive quantification of global nitrous oxide sources and sinks. Nature 2020, 586, 248–256. [Google Scholar] [CrossRef]
- Butterbach-Bahl, K.; Baggs, E.M.; Dannenmann, M.; Kiese, R.; Zechmeister-Boltenstern, S. Nitrous oxide emissions from soils: How well do we understand the processes and their controls? Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2013, 368, 20130122. [Google Scholar] [CrossRef]
- Hassan, M.U.; Aamer, M.; Mahmood, A.; Awan, M.I.; Barbanti, L.; Seleiman, M.F.; Bakhsh, G.; Alkharabsheh, H.M.; Babur, E.; Shao, J.; et al. Management Strategies to Mitigate N2O Emissions in Agriculture. Life 2022, 12, 439. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Amon, B.; Schulz, K.; Mehdi, B. Factors That Influence Nitrous Oxide Emissions from Agricultural Soils as Well as Their Representation in Simulation Mod Different letters indicate significant differences (Tukey test, p < 0.05).els: A Review. Agronomy 2021, 11, 770. [Google Scholar] [CrossRef]
- Dietrich, M.; Fongen, M.; Foereid, B. Greenhouse gas emissions from digestate in soil. Int. J. Recycl. Org. Waste Agric. 2020, 9, 1–19. [Google Scholar] [CrossRef]
- Eickenscheidt, T.; Freibauer, A.; Heinichen, J.; Augustin, J.; Drösler, M. Short-term effects of biogas digestate and cattle slurry application on greenhouse gas emissions affected by N availability from grasslands on drained fen peatlands and associated organic soils. Biogeosciences 2014, 11, 6187–6207. [Google Scholar] [CrossRef]
- Häfner, F.; Ruser, R.; Claß-Mahler, I.; Möller, K. Field Application of Organic Fertilizers Triggers N2O Emissions from the Soil N Pool as Indicated by 15N-Labeled Digestates. Front. Sustain. Food Syst. 2021, 4, 614349. [Google Scholar] [CrossRef]
- Fiedler, S.R.; Augustin, J.; Wrage-Mönnig, N.; Jurasinski, G.; Gusovius, B.; Glatzel, S. Potential short-term losses of N2O and N2 from high concentrations of biogas digestate in arable soils. SOIL 2017, 3, 161–176. [Google Scholar] [CrossRef]
- Heintze, G. N2O and CH4-emissions from energy crops—Can the use of organic fertilizers in form of biogas digestate be considered as a real alternative? Results from a three and a half year multi-site field study of energy crops fertilized with biogas digestate in so. In Proceedings of the EGU General Assembly 2016, Vienna, Austria, 17–22 April 2016. EPSC2016-16852. [Google Scholar]
- Köster, J.R.; Cárdenas, L.M.; Bol, R.; Lewicka-Szczebak, D.; Senbayram, M.; Well, R.; Giesemann, A.; Dittert, K. Anaerobic digestates lower N2O emissions compared to cattle slurry by affecting rate and product stoichiometry of denitrification—An N2O isotopomer case study. Soil Biol. Biochem. 2015, 84, 65–74. [Google Scholar] [CrossRef]
- Serdjuk, M.; Bodmer, U.; Hülsbergen, K.-J. Integration of biogas production into organic arable farming systems: Crop yield response and economic effects. Org. Agric. 2018, 8, 301–314. [Google Scholar] [CrossRef]
- Gissén, C.; Prade, T.; Kreuger, E.; Nges, I.A.; Rosenqvist, H.; Svensson, S.-E.; Lantz, M.; Mattsson, J.E.; Börjesson, P.; Björnsson, L. Comparing energy crops for biogas production—Yields, energy input and costs in cultivation using digestate and mineral fertilisation. Biomass Bioenergy 2014, 64, 199–210. [Google Scholar] [CrossRef]
- Stinner, W.; Möller, K.; Leithold, G.; Heß, J.; Rahmann, G. Biogaserzeugung im viehlosen Betrieb: Effekte auf Stickstoffmanagement, Erträge und Qualität. In Ende der Nische, Beiträge zur 8. Wissenschaftstagung Ökologischer LandbauI; Kassel University Press GmbH: Kassel, Germany, 2005. [Google Scholar]
- Stinner, W.; Möller, K.; Leithold, G. Effects of biogas digestion of clover/grass-leys, cover crops and crop residues on nitrogen cycle and crop yield in organic stockless farming systems. Eur. J. Agron. 2008, 29, 125–134. [Google Scholar] [CrossRef]
- Siegmeier, T.; Blumenstein, B.; Möller, D. Farm biogas production in organic agriculture: System implications. Agric. Syst. 2015, 139, 196–209. [Google Scholar] [CrossRef]
- The European Parliament and the Council of the European Council. Regulation (EU) 2018/848 of the European Parliament and of the Council of 30 May 2018 on Organic. Available online: https://eur-lex.europa.eu/eli/reg/2018/848/oj (accessed on 2 August 2024).
- Nkoa, R. Agricultural benefits and environmental risks of soil fertilization with anaerobic digestates: A review. Agron. Sustain. Dev. 2014, 34, 473–492. [Google Scholar] [CrossRef]
- Pampillón-González, L.; Luna-Guido, M.; Ruíz-Valdiviezo, V.M.; Franco-Hernández, O.; Fernández-Luqueño, F.; Pareds-López, O.; Hernández, G.; Dendooven, L. Greenhouse Gas Emissions and Growth of Wheat Cultivated in Soil Amended with Digestate from Biogas Production. Pedosphere 2017, 27, 318–327. [Google Scholar] [CrossRef]
- Barłóg, P.; Hlisnikovský, L.; Kunzová, E. Yield, content and nutrient uptake by winter wheat and spring barley in response to applications of digestate, cattle slurry and NPK mineral fertilizers. Arch. Agron. Soil Sci. 2019, 66, 1481–1496. [Google Scholar] [CrossRef]
- Möller, K.; Müller, T. Effects of anaerobic digestion on digestate nutrient availability and crop growth: A review. Eng. Life Sci. 2012, 12, 242–257. [Google Scholar] [CrossRef]
- Möller, K.; Stinner, W.; Deuker, A.; Leithold, G. Effects of different manuring systems with and without biogas digestion on nitrogen cycle and crop yield in mixed organic dairy farming systems. Nutr. Cycl. Agroecosyst. 2008, 82, 209–232. [Google Scholar] [CrossRef]
- Alburquerque, J.A.; de La Fuente, C.; Bernal, M.P. Chemical properties of anaerobic digestates affecting C and N dynamics in amended soils. Agric. Ecosyst. Environ. 2012, 160, 15–22. [Google Scholar] [CrossRef]
- Shi, L.; Simplicio, W.S.; Wu, G.; Hu, Z.; Hu, H.; Zhan, X. Nutrient Recovery from Digestate of Anaerobic Digestion of Livestock Manure: A Review. Curr. Pollut. Rep. 2018, 4, 74–83. [Google Scholar] [CrossRef]
- Buchen-Tschiskale, C.; Hagemann, U.; Augustin, J. Soil incubation study showed biogas digestate to cause higher and more variable short-term N2O and N2 fluxes than mineral-N. J. Plant Nutr. Soil Sci. 2020, 183, 208–219. [Google Scholar] [CrossRef]
- van Midden, C.; Harris, J.; Shaw, L.; Sizmur, T.; Pawlett, M. The impact of anaerobic digestate on soil life: A review. Appl. Soil Ecol. 2023, 191, 105066. [Google Scholar] [CrossRef]
- Möller, K. Effects of anaerobic digestion on soil carbon and nitrogen turnover, N emissions, and soil biological activity. A review. Agron. Sustain. Dev. 2015, 35, 1021–1041. [Google Scholar] [CrossRef]
- Simon, A. Langzeitwirkungen von Gärresten in Energiepflanzenfruchtfolgen auf Bodeneigenschaften und Bodenprozesse unter den Bedingungen des Ökologischen Landbaus. Ph.D. Thesis, Verlag Dr. Köster, Berlin, Germany, 2021. [Google Scholar]
- Levin, K.S.; Auerswald, K.; Reents, H.J.; Hülsbergen, K.-J. Effects of Organic Energy Crop Rotations and Fertilisation with the Liquid Digestate Phase on Organic Carbon in the Topsoil. Agronomy 2021, 11, 1393. [Google Scholar] [CrossRef]
- DüV. Düngeverordnung: Verordnung über die Anwendung von Düngemitteln, Bodenhilfsstoffen, Kultursubstraten und Pflanzenhilfsmitteln nach den Grundsätzen der Guten Fachlichen Praxis beim Düngen; DüV: Berlin, Germany, 2017. [Google Scholar]
- AHDB Cereals & Oilseeds. The Growth Stages of Cereals. Available online: https://ahdb.org.uk/knowledge-library/the-growth-stages-of-cereals (accessed on 13 April 2024).
- Vdlufa. Die Chemische Untersuchung von Futtermitteln: Eine Dokumentation; VDLUFA-Verl.: Darmstadt, Germany, 2013; ISBN 9783941273146. [Google Scholar]
- Kays, S.E.; Barton, F.E.; Windham, W.R. Predicting Protein Content by near Infrared Reflectance Spectroscopy in Diverse Cereal Food Products. J. Near Infrared Spectrosc. 2000, 8, 35–43. [Google Scholar] [CrossRef]
- Schmidt, C.; Timmermann, F. Bestimmung löslicher N-Fraktionen des Bodens in Abhängigkeit von Probenvorbereitung und Extraktionsverfahren. VDLUFA-Kongr.b. 1988, 28, 517–526. [Google Scholar]
- Prücklmaier, J.X. Feldexperimentelle Analysen zur Ertragsbildung und Stickstoffeffizienz bei Organisch-Mineralischer Düngung auf Heterogenen Standorten und Möglichkeiten zur Effizienzsteigerung durch Computer- und Sensorgestützte Düngesysteme. Ph.D. Thesis, Technische Universität München, Munich, Germany, 2020. [Google Scholar]
- Hutchinson, G.L.; Mosier, A.R. Improved Soil Cover Method for Field Measurement of Nitrous Oxide Fluxes. Soil Sci. Soc. Am. J. 1981, 45, 311–316. [Google Scholar] [CrossRef]
- De Klein, C.; Harvey, M. Nitrous Oxide Chamber Methodology Guidelines; Version 1.1 Global Research Alliance on Agricultural Greenhous Gas Emissions; Ministry for Primary Industries: Wellington, New Zealand, 2015. [Google Scholar]
- Cosentino, V.; Fernandez, P.; Aureggi, S.; Taboada, M. N2O emissions from a cultivated mollisol: Optimal time of day for sampling and the role of soil temperature. Rev. Bras. Ciência Solo 2012, 36, 1814–1819. [Google Scholar] [CrossRef]
- Reeves, S.; Wang, W. Optimum sampling time and frequency for measuring N2O emissions from a rain-fed cereal cropping system. Sci. Total Environ. 2015, 530–531, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Fuß, R. Greenhouse Gas Flux Calculation from Chamber Measurements, Package for R, Version 0.4-2; 2019. Available online: https://cran.r-project.org/web/packages/gasfluxes/gasfluxes.pdf (accessed on 15 June 2024).
- van Groenigen, J.W.; Velthof, G.L.; Oenema, O.; van Groenigen, K.J.; van Kessel, C. Towards an agronomic assessment of N2O emissions: A case study for arable crops. Eur. J. Soil Sci. 2010, 61, 903–913. [Google Scholar] [CrossRef]
- Dobbie, K.E.; McTaggart, I.P.; Smith, K.A. Nitrous oxide emissions from intensive agricultural systems: Variations between crops and seasons, key driving variables, and mean emission factors. J. Geophys. Res. Atmos. 1999, 104, 26891–26899. [Google Scholar] [CrossRef]
- Shen, J.; Treu, R.; Wang, J.; Nicholson, F.; Bhogal, A.; Thorman, R. Modeling nitrous oxide emissions from digestate and slurry applied to three agricultural soils in the United Kingdom: Fluxes and emission factors. Environ. Pollut. 2018, 243, 1952–1965. [Google Scholar] [CrossRef]
- Thomas, B.W.; Hao, X. Nitrous Oxide Emitted from Soil Receiving Anaerobically Digested Solid Cattle Manure. J. Environ. Qual. 2017, 46, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Stehfest, E.; Bouwman, L. N2O and NO emission from agricultural fields and soils under natural vegetation: Summarizing available measurement data and modeling of global annual emissions. Nutr. Cycl. Agroecosystems 2006, 74, 207–228. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Soft. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Mayerová, M.; Šimon, T.; Stehlík, M.; Madaras, M.; Koubová, M.; Smatanová, M. Long-term application of biogas digestate improves soil physical properties. Soil Tillage Res. 2023, 231, 105715. [Google Scholar] [CrossRef]
- Skinner, C.; Gattinger, A.; Krauss, M.; Krause, H.M.; Mayer, J.; Van Der Heijden, M.G.; Mäder, P. The impact of long-term organic farming on soil-derived greenhouse gas emissions. Sci. Rep. 2019, 9, 1702. [Google Scholar] [CrossRef]
- Zhou, M.; Zhu, B.; Wang, X.; Wang, Y. Long-term field measurements of annual methane and nitrous oxide emissions from a Chinese subtropical wheat-rice rotation system. Soil Biol. Biochem. 2017, 115, 21–34. [Google Scholar] [CrossRef]
- Odlare, M.; Arthurson, V.; Pell, M.; Svensson, K.; Nehrenheim, E.; Abubaker, J. Land application of organic waste—Effects on the soil ecosystem. Appl. Energy 2011, 88, 2210–2218. [Google Scholar] [CrossRef]
- Niggli, U.; Fließbach, A.; Hepperly, P.; Scialabba, N. Low Greenhouse Gas Agriculture: Mitigation and Adaptation Potential of Sustainable Farming Systems; FAO: Rome, Italy, 2009; Volume 141. [Google Scholar]
- Braun, M.; Schmid, H.; Grundler, T.; Hülsbergen, K.-J. Root-and-shoot growth and yield of different grass–clover mixtures. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2010, 144, 414–419. [Google Scholar] [CrossRef]
- Pappa, V.A.; Rees, R.M.; Watson, C.A.; Atkinson, C.; Ball, B.; Davies, D.H.K.; Rees, R.; Russell, G.; Stockdale, E.A.; Walker, R.; et al. Nitrogen transfer between clover and wheat in an intercropping experiment. In Aspects of Applied Biology 79, What Will Organic Farming Deliver? COR 2006; Association of Applied Biologists: Wellesbourne, UK, 2006. [Google Scholar]
- Oberson, A.; Frossard, E.; Bühlmann, C.; Mayer, J.; Mäder, P.; Lüscher, A. Nitrogen fixation and transfer in grass-clover leys under organic and conventional cropping systems. Plant Soil 2013, 371, 237–255. [Google Scholar] [CrossRef]
- Bleken, M.A.; Rittl, T.F.; Nadeem, S.; Hansen, S. Roots and other residues from leys with or without red clover: Quality and effects on N2O emission factors in a partly frozen soil following autumn ploughing. Sci. Total Environ. 2022, 831, 154582. [Google Scholar] [CrossRef] [PubMed]
- Niether, W.; Macholdt, J.; Schulz, F.; Gattinger, A. Yield dynamics of crop rotations respond to farming type and tillage intensity in an organic agricultural long-term experiment over 24 years. Field Crops Res. 2023, 303, 109131. [Google Scholar] [CrossRef]
- Brozyna, M.A.; Petersen, S.O.; Chirinda, N.; Olesen, J.E. Effects of grass-clover management and cover crops on nitrogen cycling and nitrous oxide emissions in a stockless organic crop rotation. Agric. Ecosyst. Environ. 2013, 181, 115–126. [Google Scholar] [CrossRef]
- Jungkunst, H.F.; Freibauer, A.; Neufeldt, H.; Bareth, G. Nitrous oxide emissions from agricultural land use in Germany—A synthesis of available annual field data. J. Plant Nutr. Soil Sci. 2006, 169, 341–351. [Google Scholar] [CrossRef]
- Lammirato, C.; Lebender, U.; Tierling, J.; Lammel, J. Analysis of uncertainty for N2O fluxes measured with the closed-chamber method under field conditions: Calculation method, detection limit, and spatial variability. J. Plant Nutr. Soil Sci. 2018, 181, 78–89. [Google Scholar] [CrossRef]
- Morris, S.G.; Kimber, S.W.L.; Grace, P.; van Zwieten, L. Improving the statistical preparation for measuring soil N2O flux by closed chamber. Sci. Total Environ. 2013, 465, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Signor, D.; Cerri, C.E.P. Nitrous oxide emissions in agricultural soils: A review. Pesqui. Agropecu. Trop. 2013, 43, 322–338. [Google Scholar] [CrossRef]
- Brown, S.E.; Wagner-Riddle, C. Assessment of random errors in multi-plot nitrous oxide flux gradient measurements. Agric. For. Meteorol. 2017, 242, 10–20. [Google Scholar] [CrossRef]
- Rees, R.M.; Augustin, J.; Alberti, G.; Ball, B.C.; Boeckx, P.; Cantarel, A.; Castaldi, S.; Chirinda, N.; Chojnicki, B.; Giebels, M.; et al. Nitrous oxide emissions from European agriculture; an analysis of variability and drivers of emissions from field experiments. Biogeosciences 2013, 10, 2671–2682. [Google Scholar] [CrossRef]
- Simon, R.O.; Hülsbergen, K.-J. Energy Balance and Energy Use Efficiency of Annual Bioenergy Crops in Field Experiments in Southern Germany. Agronomy 2021, 11, 1835. [Google Scholar] [CrossRef]
- Fouda, S. Nitrogen Availability of Biogas Residues. Ph.D. Thesis, Technische Universität München, München, Germany, 2011. [Google Scholar]
- Zilio, M.; Pigoli, A.; Rizzi, B.; Goglio, A.; Tambone, F.; Giordano, A.; Maretto, L.; Squartini, A.; Stevanato, P.; Meers, E.; et al. Nitrogen dynamics in soils fertilized with digestate and mineral fertilizers: A full field approach. Sci. Total Environ. 2023, 868, 161500. [Google Scholar] [CrossRef]
- Fouda, S.; von Tucher, S.; Lichti, F.; Schmidhalter, U. Nitrogen availability of various biogas residues applied to ryegrass. J. Plant Nutr. Soil Sci. 2013, 176, 572–584. [Google Scholar] [CrossRef]
- Li, J.; Dong, W.; Oenema, O.; Chen, T.; Hu, C.; Yuan, H.; Zhao, L. Irrigation reduces the negative effect of global warming on winter wheat yield and greenhouse gas intensity. Sci. Total Environ. 2019, 646, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Ruser, R.; Flessa, H.; Russow, R.; Schmidt, G.; Buegger, F.; Munch, J.C. Emission of N2O, N2 and CO2 from soil fertilized with nitrate: Effect of compaction, soil moisture and rewetting. Soil Biol. Biochem. 2006, 38, 263–274. [Google Scholar] [CrossRef]
- Xiong, Z.-Q.; Xing, G.-X.; Zhu, Z.-L. Nitrous Oxide and Methane Emissions as Affected by Water, Soil and Nitrogen. Pedosphere 2007, 17, 146–155. [Google Scholar] [CrossRef]
- Baggs, E.M.; Rees, R.M.; Smith, K.A.; Vinten, A.J.A. Nitrous oxide emission from soils after incorporating crop residues. Soil Use Manag. 2000, 16, 82–87. [Google Scholar] [CrossRef]
- Flessa, H.; Potthoff, M.; Loftfield, N. Greenhouse estimates of CO2 and N2O emissions following surface application of grass mulch: Importance of indigenous microflora of mulch. Soil Biol. Biochem. 2002, 34, 875–879. [Google Scholar] [CrossRef]
- Lebender, U.; Senbayram, M.; Lammel, J.; Kuhlmann, H. Impact of mineral N fertilizer application rates on N2O emissions from arable soils under winter wheat. Nutr. Cycl. Agroecosystems 2014, 100, 111–120. [Google Scholar] [CrossRef]
- Lebender, U.; Senbayram, M.; Lammel, J.; Kuhlmann, H. Effect of mineral nitrogen fertilizer forms on N2O emissions from arable soils in winter wheat production. J. Plant Nutr. Soil Sci. 2014, 177, 722–732. [Google Scholar] [CrossRef]
- Guardia, G.; Aguilera, E.; Vallejo, A.; Álvaro-Fuentes, J.; Cantero-Martínez, C.; Sanz-Cobena, A.; Barton, L.; Volpi, I.; Ibáñez, M.Á. Contribution of the postharvest period to soil N2O emissions from arable Mediterranean crops. J. Clean. Prod. 2024, 469, 143186. [Google Scholar] [CrossRef]
- Winkhart, F.; Mösl, T.; Schmid, H.; Hülsbergen, K.-J. Effects of Organic Maize Cropping Systems on Nitrogen Balances and Nitrous Oxide Emissions. Agriculture 2022, 12, 907. [Google Scholar] [CrossRef]
- Wiesmeier, M.; Poeplau, C.; Sierra, C.A.; Maier, H.; Frühauf, C.; Hübner, R.; Kühnel, A.; Spörlein, P.; Geuß, U.; Hangen, E.; et al. Projected loss of soil organic carbon in temperate agricultural soils in the 21st century: Effects of climate change and carbon input trends. Sci. Rep. 2016, 6, 32525. [Google Scholar] [CrossRef] [PubMed]
- Wiesmeier, M.; Mayer, S.; Burmeister, J.; Hübner, R.; Kögel-Knabner, I. Feasibility of the 4 per 1000 initiative in Bavaria: A reality check of agricultural soil management and carbon sequestration scenarios. Geoderma 2020, 369, 114333. [Google Scholar] [CrossRef]
- Thornton, P.E.; Lamarque, J.-F.; Rosenbloom, N.A.; Mahowald, N.M. Influence of carbon-nitrogen cycle coupling on land model response to CO2 fertilization and climate variability. Glob. Biogeochem. Cycles 2007, 21, GB4018. [Google Scholar] [CrossRef]
- Barrett, J.E.; Burke, I.C. Potential nitrogen immobilization in grassland soils across a soil organic matter gradient. Soil Biol. Biochem. 2000, 32, 1707–1716. [Google Scholar] [CrossRef]

| Parameter | Unit | 2019 | 2020 | 2021 | 2022 | ||||
|---|---|---|---|---|---|---|---|---|---|
| 00 a | DD b | 00 | DD | 00 | DD | 00 | DD | ||
| SOC | % | 0.94 | 1.10 | 0.83 | 1.02 | 1.03 | 1.11 | 0.90 | 1.20 |
| TN | % | 0.11 | 0.13 | 0.10 | 0.12 | 0.11 | 0.12 | 0.11 | 0.15 |
| C/N | 8.6 | 8.5 | 8.7 | 8.6 | 9.2 | 9.1 | 8.2 | 8.0 | |
| pH | 6.1 | 6.2 | 6.3 | 6.4 | 6.0 | 6.1 | 5.8 | 5.9 | |
| CAL-P c | mg 100 g−1 | 1.04 | 1.53 | 2.33 | 3.93 | 2.07 | 2.87 | 1.25 | 1.80 |
| CAL-K d | mg 100 g−1 | 6.03 | 10.92 | 6.09 | 12.33 | 5.53 | 9.98 | 5.72 | 8.62 |
| Cmic e | mg kg−1 | 427 | 491 | 422 | 523 | 492 | 618 | 521 | 588 |
| Bulk density | g cm−3 | 1.31 | 1.27 | 1.40 | 1.40 | 1.42 | 1.46 | 1.30 | 1.31 |
| Unit | Jan | Feb | Mar | Apr | May | Jun | Jul | Aug | Sep | Oct | Nov | Dec | Mean | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1961–1990 | ||||||||||||||
| Temperature | [°C] | −2.1 | −0.7 | 3.0 | 7.3 | 11.9 | 15.0 | 16.7 | 16.1 | 12.9 | 7.9 | 2.8 | −0.7 | 7.5 |
| Precipitation | [mm] | 43.9 | 38.9 | 43.4 | 55.5 | 89.6 | 103.7 | 98.3 | 97.1 | 64.2 | 50.5 | 54.0 | 48.4 | 787.7 |
| 1991–2020 | ||||||||||||||
| Temperature | [°C] | −0.6 | 0.4 | 4.5 | 8.8 | 13.0 | 16.3 | 18.0 | 18.0 | 13.2 | 8.7 | 3.8 | 0.5 | 8.7 |
| Precipitation | [mm] | 44.7 | 34.0 | 46.8 | 42.5 | 85.8 | 99.1 | 98.4 | 87.1 | 67.4 | 60.6 | 52.9 | 54.7 | 774.2 |
| 2019 | ||||||||||||||
| Temperature | [°C] | −0.6 | 2.2 | 6.3 | 10.1 | 10.6 | 19.6 | 19.0 | 18.7 | 13.8 | 10.2 | 4.5 | 2.2 | 9.8 |
| Precipitation | [mm] | 86.3 | 38.3 | 48.5 | 12.3 | 118 | 79.3 | 54.3 | 98.9 | 48.4 | 50.2 | 35.9 | 1.1 | 726.8 |
| 2020 | ||||||||||||||
| Temperature | [°C] | 1.3 | 4.7 | 5.0 | 10.6 | 11.8 | 16.1 | 18.5 | 18.8 | 14.3 | 9.0 | 4.2 | 1.2 | 9.6 |
| Precipitation | [mm] | 25.9 | 96.0 | 37.5 | 23.4 | 31.8 | 154.1 | 54.0 | 104.3 | 73.7 | 90.0 | 16.4 | 45.7 | 766.8 |
| 2021 | ||||||||||||||
| Temperature | [°C] | −0.7 | 2.3 | 4.1 | 6.3 | 10.5 | 18.8 | 17.9 | 16.4 | 14.5 | 8.0 | 2.8 | 2.0 | 8.6 |
| Precipitation | [mm] | 53.7 | 40.2 | 36.6 | 29.0 | 161.0 | 131.0 | 114.8 | 166.7 | 35.9 | 17.7 | 36.9 | 9.0 | 914.4 |
| 2022 | ||||||||||||||
| Temperature | [°C] | 1.2 | 3.8 | 4.6 | 7.4 | 14.8 | 18.8 | 19.6 | 19.4 | 12.7 | 11.9 | 5.2 | 1.3 | 10.1 |
| Precipitation | [mm] | 48.5 | 36.7 | 13.8 | 57.4 | 70.1 | 99.4 | 57.0 | 85.4 | 78.3 | 60.6 | 55.9 | 56.6 | 719.7 |
| Treatment * | Previous Years (2004–2018) | Years of Study (2019–2022) | Explanation |
|---|---|---|---|
| 00 | Unfertilized | Unfertilized | Control treatment |
| D0 | Digestate | Unfertilized | Analysis of the after-effects of digestate |
| 0D | Unfertilized | Digestate | Analysis of the direct effects of digestate |
| DD | Digestate | Digestate | Analysis of the long-term effects of digestate |
| Parameter | Unit | 2019 | 2020 | 2021 | 2022 |
|---|---|---|---|---|---|
| Dry matter (DM) | % | 9.55 | 9.20 | 8.30 | 8.65 |
| Tot-C | % DM | 38.85 | 38.70 | 42.03 | 40.85 |
| Tot-N | % DM | 6.11 | 7.19 | 6.89 | 6.52 |
| NH4-N | % Tot-N | 41.73 | 48.68 | 52.25 | 50.65 |
| C:N | 6.36 | 5.38 | 6.10 | 5.95 | |
| K2O-K | % DM | 8.02 | 8.82 | 9.93 | 9.47 |
| P2O5-P | % DM | 2.00 | 1.99 | 2.31 | 2.54 |
| Tot-S | % DM | 0.51 | 0.49 | 0.55 | 0.54 |
| Year | Treatment * | N Input kg ha−1 | NH4+-N Input kg ha−1 | Yield Mg ha−1 | Protein Content % DM | N Uptake kg ha−1 | N Surplus kg ha−1 | NUE % |
|---|---|---|---|---|---|---|---|---|
| 2019 | 00 | 0 | 0 | 5.3 a | 10.7 a | 105.7 a | −105.7 a | |
| D0 | 0 | 0 | 6.2 b | 10.6 a | 123.7 ab | −123.7 a | ||
| 0D | 233.4 | 97.4 | 6.9 b | 10.7 a | 138.1 b | 95.3 c | 59.2 a | |
| DD | 233.4 | 97.4 | 8.1 c | 11.4 a | 170.0 c | 63.4 b | 72.8 b | |
| 2020 | 00 | 0 | 0 | 3.8 a | 10.1 a | 73.5 a | −73.5 a | |
| D0 | 0 | 0 | 4.4 a | 10.9 a | 90.1 a | −90.1 a | ||
| 0D | 264.6 | 128.8 | 6.4 b | 11.1 a | 131.6 b | 133.0 b | 49.7 a | |
| DD | 264.6 | 128.8 | 6.9 b | 11.1 a | 142.8 b | 121.8 b | 54.0 a | |
| 2021 | 00 | 0 | 0 | 5.0 a | 10.0 a | 95.0 a | −95.0 a | |
| D0 | 0 | 0 | 6.0 a | 10.2 a | 116.3 ab | −116.3 a | ||
| 0D | 228.7 | 119.5 | 6.7 b | 11.2 a | 139.8 bc | 89.0 b | 61.1 a | |
| DD | 228.7 | 119.5 | 7.7 b | 10.9 a | 157.5 c | 71.2 b | 68.9 a | |
| 2022 | 00 | 0 | 0 | 4.6 a | 11.4 a | 95.7 a | −95.7 a | |
| D0 | 0 | 0 | 6.4 b | 11.5 a | 137.0 b | −137.0 a | ||
| 0D | 223.2 | 113.1 | 7.9 c | 11.3 a | 164.5 c | 58.7 d | 73.7 a | |
| DD | 223.2 | 113.1 | 8.4 c | 12.5 a | 190.2 d | 33.0 c | 85.2 b |
| Measured N2O-N Emissions | Modelled N2O-N Emissions | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Year of Harvest | Treatment * | Autumn and Winter | Spring and Summer | Fertilization until Harvest | Post-Harvest | Whole Year | Product- Related | Emission Factor ** | GNOC *** | Emission Factor GNOC |
| Unit | kg ha−1 | kg ha−1 | kg ha−1 | kg ha−1 | kg ha−1 | kg ha−1 | kg Mg−1 | % | kg ha−1 | % |
| 2019 | 00 | 0.64 a | 0.21 a | 0.43 a | 1.36 | |||||
| D0 | 0.96 a | 0.23 ab | 0.74 a | 1.36 | ||||||
| 0D | 1.06 b | 0.53 d | 0.53 a | 3.28 | 0.82 | |||||
| DD | 1.49 c | 0.75 c | 0.74 a | 3.28 | 0.82 | |||||
| 2020 | 00 | 1.91 | 0.39 a | 0.26 a | 0.13 a | 2.17 | 0.55 a | 1.36 | ||
| D0 | 1.16 a | 0.38 a | 0.78 a | 2.50 | 0.57 a | 1.36 | ||||
| 0D | 3.14 b | 1.92 ab | 1.22 b | 3.83 | 0.56 a | 0.63 | 3.70 | 0.88 | ||
| DD | 2.12 | 3.30 b | 2.50 b | 0.79 b | 4.62 | 0.66 a | 0.93 | 3.70 | 0.88 | |
| 2021 | 00 | 2.38 | 0.80 a | 0.53 a | 0.27 a | 2.81 | 0.52 a | 1.36 | ||
| D0 | 3.34 b | 0.78 a | 2.56 b | 2.51 | 0.42 a | 1.36 | ||||
| 0D | 1.21 ab | 0.48 a | 0.73 ab | 2.86 | 0.43 a | 0.02 | 3.24 | 0.82 | ||
| DD | 1.73 | 1.40 ab | 0.74 a | 0.66 ab | 2.47 | 0.32 a | −0.15 | 3.24 | 0.82 | |
| 2022 | 00 | 3.72 | 0.16 a | 0.14 a | 0.05 a | 3.86 | 0.82 a | 1.36 | ||
| D0 | 0.32 ab | 0.26 ab | 0.06 a | 2.30 | 0.35 a | 1.36 | ||||
| 0D | 0.42 ab | 0.38 bc | 0.04 a | 4.10 | 0.51 a | 0.11 | 3.17 | 0.81 | ||
| DD | 2.04 | 0.70 b | 0.69 c | 0.02 a | 2.73 | 0.33 a | −0.51 | 3.17 | 0.81 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Winkhart, F.; Schmid, H.; Hülsbergen, K.-J. Effects of Biogas Digestate on Winter Wheat Yield, Nitrogen Balance, and Nitrous Oxide Emissions under Organic Farming Conditions. Agronomy 2024, 14, 1739. https://doi.org/10.3390/agronomy14081739
Winkhart F, Schmid H, Hülsbergen K-J. Effects of Biogas Digestate on Winter Wheat Yield, Nitrogen Balance, and Nitrous Oxide Emissions under Organic Farming Conditions. Agronomy. 2024; 14(8):1739. https://doi.org/10.3390/agronomy14081739
Chicago/Turabian StyleWinkhart, Felizitas, Harald Schmid, and Kurt-Jürgen Hülsbergen. 2024. "Effects of Biogas Digestate on Winter Wheat Yield, Nitrogen Balance, and Nitrous Oxide Emissions under Organic Farming Conditions" Agronomy 14, no. 8: 1739. https://doi.org/10.3390/agronomy14081739
APA StyleWinkhart, F., Schmid, H., & Hülsbergen, K.-J. (2024). Effects of Biogas Digestate on Winter Wheat Yield, Nitrogen Balance, and Nitrous Oxide Emissions under Organic Farming Conditions. Agronomy, 14(8), 1739. https://doi.org/10.3390/agronomy14081739





