Biochar Improves Wax Gourd (Benincasa hispida (Thunb.) Cogn.) Yield and Quality by Regulating the Chemical Properties of Acidic Soil and Promoting Nutrient Uptake
Abstract
:1. Introduction
2. Materials and Methods
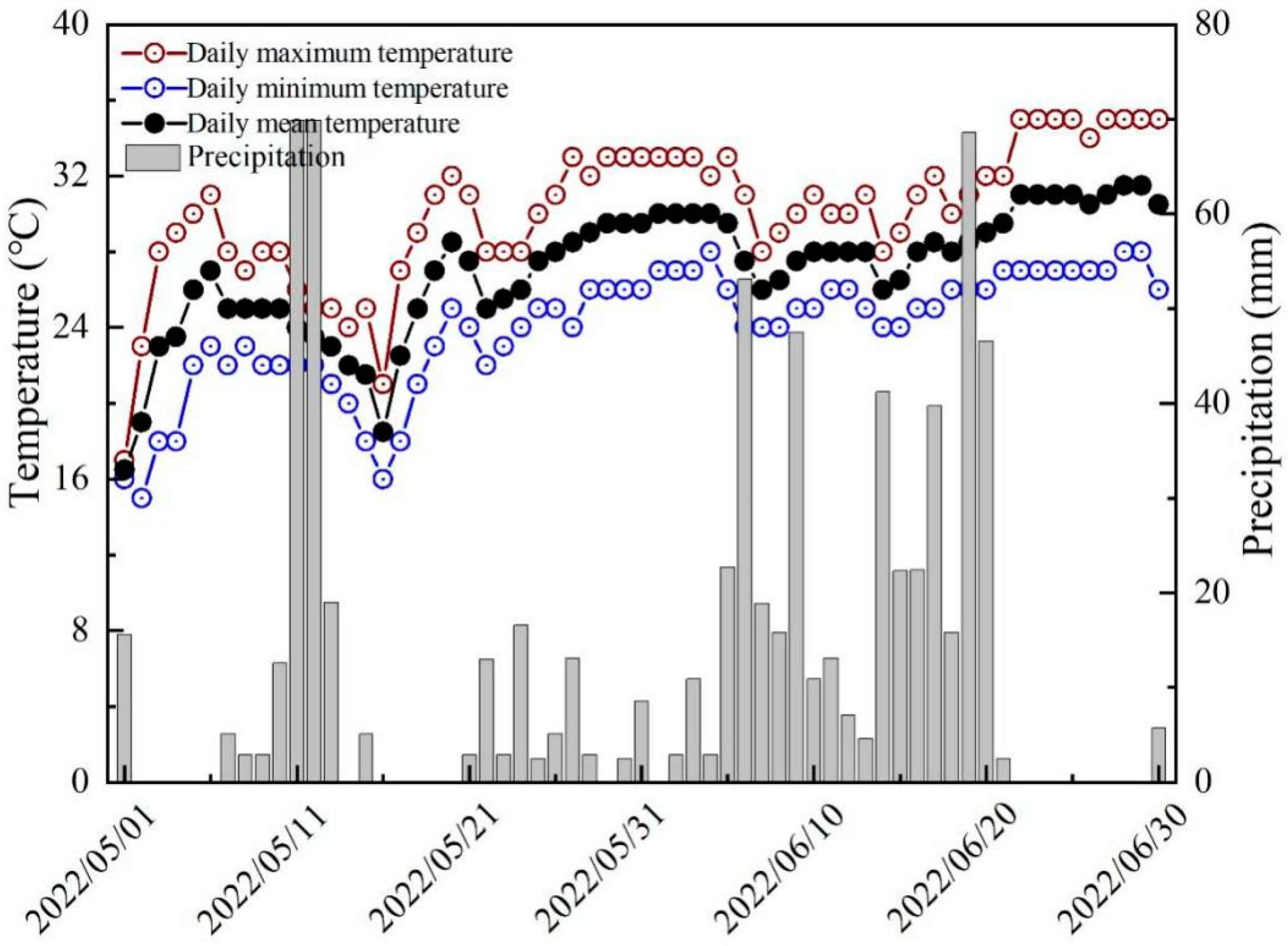
2.1. Experimental Location
2.2. Experimental Design and Field Management
2.3. Parameter Measurements and Calculations
2.3.1. Sampling and Determination at Maturity
2.3.2. Determination of Yield and Its Components
2.3.3. Soil Sample Collection and Determination at Maturity
2.3.4. Measurement of Fruit Quality
2.3.5. Statistical Analysis
3. Results
3.1. Yield and Dry Matter Accumulation
3.2. Differences in Yield Composition
3.3. Shoot Nutrient Uptake
3.4. Soil Chemical Properties (and Table 3)
3.5. Quality Differences between Treatments
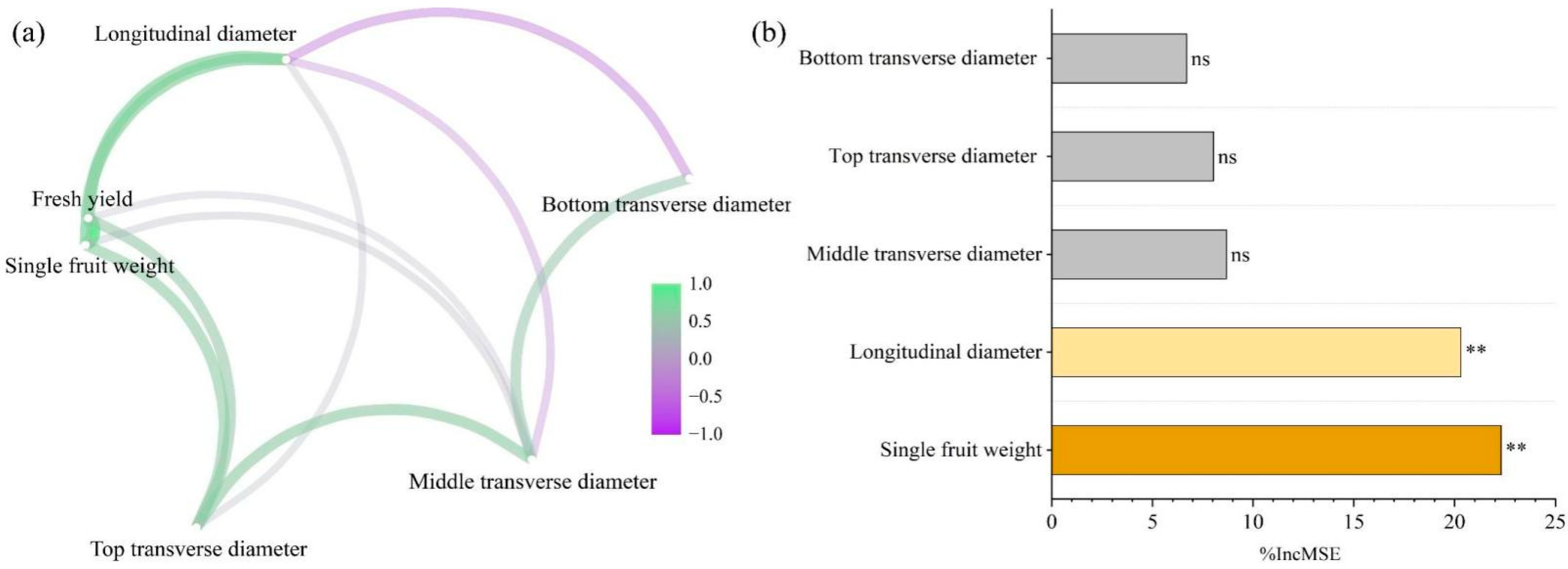
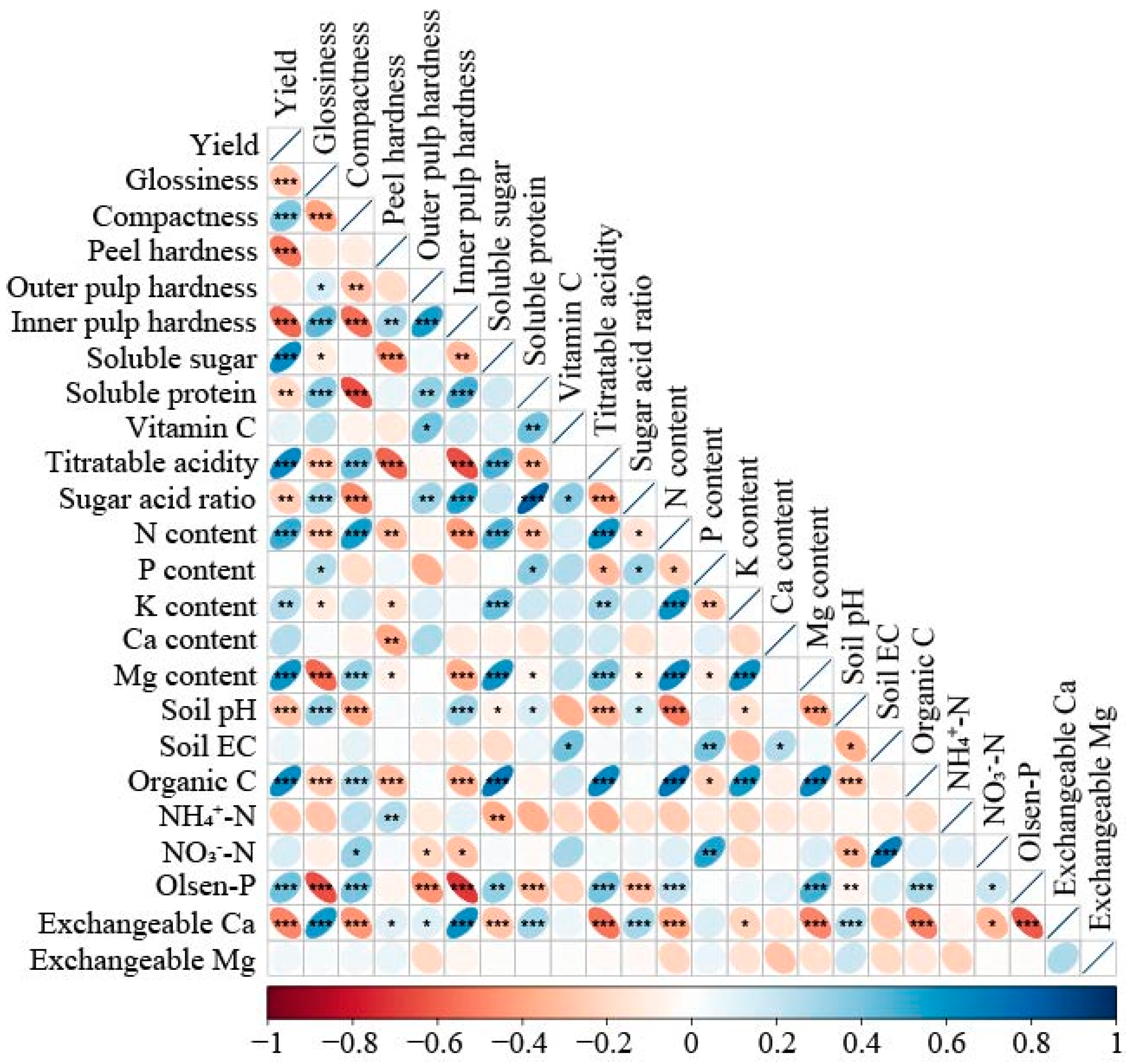
3.6. Analysis of Wax Gourd Yield and Quality Drivers
4. Discussion
4.1. Biochar Improves Wax Gourd Yield by Regulating Soil Physicochemical Properties
4.2. Differential Regulation of Soil Physicochemical Properties by Biochar Feedstock and Application Rate
4.3. Biochar Improves Wax Gourd Quality by Promoting Nutrient Absorption and Accumulation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Alemu, T.T. Nutritional contribution of fruit and vegetable for human health: A review. Int. J. Health Policy Plann. 2024, 3, 1–9. [Google Scholar]
- FAO. FAOSTAT. Database-Resources; FAO: Rome, Italy, 2022.
- Wang, X.; Dou, Z.; Shi, X.; Zou, C.; Liu, D.; Wang, Z.; Guan, X.; Sun, Y.; Wu, G.; Zhang, B.; et al. Innovative management programme reduces environmental impacts in Chinese vegetable production. Nat. Food 2021, 2, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, Q.; Cao, J.; Zhang, C.; Song, Z.; Zhang, F.; Chen, X. Reducing nitrogen leaching in a subtropical vegetable system. Agric. Ecosyst. Environ. 2017, 241, 133–141. [Google Scholar] [CrossRef]
- Wang, X.; Zou, C.; Gao, X.; Guan, X.; Zhang, W.; Zhang, Y.; Shi, X.; Chen, X. Nitrous oxide emissions in Chinese vegetable systems: A meta-analysis. Environ. Pollut. 2018, 239, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Liu, F.; Ma, X.; Guo, G.; Liu, B.; Cheng, T.; Liang, T.; Tao, W.; Chen, X.; Wang, X. Greenhouse gas emissions from vegetables production in China. J. Clean. Prod. 2021, 317, 128449. [Google Scholar] [CrossRef]
- Thompson, R.B.; Voogt, W.; Incrocci, L.; Fink, M.; Neve, S.D. Strategies for optimal fertiliser management of vegetable crops in Europe. ISHS Acta Hortic. 2018, 1192, 129–140. [Google Scholar] [CrossRef]
- Tei, F.; Neve, S.D.; Haan, J.D.; Kristensen, H.L.G. Nitrogen management of vegetable crops. Agric. Water Manag. 2020, 240, 106316. [Google Scholar] [CrossRef]
- Wang, F.; Wang, X.; Song, N. Biochar and vermicompost improve the soil properties and the yield and quality of cucumber (Cucumis sativus L.) grown in plastic shed soil continuously cropped for different years. Agric. Ecosyst. Environ. 2021, 315, 107425. [Google Scholar] [CrossRef]
- Kumar, M.; Dutta, S.; You, S.; Luo, G.; Zhang, S.; Show, P.L.; Singh, L.; Tsang, D.C.W. A critical review on biochar for enhancing biogas production from anaerobic digestion of food waste and sludge. J. Clean. Prod. 2021, 305, 127143. [Google Scholar] [CrossRef]
- Ishfaq, M.; Wang, Y.; Xu, J.; Hassan, M.U.; Yuan, H.; Liu, L.; He, B.; Ejaz, I.; White, P.J.; Cakmak, I.; et al. Improvement of nutritional quality of food crops with fertilizer: A global meta-analysis. Agron. Sustain. Dev. 2023, 43, 74. [Google Scholar] [CrossRef]
- Yan, S.; Zhang, S.; Yan, P.; Aurangzeib, M. Effect of biochar application method and amount on the soil quality and maize yield in Mollisols of Northeast China. Biochar 2023, 4, 56. [Google Scholar] [CrossRef]
- Hu, M.; Chen, J.; Liu, Y. Structural properties and adsorption performance relationship towards three categories of lignin and their derived biochar. Bioresour. Technol. 2024, 401, 130712. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.H.; Omidvar, N.; Gallart, M.; Kämper, W.; Tahmasbian, I.; Farrar, M.B.; Singh, K.; Zhou, G.; Muqadass, B.; Xu, C.; et al. Combined effects of biochar and fertilizer applications on yield: A review and meta-analysis. Sci. Total Environ. 2022, 808, 152073. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Meki, K.; Zheng, H.; Yuan, Y.; Shao, M.; Luo, X.; Li, X.; Jiang, Z.; Li, F.; Xing, B. Biochar application in remediating salt-affected soil to achieve carbon neutrality and abate climate change. Biochar 2023, 5, 45. [Google Scholar] [CrossRef]
- He, M.; Xiong, X.; Wang, L.; Hou, D.; Bolan, N.S.; Ok, Y.S.; Rinklebe, J.; Tsang, D.C.W. A critical review on performance indicators for evaluating soil biota and soil health of biochar-amended soils. J. Hazard. Mater. 2021, 414, 125378. [Google Scholar] [CrossRef] [PubMed]
- Biederman, L.A.; Harpole, W.S. Biochar and its effects on plant productivity and nutrient cycling: A meta-analysis. Glob. Chang. Biol. Bioenergy 2013, 5, 202–214. [Google Scholar] [CrossRef]
- Ye, L.; Arbestain, M.C.; Shen, Q.; Lehmann, J.; Singh, B.; Sabir, M. Biochar effects on crop yields with and without fertilizer: A meta-analysis of field studies using separate controls. Soil Use Manag. 2020, 36, 2–18. [Google Scholar] [CrossRef]
- Li, H.; Yan, T.; Fu, T.; Hao, Y.; Li, J.; Tan, Y.; Li, Z.; Peng, Y.; Chen, X.; Chang, J.; et al. Biochar combined with magnesium fertilizer improves cabbage (Brassica oleraceae L.) yield by modulating physicochemical characteristics and the bacterial community in acidic soil. Soil Use Manag. 2023, 39, 1422–1436. [Google Scholar] [CrossRef]
- Khan, T.F.; Salma, M.U.; Hossain, S.A. Impacts of different sources of biochar on plant growth characteristics. Am. J. Plant Sci. 2018, 9, 1922–1934. [Google Scholar] [CrossRef]
- Vista, S.P.; Khadka, A. Determining appropriate dose of biochar for vegetables. J. Pharmacogn. Phytochem. 2017, 6, 673–677. [Google Scholar]
- Guo, H.; Chen, L.; Wang, Y.; Li, Q.; Yi, Z. Combined application of biochar and magnesium fertilizer effectively improved the soil environment and the tea quality in southern strongly acidic tea garden. J. Soils Sediments 2023, 23, 2798–2815. [Google Scholar] [CrossRef]
- Zhang, C.; Li, X.; Yan, H.; Ullah, I.; Zuo, Z.; Li, L.; Yu, J. Effects ofirrigation quantity and biochar on soil physical properties, growth Characteristic yield and quality of greenhouse tomato. Agric. Manag. 2020, 241, 106263. [Google Scholar] [CrossRef]
- Guo, J.H.; Liu, X.J.; Zhang, Y.; Shen, J.L.; Han, W.X.; Zhang, W.F.; Christie, P.; Goulding, K.; Vitousek, P.; Zhang, F.S. Significant acidification in major chinese croplands. Science 2010, 327, 1008–1010. [Google Scholar] [CrossRef] [PubMed]
- Agegnehu, G.; Amede, T.; Erkossa, T.; Yirga, C.; Henry, C.; Tyler, R. Extent and management of acid soils for sustainable crop production system in the tropical agroecosystems: A review. Soil Plant Sci. 2021, 71, 852–869. [Google Scholar] [CrossRef]
- Marr, K.L.; Xia, Y.; Bhattarai, N.K. Allozymic, morphological, phenological, linguistic, plant use, and nutritional data of benincasa hispida (Cucurbitaceae). Econ. Bot. 2007, 61, 44–59. [Google Scholar] [CrossRef]
- Zhang, B.; Cakamak, I.; Feng, J.; Yu, C.; Chen, X.; Xie, D.; Wu, L.; Song, Z.; Cao, J.; He, Y. Magnesium deficiency reduced the yield and seed germination in wax gourd by affecting the carbohydrate translocation. Front. Plant Sci. 2020, 11, 797. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Liu, Z.; Cheng, Z.; Gou, J.; Chen, J.; Yu, W.; Wang, P. Identification and application of BhAPRR2 controlling peel colour in wax gourd (Benincasa hispida). Front. Plant Sci. 2021, 12, 716772. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, X.; Jiao, J.; Zhang, F.; Chen, X.; Li, G.; Song, Z.; Sokolowski, E.; Imas, P.; Magen, H.; et al. Towards balanced fertilizer management in south china: Enhancing wax gourd (Benincasa hispida) yield and produce quality. Sustainability 2022, 14, 5646. [Google Scholar] [CrossRef]
- Sparks, D.L.; Fendorf, S.E.; IV, C.V.T.; Carski, T.H. Kinetic methods and measurements. Methods Soil Anal. 1996, 5, 1275–1307. [Google Scholar] [CrossRef]
- Yemm, E.W.; Willis, A.J. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Deans, C.A.; Sword, G.A.; Lenhart, P.A.; Burkness, E.; Hutchison, W.D.; Behmer, S.T. Quantifying plant soluble protein and digestible carbohydrate content, using corn (Zea mays) as an exemplar. J. Vis. Exp. 2018, 138, 58164. [Google Scholar]
- Desai, A.P.; Desai, S. UV spectroscopic method for determination of vitamin C (ascorbic acid) content in different fruits in south gujarat region. Int. J. Environ. Sci. Nat. Resour. 2019, 21, 2. [Google Scholar] [CrossRef]
- Volmer, D.A.; Schultz, L.D. Determination of titratable acidity in wine using potentiometric, conductometric, and photometric methods. J. Chem. Educ. 2017, 94, 1296–1302. [Google Scholar] [CrossRef]
- Bo, X.; Zhang, Z.; Wang, J.; Guo, S.; Li, Z.; Lin, H.; Huang, Y.; Han, Z.; Kuzyakov, Y.; Zou, J. Benefits and limitations of biochar for climate-smart agriculture: A review and case study from China. Biochar 2023, 5, 77. [Google Scholar] [CrossRef]
- Liu, J.; Shu, A.; Song, W.; Shi, W.; Li, M.; Zhang, W.; Li, Z.; Liu, G.; Yuan, F.; Zhang, S.; et al. Long-term organic fertilizer substitution increases rice yield by improving soil properties and regulating soilbacteria. Geoderma 2021, 404, 115287. [Google Scholar] [CrossRef]
- Qaswar, M.; Huang, J.; Ahmed, W.; Li, D.; Liu, S.; Zhang, L.; Cai, A.; Liu, L.; Xu, Y.; Gao, J.; et al. Yield sustainability, soil organic carbon sequestration and nutrients balance under long-term combined application of manure and inorganic fertilizers in acidic paddy soil. Soil Tillage Res. 2020, 198, 104569. [Google Scholar] [CrossRef]
- Alkharabsheh, H.M.; Seleiman, M.F.; Battaglia, M.L.; Shami, A.; Jalal, R.S.; Alhammad, B.A.; Almutairi, K.F.; Al-Saif, A.M. Biochar and its broad impacts in soil quality and fertility, nutrient leaching and crop productivity: A review. Agronomy 2021, 11, 993. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Zhang, H.; Guo, L.; Chen, Y.; Heiling, M.; Zhou, B.; Mao, Y. Biochar interaction with chemical fertilizer regulates soil organic carbon mineralization and the abundance of key C-cycling-related bacteria in rhizosphere soil. Eur. J. Soil Biol. 2021, 106, 103350. [Google Scholar] [CrossRef]
- Liu, M.; Linna, C.; Ma, S.; Ma, Q.; Song, W.; Shen, M.; Song, L.; Cui, K.; Zhou, Y.; Wang, L. Biochar combined with organic and inorganic fertilizers promoted the rapeseed nutrient uptake and improved the purple soil quality. Front. Nutr. 2022, 9, 997151. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Yuan, S.; Hong, M.; Zhang, L.; Huang, Q. Mechanism of negative surface charge formation on biochar and its effect on the fixation of soil Cd. J. Hazard. Mater. 2020, 384, 121370. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Han, X.; Li, N.; Chen, K.; Yang, J.; Zhan, X.; Luo, P.; Liu, N. Combined application of biochar with fertilizer promotes nitrogen uptake in maize by increasing nitrogen retention in soil. Biochar 2021, 3, 367–379. [Google Scholar] [CrossRef]
- Xia, H.; Riaz, M.; Zhang, M.; Liu, B.; Li, Y.; El-Desouki, Z.; Jiang, C. Biochar-N fertilizer interaction increases N utilization efficiency by modifying soil C/N component under N fertilizer deep placement modes. Chemosphere 2022, 286, 131594. [Google Scholar] [CrossRef] [PubMed]
- Anand, A.; Kumar, V.; Kaushal, P. Biochar and its twin benefits: Crop residue management and climate change mitigation in India. Renew. Sustain. Energy Rev. 2022, 156, 111959. [Google Scholar] [CrossRef]
- Li, X.; Wu, D.; Liu, X.; Huang, Y.; Cai, A.; Xu, H.; Ran, J.; Xiao, J.; Zhang, W. A global dataset of biochar application effects on crop yield, soil properties, and greenhouse gas emissions. Sci. Data 2024, 11, 57. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Chen, B.; Zhu, L.; Xing, B. Effects and mechanisms of biochar-microbe interactions in soil improvement and pollution remediation: A review. Environ. Pollut. 2017, 227, 98–115. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Northup, B.K.; Rice, C.W.; Prasad, P.V.V. Biochar applications influence soil physical and chemical properties, microbial diversity, and crop productivity: A meta-analysis. Biochar 2022, 4, 8. [Google Scholar] [CrossRef]
- Lu, Y.; Gu, K.; Shen, Z.; Tang, C.; Shi, B.; Zhou, Q. Biochar implications for the engineering properties of soils: A review. Sci. Total Environ. 2023, 888, 164185. [Google Scholar] [CrossRef] [PubMed]
- Fungo, B.; Guerena, D.; Thiongo, M.; Lehmann, J.; Neufeldt, H.; Kalbitz, K. N2O and CH4 emission from soil amended with steam-activated biochar. J. Plant Nutr. Soil Sci. 2014, 177, 34–38. [Google Scholar] [CrossRef]
- Liu, Y.; Li, H.; Hu, T.; Mahmoud, A.; Li, J.; Zhu, R.; Jiao, X.; Jing, P. A quantitative review of the effects of biochar application on rice yield and nitrogen use efficiency in paddy fields: A meta-analysis. Sci. Total Environ. 2022, 830, 154792. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, A.; Ji, C.; Joseph, S.; Bian, R.; Li, L.; Pan, G.; Paz-Ferreiro, J. Biochar’s effect on crop productivity and the dependence on experimental conditions—A meta-analysis of literature data. Plant Soil 2013, 373, 583–594.10. [Google Scholar] [CrossRef]
- Zwieten, L.V.; Kimber, S.; Morris, S.; Chan, K.Y.; Downie, A.; Rust, J.; Joseph, S.; Cowe, A. Effects of biochar from slow pyrolysis of papermill waste on agronomic performance and soil fertility. Plant Soil 2010, 327, 235–246. [Google Scholar] [CrossRef]
- Guo, M.; He, Z.; Uchimiya, S.M. Introduction to biochar as an agricultural and environmental amendment. In Agricultural and Environmental Applications of Biochar: Advances and Barriers; Soil Science Society of America, Inc.: Madison, WI, USA, 2016; Volume 63. [Google Scholar] [CrossRef]
- Intani, K.; Latif, S.; Islam, M.; Müller, J. Phytotoxicity of corncob biochar before and after heat treatment and washing. Sustainability 2019, 11, 30. [Google Scholar] [CrossRef]
- Farhangi-Abriz, S.; Torabian, S.; Qin, R.; Noulas, C.; Lu, Y.; Gao, S. Biochar effects on yield of cereal and legume crops using meta-analysis. Sci. Total Environ. 2021, 775, 145869. [Google Scholar] [CrossRef]
- Rondón, M.A.; Lehmann, J.; Ramírez, J.; Hurtado, M. Biological nitrogen fixation by common beans (Phaseolus vulgaris L.) increases with biochar additions. Biol. Fertil. Soils 2007, 43, 699–708. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, Y.; Liu, S.; Li, Z.; Tan, X.; Huang, X.; Zeng, G.; Zhou, L.; Zheng, B. Biochar to improve soil fertility. A review. Agron. Sustain. Dev. 2016, 36, 36. [Google Scholar] [CrossRef]
- Cakmak, K. Agronomic biofortification of cereals with zinc: A review. Eur. J. Soil Sci. 2018, 69, 172–180. [Google Scholar] [CrossRef]
- Ishfaq, M.; Zhong, Y.; Wang, Y.; Li, X. Magnesium limitation leads to transcriptional down-tuning of auxin synthesis, transport, and signaling in the tomato root. Front. Plant Sci. 2021, 12, 802399. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Fan, Y.; Qiu, Y.; Hao, X.; Li, S.; Kang, S. Response of yield and quality of greenhouse tomatoes to water and salt stresses and biochar addition in Northwest China. Agric. Water Manag. 2022, 270, 107736. [Google Scholar] [CrossRef]
- Keabetswe, L.; Shao, G.C.; Cui, J.; Lu, J.; Stimela, T. A combination of biochar and regulated deficit irrigation improves tomato fruit quality: A comprehensive quality analysis. Folia Hortic. 2019, 31, 181–193. [Google Scholar] [CrossRef]
- Abdelghany, A.E.; Dou, Z.; Alashram, M.G.; Eltohamy, K.M.; Elrys, A.S.; Liu, X.; Wu, Y.; Cheng, M.; Fan, J.; Zhang, F. The joint application of biochar and nitrogen enhances fruit yield, quality and water-nitrogen productivity of water-stressed greenhouse tomato under drip fertigation. Agric. Water Manag. 2023, 290, 108605. [Google Scholar] [CrossRef]
- Akhtar, S.S.; Li, G.; Andersen, M.N.; Liu, F. Biochar enhances yield and quality of tomato under reduced irrigation. Agric. Water Manag. 2014, 138, 37–44. [Google Scholar] [CrossRef]
- Zeeshan, M.; Ahmad, W.; Hussain, F.; Ahamd, W.; Numan, M.; Shah, M.; Ahmad, I. Phytostabalization of the heavy metals in the soil with biochar applications, the impact on chlorophyll, carotene, soil fertility and tomato crop yield. J. Clean. Prod. 2020, 255, 120318. [Google Scholar] [CrossRef]
- Agbna, G.H.D.; Ali, A.B.; Bashir, A.K.; Eltoum, F.; Hassan, M.M. Influence of Biochar Amendment on Soil Water Characteristics and Crop Growth Enhancement Under Salinity Stress. Int. J. Eng. Work. 2017, 4, 49–54. [Google Scholar]
- Tränkner, M.; Tavakol, E.; Jákli, B. Functioning of potassium and magnesium in photosynthesis, photosynthate translocation and photoprotection. Physiol. Plant. 2018, 163, 414–431. [Google Scholar] [CrossRef] [PubMed]
- Ishfaq, M.; Wang, Y.; Yan, M.; Wang, Z.; Wu, L.; Li, C.; Li, X. Physiological essence of magnesium in plants and its widespread deficiency in the farming system of China. Front. Plant Sci. 2022, 13, 802274. [Google Scholar] [CrossRef] [PubMed]



| Treatment | Yield (t ha−1) | ||
|---|---|---|---|
| Varieties | Fertilization | 2022 | 2023 |
| Tiezhu No. 2 | FP | 125.3 ± 3.8 bc | 121.3 ± 4.5 bc |
| OPT | 118.3 ± 7.6 c | 110.2 ± 6.4 c | |
| OPT1 + T1 | 142.8 ± 10.0 ab | 145.7 ± 11.2 ab | |
| OPT1 + T2 | 134.4 ± 12.5 abc | 137.7 ± 10.6 ab | |
| OPT1 + T3 | 155.0 ± 9.9 a | 159.3 ± 12.5 a | |
| Dadao | FP | 103.9 ± 5.2 a | 108.2 ± 7.8 a |
| OPT | 101.6 ± 5.8 a | 102.5 ± 10.8 a | |
| OPT + T1 | 109.6 ± 6.8 a | 118.7 ± 6.1 a | |
| OPT + T2 | 107.5 ± 9.7 a | 113.7 ± 8.8 a | |
| OPT + T3 | 116.4 ± 4.2 a | 119.0 ± 12.5 a | |
| Treatment | Single Fruit Weight (kg) | Longitudinal Diameter (cm) | Transverse Diameter (cm) | |||
|---|---|---|---|---|---|---|
| Varieties | Fertilization | Top | Middle | Bottom | ||
| Tiezhu No. 2 | FP | 16.7 ± 0.6 bc | 90.0 ± 3.5 a | 18.7 ± 0.6 b | 16.3 ± 1.5 c | 18.3 ± 1.4 a |
| OPT | 15.8 ± 1.2 c | 86.0 ± 2.6 a | 18.0 ± 1.0 b | 16.0 ± 1.0 c | 17.3 ± 0.6 a | |
| OPT1 + T1 | 19.0 ± 1.6 ab | 88.3 ± 3.5 a | 20.3 ± 1.5 b | 18.7 ± 1.2 b | 18.3 ± 2.9 a | |
| OPT1 + T2 | 17.9 ± 2.1 abc | 89.6 ± 0.6 a | 20.0 ± 1.0 b | 17.7 ± 0.6 bc | 17.7 ± 1.5 a | |
| OPT1 + T3 | 20.7 ± 1.6 a | 85.0 ± 8.7 a | 23.7 ± 2.3 a | 21.3 ± 1.5 a | 19.0 ± 0.0 a | |
| Dadao | FP | 13.9 ± 0.9 a | 58.7 ± 2.5 bc | 19.0 ± 0.5 a | 18.2 ± 2.8 a | 19.3 ± 0.8 ab |
| OPT | 13.6 ± 1.0 a | 60.3 ± 0.6 ab | 19.3 ± 3.2 a | 19.5 ± 1.8 a | 20.0 ± 1.0 a | |
| OPT + T1 | 14.6 ± 1.1 a | 60.0 ± 1.0 abc | 18.5 ± 0.5 a | 18.7 ± 1.1 a | 19.0 ± 0.0 ab | |
| OPT + T2 | 14.3 ± 1.6 a | 56.3 ± 2.9 c | 19.3 ± 1.5 a | 19.7 ± 0.8 a | 19.5 ± 0.5 ab | |
| OPT + T3 | 15.5 ± 0.7 a | 62.7 ± 2.1 a | 19.0 ± 0.9 a | 19.0 ± 0.5 a | 18.0 ± 1.2 b | |
| Treatment | pH | EC (μS cm−1) | Organic C (g kg−1) | NH4+-N (mg kg−1) | NO3−-N (mg kg−1) | Olsen-P (mg kg−1) | Exchangeable Ca (mg kg−1) | Exchangeable Mg (mg kg−1) | |
|---|---|---|---|---|---|---|---|---|---|
| Varieties | Fertilization | ||||||||
| Tiezhu No. 2 | FP | 5.66 ± 0.07 a | 42.2 ± 17.9 a | 7.16 ± 0.9 ab | 4.66 ± 0.06 abc | 8.40 ± 3.07 bc | 70.2 ± 2.4 d | 954.2 ± 12.4 a | 94.0 ± 1.6 a |
| OPT | 5.60 ± 0.26 a | 63.3 ± 6.9 a | 6.41 ± 0.4 ab | 5.28 ± 0.92 abc | 12.43 ± 0.42 a | 91.7 ± 1.9 a | 881.0 ± 24.33 b | 92.4 ± 3.2 a | |
| OPT + T1 | 5.64 ± 0.21 a | 58.2 ± 17.4 a | 7.47 ± 0.8 a | 4.28 ± 0.11 c | 11.48 ± 1.20 a | 82.9 ± 14.5 abcd | 919.4 ± 43.6 ab | 89.3 ± 4.3 a | |
| OPT + T2 | 5.57 ± 0.34 a | 59.0 ± 15.1 a | 7.56 ± 0.8 a | 5.00 ± 0.14 ab | 11.51 ± 0.76 a | 86.6 ± 7.2 abc | 869.7 ± 40.2 b | 86.2 ± 11.2 a | |
| OPT + T3 | 5.65 ± 0.50 a | 66.1 ± 17.4 a | 6.71 ± 0.5 ab | 4.34 ± 0.24 c | 11.01 ± 1.23 a | 88.0 ± 9.4 ab | 911.4 ± 27.9 ab | 90.6 ± 4.9 a | |
| Dadao | FP | 6.04 ± 0.05 a | 44.7 ± 10.1 a | 5.72 ± 0.23 bc | 4.92 ± 0.36 abc | 6.50 ± 1.51 b | 76.9 ± 7.9 abcd | 985.4 ± 36.8 ab | 85.9 ± 11.5 a |
| OPT | 5.72 ± 0.14 a | 68.7 ± 7.9 a | 5.47 ± 0.60 c | 5.58 ± 1.01 a | 12.10 ± 0.30 a | 72.2 ± 4.4 bc | 961.4 ± 27.2 ab | 87.2 ± 9.0 a | |
| OPT + T1 | 5.69 ± 0.16 a | 70.4 ± 17.1 a | 6.81 ± 1.00 a | 4.50 ± 0.46 bc | 11.76 ± 0.34 a | 73.0 ± 7.7 bcd | 926.1 ± 62.5 b | 86.4 ± 7.5 a | |
| OPT + T2 | 5.82 ± 0.12 a | 58.1 ± 15.9 a | 6.16 ± 0.80 b | 4.58 ± 0.01 bc | 11.38 ± 1.17 a | 75.5 ± 10.4 bcd | 990.8 ± 25.5 ab | 97.1 ± 3.0 a | |
| OPT + T3 | 5.80 ± 0.19 a | 58.7 ± 12.7 a | 5.87 ± 0.49 bc | 4.55 ± 0.07 bc | 11.97 ± 0.33 a | 69.6 ± 4.6 d | 1009.2 ± 115.6 a | 96.0 ± 0.8 a | |
| Treatment | Hardness (kg cm−2) | Compactness (g cm−3) | Glossiness | Soluble Sugar (mg g−1FW) | Soluble Protein (mg g−1 FW) | Vc (mg 100g−1 FW) | Titratable Acidity (%) | Sugar–Acid Ratio | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Varieties | Fertilization | Inner Pulp | Outer Pulp | Peel | |||||||
| Tiezhu No. 2 | FP | 1.20 ± 0.31 a | 2.20 ± 0.10 a | 3.22 ± 0.26 a | 0.96 ± 0.02 a | 7.68 ± 1.18 a | 4.50 ± 0.69 ab | 242.1 ± 18.6 c | 13.15 ± 0.32 ab | 0.86 ± 0.05 a | 5.00 ± 0.25 bc |
| OPT | 0.52 ± 0.03 c | 1.12 ± 0.13 c | 3.43 ± 0.10 a | 0.98 ± 0.03 a | 5.47 ± 0.15 bc | 3.55 ± 0.08 b | 158.4 ± 11.6 d | 12.78 ± 0.63 b | 0.80 ± 0.06 ab | 3.97 ± 0.31 c | |
| OPT + T1 | 0.67 ± 0.12 bc | 1.30 ± 0.09 c | 3.17 ± 0.15 a | 0.98 ± 0.00 a | 8.50 ± 0.10 a | 5.15 ± 0.52 a | 308.4 ± 27.7 b | 13.44 ± 0.23 ab | 0.90 ± 0.06 a | 5.72 ± 0.83 b | |
| OPT + T2 | 0.92 ± 0.13 ab | 1.93 ± 0.12 b | 3.42 ± 0.24 a | 0.95 ± 0.00 a | 4.50 ± 0.20 cd | 5.10 ± 0.55 a | 392.2 ± 56.8 a | 13.61 ± 0.48 a | 0.72 ± 0.06 b | 7.17 ± 1.12 a | |
| OPT + T3 | 0.85 ± 0.05 b | 1.98 ± 0.23 ab | 3.10 ± 0.20 a | 0.95 ± 0.01 a | 5.83 ± 0.21 b | 4.94 ± 0.64 a | 293.2 ± 14.9 bc | 13.21 ± 0.27 ab | 0.90 ± 0.04 a | 5.35 ± 0.32 b | |
| Dadao | FP | 1.40 ± 0.10 a | 1.82 ± 0.18 a | 3.45 ± 0.05 a | 0.90 ± 0.02 b | 8.20 ± 1.00 ab | 3.92 ± 0.36 a | 408.1 ± 17.7 b | 12.88 ± 0.27 b | 0.55 ± 0.005 b | 6.89 ± 1.31 ab |
| OPT | 1.35 ± 0.13 a | 1.93 ± 0.20 a | 3.43 ± 0.21 ab | 0.98 ± 0.01 a | 7.50 ± 0.78 b | 2.66 ± 0.48 b | 213.4 ± 39.2 c | 13.45 ± 0.21 a | 0.56 ± 0.006 b | 5.13 ± 0.92 b | |
| OPT + T1 | 1.30 ± 0.09 a | 2.28 ± 0.53 a | 3.17 ± 0.21 b | 0.92 ± 0.02 b | 8.80 ± 0.00 ab | 4.38 ± 0.43 a | 517.3 ± 10.2 a | 13.75 ± 0.36 a | 0.80 ± 0.053 a | 8.89 ± 1.53 a | |
| OPT + T2 | 1.50 ± 0.17 a | 1.77 ± 0.10 a | 3.48 ± 0.10 a | 0.92 ± 0.02 b | 7.70 ± 0.78 b | 4.09 ± 0.51 a | 538.5 ± 66.2 a | 13.27 ± 0.09 ab | 0.55 ± 0.002 b | 9.16 ± 1.56 a | |
| OPT + T3 | 1.22 ± 0.25 a | 1.82 ± 0.08 a | 3.35 ± 0.05 ab | 0.90 ± 0.01 b | 9.47 ± 0.8 a | 4.00 ± 0.58 a | 408.1 ± 78.3 b | 13.53 ± 0.28 a | 0.56 ± 0.04 b | 6.99 ± 0.68 ab | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Hao, Y.; Li, H.; Fu, T.; Li, J.; Peng, Y.; Chang, J.; Chen, L.; Xie, D.; Chen, X.; et al. Biochar Improves Wax Gourd (Benincasa hispida (Thunb.) Cogn.) Yield and Quality by Regulating the Chemical Properties of Acidic Soil and Promoting Nutrient Uptake. Agronomy 2024, 14, 1750. https://doi.org/10.3390/agronomy14081750
Li Z, Hao Y, Li H, Fu T, Li J, Peng Y, Chang J, Chen L, Xie D, Chen X, et al. Biochar Improves Wax Gourd (Benincasa hispida (Thunb.) Cogn.) Yield and Quality by Regulating the Chemical Properties of Acidic Soil and Promoting Nutrient Uptake. Agronomy. 2024; 14(8):1750. https://doi.org/10.3390/agronomy14081750
Chicago/Turabian StyleLi, Zhen, Yongzhou Hao, Hongzhao Li, Tianhong Fu, Jing Li, Yutao Peng, Jingjing Chang, Lei Chen, Dasen Xie, Xiao Chen, and et al. 2024. "Biochar Improves Wax Gourd (Benincasa hispida (Thunb.) Cogn.) Yield and Quality by Regulating the Chemical Properties of Acidic Soil and Promoting Nutrient Uptake" Agronomy 14, no. 8: 1750. https://doi.org/10.3390/agronomy14081750







