Impacts of Biochar and Gypsum on Ammonia-Oxidizing Microorganisms in Coastal Saline Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Pot Experimental Design and Soil Collection
2.2. Determination of Potential Nitrification Rate and Analysis of Soil Properties
2.3. DNA Extraction and Quantitative Real-Time PCR of amoA Genes
2.4. Illumina MiSeq Sequencing and Phylogenetic Analysis
2.5. Statistical Analysis
3. Results
3.1. Soil Physicochemical Properties
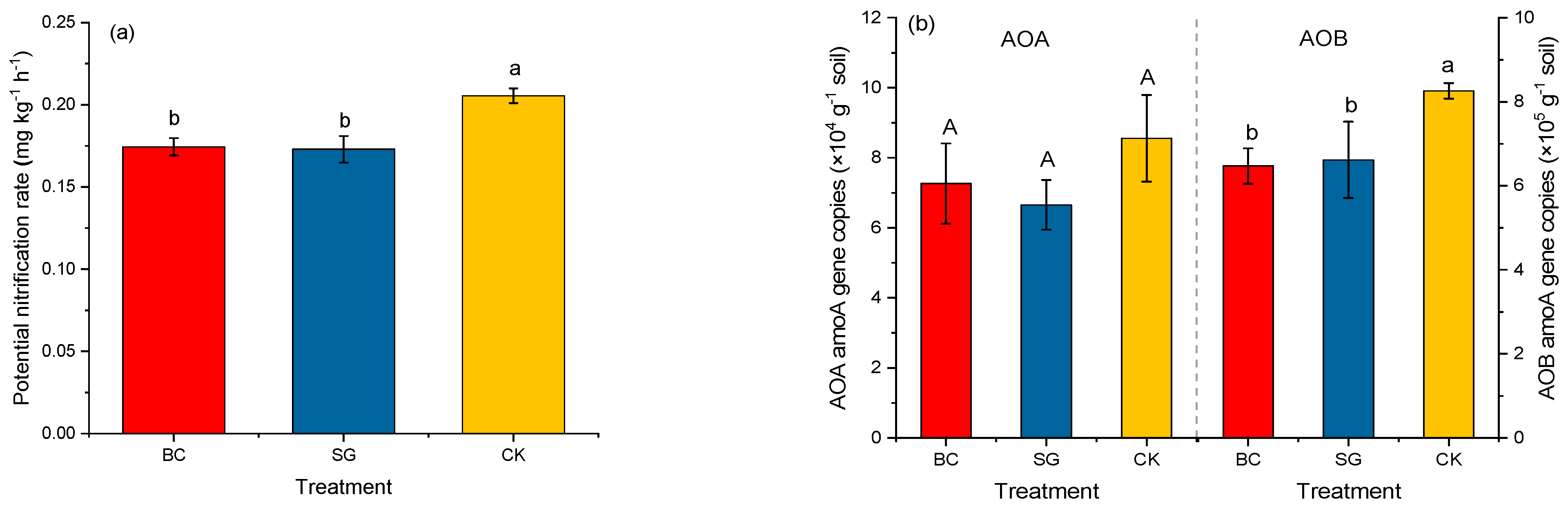
3.2. Potential Nitrification Rate and amoA Gene Copies of AOA and AOB
3.3. AOA and AOB Diversity
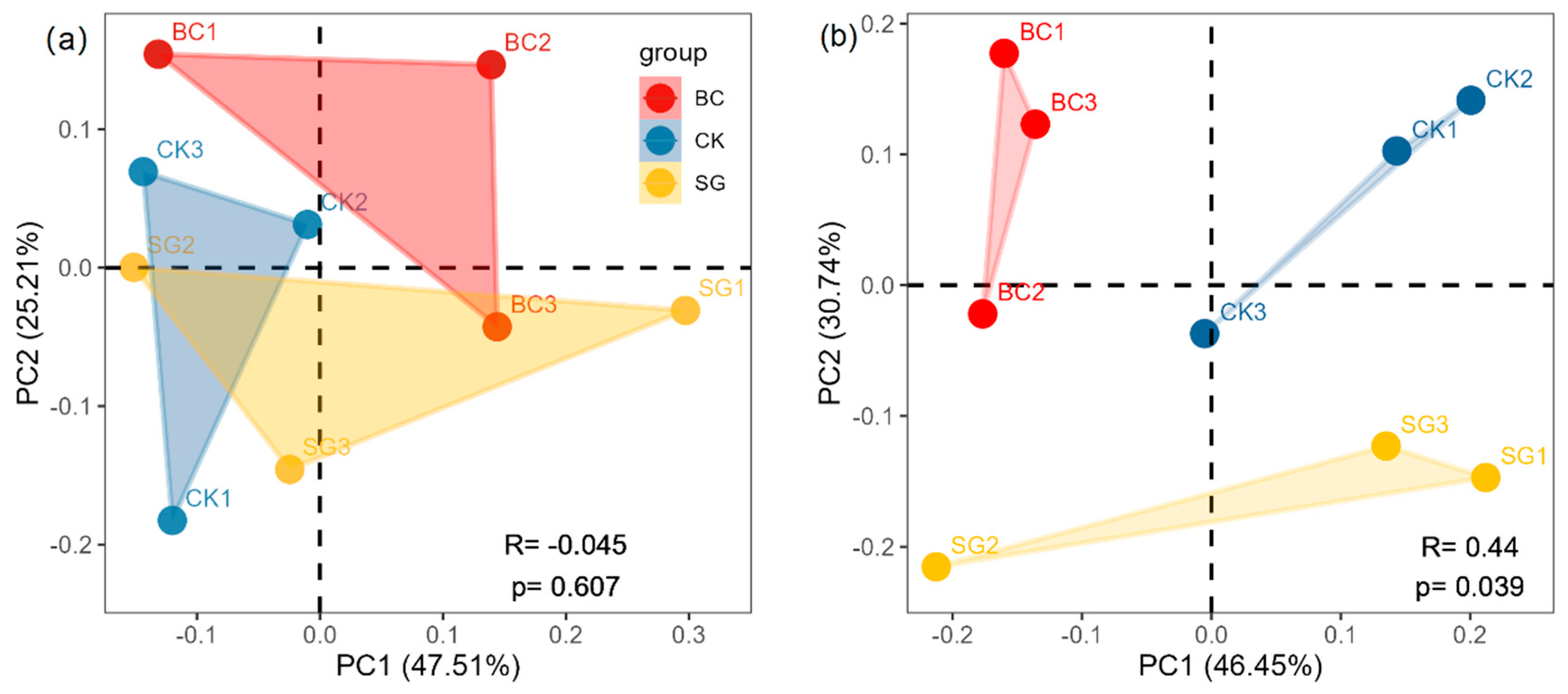
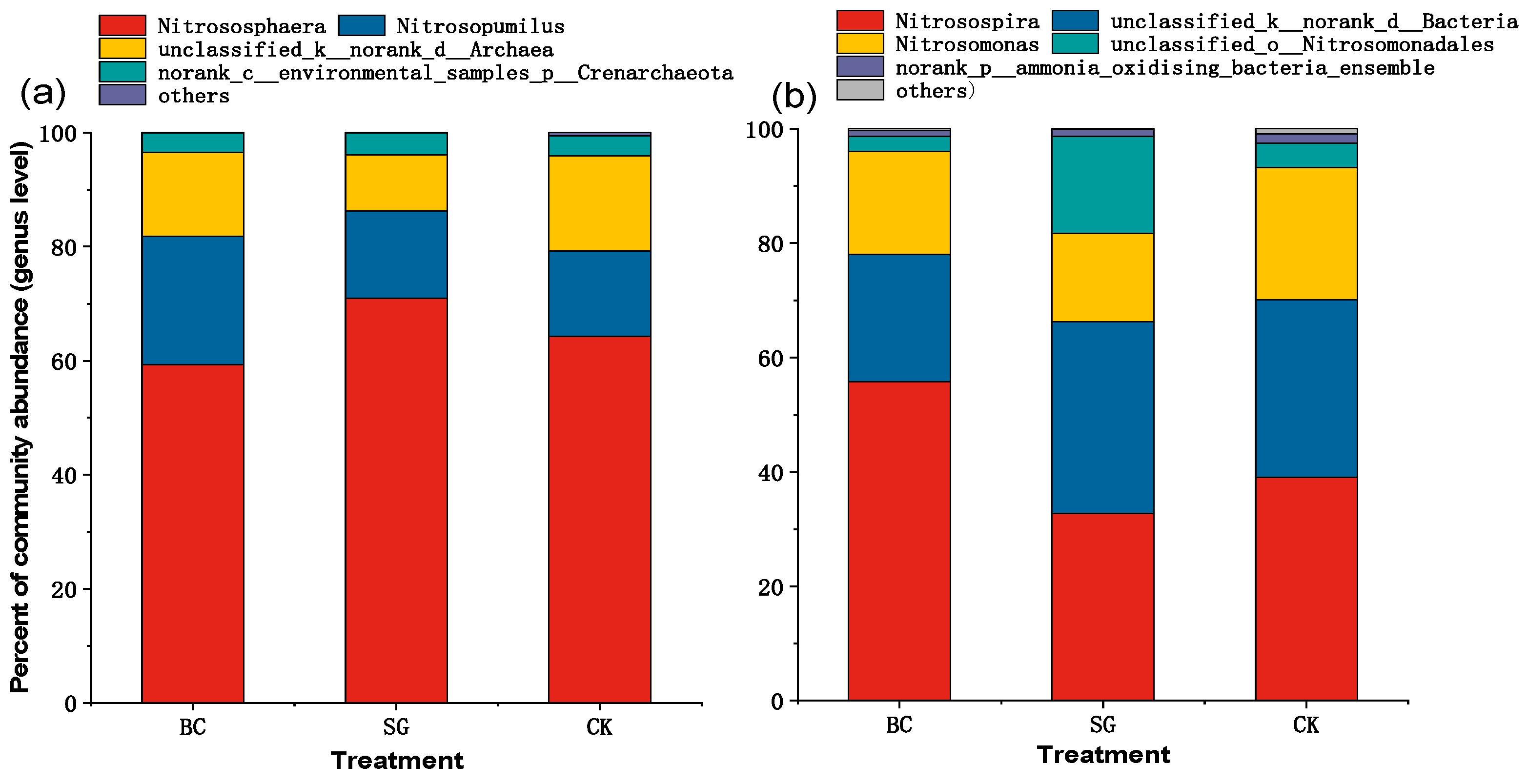
3.4. AOA and AOB Community Structure
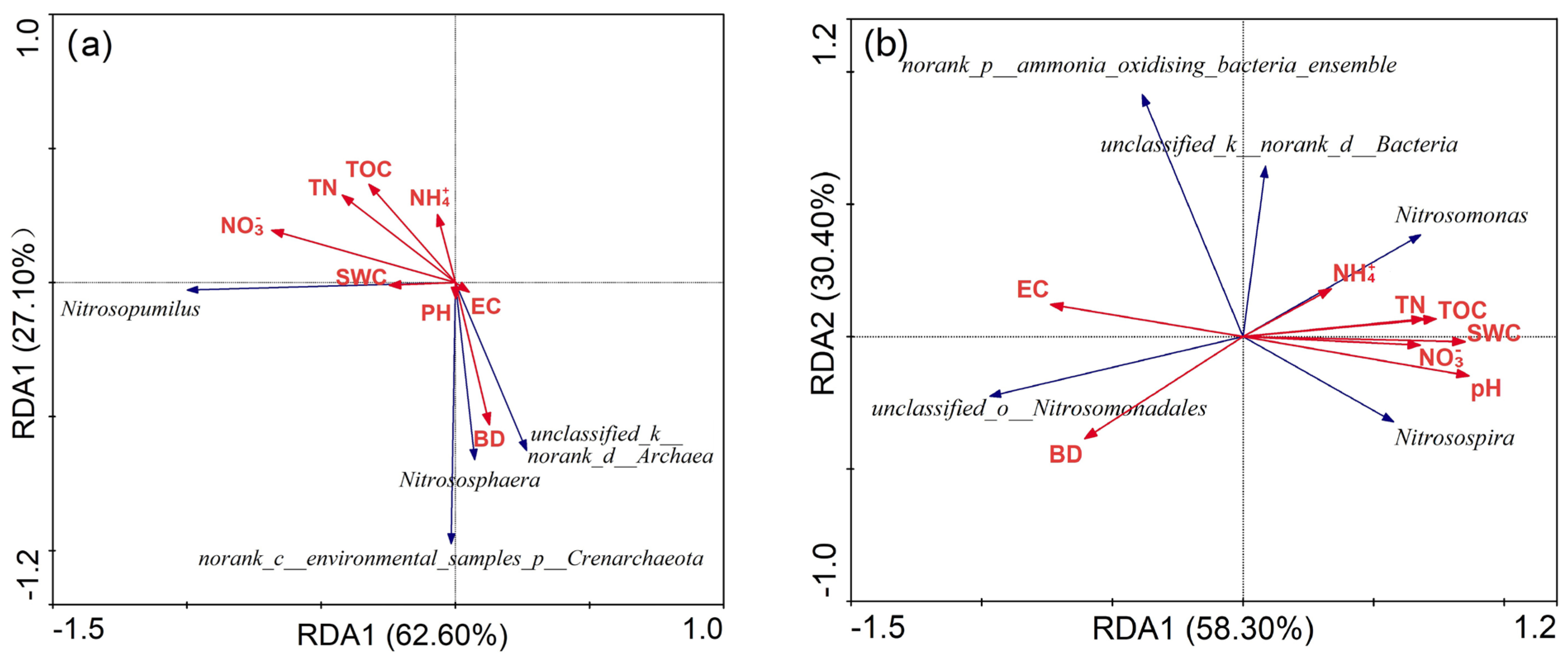
3.5. Responses of Soil Microbial Community to Soil Properties
4. Discussion
4.1. Effects of Biochar Addition on Soil Properties, Nitrification, and Ammonia-Oxidizing Microorganisms
4.2. Effects of Gypsum Addition on Soil Properties, Nitrification, and Ammonia-Oxidizing Microorganisms
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- He, L.L.; Bi, Y.C.; Zhao, J.; Pittelkow, C.M.; Zhao, X.; Wang, S.Q.; Xing, G.X. Population and community structure shifts of ammonia oxidizers after four-year successive biochar application to agricultural acidic and alkaline soils. Sci. Total Environ. 2018, 619, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.T.; Rao, Y.; Huang, S.L.; Xu, Y.; Zeng, K.Y.; Liang, X.; Ling, Q.J.; Liu, K.H.; Ma, J.M.; Yu, F.M.; et al. Impact of environmental factors on the ammonia-oxidizing and denitrifying microbial community and functional genes along soil profiles from different ecologically degraded areas in the Siding mine. J. Environ. Manag. 2023, 326, 116641. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, L.J.; Dai, T.J.; Tian, J.P.; Wen, D.H. The influence of salinity on the abundance, transcriptional activity, and diversity of AOA and AOB in an estuarine sediment: A microcosm study. Appl. Microbiol. Biot. 2015, 99, 9825–9833. [Google Scholar] [CrossRef]
- Li, Y.; Liang, Y.; Zhang, H.C.; Liu, Y.; Zhu, J.; Xu, J.; Zhou, Z.M.; Ma, J.M.; Liu, K.H.; Yu, F.M. Variation, distribution, and diversity of canonical ammonia-oxidizing microorganisms and complete-nitrifying bacteria in highly contaminated ecological restoration regions in the Siding mine area. Ecotox. Environ. Saf. 2021, 217, 112274. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.Y.; Gao, X.P.; Tenuta, M.; Kuang, W.N.; Gui, D.W.; Zeng, F.J. Manure application increased denitrifying gene abundance in a drip-irrigated cotton field. PeerJ 2019, 7, e7894. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Yang, J.S.; Li, Y.L.; Liu, X.Y.; Jin, H.F.; Li, J.F.; Yao, R.J. Effects of Soil Salinity on Nitrification and Ammonia-Oxidizing Microorganisms in Coastal Reclaimed Farmland Soil. J. Soil Sci. Plant Nutr. 2022, 22, 2743–2754. [Google Scholar] [CrossRef]
- Jin, Q.; Wang, C.; Sardans, J.; Vancov, T.; Fang, Y.Y.; Wu, L.Q.; Huang, X.T.; Gargallo-Garriga, A.; Peñuelas, J.; Wang, W.Q. Effect of soil degradation on the carbon concentration and retention of nitrogen and phosphorus across Chinese rice paddy fields. Catena 2022, 209, 105810. [Google Scholar] [CrossRef]
- Omuto, C.T.; Vargas, R.R.; El Mobarak, A.M.; Mohamed, N.; Viatkin, K.; Yigini, Y. Mapping of Salt-Affected Soils: Technical Manual; FAO: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Hsieh, C.; Chen, Y.H.; Chang, K.C.; Yang, S.Y. Transcriptome analysis reveals the mechanisms for mycorrhiza-enhanced salt tolerance in rice. Front. Plant Sci. 2022, 13, 1072171. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wall, G. Mudflat development in Jiangsu Province, China: Practices and experiences. Ocean. Coast. Manag. 2010, 53, 691–699. [Google Scholar] [CrossRef]
- Bai, Y.C.; Zang, C.Y.; Gu, M.J.; Gu, C.H.; Shao, H.B.; Guan, Y.X.; Wang, X.K.; Zhou, X.J.; Shan, Y.H.; Feng, K. Sewage sludge as an initial fertility driver for rapid improvement of mudflat salt-soils. Sci. Total Environ. 2017, 578, 47–55. [Google Scholar] [CrossRef]
- Liu, G.M.; Li, J.B.; Zhang, X.C.; Wang, X.P.; Lv, Z.Z.; Yang, J.S.; Shao, H.B.; Yu, S.P. GIS-mapping spatial distribution of soil salinity for Eco-restoring the Yellow River Delta in combination with Electromagnetic Induction. Ecol. Eng. 2016, 94, 306–314. [Google Scholar] [CrossRef]
- Zhang, G.L.; Bai, J.H.; Tebbe, C.C.; Zhao, Q.Q.; Jia, J.; Wang, W.; Wang, X.; Yu, L. Salinity controls soil microbial community structure and function in coastal estuarine wetlands. Environ. Microbiol. 2021, 23, 1020–1037. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Gupta, A.; Chandra, H.; Singh, P.; Yadav, S. Effects of salt stress on the morphology, anatomy, and gene expression of crop plants. In Physiology of Salt Stress in Plants: Perception, Signalling, Omics and Tolerance Mechanism; Wiley: New York, NY, USA, 2021; pp. 87–105. [Google Scholar]
- Thakur, R.; Yadav, S. Biofilm forming, exopolysaccharide producing and halotolerant, bacterial consortium mitigates salinity stress in Triticum aestivum. Int. J. Biol. Macromol. 2024, 262, 130049. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.X.; Yang, Y.; Guan, P.B.; Geng, L.P.; Ma, L.; Di, H.J.; Liu, W.J.; Li, B.W. Remediation of organic amendments on soil salinization: Focusing on the relationship between soil salts and microbial communities. Ecotox. Environ. Saf. 2022, 239, 113616. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.D.; Ma, L.N.; Liang, Y.; Gao, B.; Harris, W. Simultaneous Immobilization of Lead and Atrazine in Contaminated Soils Using Dairy-Manure Biochar. Environ. Sci. Technol. 2011, 45, 4884–4889. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Thangarajan, R.; Bolan, N.S.; Sarkar, B.; Khan, N.; Ok, Y.S.; Naidu, R. Biochar-induced concomitant decrease in ammonia volatilization and increase in nitrogen use efficiency by wheat. Chemosphere 2016, 142, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Jatav, H.S.; Rajput, V.D.; Minkina, T.; Singh, S.K.; Chejara, S.; Gorovtsov, A.; Barakhov, A.; Bauer, T.; Sushkova, S.; Mandzhieva, S.; et al. Sustainable Approach and Safe Use of Biochar and Its Possible Consequences. Sustainability 2021, 13, 10362. [Google Scholar] [CrossRef]
- Elbehiry, F.; Darweesh, M.; Al-Anany, F.S.; Khalifa, A.M.; Almashad, A.A.; El-Ramady, H.; El-Banna, A.; Rajput, V.D.; Jatav, H.S.; Elbasiouny, H. Using Biochar and Nanobiochar of Water Hyacinth and Black Tea Waste in Metals Removal from Aqueous Solutions. Sustainability 2022, 14, 10118. [Google Scholar] [CrossRef]
- He, L.L.; Liu, Y.; Zhao, J.; Bi, Y.C.; Zhao, X.; Wang, S.Q.; Xing, G.X. Comparison of straw-biochar-mediated changes in nitrification and ammonia oxidizers in agricultural oxisols and cambosols. Biol. Fert. Soils 2016, 52, 137–149. [Google Scholar] [CrossRef]
- Bi, Q.F.; Chen, Q.H.; Yang, X.R.; Li, H.; Zheng, B.X.; Zhou, W.W.; Liu, X.X.; Dai, P.B.; Li, K.J.; Lin, X.Y. Effects of combined application of nitrogen fertilizer and biochar on the nitrification and ammonia oxidizers in an intensive vegetable soil. AMB Express 2017, 7, 198. [Google Scholar] [CrossRef]
- Li, F.; Liang, X.; He, S.; Li, M.; Cao, Y.; Zhang, J.; Tian, G. Biochar slows gross nitrification and gasses N emission via lower autotrophic nitrification in paddy soils. J. Soils Sediments 2020, 2, 629–640. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Zong, H.Y.; Zheng, H.; Liu, G.C.; Chen, L.; Xing, B.S. Reduced nitrification and abundance of ammonia-oxidizing bacteria in acidic soil amended with biochar. Chemosphere 2015, 138, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.M.; Li, X.P.; Dick, W.A.; Chen, L.M. Remediation of saline-sodic soil with flue gas desulfurization gypsum in a reclaimed tidal flat of southeast China. J. Environ. Sci. 2016, 45, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.G.; Wang, S.J.; Li, Y.; Liu, J.; Zhuo, Y.Q.; Chen, H.X.; Wang, J.; Xu, L.Z.; Sun, Z.T. Extensive reclamation of saline-sodic soils with flue gas desulfurization gypsum on the Songnen Plain, Northeast China. Geoderma 2018, 321, 52–60. [Google Scholar] [CrossRef]
- Li, Q.L.; Guo, X.B.; Lu, Y.Y.; Shan, G.C.; Huang, J.H. Impacts of adding FGDG on the abundance of nitrification and denitrification functional genes during dairy manure and sugarcane pressmud co-composting. Waste Manag. 2016, 56, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Bossolani, J.W.; Crusciol, C.A.C.; Merloti, L.F.; Moretti, L.G.; Costa, N.R.; Tsai, S.M.; Kuramae, E.E. Long-term lime and gypsum amendment increase nitrogen fixation and decrease nitrification and denitrification gene abundances in the rhizosphere and soil in a tropical no-till intercropping system. Geoderma 2020, 375, 114476. [Google Scholar] [CrossRef]
- Zhao, Y.G.; Wang, S.J.; Li, Y.; Zhuo, Y.Q.; Liu, J. Effects of straw layer and flue gas desulfurization gypsum treatments on soil salinity and sodicity in relation to sunflower yield. Geoderma 2019, 352, 13–21. [Google Scholar] [CrossRef]
- Zhou, X.; Fornara, D.; Wasson, E.A.; Wang, D.M.; Ren, G.D.; Christie, P.; Jia, Z.J. Effects of 44 years of chronic nitrogen fertilization on the soil nitrifying community of permanent grassland. Soil. Biol. Biochem. 2015, 91, 76–83. [Google Scholar] [CrossRef]
- Yao, R.J.; Gao, Q.C.; Li, H.Q.; Wang, X.P.; Xie, W.P.; Bai, Y.C.; Zhang, X. Legacy effect of tillage practices on soil ammonia-oxidizers and comammox communities over the sunflower (Helianthus annuus L.) growing season in a salt-affected irrigation area. Appl. Soil Ecol. 2024, 196, 105283. [Google Scholar] [CrossRef]
- Lu, R. Methods of Soil and Agro-Chemical Analysis; China Agricultural Science and Technology Press: Beijing, China, 2000. [Google Scholar]
- Wang, X.; Ding, J.; Han, L.; Tan, J.; Ge, X.; Nan, Q. Biochar addition reduces salinity in salt-affected soils with no impact on soil pH: A meta-analysis. Geoderma 2024, 443, 116845. [Google Scholar] [CrossRef]
- Blanco-Canqui, H. Does biochar application alleviate soil compaction? Review and data synthesis. Geoderma 2021, 404, 115317. [Google Scholar] [CrossRef]
- Li, J.B.; Liu, G.M.; Kwak, J.Y.O.; Chang, S.X.; Gao, H.C.; Wu, Q.C.; Yang, J.S.; Chen, J.L. Reclamation of desert land to continuous cotton cropping affects soil properties and microbial communities in the desert-oasis ecotone of Xinjiang, China. J. Soil. Sediment. 2020, 20, 862–873. [Google Scholar] [CrossRef]
- Yao, R.J.; Li, H.Q.; Yang, J.S.; Zhu, W.; Yin, C.Y.; Wang, X.P.; Xie, W.P.; Zhang, X. Combined application of biochar and N fertilizer shifted nitrification rate and gene abundance of ammonia-oxidizing microorganisms in salt-affected anthropogenic-alluvial soil. Appl. Soil. Ecol. 2022, 171, 104348. [Google Scholar] [CrossRef]
- Zhu, H.; Yang, J.S.; Yao, R.J.; Wang, X.P.; Xie, W.P.; Zhu, W.; Liu, X.Y.; Cao, Y.F.; Tao, J.Y. Interactive effects of soil amendments (biochar and gypsum) and salinity on ammonia volatilization in coastal saline soil. Catena 2020, 190, 104527. [Google Scholar] [CrossRef]
- Yao, R.J.; Li, H.Q.; Yang, J.S.; Wang, X.P.; Xie, W.P.; Zhang, X. Biochar Addition Inhibits Nitrification by Shifting Community Structure of Ammonia-Oxidizing Microorganisms in Salt-Affected Irrigation-Silting Soil. Microorganisms 2022, 10, 436. [Google Scholar] [CrossRef]
- Meinhardt, K.A.; Stopnisek, N.; Pannu, M.W.; Strand, S.E.; Fransen, S.C.; Casciotti, K.L.; Stahl, D.A. Ammonia-oxidizing bacteria are the primary N2O producers in an ammonia-oxidizing archaea dominated alkaline agricultural soil. Environ. Microbiol. 2018, 20, 2195–2206. [Google Scholar] [CrossRef]
- Shi, Y.L.; Liu, X.R.; Zhang, Q.W. Effects of combined biochar and organic fertilizer on nitrous oxide fluxes and the related nitrifier and denitrifier communities in a saline-alkali soil. Sci. Total Environ. 2019, 686, 199–211. [Google Scholar] [CrossRef]
- Zou, W.X.; Lang, M.; Zhang, L.; Liu, B.; Chen, X.P. Ammonia-oxidizing bacteria rather than ammonia-oxidizing archaea dominate nitrification in a nitrogen-fertilized calcareous soil. Sci. Total Environ. 2022, 811, 151402. [Google Scholar] [CrossRef]
- Lin, Y.; Ding, W.; Liu, D.; He, T.; Yoo, G.; Yuan, J.; Chen, Z.; Fan, J. Wheat straw-derived biochar amendment stimulated N2O emissions from rice paddy soils by regulating the amoA genes of ammonia-oxidizing bacteria. Soil Biol. Biochem. 2017, 113, 89–98. [Google Scholar] [CrossRef]
- Chen, H.; Yin, C.; Fan, X.P.; Ye, M.J.; Peng, H.Y.; Li, T.Q.; Zhao, Y.H.; Wakelin, S.A.; Chu, G.X.; Liang, Y.C. Reduction of N2O emission by biochar and/or 3,4-dimethylpyrazole phosphate (DMPP) is closely linked to soil ammonia oxidizing bacteria and nosZI-N2O reducer populationsyy. Sci. Total Environ. 2019, 694, 133658. [Google Scholar] [CrossRef]
- Cui, Y.W.; Zhang, H.Y.; Ding, J.R.; Peng, Y.Z. The effects of salinity on nitrification using halophilic nitrifiers in a Sequencing Batch Reactor treating hypersaline wastewater. Sci. Rep. 2016, 6, 24825. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.J.; Zhang, X.L.; Ma, B.; Chang, S.X.; Gong, J. Biochar addition affected the dynamics of ammonia oxidizers and nitrification in microcosms of a coastal alkaline soil. Biol. Fert. Soils 2014, 50, 321–332. [Google Scholar] [CrossRef]
- Li, Y.; Wei, H.; Liu, M.; Yang, J.; Han, X. Nine years of low-dose biochar amendment suppresses nitrification rate in low-yield brown soil. Appl. Soil. Ecol. 2023, 192, 105096. [Google Scholar] [CrossRef]
- Zhang, K.L.; Chen, L.; Li, Y.; Brookes, P.C.; Xu, J.M.; Luo, Y. The effects of combinations of biochar, lime, and organic fertilizer on nitrification and nitrifiers. Biol. Fert. Soils 2017, 53, 77–87. [Google Scholar] [CrossRef]
- Zhang, W.C.; Zhang, W.X.; Zhao, Y.G.; Wang, S.J.; Liu, J.; Li, Y. Water regime enhances the effects of flue gas desulfurization gypsum on the reclamation of highly saline-sodic soil. Land. Degrad. Dev. 2023, 34, 981–991. [Google Scholar] [CrossRef]
- Amezketa, E.; Aragüés, R.; Gazol, R. Efficiency of Sulfuric Acid, Mined Gypsum, and Two Gypsum By-Products in Soil Crusting Prevention and Sodic Soil Reclamation. Agron. J. 2005, 97, 983–989. [Google Scholar] [CrossRef]
- Egbeagu, U.U.; Liu, W.Y.; Zhang, J.N.; Sun, L.; Bello, A.; Wang, B.; Deng, L.T.; Sun, Y.; Han, Y.; Zhao, Y.; et al. The activity of ammonia-oxidizing bacteria on the residual effect of biochar-compost amended soils in two cropping seasons. Biochem. Eng. J. 2023, 191, 108778. [Google Scholar] [CrossRef]

| EC (μs cm−1) | pH | TOC (g kg−1) | TN (g kg−1) | NH4+ (mg kg−1) | NO3− (mg kg−1) | SWC (g g−1) | BD (g cm−3) | |
|---|---|---|---|---|---|---|---|---|
| BC | 459.43 ± 27.54 c | 9.10 ± 0.04 a | 11.25 ± 0.97 a | 0.61 ± 0.03 a | 3.79 ± 0.36 a | 6.55 ± 1.14 a | 0.27 ± 0.02 a | 1.21 ± 0.02 b |
| SG | 646.33 ± 14.94 a | 8.71 ± 0.06 b | 8.82 ± 0.28 b | 0.58 ± 0.01 ab | 3.55 ± 0.27 a | 5.63 ± 0.48 a | 0.24 ± 0.01 a | 1.25 ± 0.02 a |
| CK | 515.67 ± 20.79 b | 8.99 ± 0.06 a | 8.47 ± 0.40 b | 0.56 ± 0.01 b | 2.67 ± 0.20 b | 6.27 ± 0.93 a | 0.25 ± 0.00 a | 1.28 ± 0.01 a |
| PNR | AOA | AOB | |
|---|---|---|---|
| PNR | 1 | ||
| AOA | 0.583 | 1 | |
| AOB | 0.707 * | 0.597 | 1 |
| Soil Properties | AOA | AOB | ||||
|---|---|---|---|---|---|---|
| OTUs | Shannon | Chao | OTUs | Shannon | Chao | |
| EC | −0.348 | −0.142 | −0.067 | 0.082 | 0.011 | 0.104 |
| pH | 0.368 | −0.095 | 0.067 | 0.094 | −0.034 | 0.075 |
| TOC | 0.025 | −0.203 | 0.127 | −0.127 | −0.726 * | −0.165 |
| TN | 0.101 | −0.477 | 0.235 | 0.036 | −0.859 * | 0.005 |
| NH4+ | −0.276 | −0.241 | −0.074 | −0.094 | −0.690 * | −0.15 |
| NO3− | 0.435 | −0.296 | 0.08 | −0.019 | −0.436 | 0.022 |
| BD | 0.322 | −0.397 | 0.144 | 0.261 | −0.472 | 0.231 |
| SWC | 0.172 | 0.178 | 0.108 | 0.276 | 0.665 | 0.333 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, H.; Liu, Y.; Yao, R. Impacts of Biochar and Gypsum on Ammonia-Oxidizing Microorganisms in Coastal Saline Soil. Agronomy 2024, 14, 1756. https://doi.org/10.3390/agronomy14081756
Zhu H, Liu Y, Yao R. Impacts of Biochar and Gypsum on Ammonia-Oxidizing Microorganisms in Coastal Saline Soil. Agronomy. 2024; 14(8):1756. https://doi.org/10.3390/agronomy14081756
Chicago/Turabian StyleZhu, Hai, Yuxing Liu, and Rongjiang Yao. 2024. "Impacts of Biochar and Gypsum on Ammonia-Oxidizing Microorganisms in Coastal Saline Soil" Agronomy 14, no. 8: 1756. https://doi.org/10.3390/agronomy14081756
APA StyleZhu, H., Liu, Y., & Yao, R. (2024). Impacts of Biochar and Gypsum on Ammonia-Oxidizing Microorganisms in Coastal Saline Soil. Agronomy, 14(8), 1756. https://doi.org/10.3390/agronomy14081756





