Tillage Practices Effect on Root Distribution and Variation of Soil CO2 Emission under Different Cropping Strategies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Location
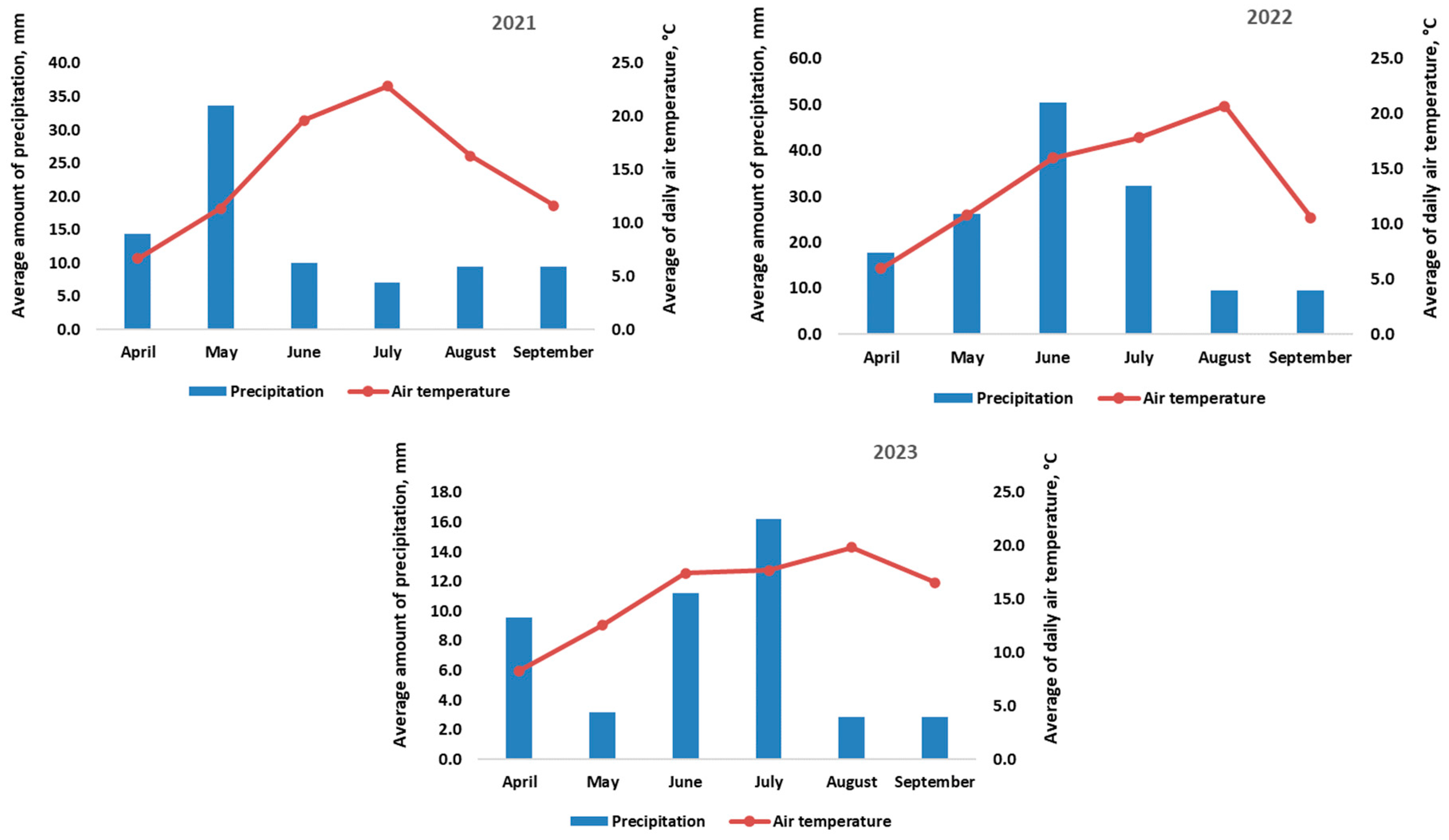
2.2. Meteorological Conditions
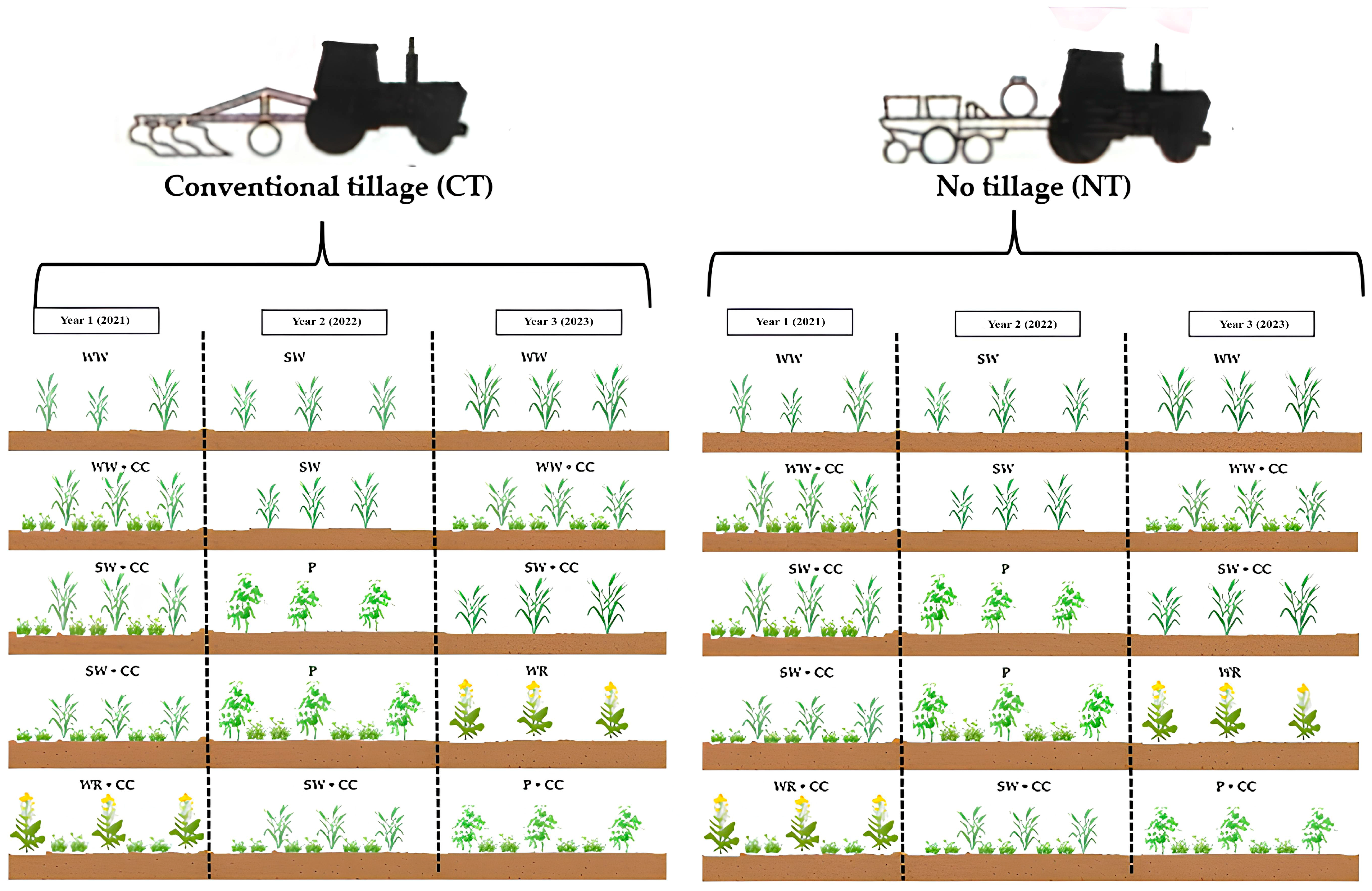
2.3. Experimental Design and Treatments
2.4. Measurement of Soil CO2 Emissions
2.5. Investigations of Root Parameters
2.6. Statistical Analysis
3. Results and Discussion
3.1. Changes in Root Parameters under Different Cropping Ant Tillage Strategies
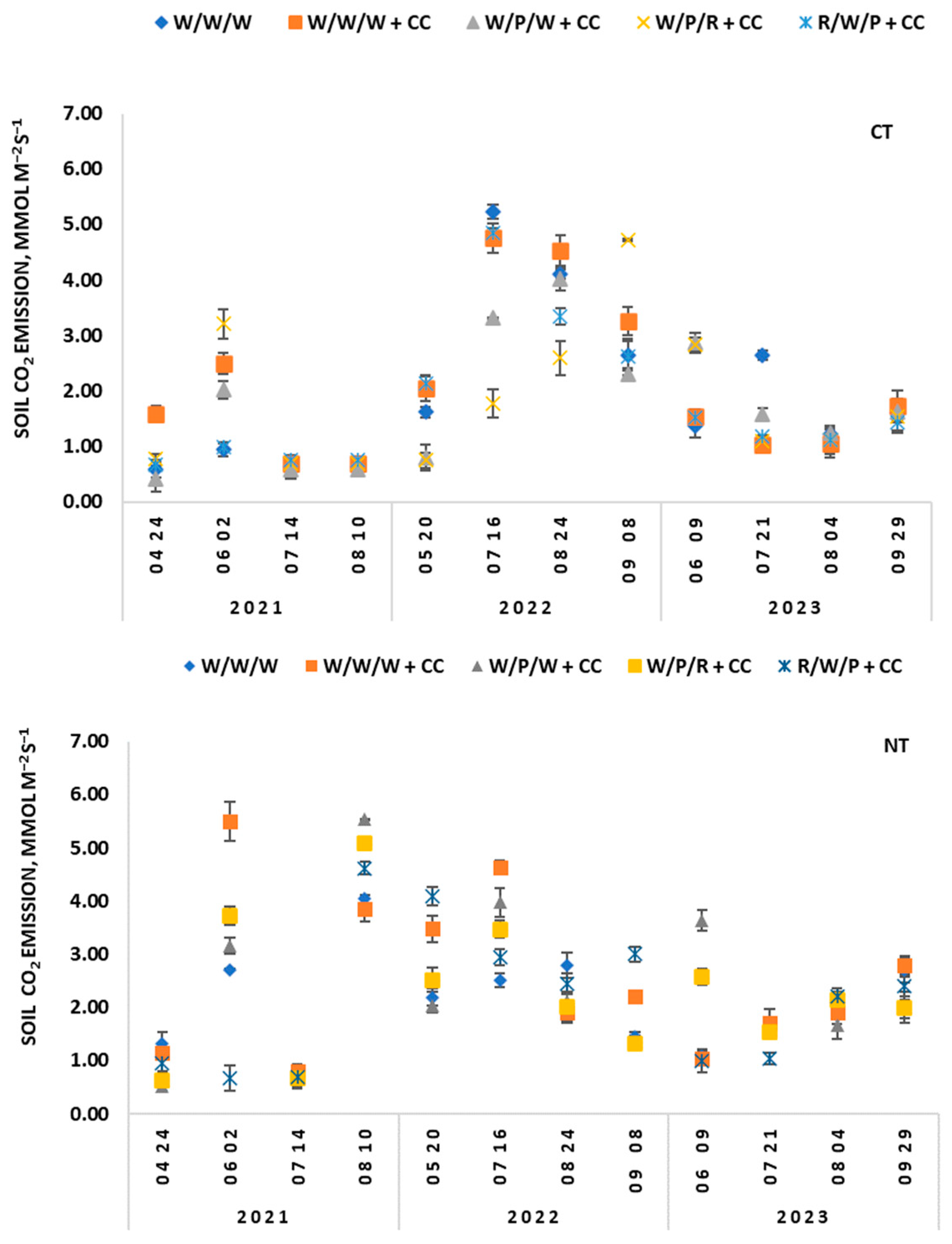
3.2. Soil CO2 Emissions in Response to Tillage and Different Cropping Strategies
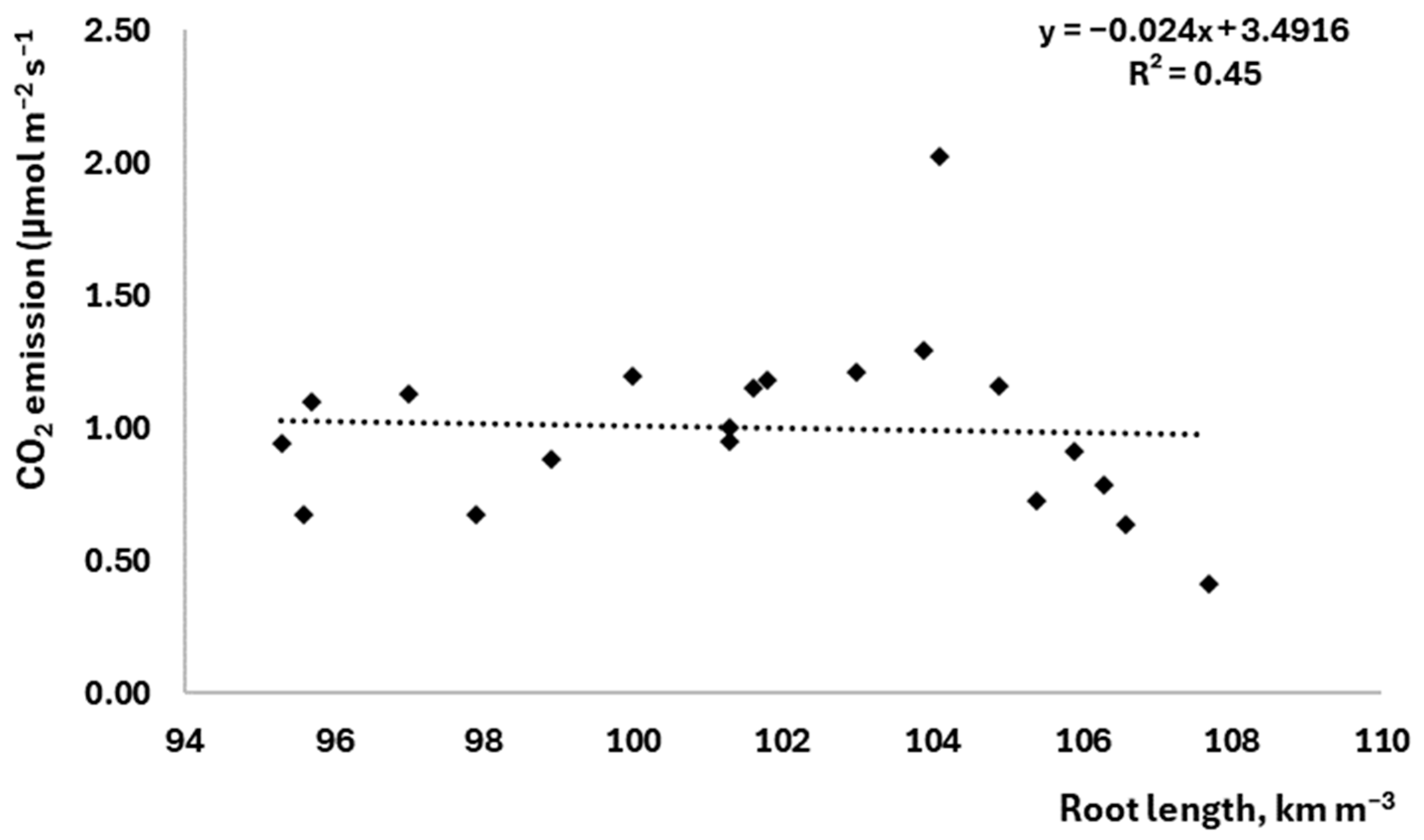
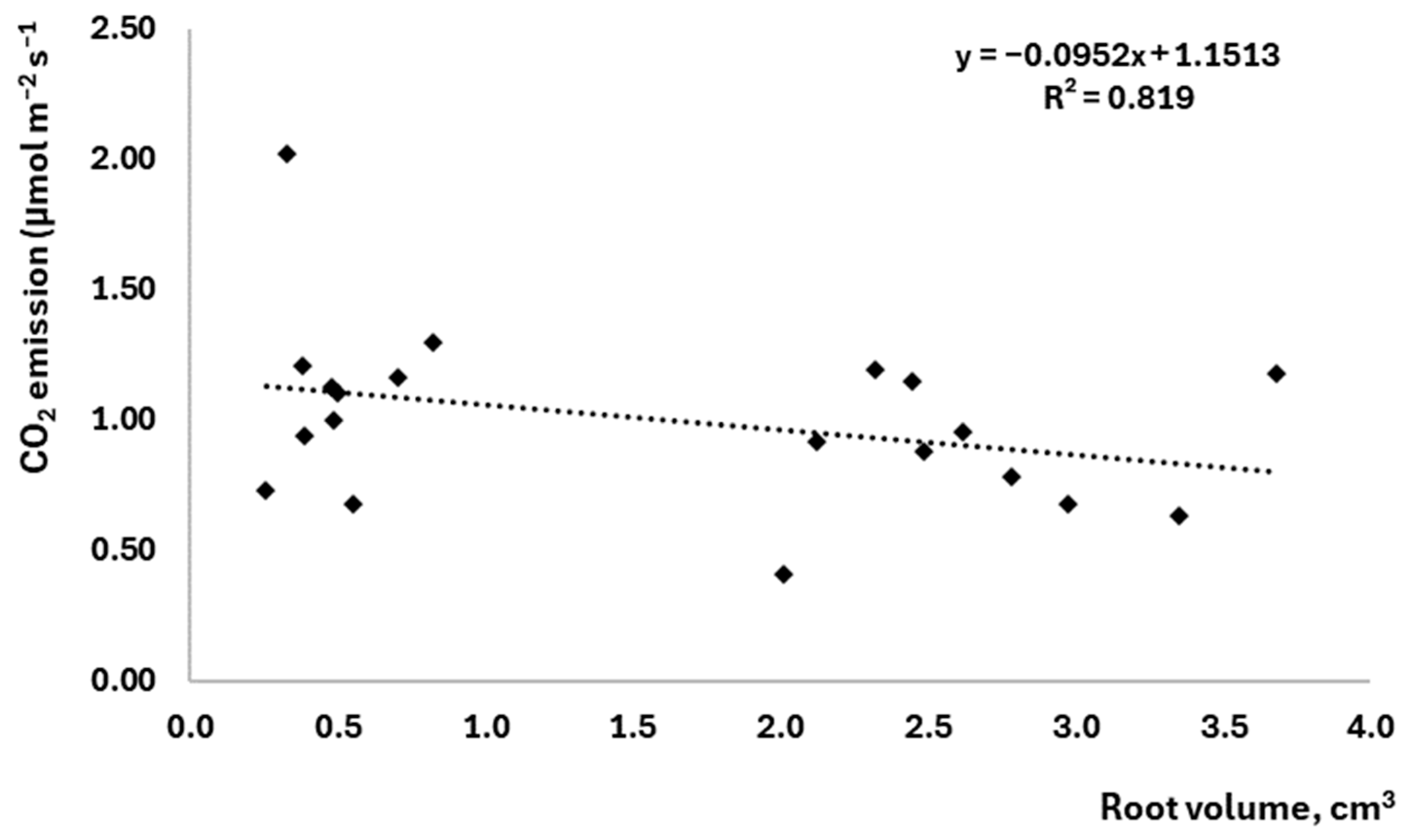
3.3. The Effect of Root Parameters on Soil CO2 Emissions
4. Conclusions
- The tillage method was identified as one of the main factors determining CO2 emissions. Reducing the intensification of tillage promoted the release of carbon dioxide from the soil.
- The inclusion of legumes and catch crops in the rotation promoted CO2 emissions from the soil.
- Soil depth, type of cropping systems and inclusion of CC significantly decreased the root length and root volume. The root volume was significantly lower in the rotations where the catch crop (CC) was grown each year (R/W/P + CC) compared with the rotation where CC where only wheat was grown—(W/W/W).
- Root length and root volume had a positive effect on soil CO2 emissions, suggesting that root activity is a major factor in the production of CO2 emissions from the soil.
- From a practical viewpoint, the results showed that cropping strategies diversification with the inclusion of CC every year (R/W/P + CC) demonstrates the possibility of reducing CO2 emission and improving root network parameters compared to monoculture. This informs us of the necessity of crop rotation implementation under moderate climatic conditions.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Arora, N.K. Impact of Climate Change on Agriculture Production and Its Sustainable Solutions. Environ. Sustain. 2019, 2, 95–96. [Google Scholar] [CrossRef]
- Smith, P.; Bustamenta, M.; Krug, T.; Nabuurs, G.-J.; Molodovskaya Canada, M.; Bustamante, M.; Ahammad, H.; Clark, H.; Dong, H.; Elsiddig, E.A.; et al. IPCC Fifth Assessment Report: Agriculture, Forestry and Other Land Use (AFOLU). In Climate Change 2014: Mitigation of Climate Change. Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Hopkins, F.; Gonzalez-Meler, M.A.; Flower, C.E.; Lynch, D.J.; Czimczik, C.; Tang, J.; Subke, J.A. Ecosystem-Level Controls on Root-Rhizosphere Respiration. New Phytol. 2013, 199, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, W.; Sun, T.; Chen, L.; Pang, X.; Wang, Y.; Xiao, F. N and P Fertilization Reduced Soil Autotrophic and Heterotrophic Respiration in a Young Cunninghamia Lanceolata Forest. Agric. For. Meteorol. 2017, 232, 66–73. [Google Scholar] [CrossRef]
- Yan, M.; Guo, N.; Ren, H.; Zhang, X.; Zhou, G. Autotrophic and Heterotrophic Respiration of a Poplar Plantation Chronosequence in Northwest China. For. Ecol. Manag. 2015, 337, 119–125. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Chang, S.X.; Liang, X.; Qin, H.; Chen, J.; Xu, Q. Linking Soil Fungal Community Structure and Function to Soil Organic Carbon Chemical Composition in Intensively Managed Subtropical Bamboo Forests. Soil Biol. Biochem. 2017, 107, 19–31. [Google Scholar] [CrossRef]
- Subke, J.; Inglima, I.; Cotrufo, M.F. Trends and Methodological Impacts in Soil CO2 Efflux Partitioning: A Metaanalytical Review. Glob. Chang. Biol. 2006, 12, 921–943. [Google Scholar] [CrossRef]
- Bond-Lamberty, B.; Wang, C.; Gower, S.T. A Global Relationship between the Heterotrophic and Autotrophic Components of Soil Respiration? Glob. Chang. Biol. 2004, 10, 1756–1766. [Google Scholar] [CrossRef]
- Saurette, D.D.; Chang, S.X.; Thomas, B.R. Autotrophic and Heterotrophic Respiration Rates across a Chronosequence of Hybrid Poplar Plantations in Northern Alberta. Can. J. Soil Sci. 2008, 88, 261–272. [Google Scholar] [CrossRef]
- Tu, L.-H.; Hu, T.-X.; Zhang, J.; Li, X.-W.; Hu, H.-L.; Liu, L.; Xiao, Y.-L. Nitrogen Addition Stimulates Different Components of Soil Respiration in a Subtropical Bamboo Ecosystem. Soil Biol. Biochem. 2013, 58, 255–264. [Google Scholar] [CrossRef]
- Atarashi-Andoh, M.; Koarashi, J.; Ishizuka, S.; Hirai, K. Seasonal Patterns and Control Factors of CO2 Effluxes from Surface Litter, Soil Organic Carbon, and Root-Derived Carbon Estimated Using Radiocarbon Signatures. Agric. For. Meteorol. 2012, 152, 149–158. [Google Scholar] [CrossRef]
- Lehmann, J.; Bossio, D.A.; Kögel-Knabner, I.; Rillig, M.C. The Concept and Future Prospects of Soil Health. Nat. Rev. Earth Environ. 2020, 1, 544–553. [Google Scholar] [CrossRef]
- Kraamwinkel, C.T.; Beaulieu, A.; Dias, T.; Howison, R.A. Planetary Limits to Soil Degradation. Commun. Earth Environ. 2021, 2, 249. [Google Scholar] [CrossRef]
- King, A.E.; Blesh, J. Crop Rotations for Increased Soil Carbon: Perenniality as a Guiding Principle: Perenniality. Ecol. Appl. 2018, 28, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.P.; Setia, R.; Wiesmeier, M.; Kunhikrishnan, A. Agricultural Management Practices and Soil Organic Carbon Storage. In Soil Carbon Storage: Modulators, Mechanisms and Modeling; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Abdalla, K.; Chivenge, P.; Ciais, P.; Chaplot, V. No-Tillage Lessens Soil CO2 Emissions the Most under Arid and Sandy Soil Conditions: Results from a Meta-Analysis. Biogeosciences 2016, 13, 3619–3633. [Google Scholar] [CrossRef]
- Kumara, T.K.; Kandpal, A.; Pal, S. A meta-Analysis of Economic and Environmental Benefits of Conservation Agriculture in South Asia. J. Environ. Manag. 2020, 269, 110773. [Google Scholar] [CrossRef] [PubMed]
- Moussadek, R.; Mrabet, R.; Dahan, R.; Douaik, A.; Verdoodt, A.; van Ranst, E. Effect of Tillage Practices on the Soil Carbon Dioxide Flux during Fall and Spring Seasons in a Mediterranean Vertisol. J. Soil Sci. Environ. Manag. 2011, 2, 362–369. [Google Scholar]
- Valkama, E.; Kunypiyaeva, G.; Zhapayev, R.; Karabayev, M.; Zhusupbekov, E.; Perego, A.; Schillaci, C.; Sacco, D.; Moretti, B.; Grignani, C.; et al. Can Conservation Agriculture Increase Soil Carbon Sequestration? A Modelling Approach. Geoderma 2020, 369, 114298. [Google Scholar] [CrossRef]
- Jat, H.S.; Datta, A.; Choudhary, M.; Yadav, A.K.; Choudhary, V.; Sharma, P.C.; Gathala, M.K.; Jat, M.L.; McDonald, A. Effects of Tillage, Crop Establishment and Diversification on Soil Organic Carbon, Aggregation, Aggregate Associated Carbon and Productivity in Cereal Systems of Semi-Arid Northwest India. Soil Tillage Res. 2019, 190, 128–138. [Google Scholar] [CrossRef]
- Qin, R.; Stamp, P.; Richner, W. Impact of Tillage on Root Systems of Winter Wheat. Agron. J. 2004, 96, 1523–1530. [Google Scholar] [CrossRef]
- Ghimire, R.; Norton, J.B.; Stahl, P.D.; Norton, U. Soil Microbial Substrate Properties and Microbial Community Responses under Irrigated Organic and Reduced-Tillage Crop and Forage Production Systems. PLoS ONE 2014, 9, e103901. [Google Scholar] [CrossRef]
- Van Kessel, C.; Venterea, R.; Six, J.; Adviento-Borbe, M.A.; Linquist, B.; van Groenigen, K.J. Climate, Duration, and N Placement Determine N2O Emissions in Reduced Tillage Systems: A Meta-Analysis. Glob. Chang. Biol. 2013, 19, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, S.; Pu, C.; Zhang, X.; Xue, J.; Zhang, R.; Wang, Y.; Lal, R.; Zhang, H.; Chen, F. Methane and Nitrous Oxide Emissions under No-Till Farming in China: A Meta-Analysis. Glob. Chang. Biol. 2016, 22, 1372–1384. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.W.; Peters, G.P.; Gasser, T.; Andrew, R.M.; Schwingshackl, C.; Gütschow, J.; Houghton, R.A.; Friedlingstein, P.; Pongratz, J.; Le Quéré, C. National Contributions to Climate Change Due to Historical Emissions of Carbon Dioxide, Methane, and Nitrous Oxide Since 1850. Sci. Data 2023, 10, 155. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Lu, X.; Tanveer, S.K.; Wen, X.; Liao, Y. Effects of Tillage Management on soil CO2 Emission and Wheat Yield under rain-Fed Conditions. Soil Res. 2016, 54, 38. [Google Scholar] [CrossRef]
- Oorts, K.; Merckx, R.; Gréhan, E.; Labreuche, J.; Nicolardot, B. Determinants of Annual Fluxes of CO2 and N2O in Long-Term No-Tillage and Conventional Tillage Systems in northern France. Soil Tillage Res. 2007, 95, 133–148. [Google Scholar] [CrossRef]
- Yao, Z.; Zheng, X.; Wang, R.; Xie, B.; Butterbach-Bahl, K.; Zhu, J. Nitrous Oxide and Methane Fluxes from a Rice–Wheat Crop Rotation under Wheat Residue Incorporation and No-Tillage Practices. Atmos. Environ. 2013, 79, 641–649. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, J.; Liu, T.; Cao, C.; Li, C. Effects of Nitrogen Fertilizer Sources and Tillage Practices on Greenhouse Gas Emissions in Paddy Fields of Central China. Atmos. Environ. 2016, 144, 274–281. [Google Scholar] [CrossRef]
- Kim, S.Y.; Gutierrez, J.; Kim, P.J. Unexpected Stimulation of CH4 Emissions under Continuous No-Tillage System in Mono-Rice Paddy Soils during Cultivation. Geoderma 2016, 267, 34–40. [Google Scholar] [CrossRef]
- He, J.; Li, H.; Rasaily, R.G.; Wang, Q.; Cai, G.; Su, Y.; Qiao, X.; Liu, L. Soil Properties and Crop Yields after 11 Years of no Tillage Farming in Wheat–Maize Cropping System in North China Plain. Soil Tillage Res. 2011, 113, 48–54. [Google Scholar] [CrossRef]
- Plaza-Bonilla, D.; Cantero-Martínez, C.; Bareche, J.; Arrúe, J.L.; Álvaro-Fuentes, J. Soil Carbon Dioxide and Methane Fluxes as Affected by Tillage and N Fertilization in Dryland Conditions. Plant Soil 2014, 381, 111–130. [Google Scholar] [CrossRef]
- Mrabet, R. No-Tillage Agriculture in West Asia and North Africa. In Rainfed Farming Systems; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Desoky, E.-S.M.; Mansour, E.; Yasin, M.A.T.; El-Sobky, E.-S.E.A.; Rady, M.M. Improvement of Drought tolerance in Five Different Cultivars of Vicia Faba with Foliar Application of Ascorbic Acid or Silicon. Span. J. Agric. Res. 2020, 18, e0802. [Google Scholar] [CrossRef]
- Frasier, I.; Quiroga, A.; Noellemeyer, E. Effect of Different Cover Crops on C and N Cycling in Sorghum NT Systems. Sci. Total Environ. 2016, 562, 628–639. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Cobena, A.; García-Marco, S.; Quemada, M.; Gabriel, J.L.; Almendros, P.; Vallejo, A. Do Cover Crops Enhance N2O, CO2 or CH4 Emissions from Soil in Mediterranean Arable Systems? Sci. Total Environ. 2014, 466–467, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Skinner, C.; Gattinger, A.; Krauss, M.; Krause, H.-M.; Mayer, J.; van der Heijden, M.G.A.; Mäder, P. The Impact of Long-Term Organic Farming on Soil-Derived Greenhouse Gas Emissions. Sci. Rep. 2019, 9, 1702. [Google Scholar] [CrossRef] [PubMed]
- Youngerman, C.Z.; Ditommaso, A.; Curran, W.S.; Mirsky, S.B.; Ryan, M.R. Corn Density Effect on Interseeded Cover Crops, Weeds, and Grain Yield. Agron. J. 2018, 110, 2478–2487. [Google Scholar] [CrossRef]
- Cueva, A.; Bullock, S.H.; López-Reyes, E.; Vargas, R. Potential Bias of Daily Soil CO2 Efflux Estimates Due to Sampling Time. Sci. Rep. 2017, 7, 11925. [Google Scholar] [CrossRef]
- Bouma, T.J.; Nielsen, K.L.; Koutstaal, B. Sample Preparation and Scanning Protocol for Computerised Analysis of Root Length and Diameter. Plant Soil 2000, 218, 185–196. [Google Scholar] [CrossRef]
- Seethepalli, A.; Dhakal, K.; Griffiths, M.; Guo, H.; Freschet, G.T.; York, L.M. RhizoVision Explorer: Open-Source Software for Root Image Analysis and Measurement Standardization. AoB Plants 2021, 13, plab056. [Google Scholar] [CrossRef]
- Shi, X.; Yang, X.; Drury, C.; Reynolds, W.; McLaughlin, N.; Zhang, X. Impact of Ridge Tillage on soil Organic Carbon and Selected Physical Properties of a Clay Loam in Southwestern Ontario. Soil Tillage Res. 2012, 120, 1–7. [Google Scholar] [CrossRef]
- Li, S.; Jiang, X.; Wang, X.; Wright, A.L. Tillage Effects on Soil Nitrification and the Dynamic Changes in Nitrifying Microorganisms in a Subtropical Rice-Based Ecosystem: A Long-Term Field Study. Soil Tillage Res. 2015, 150, 132–138. [Google Scholar] [CrossRef]
- Säle, V.; Aguilera, P.; Laczko, E.; Mäder, P.; Berner, A.; Zihlmann, U.; van der Heijden, M.G.; Oehl, F. Impact of Conservation Tillage and Organic Farming on the Diversity of Arbuscular Mycorrhizal Fungi. Soil Biol. Biochem. 2015, 84, 38–52. [Google Scholar] [CrossRef]
- Feiziene, D.; Kadžiene, G. The Influence of Soil Organic Carbon, Moisture and Temperature on Soil Surface CO2 Emission in the 10 Th Year of Different Tillage-Fertilisation Management. Zemdirbyste 2008, 95, 29–45. [Google Scholar]
- Velykis, A.; Satkus, A. The Impact of Tillage, Ca-Amendment and Cover Crop on the Physical State of a Clay Loam Soil. Zemdirbyste-Agriculture 2018, 105, 3–10. [Google Scholar] [CrossRef]
- Munkholm, L.J.; Hansen, E.M.; Olesen, J.E. The Effect of Tillage Intensity on Soil Structure and Winter Wheat Root/Shoot Growth. Soil Use Manag. 2008, 24, 392–400. [Google Scholar] [CrossRef]
- Bécel, C.; Vercambre, G.; Pagès, L. Soil Penetration Resistance, a Suitable Soil Property to Account for Variations in Root Elongation and Branching. Plant Soil 2012, 353, 169–180. [Google Scholar] [CrossRef]
- Bodner, G.; Leitner, D.; Kaul, H.-P. Coarse and Fine Root Plants Affect Pore Size Distributions Differently. Plant Soil 2014, 380, 133–151. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Weil, R.R. Root Growth and Yield of Maize as Affected by Soil Compaction and COVER crops. Soil Tillage Res. 2011, 117, 17–27. [Google Scholar] [CrossRef]
- Correa, J.; Postma, J.A.; Watt, M.; Wojciechowski, T. Soil Compaction and the Architectural Plasticity of Root Systems. J. Exp. Bot. 2019, 70, 6019–6034. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Nest, T.V.; Ruysschaert, G.; D’hose, T.; Cornelis, W.M. Short-Term Effects of Cover Crops and Tillage Methods on Soil Physical Properties and Maize Growth in a Sandy Loam soil. Soil Tillage Res. 2019, 192, 76–86. [Google Scholar] [CrossRef]
- Kolb, E.; Legué, V.; Bogeat-Triboulot, M.-B. Physical Root-Soil Interactions. Phys. Biol. 2017, 14, 065004. [Google Scholar] [CrossRef]
- Tubeileh, A.; Groleau-Renaud, V.; Plantureux, S.; Guckert, A. Effect of Soil Compaction on Photosynthesis and Carbon Partitioning within a Maize–Soil System. Soil Tillage Res. 2003, 71, 151–161. [Google Scholar] [CrossRef]
- Veršulienė, A.; Kadžienė, G.; Kochiieru, M.; Pranaitienė, S.; Meškauskienė, L.; Auškalnienė, O. Response of Spring Barley Root and Soil Physical Properties to Changes under Cover Crop and Different Tillage. Zemdirbyste 2022, 109, 291–296. [Google Scholar] [CrossRef]
- Page, K.L.; Bell, M.; Dalal, R.C. Changes in Total Soil Organic Carbon Stocks and Carbon Fractions in Sugarcane Systems as Affected by Tillage and Trash Management in Queensland, Australia. Soil Res. 2013, 51, 608–614. [Google Scholar] [CrossRef]
- Mangalassery, S.; Mooney, S.; Sparkes, D.; Fraser, W.; Sjögersten, S. Impacts of Zero Tillage on Soil Enzyme Activities, Microbial Characteristics and Organic Matter Functional Chemistry in Temperate Soils. Eur. J. Soil Biol. 2015, 68, 9–17. [Google Scholar] [CrossRef]
- Sheehy, J.; Regina, K.; Alakukku, L.; Six, J. Impact of No-Till and Reduced Tillage on Aggregation and Aggregate-Associated Carbon in Northern European Agroecosystems. Soil Tillage Res. 2015, 150, 107–113. [Google Scholar] [CrossRef]
- Shahidi, B.; Dyck, M.; Malhi, S. Carbon Dioxide Emissions from Tillage of Two Long-Term No-Till Canadian Prairie Soils. Soil Tillage Res. 2014, 144, 72–82. [Google Scholar] [CrossRef]
- Fuentes, M.; Hidalgo, C.; Etchevers, J.; De León, F.; Guerrero, A.; Dendooven, L.; Verhulst, N.; Govaerts, B. Conservation Agriculture, Increased Organic Carbon in the Top-Soil Macro-Aggregates and Reduced Soil CO2 Emissions. Plant Soil 2012, 355, 183–197. [Google Scholar] [CrossRef]
- Vinten, A.J.A.; Ball, B.C.; O’Sullivan, M.F.; Henshall, J.K. The Effects of Cultivation Method, Fertilizer Input and Previous Sward Type on Organic C and N Storage and Gaseous Losses under Spring and Winter Barley Following Long-Term Leys. J. Agric. Sci. 2002, 139, 231–243. [Google Scholar] [CrossRef]
- Jans, W.W.; Jacobs, C.M.; Kruijt, B.; Elbers, J.A.; Barendse, S.; Moors, E.J. Carbon Exchange of a maize (Zea mays L.) Crop: Influence of Phenology. Agric. Ecosyst. Environ. 2010, 139, 316–324. [Google Scholar] [CrossRef]
- Kan, Z.; Liu, W.; Lal, R.; Dang, Y.P.; Zhao, X.; Zhang, H. Mechanisms of Soil Organic Carbon Stability and Its Response to No-till: A Global Synthesis and Perspective. Glob. Chang. Biol. 2022, 28, 693–710. [Google Scholar] [CrossRef]
- Jayarathne, J.; Deepagoda, T.C.; Clough, T.J.; Thomas, S.; Elberling, B.; Smits, K.M. Effect of Aggregate Size Distribution on Soil Moisture, Soil-Gas Diffusivity, and N2O Emissions from a Pasture Soil. Geoderma 2021, 383, 114737. [Google Scholar] [CrossRef]
- Cheng-Fang, L.; Dan-Na, Z.; Zhi-Kui, K.; Zhi-Sheng, Z.; Jin-Ping, W.; Ming-Li, C.; Cou-Gui, C. Effects of Tillage and Nitrogen Fertilizers on CH4 and CO2 Emissions and Soil Organic Carbon in Paddy Fields of Central China. PLoS ONE 2012, 7, e34642. [Google Scholar] [CrossRef] [PubMed]
- Ussiri, D.A.; Lal, R. Long-Term Tillage Effects on Soil Carbon Storage and Carbon Dioxide Emissions in Continuous Corn Cropping System from an Alfisol in Ohio. Soil Tillage Res. 2009, 104, 39–47. [Google Scholar] [CrossRef]
- Shakoor, A.; Shahbaz, M.; Farooq, T.H.; Sahar, N.E.; Shahzad, S.M.; Altaf, M.M.; Ashraf, M. A Global Meta-Analysis of Greenhouse Gases Emission and Crop Yield under No-Tillage as Compared to Conventional Tillage. Sci. Total Environ. 2021, 750, 142299. [Google Scholar] [CrossRef] [PubMed]
- Austin, E.E.; Wickings, K.; McDaniel, M.D.; Robertson, G.P.; Grandy, A.S. Cover Crop Root Contributions to Soil Carbon in a No-Till Corn Bioenergy Cropping System. GCB Bioenergy 2017, 9, 1252–1263. [Google Scholar] [CrossRef]
- Wortman, S.E.; Francis, C.A.; Lindquist, J.L. Cover Crop Mixtures for the Western Corn Belt: Opportunities for Increased Productivity and Stability. Agron. J. 2012, 104, 699–705. [Google Scholar] [CrossRef]
- Finney, D.M.; White, C.M.; Kaye, J.P. Biomass Production and Carbon/Nitrogen Ratio Influence Ecosystem Services from Cover Crop Mixtures. Agron. J. 2016, 108, 39–52. [Google Scholar] [CrossRef]
- Noland, R.L.; Wells, M.S.; Sheaffer, C.C.; Baker, J.M.; Martinson, K.L.; Coulter, J.A. Establishment and Function of Cover Crops Interseeded into Corn. Crop Sci. 2018, 58, 863–873. [Google Scholar] [CrossRef]
- Chirinda, N.; Olesen, J.E.; Porter, J.R.; Schjønning, P. Soil Properties, Crop Production and Greenhouse Gas Emissions from Organic and Inorganic Fertilizer-Based Arable Cropping Systems. Agric. Ecosyst. Environ. 2010, 139, 584–594. [Google Scholar] [CrossRef]
- Ferrara, R.M.; Campi, P.; Muschitiello, C.; Leogrande, R.; Vonella, A.V.; Ventrella, D.; Rana, G. Soil Respiration during Three Cropping Cycles of Durum Wheat under Different Tillage Conditions in a Mediterranean Environment. Soil Use Manag. 2022, 38, 1547–1563. [Google Scholar] [CrossRef]
- Vicente-Vicente, J.L.; Gómez-Muñoz, B.; Hinojosa-Centeno, M.B.; Smith, P.; Garcia-Ruiz, R. Carbon Saturation and Assessment of Soil Organic Carbon Fractions in Mediterranean Rainfed Olive Orchards under Plant Cover Management. Agric. Ecosyst. Environ. 2017, 245, 135–146. [Google Scholar] [CrossRef]
- Tiemann, L.K.; Grandy, A.S.; Atkinson, E.E.; Marin-Spiotta, E.; McDaniel, M.D. Crop Rotational Diversity Enhances Belowground Communities and Functions in an Agroecosystem. Ecol. Lett. 2015, 18, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Gómez, J.A. Sustainability Using Cover Crops in Mediterranean Tree Crops, Olives and Vines—Challenges and Current Knowledge. Hung. Geogr. Bull. 2017, 66, 13–28. [Google Scholar] [CrossRef]
- Shibistova, O.; Lloyd, J.; Evgrafova, S.; Savushkina, N.; Zrazhevskaya, G.; Arneth, A.; Knohl, A.; Kolle, O.; Schulze, E.-D. Seasonal and Spatial Variability in soil CO2 Efflux Rates for a Central Siberian Pinus Sylvestris Forest. Tellus B Chem. Phys. Meteorol. 2002, 54, 552–567. [Google Scholar] [CrossRef]
- Kochiieru, M.; Feiza, V.; Feiziene, D.; Volungevicius, J.; Deveikyte, I.; Seibutis, V.; Pranaitiene, S. The Effect of Environmental Factors and Root System on CO2 Efflux in Different Types of Soil and Land Uses. Zemdirbyste 2021, 108, 3–10. [Google Scholar] [CrossRef]
| Year | 2021 | 2022 | 2023 | Long-Term Mean (1924–2021) |
|---|---|---|---|---|
| Annual mean air temperature, °C | 7.4 | 8.0 | 8.8 | 6.6 |
| Difference from long-term mean, °C | +0.8 | +1.4 | +2.2 | − |
| Average air temperature during the plant’s vegetation period, °C | 14.7 | 13.6 | 15.4 | 12.8 |
| Difference from long-term mean, °C | +1.9 | +0.8 | +2.6 | − |
| Total annual precipitation, mm | 573.0 | 627.9 | 504.7 | 570.0 |
| Difference from long-term mean, mm | +3.0 | +57.9 | −65.3 | − |
| Total amount of precipitation during the plant’s vegetation period, mm | 374.4 | 452.9 | 225.4 | 410 |
| Difference from long-term mean, mm | −35.6 | +42.9 | −184.6 | − |
| Cropping Strategy | 2021 | 2022 | 2023 | Share (%) of Poaceae, Fabaceae and Brassicaceae in the Rotation | Catch Crops (CC) | The Number of Times CC Grown per Rotation |
|---|---|---|---|---|---|---|
| W/W/W | W. wheat | S. wheat | W. wheat | 100 + 0 + 0 | − | 0 |
| W/W/W + CC | W. wheat cc | S. wheat | W. wheat cc | 100 + 0 + 0 | + | 2 |
| W/P/W + CC | S. wheat cc | Pea | S. wheat | 75 + 25 + 0 | + | 1 |
| W/P/R + CC | S. wheat cc | Pea cc | W. rape | 50 + 25 + 25 | + | 2 |
| R/W/P + CC | W. rape cc | S. wheat cc | Pea cc | 50 + 25 + 25 | + | 3 |
| Tillage (Factor A) | Soil Depth (Factor B) | Cropping Strategies (Factor C) | Root Length, km m−3 | Root Diameter, mm | Root Volume, cm3 |
|---|---|---|---|---|---|
| CT | 104.6 a | 0.41 a | 1.41 a | ||
| NT | 104.4 a | 0.42 a | 1.39 a | ||
| 0–10 cm | 104.8 a | 0.44 a | 2.35 a | ||
| 10–20 cm | 104.2 a | 0.37 a | 1.44 b | ||
| W/W/W | 103.4 ab | 0.39 a | 1.88 a | ||
| W/W/W + CC | 104.5 ab | 0.38 a | 1.56 b | ||
| W/P/W + CC | 105.8 ab | 0.41 a | 1.21 a | ||
| W/P/R + CC | 106.0 b | 0.42 a | 1.56 b | ||
| R/W/P + CC | 102.9 a | 0.44 a | 1.48 b | ||
| Actions and Interactions | |||||
| A | n.s. | n.s. | n.s. | ||
| B | n.s. | n.s. | ** | ||
| C | * | n.s. | ** | ||
| A × B | n.s. | n.s. | ** | ||
| A × C | n.s. | n.s. | n.s. | ||
| B × C | n.s. | n.s. | ** | ||
| A × B × C | n.s. | n.s. | n.s. | ||
| Year (Factor A) | Tillage (Factor B) | Cropping Strategies (Factor C) | CO2 Emissions (µmol m−2 s−1) | Actions | ||
|---|---|---|---|---|---|---|
| A | B | C | ||||
| 2021 | 1.66 a | n.s. | ||||
| 2022 | 1.77 b | |||||
| 2023 | 2.25 ab | |||||
| CT | 1.66 a | ** | ||||
| NT | 2.13 b | |||||
| W/W/W | 1.75 ab | * | ||||
| W/W/W + CC | 1.89 ab | |||||
| W/P/W + CC | 2.27 a | |||||
| W/P/R + CC | 2.17 a | |||||
| R/W/P + CC | 1.68 b | |||||
| Interactions | ||||||
| A × B | n.s. | |||||
| A × C | n.s. | |||||
| B × C | n.s. | |||||
| A × B × C | n.s. | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buivydienė, A.; Deveikytė, I.; Veršulienė, A.; Feiza, V. Tillage Practices Effect on Root Distribution and Variation of Soil CO2 Emission under Different Cropping Strategies. Agronomy 2024, 14, 1768. https://doi.org/10.3390/agronomy14081768
Buivydienė A, Deveikytė I, Veršulienė A, Feiza V. Tillage Practices Effect on Root Distribution and Variation of Soil CO2 Emission under Different Cropping Strategies. Agronomy. 2024; 14(8):1768. https://doi.org/10.3390/agronomy14081768
Chicago/Turabian StyleBuivydienė, Agnė, Irena Deveikytė, Agnė Veršulienė, and Virginijus Feiza. 2024. "Tillage Practices Effect on Root Distribution and Variation of Soil CO2 Emission under Different Cropping Strategies" Agronomy 14, no. 8: 1768. https://doi.org/10.3390/agronomy14081768







