Seasonal Photosynthetic and Water Relation Responses of Three Cool Temperate Garden Shrubs to Drought Stress
Abstract
1. Introduction
- (a)
- Is there seasonality in the effect of drought stress on the leaf Ψ and photosynthetic parameters in the shrubs used for greening?
- (b)
- What are the differences in adaptive and responsive mechanisms of different shrubs used for greening to water deficit during the growing season?
2. Materials and Methods
2.1. Study Site
2.2. Plant Materials and Irrigation Treatments
2.3. Measurement of the Photosynthetic Parameters
2.4. Soil Moisture Content in Response to Drought Stress
2.5. Determination of the Level of Plant Stress
2.6. Statistical Analysis
3. Results
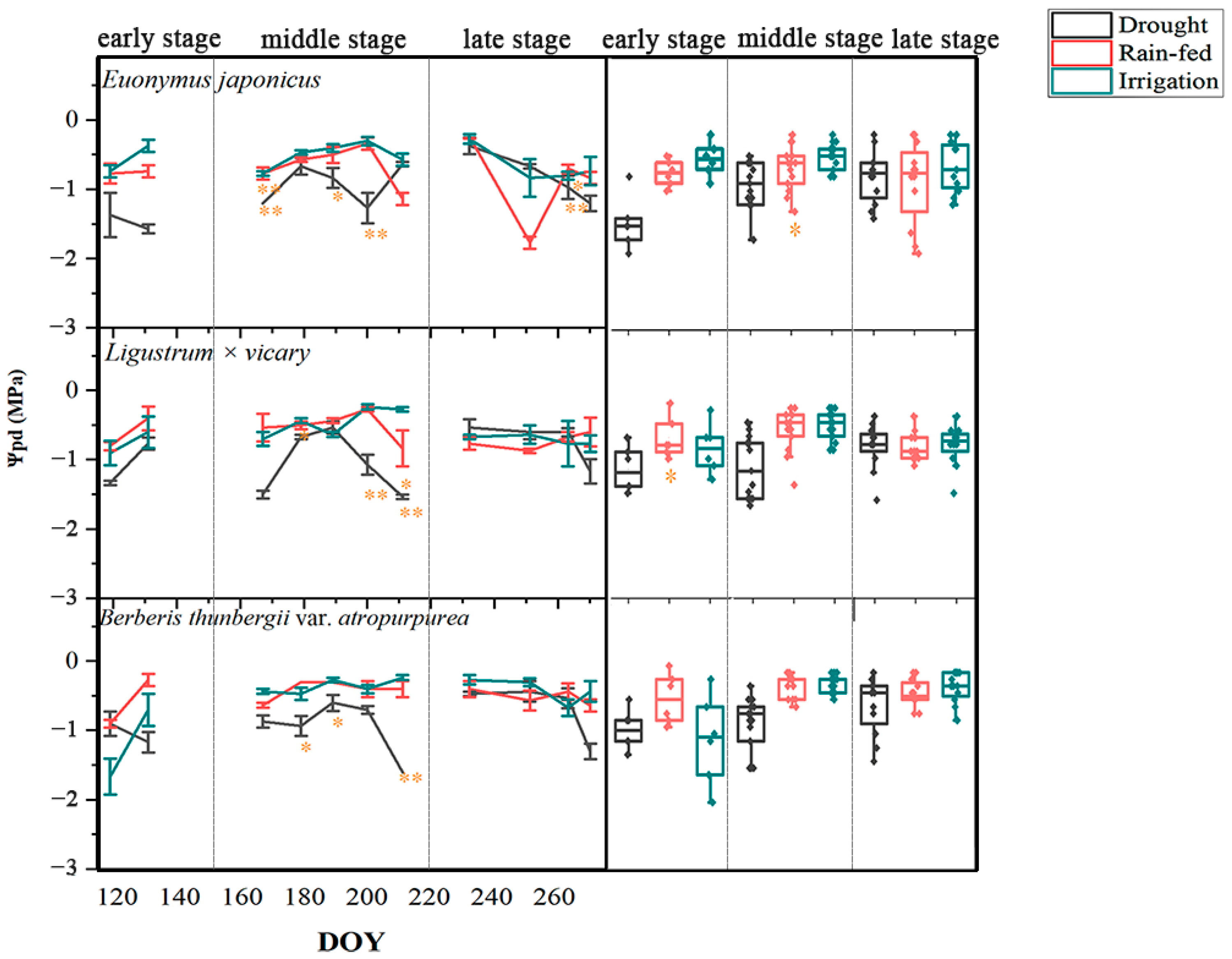
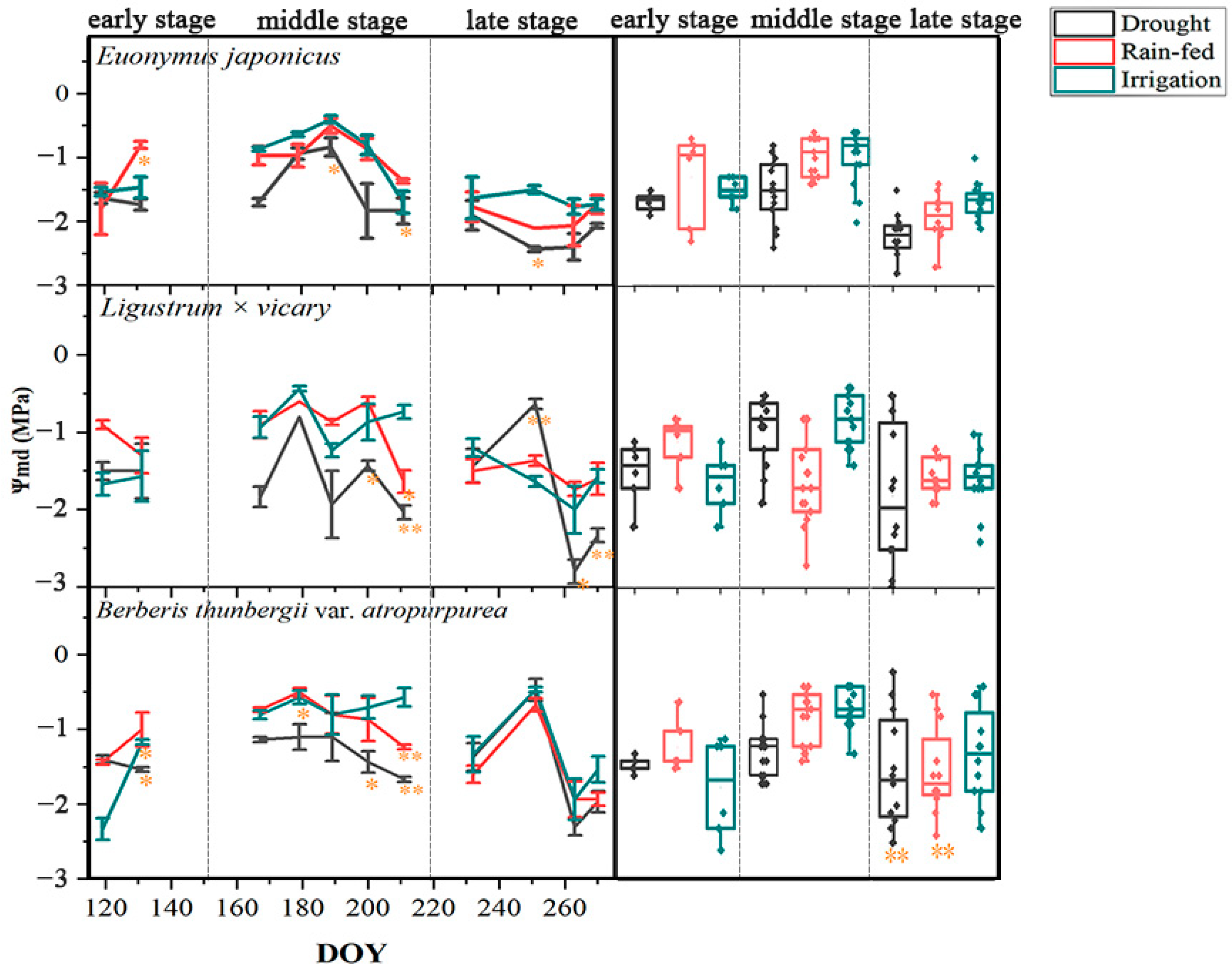
3.1. Responses of the Leaf Water Potential
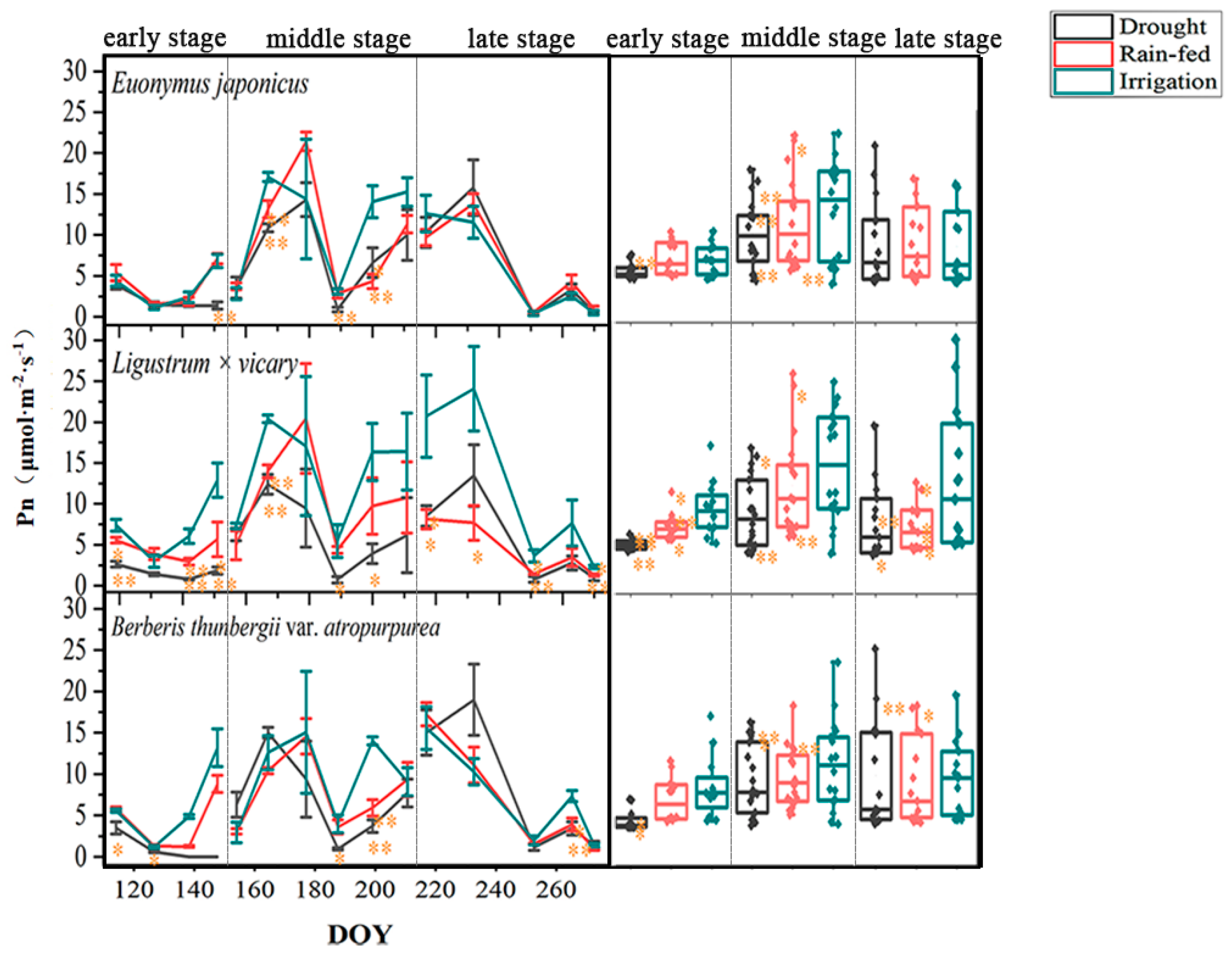
3.2. Response of the Photosynthetic Rate
3.3. Response of Stomatal Conductance
3.4. Relationships between the Photosynthetic Parameters and Leaf Water Potentials
4. Discussion
4.1. Seasonal Differences in the Response of Three Shrubs to Water Deficit
4.2. Differences in the Acclimation and Mechanisms of Responses of Three Shrubs to Water Deficits in the Growing Season
5. Conclusions and Prospects
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations. United Nations 2023 Water Conference Mid-Term Review of the Water Action Decade: Key Messages from the United Nations Regional Commissions; United Nations: Bangkok, Thailand, 2023. [Google Scholar]
- Balting, D.F.; AghaKouchak, A.; Lohmann, G.; Ionita, M. Northern Hemisphere drought risk in a warming climate. NPJ Clim. Atmos. Sci. 2021, 4, 61. [Google Scholar] [CrossRef]
- Chiang, F.; Mazdiyasni, O.; Agha Kouchak, A. Evidence of anthropogenic impacts on global drought frequency, duration, and intensity. Nat. Commun. 2021, 12, 2754. [Google Scholar] [CrossRef]
- Ghafari, S.; Kaviani, B.; Sedaghathoor, S.; Sadegh, M. Urban Forestry & Urban Greening Ecological Potentials of Trees, Shrubs and Hedge Species for Urban Green Spaces by Multi Criteria Decision Making. Urban For. Urban Green. 2020, 55, 126824. [Google Scholar] [CrossRef]
- Meineke, E.K.; Davies, T.J. Museum Specimens Provide Novel Insights into Changing Plant–herbivore Interactions. Philos. Trans. R. Soc. B Biol. Sci. 2019, 374, 20170393. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Basra, S.M.A.; Fujita, D.B. Plant Drought Stress: Effects, Mechanisms and Management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef]
- Vilagrosa, A.; Hernández, E.I.; Luis, V.C.; Cochard, H.; Pausas, J.G. Physiological Differences Explain the Co-Existence of Different Regeneration Strategies in Mediterranean Ecosystems. New Phytol. 2014, 201, 1277–1288. [Google Scholar] [CrossRef] [PubMed]
- Toscano, S.; Scuderi, D.; Giuffrida, F.; Romano, D. Responses of Mediterranean Ornamental Shrubs to Drought Stress and Recovery. Sci. Hortic. 2014, 178, 145–153. [Google Scholar] [CrossRef]
- Long, M. Comparative Study on the Structure and Function of Shrub Leaves in the Traffic Separation Green Belt on East University Road in Nanning City; Guangxi University: Guangxi, China, 2020; (In Chinese). [Google Scholar] [CrossRef]
- Qi, Y.; Wei, W.; Chen, C.; Chen, L. Plant Root-Shoot Biomass Allocation over Diverse Biomes: A Global Synthesis. Glob. Ecol. Conserv. 2019, 18, e00606. [Google Scholar] [CrossRef]
- Song, S.; Leng, H.; Feng, S.; Meng, C.; Luo, B.; Zhao, L.; Zhang, C. Biomass Allocation Pattern of Urban Shrubs in the Yangtze River Delta Region, China–A Field Observation of 13 Shrub Species. Urban For. Urban Green. 2021, 63, 127228. [Google Scholar] [CrossRef]
- Bradford, K.J.; Hsiao, T.C. Physiological Responses to Moderate Water Stress. In Physiologcal Plant Ecology II; Springer: Berlin/Heidelberg, Germany, 1982; pp. 263–324. [Google Scholar] [CrossRef]
- Hsiao, T.C. Plant responses to water stress. Ann. Rev. Plant Physiol. 1973, 24, 519–570. [Google Scholar] [CrossRef]
- Jones, H.G. Partitioning Stomatal and Non-stomatal Limitations to Photosynthesis. Plant. Cell Environ. 1985, 8, 95–104. [Google Scholar] [CrossRef]
- Anjum, S.A.; Xie, X.Y.; Wang, L.C.; Saleem, M.F.; Man, C.; Lei, W. Morphological, physiological and biochemical responses of plants to drought stress. Afr. J. Agric. Res. 2011, 6, 2026–2032. [Google Scholar] [CrossRef]
- Campbell, D.R.; Wu, C.A.; Travers, S.E. Photosynthetic and Growth Responses of Reciprocal Hybrids to Variation in Water and Nitrogen Availability. Am. J. Bot. 2010, 97, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, O.; Singh, P.C.; Bhatia, R. A review on drought stress in plants: Implications, mitigation and the role of plant growth promoting rhizobacteria. Resour. Environ. Sustain. 2021, 5, 100032. [Google Scholar] [CrossRef]
- Pereira, L.S.; Oweis, T.; Zairi, A. Irrigation Management under Water Scarcity. Agric. Water Manag. 2002, 57, 175–206. [Google Scholar] [CrossRef]
- Mitchell, P.-D.; Ke, G. Effects of regulating water deficit on tree growth, flowering, fruit development and yield of pear trees. Fujian Fruit Tree 1985, 3, 43–44. (In Chinese) [Google Scholar]
- Leib, B.G.; Caspari, H.W.; Redulla, C.A.; Andrews, P.K.; Jabro, J.J. Partial rootzone drying and deficit irrigation of ‘Fuji’ apples in a semi-arid climate. Irrig. Sci. 2006, 24, 85–99. [Google Scholar] [CrossRef]
- Tribulato, A.; Toscano, S.; Di Lorenzo, V.; Romano, D. Effects of Water Stress on Gas Exchange, Water Relations and Leaf Structure in Two Ornamental Shrubs in the Mediterranean Area. Agronomy 2019, 9, 381. [Google Scholar] [CrossRef]
- Chen, X.; Qi, Z.; Gui, D.; Sima, M.W.; Zeng, F.; Li, L.; Li, X.; Feng, S. Responses of Cotton Photosynthesis and Growth to a New Irrigation Control Method under Deficit Irrigation. Field Crop. Res. 2022, 275, 108373. [Google Scholar] [CrossRef]
- Jung, M.; Reichstein, M.; Ciais, P.; Seneviratne, S.I.; Sheffield, J.; Goulden, M.L.; Bonan, G.; Cescatti, A.; Chen, J.; De Jeu, R.; et al. Recent Decline in the Global Land Evapotranspiration Trend Due to Limited Moisture Supply. Nature 2010, 467, 951–954. [Google Scholar] [CrossRef]
- Kljun, N.; Black, T.A.; Griffis, T.J.; Barr, A.G.; Gaumont-Guay, D.; Morgenstern, K.; McCaughey, J.H.; Nesic, Z. Response of Net Ecosystem Productivity of Three Boreal Forest Stands to Drought. Ecosystems 2007, 10, 1039–1055. [Google Scholar] [CrossRef]
- Richardson, A.D.; Black, T.A.; Ciais, P.; Delbart, N.; Friedl, M.A.; Gobron, N.; Hollinger, D.Y.; Kutsch, W.L.; Longdoz, B.; Luyssaert, S.; et al. Influence of Spring and Autumn Phenological Transitions on Forest Ecosystem Productivity. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 3227–3246. [Google Scholar] [CrossRef]
- Piao, S.; Ciais, P.; Friedlingstein, P.; Peylin, P.; Reichstein, M.; Luyssaert, S.; Margolis, H.; Fang, J.; Barr, A.; Chen, A.; et al. Net Carbon Dioxide Losses of Northern Ecosystems in Response to Autumn Warming. Nature 2008, 451, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Yousfi, S.; Marin, J.F.; Parra, L.; Lloret, J.; Mauri, P.V. Remote sensing devices as key methods in the advanced turfgrass phenotyping under different water regimes. Agric. Water Manag. 2022, 266, 107581. [Google Scholar] [CrossRef]
- Miller, D.L.; Alonzo, M.; Roberts, D.A.; Tague, C.L.; McFadden, J.P. Drought response of urban trees and turfgrass using airborne imaging spectroscopy. Remote Sens. Environ. 2020, 240, 111646. [Google Scholar] [CrossRef]
- De Micco, V.; Aronne, G. Occurrence of Morphological and Anatomical Adaptive Traits in Young and Adult Plants of the Rare Mediterranean Cliff Species Primula palinuri Petagna. Sci. World J. 2012, 2012, 471814. [Google Scholar] [CrossRef]
- Robinson, T.M.P.; La Pierre, K.J.; Vadeboncoeur, M.A.; Byrne, K.M.; Thomey, M.L.; Colby, S.E. Seasonal, Not Annual Precipitation Drives Community Productivity across Ecosystems. Oikos 2013, 122, 727–738. [Google Scholar] [CrossRef]
- Bates, J.D.; Svejcar, T.; Miller, R.F.; Angell, R.A. The Effects of Precipitation Timing on Sagebrush Steppe Vegetation. J. Arid Environ. 2006, 64, 670–697. [Google Scholar] [CrossRef]
- De Boeck, H.J.; Dreesen, F.E.; Janssens, I.A.; Nijs, I. Whole-System Responses of Experimental Plant Communities to Climate Extremes Imposed in Different Seasons. New Phytol. 2011, 189, 806–817. [Google Scholar] [CrossRef]
- Mulhouse, J.M.; Hallett, L.M.; Collins, S.L. The Influence of Seasonal Precipitation and Grass Competition on 20 Years of Forb Dynamics in Northern Chihuahuan Desert Grassland. J. Veg. Sci. 2017, 28, 250–259. [Google Scholar] [CrossRef]
- Munson, S.M.; Lauenroth, W.K. Controls of Vegetation Structure and Net Primary Production in Restored Grasslands. J. Appl. Ecol. 2014, 51, 988–996. [Google Scholar] [CrossRef]
- Leuschner, C.; Wedde, P.; Lübbe, T. The Relation between Pressure–volume Curve Traits and Stomatal Regulation of Water Potential in Five Temperate Broadleaf Tree Species. Ann. For. Sci. 2019, 76, 60. [Google Scholar] [CrossRef]
- Zhou, J. Change of biodiversity conservation concept in the era of ecological civilisation. People’s Forum-Acad. Front. 2022, 4, 16–23. (In Chinese) [Google Scholar] [CrossRef]
- Zhou, Y. Potential Annual Water Consumption of Green Plants in Beijing; Beijing Forestry University: Beijing, China, 2020. (In Chinese) [Google Scholar]
- Hou, L.W.; Lu, S.W.; Li, S.N. Effects of drought on dry mass partitioning and root distribution of three shrub species. J. Plant Resour. Environ. 2023, 32, 34–42. (In Chinese) [Google Scholar]
- Xu, X.; Liu, H.; Wang, W.; Hu, G.; Wu, X.; Song, Z. Effects of Manipulated Precipitation on Aboveground Net Primary Productivity of Grassland Fields: Controlled Rainfall Experiments in Inner Mongolia, China. Land Degrad. Dev. 2021, 32, 1981–1992. [Google Scholar] [CrossRef]
- Yahdjian, L.; Sala, O.E. A Rainout Shelter Design for Intercepting Different Amounts of Rainfall. Oecologia 2002, 133, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, T.C.; Acevedo, E. Plant responses to water deficits, water-use efficiency, and drought resistance. Dev. Agric. Manag. For. Ecol. 1975, 1, 59–84. [Google Scholar] [CrossRef]
- Huang, B. Recent Advances in Drought and Heat Stress Physiology of Turfgrass—A Review. Acta Hortic. 2004, 661, 185–192. [Google Scholar] [CrossRef]
- Nayyar, H.; Gupta, D. Differential Sensitivity of C3 and C4 Plants to Water Deficit Stress: Association with Oxidative Stress and Antioxidants. Environ. Exp. Bot. 2006, 58, 106–113. [Google Scholar] [CrossRef]
- Hessini, K.; Wasli, H.; Al-Yasi, H.M.; Ali, E.F.; Issa, A.A.; Hassan, F.A.S.; Siddique, K.H.M. Graded Moisture Deficit Effect on Secondary Metabolites, Antioxidant, and Inhibitory Enzyme Activities in Leaf Extracts of Rosa damascena Mill. var. trigentipetala. Horticulturae 2022, 8, 177. [Google Scholar] [CrossRef]
- Scarascia-Mugnozza, G.; De Angelis, P.; Matteucci, G.; Valentini, R. Long-Term Exposure to Elevated [CO2] in a Natural Quercus Ilex L. Community: Net Photosynthesis and Photochemical Efficiency of PSII at Different Levels of Water Stress. Plant Cell Environ. 1996, 19, 643–654. [Google Scholar] [CrossRef]
- Luo, Q.H.; Ning, H.S.; He, M.; Ji, X.M.; Lei, C.Y. Ecophysiological responses of five sandy shrubs to drought stress. Sci. Silvae Sin. 2017, 53, 29–42. (In Chinese) [Google Scholar]
- Escobar-Gutiérrez, A.J.; Zipperlin, B.; Carbonne, F.; Moing, A.; Gaudillere, J.P. Photosynthesis, carbon partitioning and metabolite content during drought stress in peach seedlings. Funct. Plant Biol. 1998, 25, 197–205. [Google Scholar] [CrossRef]
- Rieger, M.; Lo Bianco, R.; Okie, W.R. Response of Prunus Ferganensis, Prunus Persica and Two Interspecific Hybrids to Moderate Drought Stress. Tree Physiol. 2003, 23, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Mellisho, C.D.; Cruz, Z.N.; Conejero, W.; Ortuño, M.F.; Rodríguez, P. Mechanisms for Drought Resistance in Early Maturing Cvar Flordastar Peach Trees. J. Agric. Sci. 2011, 149, 609–616. [Google Scholar] [CrossRef]
- Haider, M.S.; Kurjogi, M.M.; Khalil-ur-Rehman, M.; Pervez, T.; Songtao, J.; Fiaz, M.; Jogaiah, S.; Wang, C.; Fang, J. Drought Stress Revealed Physiological, Biochemical and Gene-Expressional Variations in ‘Yoshihime’ Peach (Prunus persica L.) Cultivar. J. Plant Interact. 2018, 13, 83–90. [Google Scholar] [CrossRef]
- Lansac, A.R.; Zaballos, J.P.; Martin, A. Seasonal Water Potential Changes and Proline Accumulation in Mediterranean Shrubland Species. Vegetatio 1994, 113, 141–154. [Google Scholar] [CrossRef]
- Saxe, H.; Cannell, M.G.R.; Johnsen, Ø.; Ryan, M.G.; Vourlitis, G. Tree and Forest Functioning in Response to Global Warming. New Phytol. 2001, 149, 369–399. [Google Scholar] [CrossRef] [PubMed]
- Arend, M.; Brem, A.; Kuster, T.M.; Günthardt-Goerg, M.S. Seasonal Photosynthetic Responses of European Oaks to Drought and Elevated Daytime Temperature. Plant Biol. 2013, 15, 169–176. [Google Scholar] [CrossRef]
- Noordermeer, D.; Velasco, V.M.E.; Ensminger, I. Autumn Warming Delays the Downregulation of Photosynthesis and Does Not Increase the Risk of Freezing Damage in Interior and Coastal Douglas-Fir. Front. For. Glob. Change 2021, 4, 1–14. [Google Scholar] [CrossRef]
- Abrams, M.D. Adaptations and Responses to Drought in Quercus Species of North America. Tree Physiol. 1990, 7, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Zhang, X.; Li, X.; Foetzki, A.; Runge, M. Seasonal variation of water potential of tamarisk and poplar at the southern edge of Taklamakan Desert. Chin. J. Appl. Ecol. 2005, 16, 1389–1393. (In Chinese) [Google Scholar]
- Sanchez-Diaz, M.F.; Kramer, P.J. Behavior of Corn and Sorghum under Water Stress and during Recovery. Plant Physiol. 1971, 48, 613–616. [Google Scholar] [CrossRef]
- Hou, L.W. Response of Biomass Allocation and Root Distribution of Three Greening Shrubs to Drought at Different Periods of the Growing Season; Shenyang Agricultural University: Shenyang, China, 2023. (In Chinese) [Google Scholar]
- Leigh, A.; Sevanto, S.; Close, J.D.; Nicotra, A.B. The Influence of Leaf Size and Shape on Leaf Thermal Dynamics: Does Theory Hold up under Natural Conditions? Plant Cell Environ. 2017, 40, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Hassan, F.; Al-Yasi, H.; Ali, E.F.; Alamer, K.; Hessini, K.; Attia, H.; El-Shazly, S. Mitigation of salt stress effects by moringa leaf extract or salicylic acid through motivating antioxidant machinery in damask rose. Can. J. Plant Sci. 2021, 101, 157–165. [Google Scholar] [CrossRef]
- Reich, P.B. The World-Wide “fast-Slow” Plant Economics Spectrum: A Traits Manifesto. J. Ecol. 2014, 102, 275–301. [Google Scholar] [CrossRef]
- Xu, G.; Chen, T.; Liu, S.; Ma, J.; Li, Y. Within-crown plasticity of hydraulic properties influence branch dieback patterns of two woody plants under experimental drought conditions. Sci. Total Environ. 2022, 854, 158802. [Google Scholar] [CrossRef]
- Tardieu, F.; Simonneau, T. Variability among Species of Stomatal Control under Fluctuating Soil Water Status and Evaporative Demand: Modelling Isohydric and Anisohydric Behaviours. J. Exp. Bot. 1998, 49, 419–432. [Google Scholar] [CrossRef]
- Luo, D.D.; Wang, C.K.; Jin, Y. Plant Water-Regulation Strategies: Isohydric versus Anisohydric Behavior. Chin. J. Plant Ecol. 2017, 41, 1020–1032. [Google Scholar] [CrossRef]
- Martínez-Vilalta, J.; Poyatos, R.; Aguadé, D.; Retana, J.; Mencuccini, M. A New Look at Water Transport Regulation in Plants. New Phytol. 2014, 204, 105–115. [Google Scholar] [CrossRef]
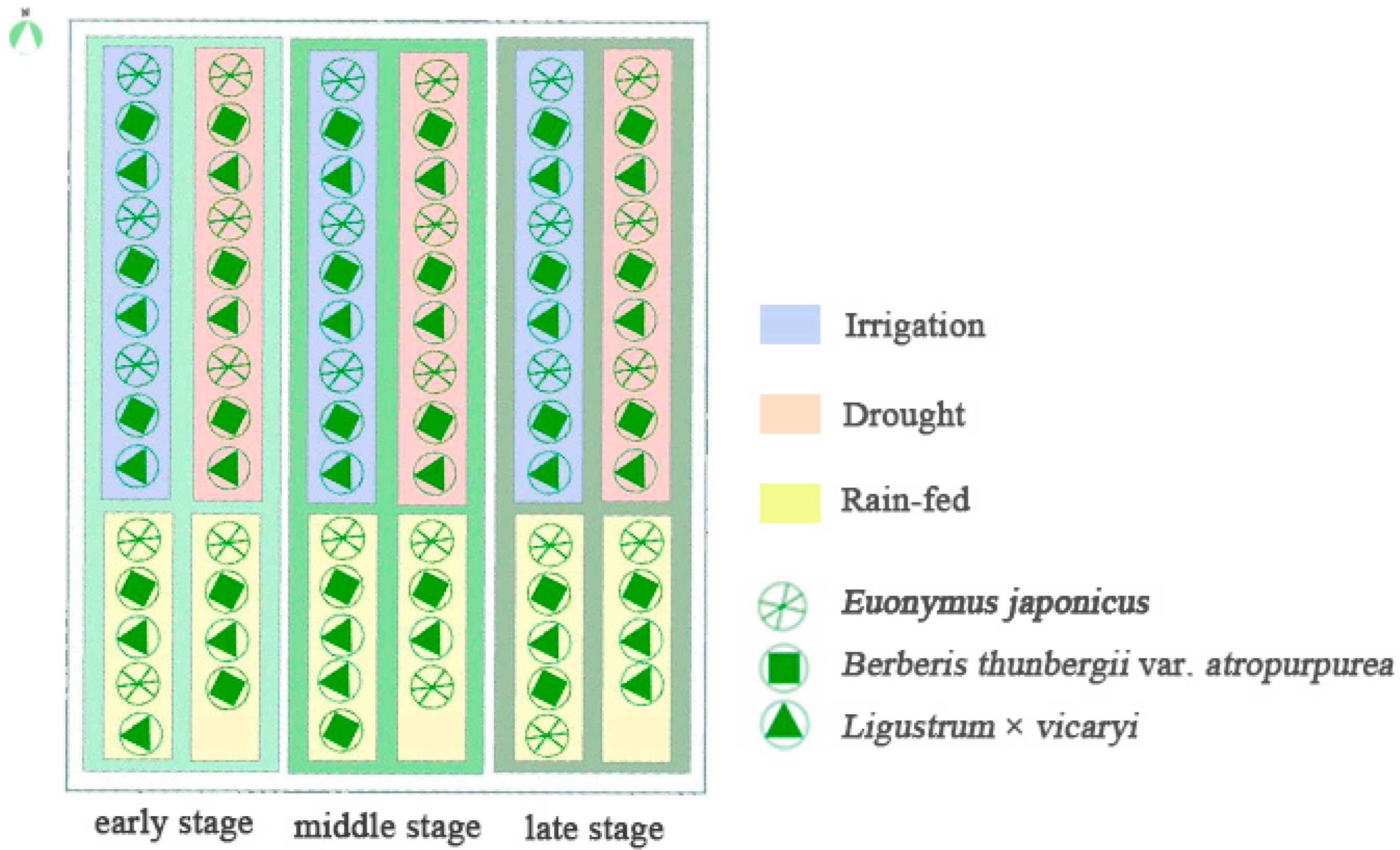
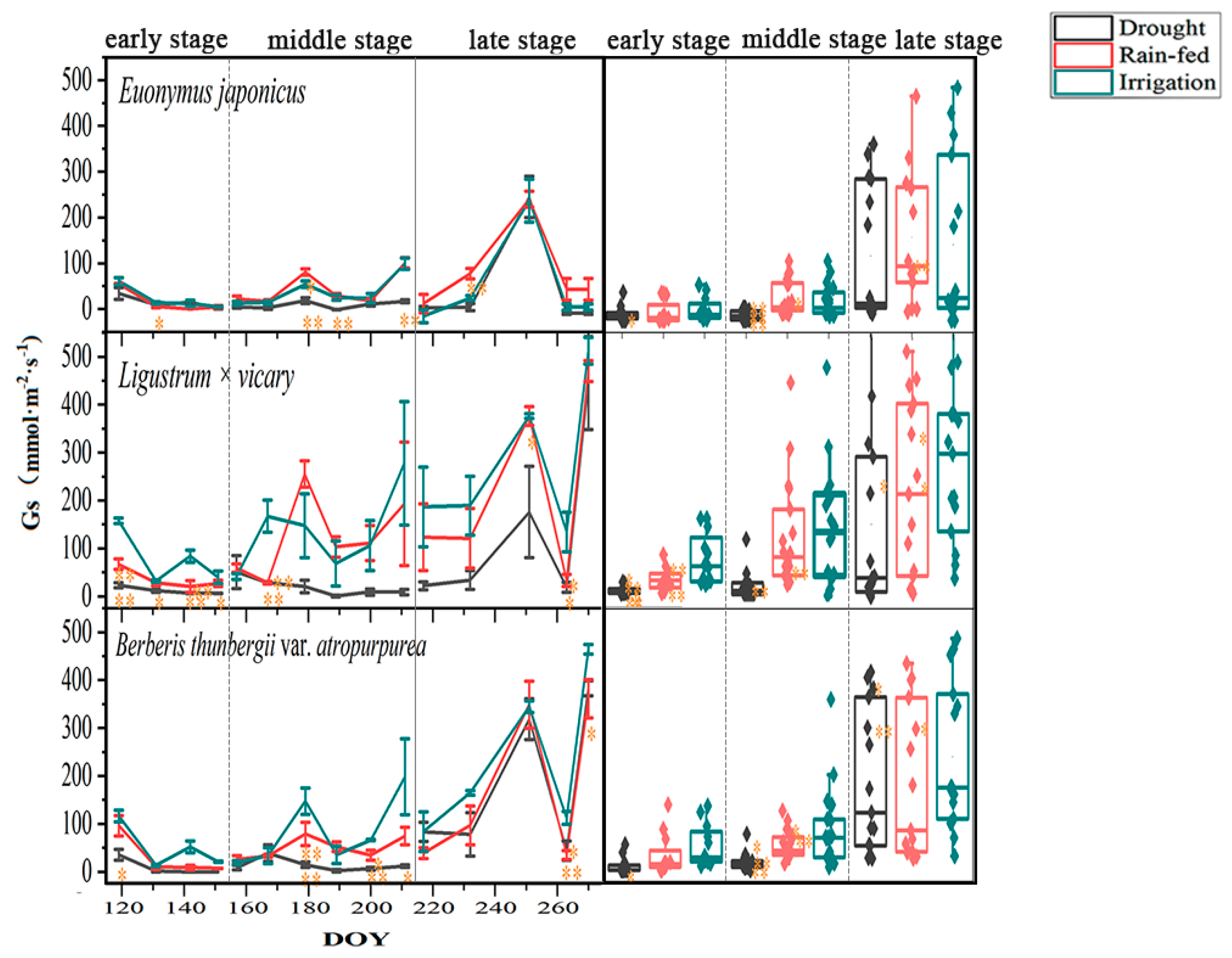
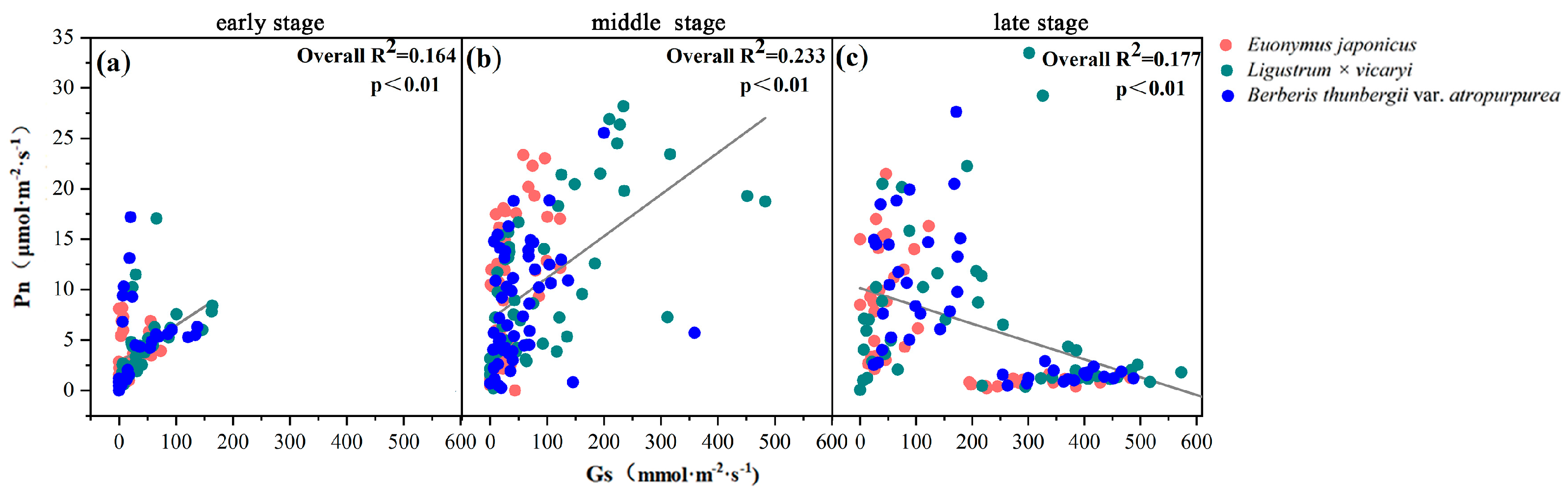
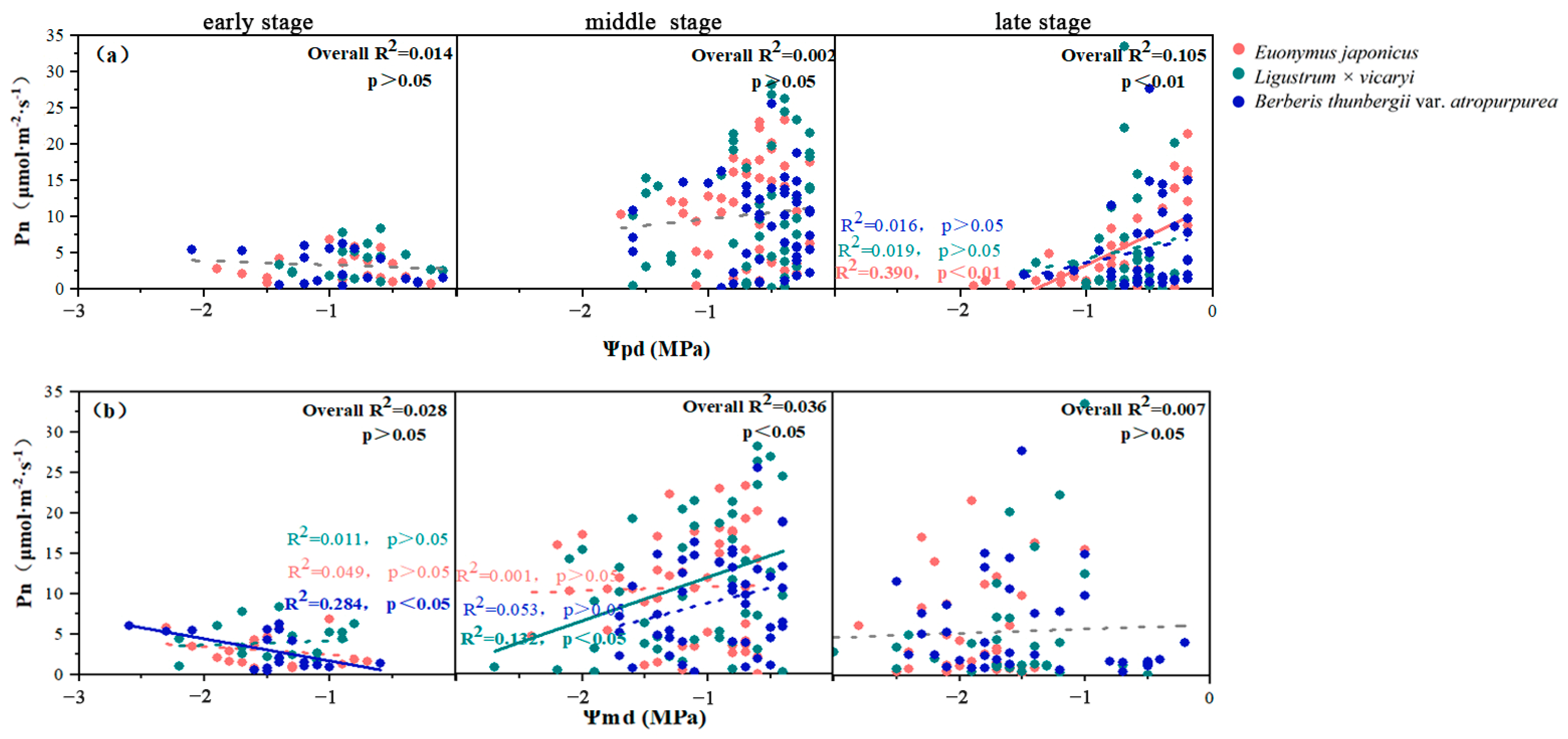
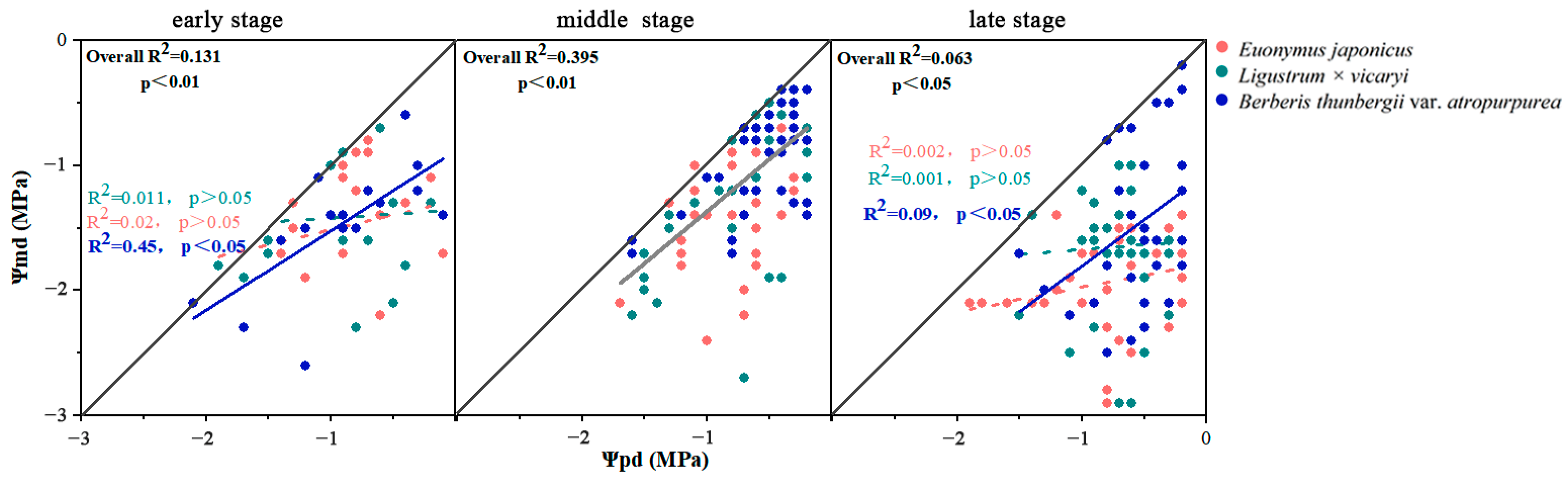
| Drought | Rain-Fed | Irrigation | |
|---|---|---|---|
| Early stage (1 April–31 May) | 15.91 ± 9.88 | 20.17 ± 7.78 | 31.07 ± 4.84 |
| Middle stage (1 June–31 July) | 12.20 ± 5.08 | 25.29 ± 3.71 | 26.66 ± 2.99 |
| Late stage (1 August–30 September) | 16.04 ± 7.14 | 26.54 ± 1.30 | 30.03 ± 1.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Lu, S.; Li, S.; Li, B.; Hou, L.; Zhao, N.; Xu, X. Seasonal Photosynthetic and Water Relation Responses of Three Cool Temperate Garden Shrubs to Drought Stress. Agronomy 2024, 14, 1772. https://doi.org/10.3390/agronomy14081772
Li J, Lu S, Li S, Li B, Hou L, Zhao N, Xu X. Seasonal Photosynthetic and Water Relation Responses of Three Cool Temperate Garden Shrubs to Drought Stress. Agronomy. 2024; 14(8):1772. https://doi.org/10.3390/agronomy14081772
Chicago/Turabian StyleLi, Jiaying, Shaowei Lu, Shaoning Li, Bin Li, Liwei Hou, Na Zhao, and Xiaotian Xu. 2024. "Seasonal Photosynthetic and Water Relation Responses of Three Cool Temperate Garden Shrubs to Drought Stress" Agronomy 14, no. 8: 1772. https://doi.org/10.3390/agronomy14081772
APA StyleLi, J., Lu, S., Li, S., Li, B., Hou, L., Zhao, N., & Xu, X. (2024). Seasonal Photosynthetic and Water Relation Responses of Three Cool Temperate Garden Shrubs to Drought Stress. Agronomy, 14(8), 1772. https://doi.org/10.3390/agronomy14081772






