Comprehensive Perspective on Contamination Identification, Source Apportionment, and Ecological Risk Assessment of Heavy Metals in Paddy Soils of a Tropical Island
Abstract
:1. Introduction
2. Materials and Methods
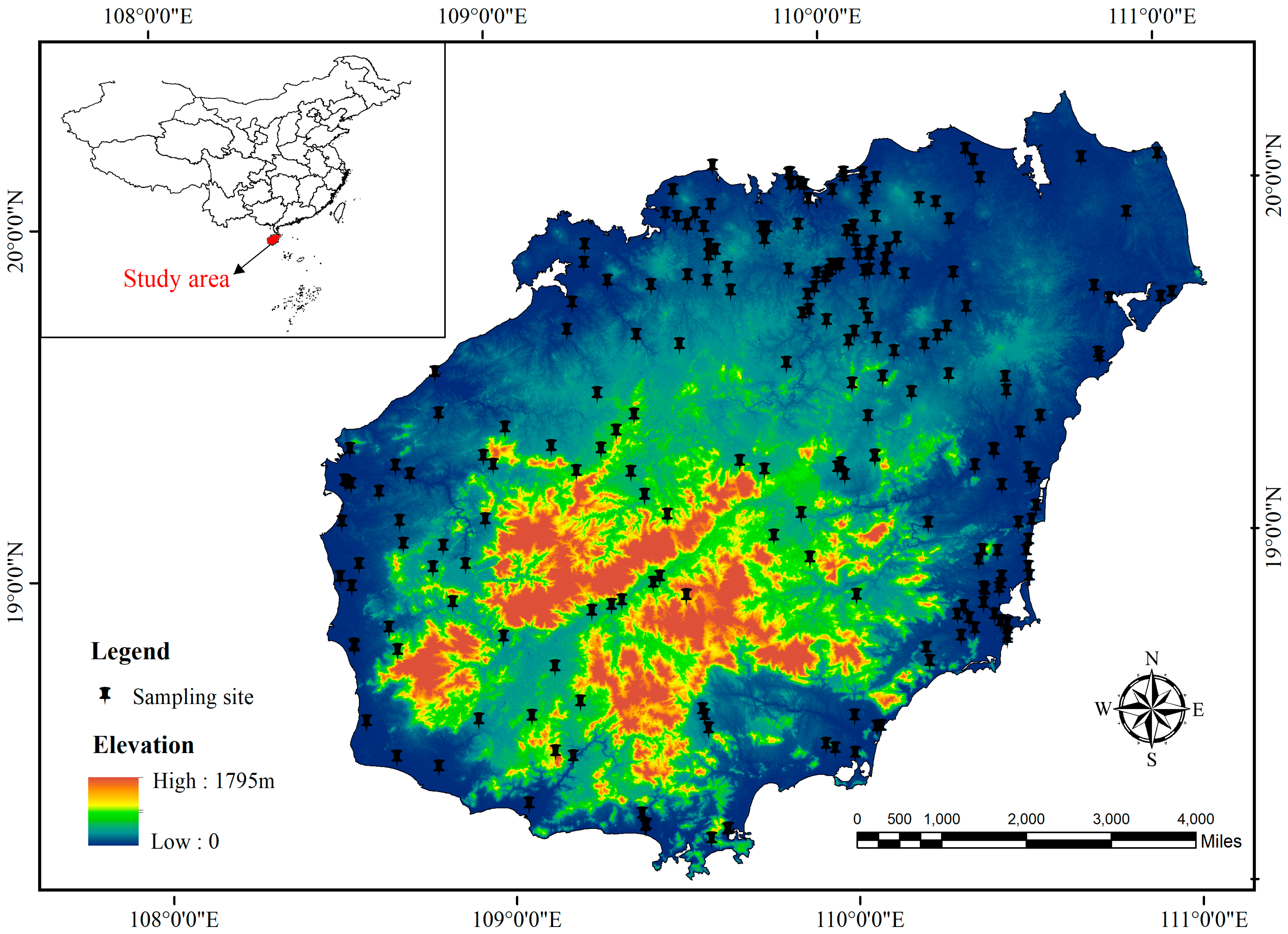
2.1. Study Area
2.2. Data Sources and Chemical Analysis
2.3. Contamination Level of Heavy Metals in Paddy Soils
2.4. Contamination Source Apportionment
2.4.1. Regression Model
2.4.2. PCA Analysis
2.4.3. APCS-MLR Model
2.5. Risk Assessments
2.5.1. Potential Ecological Risk
2.5.2. Risk Allocation among Soil Microorganisms and Vegetation
2.5.3. Risk Flow Mechanism among Ecological Components
3. Results and Discussion
3.1. Soil Physicochemical Properties and Heavy Metal Concentrations
3.2. Contamination Level of Heavy Metals in Paddy Soils
3.3. Source Apportionment
3.3.1. Correlation Analysis
3.3.2. PCA Analysis
3.3.3. Source Apportionment Based on APCS-MLR Model
3.4. Risk Assessment
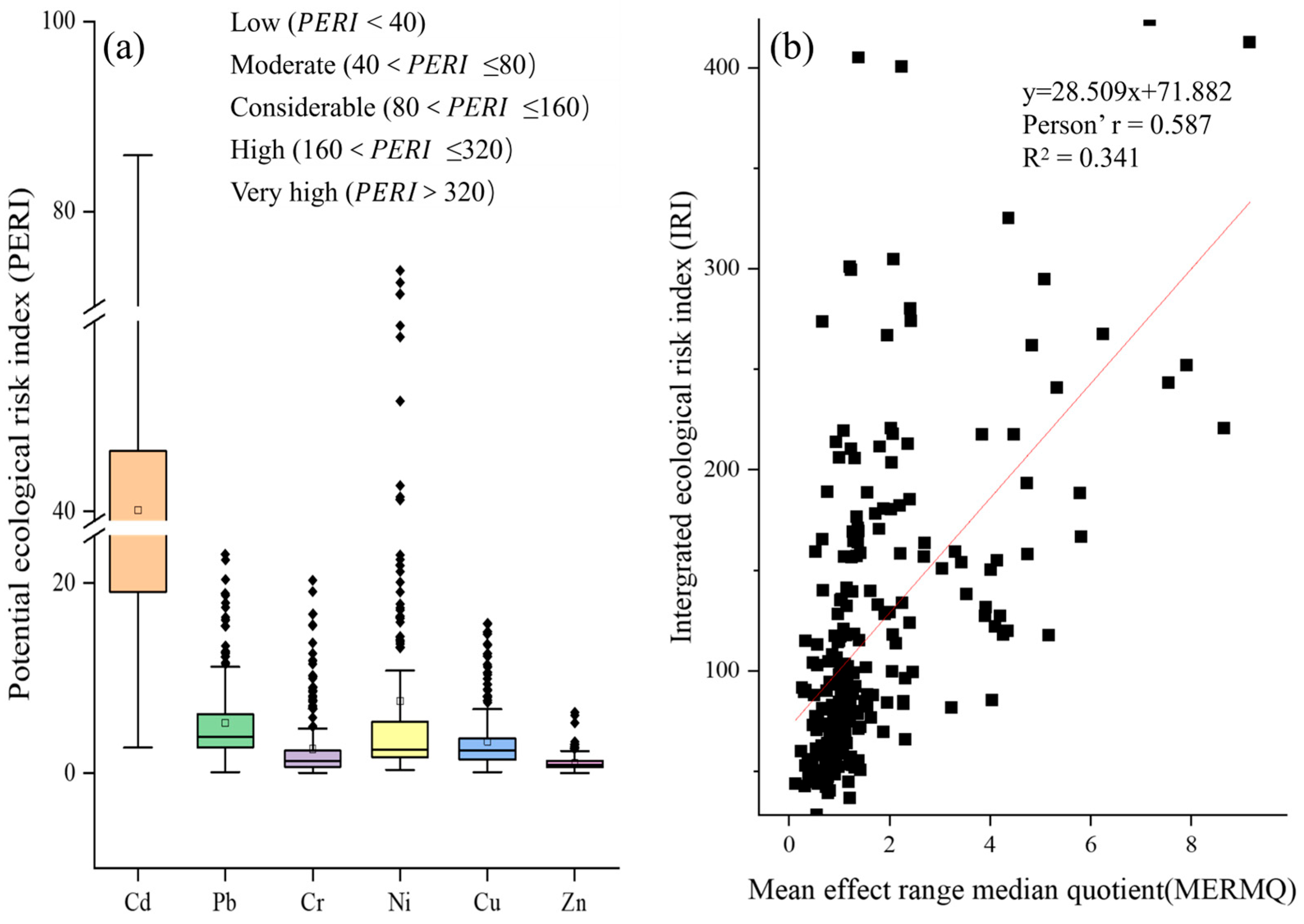
3.4.1. Potential Ecological Risk of Heavy Metals in Paddy Soil
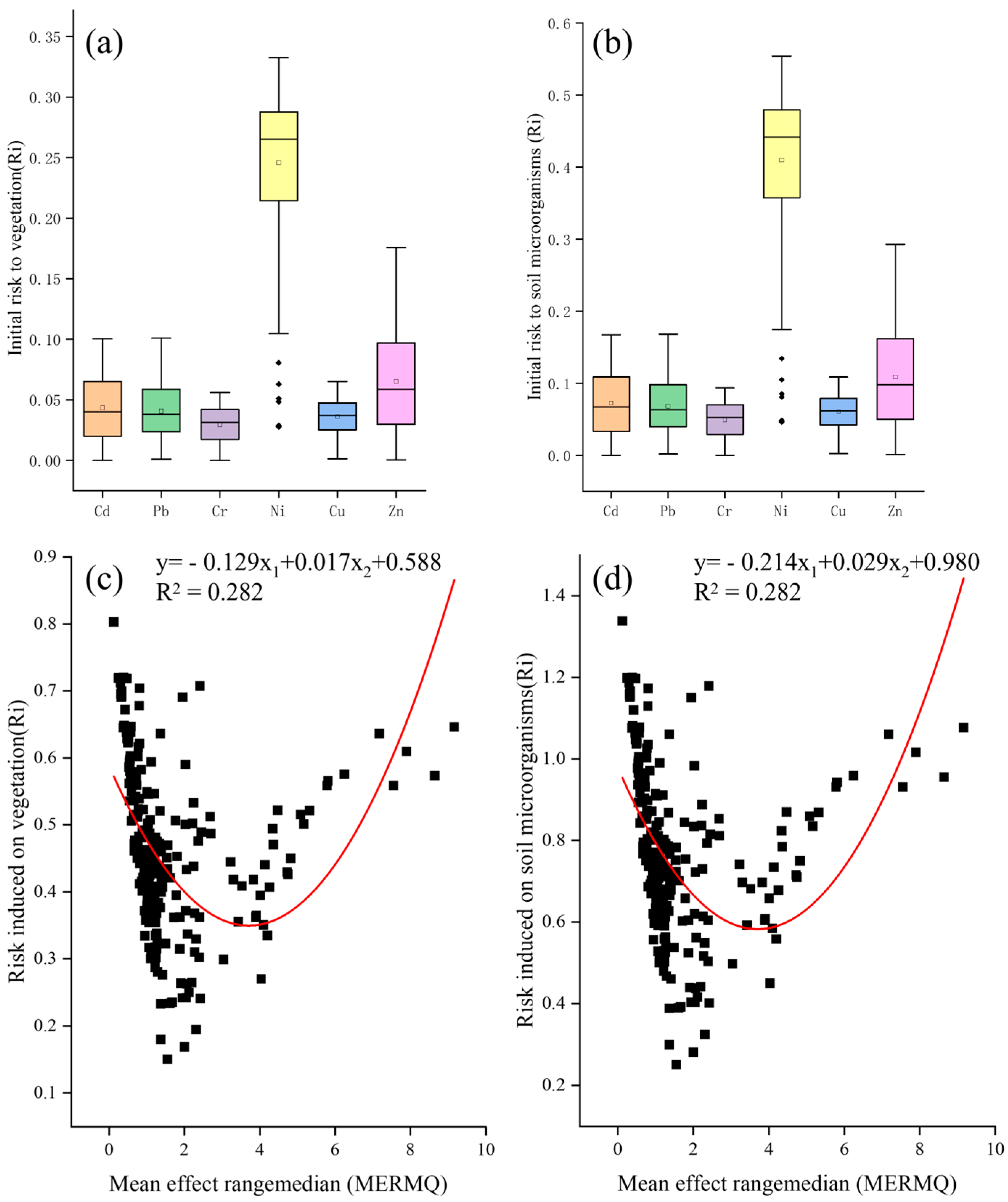
3.4.2. Risk Allocation among Soil Microorganisms and Vegetation
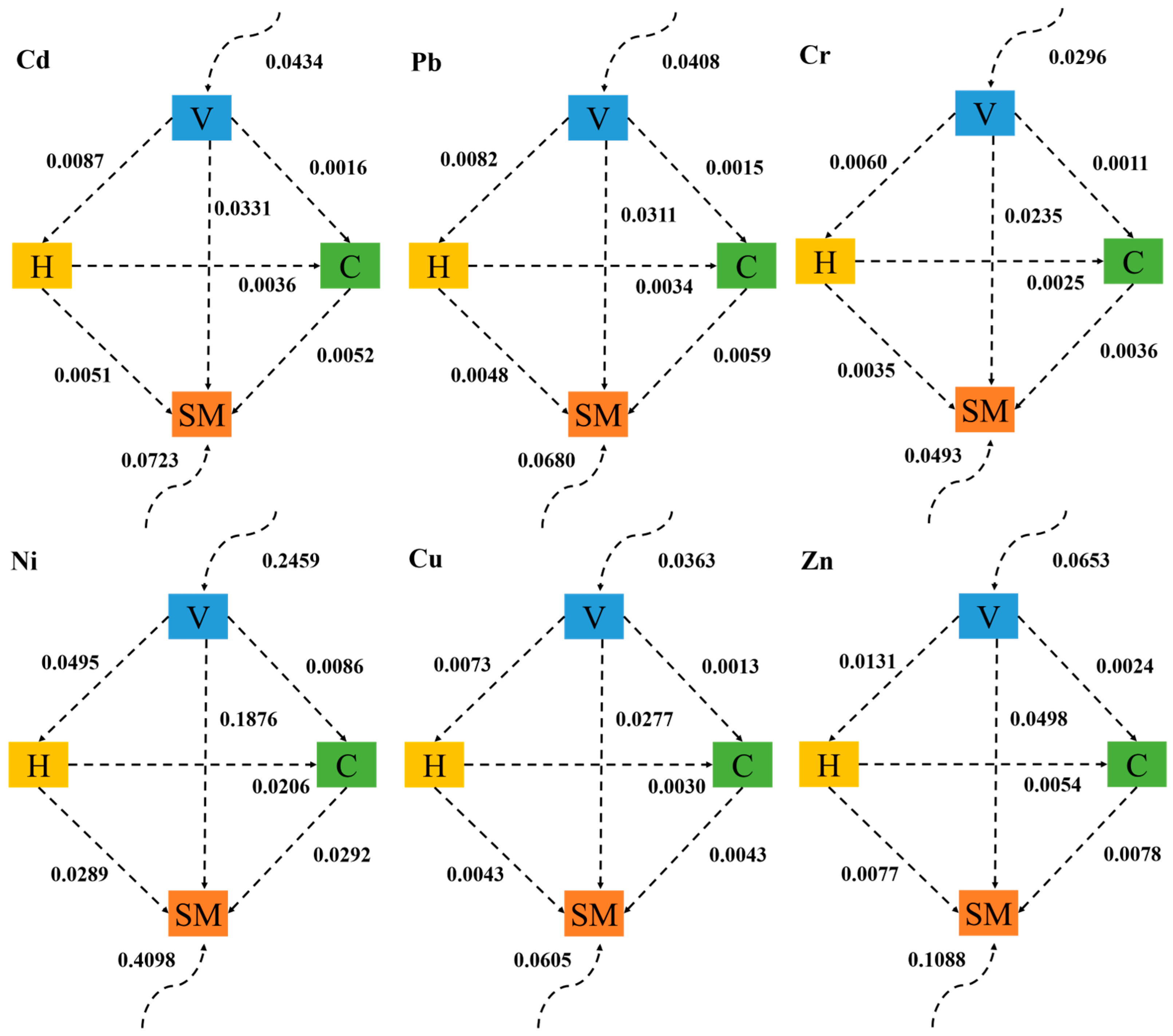
3.4.3. Risk Flow Mechanism among Ecological Components
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, Y.; Li, X.H.; Liu, Y.S. Cultivated land protection and rational use in China. Land Use Policy 2021, 106, 105454. [Google Scholar] [CrossRef]
- Hua, Y.N.; He, K.L.; Sun, Z.H.; Chen, G.; Cheng, H.F. Quantitative source apportionment of heavy metal(loid)s in the agricultural soils of an industrializing region and associated model uncertainty. J. Hazard. Mater. 2020, 391, 122244. [Google Scholar] [CrossRef]
- Xu, Y.F.; Bi, R.T.; Li, Y.H. Effects of anthropogenic and natural environmental factors on the spatial distribution of trace elements in agricultural soils. Ecotoxicol. Environ. Saf. 2023, 249, 114436. [Google Scholar] [CrossRef]
- Li, R.; Zhang, R.; Yang, Y.; Li, Y. Accumulation characteristics, driving factors, and model prediction of cadmium in soil-highland barley system on the Tibetan Plateau. J. Hazard. Mater. 2023, 453, 131407. [Google Scholar] [CrossRef]
- Sheng, D.R.; Meng, X.H.; Wen, X.H.; Wu, J.; Yu, H.J.; Wu, M. Contamination characteristics, source identification, and source-specific health risks of heavy metal (loid) s in groundwater of an arid oasis region in Northwest China. Sci. Total Environ. 2022, 84, 1156733. [Google Scholar] [CrossRef]
- Zhou, M.G.; Li, Y.H. Spatial distribution and source identification of potentially toxic elements in Yellow River Delta soils, China: An interpretable machine-learning approach. Sci. Total Environ. 2024, 912, 169092. [Google Scholar] [CrossRef] [PubMed]
- Chai, L.; Wang, Y.H.; Wang, X.; Ma, L.; Cheng, Z.X.; Su, L.M.; Liu, M.X. Quantitative source apportionment of heavy metals in cultivated soil and associated model uncertainty. Ecotoxicol. Environ. Saf. 2021, 215, 112150. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q.Y.; Zhao, R.; Pan, N.H.; Wang, F.F.; Yang, Y.Y.; Luo, H.P. Source apportionment of heavy metals in farmland soil of Wuwei, China: Comparison of three receptor models. J. Clean. Prod. 2019, 237, 117792. [Google Scholar] [CrossRef]
- Jafarabadi, R.A.; Bakhtiyari, R.A.; Toosi, S.A.; Jadot, C. Spatial distribution, ecological and health risk assessment of heavy metals in marine surface sediments and coastal seawaters of fringing coral reefs of the Persian gulf, Iran. Chemosphere 2017, 185, 1090–1111. [Google Scholar] [CrossRef]
- Srivastava, A.; Sengupta, B.; Dutta, S.A. Source apportionment of ambient VOCs in Delhi City. Sci. Total Environ. 2005, 343, 207–220. [Google Scholar] [CrossRef]
- Deng, J.; Zhang, Y.; Qiu, Y.; Zhang, H.; Du, W.; Xu, L.; Hong, Y.; Chen, Y.; Chen, J. Source apportionment of PM2.5 at the Lin’an regional background site in China with three receptor models. Atmos. Res. 2018, 202, 23–32. [Google Scholar] [CrossRef]
- Lei, M.; Li, K.; Guo, G.; Ju, T. Source-specific health risks apportionment of soil potential toxicity elements combining multiple receptor models with Monte Carlo simulation. Sci. Total Environ. 2022, 817, 152899. [Google Scholar] [CrossRef]
- Men, C.; Liu, R.M.; Wang, Q.R.; Miao, Y.X.; Wang, Y.F.; Jiao, L.J.; Li, L.; Cao, L.P.; Shen, Z.R.; Li, Y.; et al. Spatial-temporal characteristics, source-specific variation and uncertainty analysis of health risks associated with heavy metals in road dust in Beijing, China. Environ. Pollut. 2021, 278, 116866. [Google Scholar] [CrossRef]
- Men, C.; Liu, R.M.; Wang, Y.F.; Cao, L.P.; Jiao, L.J.; Li, L.; Shen, Z.Y. A four-way model (FEST) for source apportionment: Development, verification, and application. J. Hazard. Mater. 2022, 426, 128009. [Google Scholar] [CrossRef]
- Zhang, H.; Cheng, S.Q.; Li, H.F.; Fu, K.; Xu, Y. Groundwater pollution source identification and apportionment using PMF and PCA-APCS-MLR receptor models in a typical mixed land-use area in Southwestern China. Sci. Total Environ. 2020, 741, 140383. [Google Scholar] [CrossRef]
- Wei, R.F.; Meng, Z.R.; Zerizghi, T.; Luo, J.; Guo, Q.J. A comprehensive method of source apportionment and ecological risk assessment of soil heavy metals: A case study in Qingyuan city, China. Sci. Total Environ. 2023, 882, 163555. [Google Scholar] [CrossRef]
- Rashid, A.; Schutte, B.J.; Ulery, A.; Deyholos, M.K.; Sanogo, S.; Lehnhoff, E.A.; Beck, L. Heavy Metal Contamination in Agricultural Soil: Environmental Pollutants Affecting Crop Health. Agronomy 2023, 13, 1521. [Google Scholar] [CrossRef]
- Tóth, G.; Hermann, T.; Silva, M.R.D.; Montanarella, L. Heavy metals in agricultural soils of the European Union with implications for food safety. Environ. Int. 2016, 88, 299–309. [Google Scholar] [CrossRef]
- Radim, V. Heavy Metal Pollution and Its Effects on Agriculture. Agronomy 2021, 11, 1719. [Google Scholar] [CrossRef]
- Jiang, H.-H.; Cai, L.M.; Hu, G.C.; Wen, H.H.; Luo, J.; Xu, H.Q.; Chen, L.-G. An integrated exploration on health risk assessment quantification of potentially hazardous elements in soils from the perspective of sources. Ecotoxicol. Environ. Saf. 2021, 208, 111489. [Google Scholar] [CrossRef]
- Hiroki, M. Effects of heavy metal contamination on soil microbial population. Soil Sci. Plant Nutr. 1992, 38, 141–147. [Google Scholar] [CrossRef]
- McIntyre, T.; Whiting, M.J. Increased metal concentrations in Giant Sungazer Lizards (Smaug giganteus) from mining areas in South Africa. Arch. Environ. Contam. Toxicol. 2012, 63, 574–585. [Google Scholar] [CrossRef] [PubMed]
- IARC—International Agency for Research on Cancer. Agents Classified by the IARC Monographs. 2011, pp. 1–102. Available online: https://monographs.iarc.fr/agents-classified-by-the-iarc/ (accessed on 20 October 2023).
- Yang, S.Y.; He, M.J.; Zhi, Y.Y.; Chang, S.X.; Gu, B.J.; Liu, X.M.; Xu, J.M. An integrated analysis on source-exposure risk of heavy metals in agricultural soils near intense electronic waste recycling activities. Environ. Int. 2019, 133, 105239–105248. [Google Scholar] [CrossRef]
- Mama, C.; Nnaji, C.; Emenike, P.; Chibueze, C. Potential environmental and human health risk of soil and roadside dust in a rapidly growing urban settlement. Int. J. Environ. Sci. Technol. 2020, 17, 2385–2400. [Google Scholar] [CrossRef]
- Ma, W.; Tai, L.; Qiao, Z.; Zhong, L.; Wang, Z.; Fu, K.; Chen, G. Contamination source apportionment and health risk assessment of heavy metals in soil around municipal solid waste incinerator: A case study in North China. Sci. Total Environ. 2018, 631, 348–357. [Google Scholar] [CrossRef]
- Xu, X.L.; Liu, J.Y.; Zhang, S.W.; Li, R.D.; Yan, C.Z.; Wu, S.X. China Multi Period Land Use Remote Sensing Monitoring Dataset (CNLUCC). Resource and Environmental Science Data Registration and Publishing System. 2018. Available online: https://www.resdc.cn/DOI/doi.aspx?DOIid=54 (accessed on 10 March 2024). [CrossRef]
- Zang, Z.F.; Li, Y.H.; Liu, S.H.; Li, H.R.; Hao, Z.; Xu, Y.F. Assessment of the heavy metal pollution and health risks of rice cultivated in Hainan Island, China. Environ. Forensics 2021, 22, 63–74. [Google Scholar] [CrossRef]
- Guo, Y.; Yang, Y.; Li, R.X.; Liao, X.Y.; Li, Y.H. Distribution of cadmium and lead in soil-rice systems and their environmental driving factors at the island scale. Ecotoxicol. Environ. Saf. 2023, 265, 115530. [Google Scholar] [CrossRef]
- Guo, Y.; Yang, Y.; Li, R.X.; Liao, X.Y.; Li, Y.H. Cadmium accumulation in tropical island paddy soils: From environment and health risk assessment to model prediction. J. Hazard. Mater. 2024, 465, 133212. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.F.; Hao, Z.; Li, Y.H.; Li, H.R.; Wang, L.; Zang, Z.F.; Liao, X.Y.; Zhang, R. Distribution of selenium and zinc in soil-crop system and their relationship with environmental factors. Chemosphere 2020, 242, 125289. [Google Scholar] [CrossRef]
- USEPA—United States Environmental Protection Agency. Exposure Factors Handbook, Final ed.; Environmental Protection Agency: Washington, DC, USA, 2011. [Google Scholar]
- Fischer, G.; Nachtergaele, F.; Prieler, S.; van Velthuizen, H.T.; Verelst, L.; Wiberg, D. Global Agro-Ecological Zones Assessment for Agriculture; IIASA: Laxenburg, Austria; FAO: Rome, Italy, 2008. [Google Scholar]
- Hakanson, L. An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Qu, M.; Li, W.; Zhang, C. Spatial distribution and uncertainty assessment of potential ecological risks of soil heavy metals using sequential Gaussian simulation. Hum. Ecol. Risk Assess. 2014, 20, 764–778. [Google Scholar] [CrossRef]
- Swaine, D.J. Why trace elements are important. Fuel Process. Technol. 2000, 65, 21–33. [Google Scholar] [CrossRef]
- Förstner, U.; Ahlf, W.; Calmano, W.; Kersten, M. Sediment criteria development. In Sediments and Environmental Geochemistry; Springer: Berlin/Heidelberg, Germany, 1990; pp. 311–338. [Google Scholar]
- GB15618-2018; MEE of the PRC and SAMR of the People’s Republic of China, Soil Environmental Quality Risk Control Standard for Soil Contamination of Agricultural Land. China Environmental Science Press: Beijing, China, 2018.
- Kaiser, H.F. An index of factorial simplicity. Psychometrika 1974, 39, 31–36. [Google Scholar] [CrossRef]
- Guo, G.H.; Wang, Y.T.; Zhang, D.G.; Lei, M. Source-specific ecological and health risks of potentially toxic elements in agricultural soils in southern Yunnan province and associated uncertainty analysis. J. Hazard. Mater. 2021, 417, 126144. [Google Scholar] [CrossRef]
- Ma, M.L.; Li, Z.Y.; Zhou, M.; Luo, Y.Z.; Zeng, S.Q.; Chen, W.Y.; Lin, S. Soil Environmental Background Values in Hainan Province; Guangdong Provincial Environmental Monitoring Center, Department of Geography, Sun Yat-sen University, Hainan Provincial Environmental Monitoring Center: Guangzhou, China, 1991; pp. 14–15. (In Chinese) [Google Scholar]
- Zhang, J.J.; Ma, C.M.; Kuang, H.; Zhou, A.G. Assessment of heavy metals pollution in soil of Qingdao based on matter-element extension model. China Environ. Sci. 2017, 37, 661–668. [Google Scholar]
- Hu, M.J.; Li, C.Y.; Li, N.N.; Ji, T.Q.; Zheng, D.Y. Using the matter-element extension model to assess heavy metal pollution in topsoil in parks in the main district park of Lanzhou City. Environ. Sci. 2021, 42, 2457–2468. [Google Scholar]
- Weissmannová, H.D.; Pavlovský, J. Indices of soil contamination by heavy metals– methodology of calculation for pollution assessment (minireview). Environ. Monit. Assess. 2017, 189, 616. [Google Scholar] [CrossRef]
- Lu, J.; Lu, H.; Wang, W.; Feng, S.; Lei, K. Ecological risk assessment of heavy metal contamination of mining area soil based on land type changes: An information network environ analysis. Ecol. Model. 2021, 455, 109633. [Google Scholar] [CrossRef]
- Zerizghi, T.; Guo, Q.J.; Tian, L.Y.; Wei, R.F.; Zhao, C.Q. An integrated approach to quantify ecological and human health risks of soil heavy metal contamination around coal mining area. Sci. Total Environ. 2022, 814, 152653. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Fath, B.D.; Chen, B. Information-based network environ analysis: A system perspective for ecological risk assessment. Ecol. Indic. 2011, 11, 1664–1672. [Google Scholar] [CrossRef]
- Fath, B.D.; Patten, B.C. Review of the foundations of network environ analysis. Ecosystems 1999, 2, 167–179. [Google Scholar] [CrossRef]
- Tang, P.Z.; Liu, J.Z.; Lu, H.W.; Wang, Z.; He, L. Information-based network environ analysis for ecological risk assessment of heavy metals in soils. Ecol. Model. 2017, 344, 17–28. [Google Scholar] [CrossRef]
- Shi, T.R.; Zhang, Y.Y.; Gong, Y.W.; Ma, J.; Wei, H.Y.; Xu, X.; Zhao, L.; Hou, H. Status of cadmium accumulation in agricultural soils across China (1975–2016): From temporal and spatial variations to risk assessment. Chemosphere 2019, 230, 136–143. [Google Scholar] [CrossRef] [PubMed]
- CNEMC—China National Environmental Monitoring Centre. Soil Elements Background Values in China; China Environmental Science Press: Beijing, China, 1990. (In Chinese) [Google Scholar]
- Zhang, P.Y.; Qin, C.Z.; Hong, X.; Kang, G.H.; Qin, M.Z.; Yang, D.; Pang, B.; Li, Y.Y.; He, J.J.P.; Dick, R. Risk assessment and source analysis of soil heavy metal pollution from lower reaches of Yellow River irrigation in China. Sci. Total Environ. 2018, 633, 1136–1147. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Gupta, S. An Overview of Selenium Uptake, Metabolism, and Toxicity in Plants. Front. Plant Sci. 2016, 7, 2074. [Google Scholar] [CrossRef] [PubMed]
- Hajek, A.E.; Eilenberg, J. Natural Enemies: An Introduction to Biological Control; Cambridge University Press: Cambridge, UK, 2018. [Google Scholar]
- Ihedioha, J.N.; Ujam, O.T.; Nwuche, C.O.; Ekere, N.R.; Chime, C.C. Assessment of heavy metal contamination of rice grains (Oryza sativa) and soil from Ada field, Enugu, Nigeria: Estimating the human healtrisk. Hum. Ecol. Risk Assess. Int. J. 2016, 22, 1665–1677. [Google Scholar] [CrossRef]
- Liang, J.; Feng, C.; Zeng, G.; Gao, X.; Zhong, M.; Li, X.; Li, X.; He, X.; Fang, Y. Spatial distribution and source identification of heavy metals in surface soils in a typical coal mine city, Lianyuan, China. Environ. Pollut. 2017, 225, 681. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Stewart, B.A.; Zhang, F. Long-term experiments for sustainable nutrient management in China. A review. Agron. Sustain. Dev. 2011, 31, 397–414. [Google Scholar] [CrossRef]
- Arunakumara, K.K.I.U.; Walpola, B.C.; Yoon, M.-H. Current status of heavy metal contamination in Asia’s rice lands. Rev. Environ. Sci. Biotechnol. 2013, 12, 355–377. [Google Scholar] [CrossRef]
- Wuana, R.A.; Okieimen, F.E. Heavy Metals in Contaminated Soils: A Review of Sources, Chemistry, Risks and Best Available Strategies for Remediation. Int. Sch. Res. Not. 2011, 2011, e402647. [Google Scholar] [CrossRef]
- Luo, L.; Ma, Y.; Zhang, S.; Wei, D.; Zhu, Y.-G. An inventory of trace element inputs to agricultural soils in China. J. Environ. Manag. 2009, 90, 2524–2530. [Google Scholar] [CrossRef] [PubMed]
- Rosen, G.D.; Roberts, P.A. Comprehensive Survey of the Response of Growing Pigs to Supplementary Copper in Feed; Field Investigations and Nutrition Service Ltd.: London, UK, 1996. [Google Scholar]
- Holm, A. E. Coli Associated Diarrhoea in Weaner Pigs: Zinc Oxide Added to the Feed as a Preventive Measure. In Proceedings of the International Pig Veterinary Society, Congress, Lausanne, Switzerland, 1–5 July 1990. [Google Scholar]
- Mondal, M.K.; Das, T.K.; Biswas, P.; Samanta, C.C.; Bairagi, B. Influence of dietary inorganic and organic copper salt and level of soybean oil on plasma lipids, metabolites, and mineral balance of broiler chickens. Anim. Feed Sci. Technol. 2007, 139, 212–233. [Google Scholar] [CrossRef]
- Gupta, D.K.; Chatterjee, S.; Datta, S.; Veer, V.; Walther, C. Role of phosphate fertilizers in heavy metal uptake and detoxification of toxic metals. Chemosphere 2014, 108, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Wang, Z.; Shen, G.; Chen, X.; Tang, Z.; Guo, C.; Gu, H.; Fu, K. Heavy metals accumulation in soil after 4 years of continuous land application of swine manure: A field-scale monitoring and modeling estimation. Chemosphere 2018, 210, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Xiao, Z.; Zhang, G.; Wang, A.; Li, Z.; Liu, Y.; Wang, H.; Zeng, Q.; Liang, Y.; Zou, D. Speciation and bioavailability of heavy metals in pyrolytic biochar of swine and goat manures. J. Anal. Appl. Pyrolysis 2018, 132, 82–93. [Google Scholar] [CrossRef]
- Wang, S.; Cai, L.-M.; Wen, H.-H.; Luo, J.; Wang, Q.-S.; Liu, X. Spatial distribution and source apportionment of heavy metals in soil from a typical county-level city of Guangdong Province, China. Sci. Total Environ. 2019, 655, 92–101. [Google Scholar] [CrossRef]
- Jiang, H.H.; Cai, L.M.; Wen, H.H.; Luo, J. Characterizing pollution and source identification of heavy metals in soils using geochemical baseline and PMF approach. Sci. Rep. 2020, 10, 6460. [Google Scholar] [CrossRef]
- Jiang, H.H.; Cai, L.M.; Wen, H.H.; Hu, G.C.; Chen, L.G.; Luo, J. An integrated approach to quantifying ecological and human health risks from different sources of soil heavy metals. Sci. Total Environ. 2020, 701, 134466. [Google Scholar] [CrossRef] [PubMed]
- Men, C.; Liu, R.M.; Xu, L.B.; Wang, Q.R.; Guo, L.J.; Miao, Y.X.; Shen, Z.Y. Source-specific ecological risk analysis and critical source identification of heavy metals in road dust in Beijing, China. J. Hazard. Mater. 2020, 388, 121763–121775. [Google Scholar] [CrossRef]
- Zhang, J.; Hua, P.; Krebs, P. Influences of land use and antecedent dry-weather period on pollution level and ecological risk of heavy metals in road-deposited sediment. Environ. Pollut. 2017, 228, 158–168. [Google Scholar] [CrossRef]
- Giller, K.E.; Witter, E.; McGrath, S.P. Assessing risks of heavy metal toxicity in agricultural soils: Do microbes matter? Hum. Ecol. Risk Assess. Int. J. 1999, 5, 683–689. [Google Scholar] [CrossRef]
- Fath, B.D. Distributed control in ecological networks. Ecol. Model. 2004, 179, 235–245. [Google Scholar] [CrossRef]
- Li, J.; Chen, Y.; Peng, H.; He, L. Dynamic rule of ecological risk transmission among ecological communities based on network environmental analysis. Sci. Total Environ. 2021, 781, 146729. [Google Scholar] [CrossRef]
- Li, J.; Chen, Y.Z.; Peng, H.; He, L. A system-scale environmental risk analysis with considering a conceptual conversion from material/energy flow to information flow under uncertainties. J. Environ. Manag. 2021, 300, 113775. [Google Scholar] [CrossRef] [PubMed]
- Fath, B.D.; Scharler, U.M.; Ulanowicz, R.E.; Hannon, B. Ecological network analysis: Network construction. Ecol. Model. 2007, 208, 49–55. [Google Scholar] [CrossRef]
- Wang, A.C.; Li, X.; Hao, X.L.; Luo, X.S.; Chen, W.L.; Huang, Q.Y. Ammonia level influences the assembly of dissimilatory nitrate reduction to ammonia bacterial community in soils under different heavy metal remediation treatments. Sci. Total Environ. 2022, 838, 156393. [Google Scholar] [CrossRef]


| Max | Min | Average | Median | SD | CV. (%) | Background Value [51] | Risk Screening Value [38] | % of SER | |
|---|---|---|---|---|---|---|---|---|---|
| pH | 8.90 | 4.40 | 5.75 | 5.20 | 1.20 | 20.92 | - | - | - |
| OC | 6.74 | 0.40 | 1.33 | 1.13 | 0.76 | 56.93 | - | - | - |
| CEC | 32.00 | 1.00 | 10.80 | 9.00 | 5.86 | 54.24 | - | - | - |
| Cd | 1.60 | 0.02 | 0.29 | 0.20 | 0.28 | 93.79 | 0.22 | 0.3 | 29.26% |
| Pb | 211.93 | 1.03 | 43.05 | 31.15 | 36.24 | 84.18 | 40.6 | 80 | 11.35% |
| Cr | 662.14 | 1.70 | 82.94 | 41.38 | 112.65 | 135.82 | 65.2 | 250 | 9.61% |
| Ni | 313.54 | 1.57 | 32.23 | 10.65 | 54.30 | 168.48 | 42.5 | 60 | 15.72% |
| Cu | 121.16 | 0.88 | 25.82 | 18.41 | 24.25 | 93.93 | 38.38 | 50 | 13.10% |
| Zn | 575.70 | 3.20 | 99.15 | 81.53 | 74.65 | 75.29 | 89.7 | 200 | 6.55% |
| Mn | 2481.15 | 47.27 | 596.62 | 483.68 | 470.92 | 78.93 | 626 | - | - |
| Fe | 20.82 | 0.19 | 3.98 | 2.67 | 3.61 | 90.63 | - | - | - |
| P | 5496.43 | 78.26 | 1133.91 | 929.82 | 881.50 | 77.74 | - | - | - |
| Principal Component Load | |||
|---|---|---|---|
| PC1 | PC2 | PC3 | |
| Cd | 0.158 | −0.034 | 0.861 |
| Pb | 0.054 | 0.129 | 0.882 |
| Cr | 0.910 | 0.141 | 0.038 |
| Ni | 0.926 | 0.090 | 0.182 |
| Cu | 0.740 | 0.499 | 0.210 |
| Zn | 0.302 | 0.845 | 0.137 |
| Mn | 0.729 | 0.472 | 0.058 |
| Fe | 0.876 | 0.281 | 0.076 |
| P | 0.188 | 0.907 | −0.038 |
| Percentage of variance (%) | 54.072 | 16.261 | 12.410 |
| Percentage of accumulation (%) | 54.072 | 70.332 | 82.742 |
| Heavy Metals | Background Level | Polluted | ΔIx | Probability of Risk Occurrence (Px) |
|---|---|---|---|---|
| Cd | 0.22 | 0.3 | 0.393 | 0.368 |
| Pb | 40.60 | 80 | 0.394 | 0.345 |
| Cr | 65.20 | 250 | 0.514 | 0.192 |
| Ni | 42.50 | 60 | 0.712 | 1.151 |
| Cu | 38.38 | 50 | 0.544 | 0.222 |
| Zn | 89.70 | 200 | 0.358 | 0.607 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Yang, Y.; Li, Y. Comprehensive Perspective on Contamination Identification, Source Apportionment, and Ecological Risk Assessment of Heavy Metals in Paddy Soils of a Tropical Island. Agronomy 2024, 14, 1777. https://doi.org/10.3390/agronomy14081777
Guo Y, Yang Y, Li Y. Comprehensive Perspective on Contamination Identification, Source Apportionment, and Ecological Risk Assessment of Heavy Metals in Paddy Soils of a Tropical Island. Agronomy. 2024; 14(8):1777. https://doi.org/10.3390/agronomy14081777
Chicago/Turabian StyleGuo, Yan, Yi Yang, and Yonghua Li. 2024. "Comprehensive Perspective on Contamination Identification, Source Apportionment, and Ecological Risk Assessment of Heavy Metals in Paddy Soils of a Tropical Island" Agronomy 14, no. 8: 1777. https://doi.org/10.3390/agronomy14081777





