Abstract
Sustainable management of potassium nutrition in alfalfa crop production is one of the major key factors for achieving optimum seed and biomass yields. An inappropriate supply of mineral potassium nutrition in alfalfa production could result in a decrease in biomass and grain yield production, leading to luxury consumption with cost implications. Alfalfa (Medicago sativa L.) is a perennial leguminous forage crop known for its high protein content, nutritive value, biomass yield production, soil-improving abilities, and livestock feed. Potassium nutrition plays a crucial role in alfalfa production by influencing several physiological processes essential for biomass yield, growth, development, photosynthesis, nutrient uptake, and stress tolerance. Although several studies have been conducted regarding the role of potassium nutrition in agriculture productivity, only limited research has focused on crop-specific impacts. Therefore, this paper reviews (i) the significant role potassium nutrition plays in alfalfa production along with its implications for quality, yield, growth, and resistance to abiotic stress; (ii) the factors affecting the availability, absorption, and transport of potassium; (iii) the source of potassium and the consequences of inadequate availability; and (iv) highlights some strategies for mitigating potassium nutrient deficiency to optimize alfalfa productivity and sustainability in agricultural systems.
1. Introduction
Alfalfa (Medicago sativa L.) is an extensively planted significant perennial leguminous forage crop, due to its excellent productivity, good adaptability, favorable quality characteristics, and versatility. It is currently still mainly used as animal feed, but its high protein content is increasingly being considered for human consumption [1,2]. Around 30 million hectares of alfalfa were planted globally; 41% in North America, 25% in Europe, 23% in South America, 8% in Asia, and 3% in Africa and Oceania [3]. It is used for grazing, hay, and silage, as well as green manure and cover crops. Alfalfa leaves are an excellent source of protein, having a dry matter crude protein content of 26% to 30%, while the dry matter crude protein content of the stems is considerably lower, 10–12% [4,5]. Native to warmer temperate regions, alfalfa is prized for its deep root system, which not only prevents soil erosion, but also increases soil fertility with nitrogen [6,7]. It is extremely durable, especially against droughts, because of its deep root structure and perennial crowns that store carbohydrates as an energy reserve. Alfalfa stands require special attention to fertility maintenance for years of high output since a constant supply of nutrients throughout the growing season is essential for high-yield, high-quality forage production [8].
Potassium (K) has been known to play a vital role in enhancing productivity and sustainable production of alfalfa [9,10,11]. Among the advantages of potassium nutrition is the activation of enzymes that affect the production of protein, starch, and adenosine triphosphate (ATP) [12], as well as the assistance in regulating the opening and closing of stomata that control the exchange of water vapor, carbon dioxide, and oxygen [13]. Potassium is characterized by its ability to mitigate various stresses, including abiotic (e.g., drought, salinity, nutrient imbalance) and biotic stress (pests, diseases, pathogens) [8,9]. It also improves the flow of water, minerals, and carbohydrates, decreases water loss and wilting, enhances drought tolerance, and maintains turgor in plants [14]. Its availability is necessary for enzyme production, photosynthetic processes, osmotic pressure control, stomatal movement, protein synthesis, phloem transport, energy transfer, maintaining cation-anion balance in the soil, and improving stress tolerance [15,16,17]. Potassium is associated with changes in respiration, photosynthesis, translocation, and enzyme system activities in alfalfa crop productivity, making K fertilization crucial for improving the growth and yield of alfalfa. Berg et al. [18] revealed an increase in below-crown root mass of alfalfa under K fertilization but a decrease in nutritional value. According to Elgharably and Benes [19], the addition of K mineral fertilizer causes alfalfa N-fixation to increase to a maximum of 300% on sandy soils, thereby improving growth, development, yield, cell wall formation, and tolerance to abiotic stress. Other studies found that root formation and growth were equally hindered by excess and deficiency of K [16]. These findings clearly show that providing an optimum supply of potassium is an important requirement for alfalfa production in order to maintain high-quality and profitable yields. Although the role of K nutrition in crop productivity has been well documented, very few reviews have focused exclusively on the significant role potassium plays in alfalfa (Medicago sativa L.) as a perennial leguminous crop. In this paper, we discuss the significant role of potassium (K) in alfalfa production under abiotic conditions and its influence on the growth and yield of alfalfa, and factors affecting the availability, uptake, and transport of K in alfalfa production. Such knowledge is necessary to understand how potassium contributes to sustaining the productivity and quality of alfalfa.
2. Effects of Potassium on Growth, Yield, and Yield Quality of the Alfalfa Plant
Potassium (K) is regarded as a macronutrient crucial in improving crop growth and yield parameters [20]. Annual potassium (K) losses from perennial crops like alfalfa are substantial, so when K is applied insufficiently, it can lead to a lower yield [9]. Berg et al. reported that poor K fertilization reduces the number of nodules as well as the quantity of C transported to nodulated roots and photosynthesis [21]. Across different cultivars, there was a consistent increase in yield with K fertilizer applied at rates higher than those that maximized output. The response from potassium additions is first noticed, according to Berg et al. [18], when the rate of regrowth is quicker after a harvest. Islam and Baidoo [22] reported that K boosts the amount of carbohydrate held in alfalfa roots, which hastens the growth process. Longer stand life is produced by the alfalfa’s ability to compete more fiercely against weeds and insects due to its quick rate of regeneration. Another aspect of potassium fertilization is attributed to the possibility of increased disease resistance [23]. In their 1995 study, Meyer and Mathews [23] found no statistically significant difference between the values in the alfalfa forage for the first five harvests following the application of the two potassium fertilizer sources: potassium chloride and potassium sulfate. The average annual dry matter (DM) yield, according to a study by Lloveras et al. [24], was 21.5 t ha−1, and it showed a small linear response to K fertilizer, even if it marginally boosted DM yields for alfalfa under irrigation in this very demanding Mediterranean soil. Berg et al. [18] observed no influence of K fertilization on the alfalfa population or shoot per plant . Other research on the impact of applying potassium fertilizer to alfalfa herbage yield has produced some contradicting results. For example, Kitchen et al. [25] reported a considerable increase in herbage yield after applying potassium, whereas Elouear et al. [26] found a decrease in herbage yield.
Alfalfa stands generally produce the highest yields during their first two or three years of production, after which they start to decline. Increasing the longevity of the crop improves the economics of production. Numerous studies suggested that many key determinants are associated with limiting the length of alfalfa use in intensive crop production systems [27,28,29,30]. Alfalfa’s capacity to survive the winter may be affected by potassium’s effects on plant health [8,22,31]. It is essential for various processes, such as rapid regrowth, photosynthesis, nitrogen fixation, efficient water use by plants, suppression of invasive weeds and grass, and enhancement of winter hardiness [25,32]. The longevity of alfalfa stands is influenced by a number of factors other than nutrient supply and their interaction. These determinants include location, soil textural class [28], harvesting frequency [30,33], pest and disease incidence [29], availability of water [27], genetic properties, environmental conditions, and production technologies [34]. Ayars et al. [27] indicated that the quality of groundwater, salinity, and depth all influence longevity and crop mineral absorption of alfalfa use in intensive agriculture. Milić et al. [34] revealed that developing harvesting techniques to boost the profitability of alfalfa cultivation, the influence of varietal selection on yield, and growth performance evaluation in intensive cutting management is crucial. Alfalfa yield and its quality parameters can be improved without adversely affecting alfalfa stand persistence by improving harvesting management and raising harvesting frequency for determining the length of alfalfa use in intensive agriculture systems. In addition to effective fertilization techniques or strategies, proper irrigation techniques and pest management strategies like agronomic practices could be crucial in sustaining alfalfa stands in intensive agriculture [6,35].
Lissbrant et al. [36] and Putman et al. [37] stated that while fertilizer use for alfalfa might enhance yields, the plants’ morphology and physiology may change, which could reduce the fodder quality such as high dry matter, sustainable level of crude protein, high palatability, high digestibility, and low lignin content. Low leaf-to-stem ratios usually indicate worse forage quality because P and K fertilization can make shoots grow higher and thicker, effectively reducing the number of leaves compared to the amount of stem tissue [36]. K fertilizer is more concentrated in plant tissue when it is applied excessively, and this alters the nutritional balance of alfalfa as a feed source for animals [38,39]. The concentration of K in the above- and below-ground biomass rose, indicating a luxury K use [40]. According to a 2019 study by Jungers et al. [40], the potassium fertilizer increased K content, decreasing the forage nutritional value. The maximum tolerable level (MTL) for potassium is 2% of diet DM (dry matter), although cattle can tolerate higher (more than 3% of DM) potassium in early spring pastures or if magnesium supplementation is increased in case of lactating cows [41,42]. If potassium concentration in the diet exceeds 3.5% (increased potassium (K) concentration of herbage, together with reduced calcium (Ca) and magnesium (Mg) concentration of plant [31], the K interferes with the uptake of Ca and Mg [43,44], compromising calcium homeostasis [45]. Ritter [46] suggested that the approximately 2.2 critical K/Ca + Mg ratio in animal feed is crucial to prevent health problems, like milk fever in dairy cows. Fortunately, plant K level rarely reaches a concerning level [47]. A study by James et al. [48] examined the effect of potassium and phosphorus deficiency on the elemental composition of alfalfa herbage, and according to their findings, the cultivar exhibited a noteworthy decrease in the concentration of elemental components including B, Cu, and Mn in the herbage when exposed to potassium deficiency. Potassium additions usually result in a drop in protein content and only occasionally accumulation of vital components for animal feed, such as protein and total digestible nutrients (TDNs) [18,22,31]. Furthermore, a drop in crop quality can also result from low potassium availability [32].
Several important causes can be blamed for these discrepancies. Crop response to potassium fertilization can be significantly impacted by changes in experimental conditions, including soil type, crop species, climate, and application rates. Because of these intrinsic variances, research carried out under various situations may produce results that are not entirely consistent. Since potassium interacts with nitrogen, phosphorus, and other important components in the soil-plant system, the intricate relationships between potassium and other nutrients may be a contributing factor to these discrepancies. Table 1 lists some of the findings from other studies on the effects of potassium fertilization on alfalfa productivity.

Table 1.
Impact of potassium fertilization on growth, yield, and quality of the alfalfa plant.
3. Potassium’s Significance in Alfalfa Production under Abiotic Stress Condition
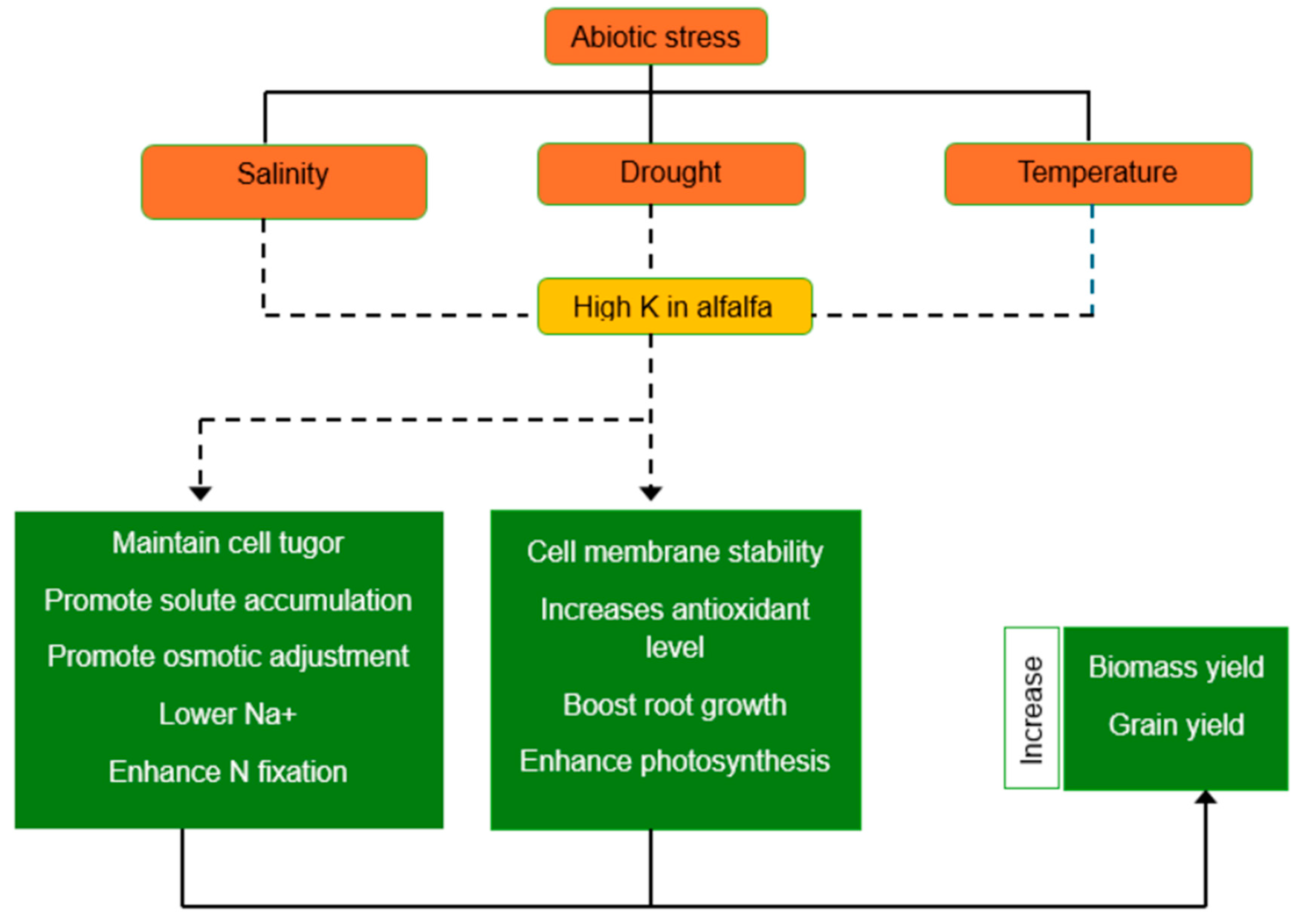
Potassium (K+) is essential for plants to respond physiologically and morphologically, especially in the context of adapting to abiotic stress. It has a role in cellular signaling, stress responses, reproductive growth, and maintaining nutritional balance. Photosynthesis, osmoprotection, stomatal control, nutrient translocation, water-nutrient absorption, and enzyme activation are all aided by K+. The availability of K in alfalfa encourages the fixation of nitrogen [8,56]. The addition of potassium contributes to osmotic adjustment by accumulating with other solutes to sustain metabolic activities under stress. K+ also enhances water-use efficiency and turgor potential, helping alfalfa cope with abiotic stresses such as drought and salinity [9]. Its role in coordinating with other nutrients improves plant resilience to adverse environmental conditions, making it essential for sustainable agriculture in the face of climate challenges. The role of K in enhancing alfalfa productivity under abiotic stress is shown in Figure 1.
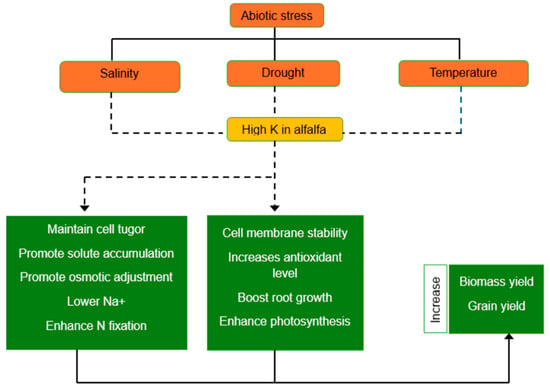
Figure 1.
Role of potassium (K) in alfalfa productivity under abiotic stress condition. (Image by authors). Dotted arrow shows the K role in plant defence mechanism; continous arrow denotes plant adaptation.
3.1. Salinity
One of the biggest global barriers to alfalfa plant growth and development that results in significant crop productivity loss is salinity. Due to an excess of potassium (K+) efflux and sodium (Na+) ion influx, salinity stress impairs the metabolic and cellular functions of plants [57,58]. Several studies have reported that treating alfalfa with K nanoparticles results in less sensitivity to salt stress regarding N-fixing alfalfa plants compared to NO3-fed plants [38,59,60]. Potassium nanofertilizers can be natural or engineered nanomaterials [61]. Natural nanoparticles can be produced from photochemical reaction processes, volcanic eruptions, forest fires, erosion, plants, animals, or microorganisms. In green nanotechnology, different plant species and microorganisms (bacteria, algae, and fungi) are used for the synthesis of nanoparticles. Application of K2SO4 nanoparticles can increase the Na+/K+ ratio and concentration of other elements in alfalfa plant tissue, including phosphorus, copper, zinc, and manganese. Additionally, Ramos-Ulate et al. [38] and Zulfiqar and Ashraf [59] revealed that by raising the concentration of catalase, proline, and antioxidant enzymes and reducing electrolyte leakage, the addition of K2SO4 nanoparticles, a K source, is essential to the alfalfa plant’s physiological response to salt stress. K+ modulates photosynthetic carbon fixation and nutrient and water transpiration by regulating stomata dynamics and functionalities, which makes it very important in maintaining photosynthate translocation in alfalfa plants [16,62,63]. Boosting the alfalfa plant defense mechanism against salinity by the activity of improved RubiSCo and carbohydrate metabolism is also a practice associated with K+ [64]. Studies proposed that K+ concentration in cytosolic could be vital in tolerating salt stress conditions at the early growth phase and could facilitate recovery [65]. Consequently, K deficit under salt stress conditions could be detrimental to alfalfa plant establishment. K+ has been noted to decrease reactive oxygen space (ROS) generation under salinity conditions and trigger activation of stress-related genes, leading to the up-regulation of both non- and enzymatic antioxidants, which then improve alfalfa growth [57,66]. This is possible due to the alterations in the antioxidant metabolism and maintenance of the Na+/K+ ratio, therefore, the addition of K could be significant to overcome the impact of salinity that comes along with oxidative stress. K+ may activate transcription factors that induce genes responding to stress, which in turn stimulates the antioxidant defense system and enhances stress tolerance [67]. Ahanger et al. [67] found that K+ ions greatly reduce the harm caused by oxidative stress under salinity during the pre-flowering and blooming stages.
3.2. Drought
The unavailability of soil water is the main factor that limits plant growth and productivity in arid and semi-arid locations. Sustaining a favorable water status is crucial for alfalfa production in order to ensure plant life during drought stress. Several studies have shown that plant species’ resistance to drought is positively correlated with leaf osmotic adjustment [68,69]. Potassium ion, one of the most significant inorganic osmotica in plants, is necessary even in drought-like conditions for the development of the osmotic adjustment ability [70]. Cell turgor recovery in osmotically induced stress is controlled by increased absorption of K+, Cl−, and Na+ by root cells, which is largely mediated by voltage-gated K+ transporters at the cellular plasma membrane. Sufficient K also encourages solute build up, which reduces osmotic potential and aids the preservation of plant cell turgor in the face of osmotic stress [71]. Continuous drought stress on plants can result in the formation of reactive oxygen space (ROS), which damages leaves [72] and eventually lowers agricultural output. K absorption was limited during drought stress due to restrictions on root growth and the rates at which K+ diffused from the soil towards the roots. To boost crop tolerance to stress in low-moisture soils, Wang et al. [15] recommend encouraging deeper roots, larger absorption surfaces, and enhanced water retention in plant tissues. Using K fertilizer in conjunction with other mineral nutrients like P and N, which also act as root signaling agents, may promote deeper roots [73]. Adequate K concentrations can increase total dry matter accumulation in drought-stressed plants [74]. Given that K+ regulates stomata and leads to more intense photosynthesis, and that K is also required for the transfer of photoassimilates to promote root growth, enhancing root development and resulting in greater water uptake [75,76]. Appropriate K fertilization increases plant total dry mass and leaf area while also improving water retention in plant tissues under drought stress, according to Egilla et al. [77]. Growing data indicate that plant drought tolerance depends on the maintenance of membrane stability and integrity under drought stress [78,79]. According to a study by Waraich [80], the effects of drought stress significantly decreased the stability of cell membranes. It was determined that K’s role in boosting cell membrane integrity and osmotic adjustment capability was the primary reason for this decrease. Moreover, the ability of alfalfa plants to withstand drought stress may be improved by appropriate K status, promote osmotic adjustment, maintain increased turgor pressure and relative water content, and reduce osmotic potential.
3.3. Temperature Stress
Extreme temperature conditions prevent plant growth and development, which limits crop yield. It has an impact on plants by directly suppressing metabolic processes and subtly affecting osmotic, oxidative, and other stressors brought on by cold [81,82,83]. The destruction of photosynthetic processes and decreased activity of antioxidant enzymes under conditions of cold stress might lead to a build-up of reactive oxygen species (ROS) [84]. K2O application increased the activity of superoxide dismutase (SOD), catalase (CAT), and peroxidase (POD), under low-temperature stress treatment (+4, −10, −20, and −30 °C). The application rate of 200 kg K2O·ha−1 resulted in the strongest cold resistance of alfalfa plants, which was also probably related to the significantly increased soluble sugar, sucrose, fructose, and starch content of the alfalfa crown [85,86]. Greater frost damage in K-deficient plants is associated with water deficit due to the freezing-induced dehydration of cells and chilling-induced restriction of water uptake [87]. Frost damage and leaf K concentration were found to be significantly inversely correlated, and an adequate K supply can significantly boost frost resistance [88]. According to other experiments, alfalfa that received enough K could withstand late frost without evident harm, but much of the crop cultivated on K-deficient soil perished [16,89,90]. This result may be explained by the control over osmotic and water potential, as well as the decrease in electrolyte leakage brought on by cold stress [91]. The plant was prevented from freezing by high K+ concentrations that lowered the freezing point of the cell fluid. Furthermore, a certain cytosol K+ content is required for the enzyme activities that regulate frost resistance [92]. The ratio of unsaturated to saturated fatty acids in the cell membrane is essential for plants in order to be tolerant to cold stress, and the higher the ratio, the more resistant the tissue is [93]. By boosting enzyme activity connected to lipid synthesis, the proper application of K fertilizer can optimize the fatty acid components. Chen et al. [94] published that optimum potassium fertilization could increase the unsaturated fatty acid content and decrease the saturated fatty acid content in rice. Cold stress increases the amount of unsaturated fatty acids, which causes changes in the lipid–protein mix and fatty acid unsaturation of the cell membrane. It can lead to a reduction in membrane fluidity, as the plasma membrane is the primary site for sensing temperature changes [95,96,97]. The transfer of ions, water, and metabolites may be further impacted by a decrease in membrane fluidity. Increasing membrane permeability, phospholipid levels, and cell biophysical and biochemical features all improve plant frost resistance when K is added [88]. Enhanced yield output was achieved by reducing chilling damage and enhancing cold resistance in tissues with higher K contents [88,98].
4. Deficiency of Potassium in Alfalfa Production
Alfalfa takes up a higher amount of K through its life cycle than the remaining macronutrients [18,22]. However, inadequate or lack of K availability will have a lot of effect on alfalfa production. Potassium deficit may result from low soil oxygen levels, excessive liming or calcium-rich fields, low soil pH, or real soil deficiency. In the case of K deficiency, the growth rate of alfalfa is first reduced and the plant seems stunted. Potassium-deficient plants tend to wilt on dry, light days [99]. As potassium is easily mobilized in the plant, the first visible symptoms appear on older leaves [100]. Early indications of potassium deficiency are the small white necrotic spots appearing on the edges of the lower leaves, then in advanced cases, yellowing (chlorosis) at the margins of older leaves and necrosis (death) on the lower portion of the plant are visible [100]. A stocky appearance and short internodes indicate weak plants. Tiny leaf blades and slowed growth are visible in younger leaves [101]. In addition, leaves may have a copper metallic shine, seem wavy, or be dark to bluish-green. Potassium, in contrast to other nutrients, does not condense into compounds in plants, leaving it free to control many essential functions such as photosynthesis, starch formation, water-use efficiency, enzyme activation, and protein synthesis. Inadequate or insufficient K will have a major impact on these functions [102,103]. Potassium-deficient plants form their roots slowly and grow slowly [104], resulting in weak stalks and frequent lodgings [82,83]. Potassium-deficient plants have poor yield quality and decreased susceptibility to disease and moisture stress [56,104]. Studies conducted by Oregon State University showed that only 90% of the maximum yield of alfalfa was obtained when soil K was under 150 mg kg−1. They suggested that soil K content must be monitored annually to know the most critical value for K in alfalfa productivity. They also proposed that when soil test K is below 150 mg kg−1 and hay, or tissue test values are below 2% it is likely that fertilizer K addition is needed [11]. Sun et al. [105] suggested that nutrient supply should be only considered when the concentration in the plant drops to 1.7% K. Therefore, soil and tissue tests must be monitored regularly to notice the critical stage of the K deficit to recommend the K necessary to ensure optimum growth and yield.
5. Mitigating Potassium Deficiency for Optimized Alfalfa Production
The availability of the mineral K nutrient is crucial for enhancing both physiological processes and crop growth and development. The importance of potassium for the development, productivity, quality, and abiotic stress tolerance of alfalfa has been discussed in detail in previous chapters. This shows that without enough potassium, plants will have underdeveloped roots, slow growth, limited seed production, decreased yields, and yield quality. Plant development and physiological activities will be hampered by restricted potassium nutrition availability [100,101,102]. To mitigate the potassium deficit, different strategies could be employed to enhance alfalfa crop production under potassium deficiency conditions. These strategies include;
- Encouraging the use of biostimulants/biofertilizers in alfalfa crop production. Biostimulants/biofertilizers are compounds that promote plant growth and development; therefore, they will improve the alfalfa plants’ uptake and usage of potassium [38,54].
- Selecting alfalfa genotypes that have a higher tolerance or efficiency in potassium uptake can help mitigate the effects of potassium deficiency on alfalfa production [48,106].
- Rotating alfalfa with potassium-rich crops can help replenish soil potassium levels and reduce the impact of deficiency on alfalfa production [2,107,108].
- Conducting soil analysis to examine the quantities or level of potassium available and accessible for plant uptake could help determine the extent of deficiency and guide proper corrective measures [109,110].
- The addition of organic materials such as farmyard manure (FYM), compost, crop residues, and biochar in the soil could improve soil structure and potassium availability, enhancing root growth and mineral nutrient element uptake [108,111,112].
- Better soil pH management should be employed to ensure the pH remains within the optimum range, especially between 6 and 7 for potassium availability. Soils with low pH values (acidic soil) tend to bind potassium, decreasing the soil’s ability to supply potassium for plant uptake [113,114,115].
- Better management of nutrients could address potassium deficits in alfalfa by using balanced fertilization techniques that include potassium addition [48,60,116,117].
Improving potassium deficit and soil health are crucial strategies for maintaining mineral nutrients and ensuring sustainable crop productivity. Alfalfa should receive the proper dosage of potassium fertilizers to reduce the likelihood of leaching from the root zone or excessive consumption. Table 2 lists the percentage (%) of a few potassium concentrations of various commercial chemical fertilizers.

Table 2.
Potassium content in some chemical fertilizers.
6. Sources of Potassium in Soil
In soils, potassium is primarily found in inorganic forms. It can be found in soil solutions, adsorbed to or released from clay particle surfaces, bonded in silicates, and exchangeable form [109]. The main sources of K are the rocks mica and feldspar, which release this element during weathering [118]. K exists in the soil in four different forms in dynamic equilibrium with each other [119,120,121]:
- Mineral or structural form: A major portion of soil K, accounts for 90–98% of the total K in the soil in the mineral structure
- Non-exchangeable (fixed) form or relatively unavailable form: The difference from the mineral form is that it is not bonded within the crystal structure of soil mineral particles. It is present within clay minerals in a 2:1 ratio
- Exchangeable form or slowly available form: Contributes 1–10% of the K in soil. Exchangeable potassium is approximately 90% of the available K. Usually the ratio of exchangeable K to total K is under 2%
- Water soluble form: Only accounts for 0.1–2% of the total potassium in soil and is made up of potassium dissolved in the soil and held in the exchange position of the clay and organic matter.
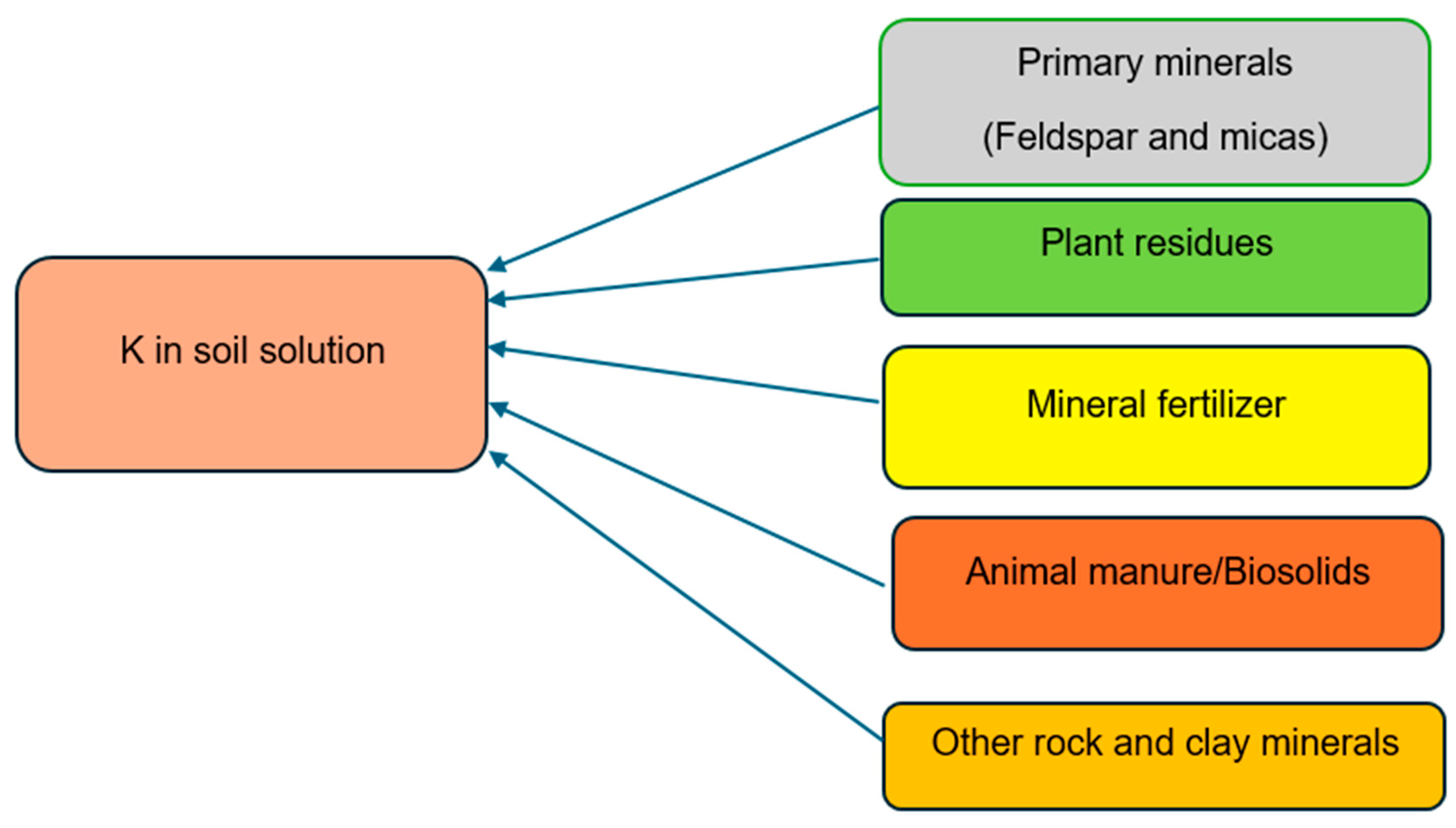
Chemical fertilizers, organic manure, and plant residues are also sources of this element. Organic manure is rich in nutrients, about 80–90% potassium (K) in feed is excreted from the animals in the manure. Li et al. and McLaughlin et al. [122,123] reported that some layered-aluminosilicate clay minerals may have the ability to absorb potassium both on their exterior surfaces and within the layers of their crystal lattices, leading to the immobilization (or fixing) of potassium ions. Generally, potassium ranges between 0.4 and 30 g kg−1 in mineral soils, with agricultural soils containing between 10 and 20 g kg−1 [109]. In the top 0.2 m of the soil profile, total K levels in soils typically range between 10,000 and 50,000 kg ha−1 [118], while other sources [26,124] state that the total amount of K in soils varies from 3000 to 100,000 kg ha−1 in the top 20 cm of the soil profile. Only around 2% of this total K content is in the soil solution and in exchangeable phases, with the remaining 98% bound in minerals [118]. The amount of K in soil reflects its parent material, weathering level, K fertilizer additions, and losses from crop removal, erosion, and leaching (Figure 2). Shale, sandstone, limestone, and igneous rocks in the lithosphere all have average K contents of around 26, 27, 11, and 2.7 g kg−1, respectively [110]. Approximately 15% of the whole lithosphere is made up of feldspars [125]. The likelihood of providing K to crops depends on the rate of weathering and the relative proportions of the minerals in the soil [126].
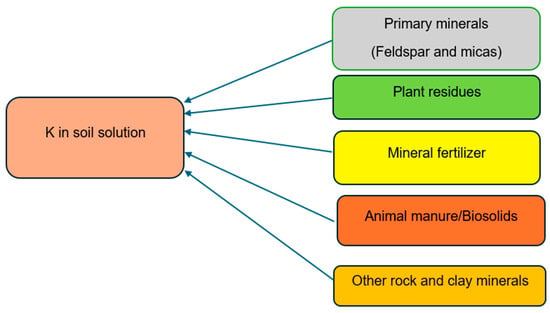
Figure 2.
Sources of potassium (K) in soil (image by authors).
7. Availability, Uptake, and Transport of Potassium in the Alfalfa Plant
The developmental phases of plants determine the specific K+ requirements for growth, consequently, agricultural plants have different K+ uptake patterns. The early vegetative growth stage is when most crop plants, including alfalfa, use up a sizable portion of their K+ requirement. Therefore, fixing K in the soil plays a crucial role in ensuring that K is readily available for uptake by alfalfa [22]. K is often conserved as a result of K fixation, making it unavailable for a very long time [8,127]. The likelihood of providing K to crops depends on the rate of weathering and the relative proportions of the minerals in the soil [90,113]. Alfalfa can use more of the available K for growth, thus some of it is not completely lost to the plant. A deficiency of K+ during the vegetative stage may make the plant susceptible to various stresses [128]. Plants typically only have access to a very small fraction of the soil potassium content. In normal soils, the presence of Ca2+ and Mg2+ hinders the actual plant uptake of soil solution K+ [114]. Over time, K+ fixed by expandable clays may become accessible, particularly at a very slow rate of dissolution. The rate at which potassium is absorbed by alfalfa roots as an ion (K+) from the soil solution is directly correlated with its concentration. While no structural role for potassium has been identified, it is characterized by high mobility at all levels within the plant –individual cells, tissues, and long-distance transport via the xylem and phloem [129]. Soil characteristics, including moisture content, temperature, and aeration have an impact on this. Alfalfa roots can reach deeper soil layers and absorb more K, which strengthens the plant’s photosynthetic system and accelerates its physiological and biochemical processes to promote growth and development [110]. However, more studies are needed on the structural function of potassium to shed more light on its mobility for better K uptake and utilization rates in alfalfa productivity.
8. Factors Affecting Potassium Availability and Absorption in the Alfalfa Plant
Plant roots can absorb potassium more readily than any other cation. Although it is absorbed similarly extensively to calcium and magnesium, potassium is normally present in the root medium in much lesser amounts. Potassium is typically utilized as the indicator ion in research studies of the absorption process. The process of absorbing potassium into the cytoplasm or vacuole of the root cells is known as active absorption, which requires the expenditure of metabolic energy [130,131]. Potassium content in the root medium is correlated with the amount of potassium that will be absorbed to maintain the highest possible rate of growth for the plant. The availability and uptake of potassium (K) at the plant root zone are directly impacted by the concentration gradient between the soil and the roots, the speed at which potassium diffuses from the soil to the root surfaces, and the surface area of the roots [132,133]. By improving plant metabolism, lowering stress, and controlling water loss, an adequate supply of potassium fertilizer greatly boosts alfalfa productivity [60,134,135]. However, alfalfa crops are more difficult to access potassium due to several factors including soil temperature, aeration, moisture content, tillage technique, and fertilizer management methods that impede availability and uptake.
8.1. Soil Moisture
Alfalfa’s ability to access and absorb potassium depends on the moisture level of the soil. K is transported and becomes more readily available to plant roots when the soil contains more moisture. According to research, K fertilization frequently yields better results in dry years [136]. Moisture is required for K+ to enter the soil and travel to the roots of the alfalfa plants for uptake. Consequently, as the soil dries out, it becomes harder to follow the path for K+ diffusion, which restricts and lowers K+ movement in the soil [137]. Under these circumstances, K is less easily absorbed by plants, especially at the root zone [138]. The type and quantity of K in the subsoil along with the particular rooting pattern influenced by soil moisture affect alfalfa’s ability to absorb K. Nonetheless, elevating the soil moisture level to approximately 30% enhances potassium diffusion within the soil, hence augmenting the quantity of K+ accessible for assimilation by alfalfa [22,108].
8.2. Soil Temperature
The normal soil temperature for bioactivity is between 10 and 24.1 °C, which is beneficial for the regular life activities of the earth’s biota and provides a correct breakdown of organic matter, increased nutrient mineralization, intake of soluble compounds, and metabolism [111]. K availability is dependent on changes in soil temperature, which have an impact on K absorption. Low temperatures typically slow down plant operations by altering root activity, soil water, and aqueous solution structure [112]. As a result, there is less K available for the uptake and growth of alfalfa. According to Marschner [116], K influx at 15 °C was almost half that at 29 °C, root development was eight times larger at 29 °C than at 15 °C, and K concentration in the shoot was 8.1% at 29 °C and 3.7% at 15 °C due to variations in K availability and root activity. An effective approach to addressing issues with K availability and uptake caused by cold weather is to provide high K levels. As soil temperature rises, plant activities, physiological processes, and root activity increase [139,140]. K absorption also increases with this rise in physiological activity; however, it is important to keep in mind that the ideal soil temperature range for absorption is between 15.5 and 26.7 °C.
8.3. Soil Aeration
Several studies stated that both root respiration and K uptake require air [141,142,143,144]. The decrease in root activity and subsequent K absorption occurs when the soil moisture content reaches saturation. Oxygen concentrations in saturated soils are very low. According to Li et al. [145], to produce ATP from carbohydrates in plant roots, there must be enough oxygen present. About 38 mol ATP may be produced from 1 mol of glucose via aerobic respiration; however, only 2 mol of ATP may be generated in anaerobic conditions [145], preventing plant roots from getting sufficient energy to sustain their health and proper physiological function [144]. A sufficient amount of oxygen (O2) and metabolic energy from the soil are necessary for plant root respiration, which is essential for K absorption, and proper root function. Reduced soil air space caused by compacted or wet soils lowers O2 supply and limits root growth [146,147]. In poorly aerated, compacted soils, impeded root development, the smaller root surface area also contributes to reduced nutrient uptake and weaker plant growth. Potassium fertilizer application can reduce yield loss slightly, but cannot fully compensate for the yield loss [148].
8.4. Soil pH, Clay Minerals and Cation Exchange Capacity
Studies indicated that due to competition between K+, Ca2+, and Mg2+ for plant uptake in acidic soils, high levels of Al3+ and Mn2+ generate an unfavorable root environment for K uptake [149,150]. Increasing soil pH reduces exchangeable Al³⁺, which lessens competition and makes it easier for K+ to outcompete Ca2+ and Mg2+ for open exchange sites. This enhances the availability of K, leading to increased K+ absorption by alfalfa and improved growth [151,152]. Moreover, a higher amount of K is present in soils containing vermiculite, montmorillonite, or mica than in soils mostly composed of weathered kaolinite clays, which are deficient in K [153]. The abundance of clay minerals that contain K increases the availability of K in the soil. The term “cation exchange capacity” (CEC) refers to the soil’s ability to contain K and other cations and store them for plant uptake [154]. The two main elements of soil that affect CEC are clay minerals and soil organic matter (OM). When soil CEC grows, a specific quantity of exchangeable K equilibrates with less K+ in solution. Fine-textured soils normally contain more exchangeable K and have higher CEC than coarse-textured soils [154,155,156]. Variations in CEC resulting from the concentration of soil clay minerals are specific to a given region, it is not always true that finer textured soils will provide access to more K than coarser textured soils.
8.5. Genetic Variation
Genetically Medicago genus is classified into more than 60 species with one-third being perennial and two-thirds being annual [106]. In the most important species of the genus, Medicago sativa L., there can be significant differences in nutrient response between cultivated genotypes. These changes result from cultivar development, including the root system, root density, and associated metabolic rates, which impact the K available for alfalfa to absorb [22,76]. The cation exchange properties of the roots have a direct bearing on variations in K uptake [155]. Cultivars with relatively low root CEC absorb more K+ and less Ca2+, while cultivars with high root CEC absorb less K+ and more Ca2+ [8,157]. This is because low CEC is associated with reduced Ca requirement. As a result, the amount of K+ that is accessible to the plant will either increase or decrease. The developmental stages of the crop also vary, according to [158,159], the metabolic rates of young alfalfa plants are higher than older plants. Therefore, alfalfa is particularly active in absorbing high levels of K during the early growth phases compared to the amounts absorbed at the advanced growth stages to speed up its physiological and biochemical plant processes for growth and development [160]. However, since the roots of alfalfa may not have sufficiently grown to reach such depths, K in the deeper portions of the soil is not accessible for absorption by the plant during the early growth stage. According to research by [135], there is an increasing need for K to support high growth and yields in the alfalfa population. The alfalfa plants compete with one another, which causes an increase in K uptake. As a result, future plant uptake of K is limited by the amount of accessible K. Similar to this, as alfalfa production and growth increase, so does K intake, which reduces the amount of K that is available in the soil for further uptake, especially in soils with low K stocks [161,162]. In comparison to later years, ref. [7] claims that potassium fertilizer had less of an impact on alfalfa fodder during the establishment year. To enhance K availability and uptake for alfalfa growth, particularly in the early growth stage, it is imperative to raise the solution K by applying K fertilizer at the soil surface [9,22,163]. Genetic improvements in alfalfa increase the plant’s efficiency of absorbing K, which facilitates the development of novel cultivars with great production potential due to their unique traits.
8.6. Management Practices
The many beneficial effects of potassium have been analyzed in depth in previous chapters. Alfalfa requires balanced nutrition, and although potassium has relatively little influence on improving stand establishment, the alfalfa stand persistence is highly dependent on adequate potassium supply. The aim of the nutrient management practice is to keep potassium nutrient levels in the optimum range during the life of the alfalfa stand (in general for four years) [36]. Under varying ecological and agrotechnical conditions, the amount of potassium nutrients that need to be applied to alfalfa stands to achieve adequate potassium supply, can vary widely. It is not enough to simply consider—as is often the practice—the crop K removal rates. We need to know the expected yield, soil potassium level, and soil cation exchange capacity (CEC) [155].
Soil tests are a reliable method for preventing nutrient deficiencies or excess K application, soil K accumulation, and luxury K consumption by alfalfa. The proportion of K in the soil to other plant nutrients has a major impact on the availability and uptake of K. When potassium content in soils is in the optimum range, the K fertilizer amount is equal to the anticipated crop removals but when potassium content in the soil is high, then K fertilizer could be half of the anticipated removal, or at excessively high range no K fertilizer is needed. When enough K is present in the soil, the addition of other essential components that are K-deficient contributes to the creation of a favorable soil environment that improves the availability of K for uptake [164].
The availability of K and the specific cation adsorbed to the CEC can affect the amount of K leached from the soil [165]. K treatment may not yield the intended results if soil macronutrient and other micronutrient availability are inadequate because K needs other necessary nutrients to be in balance for its full potential to regulate a plant’s physiological activities. When K levels are high relative to other plant nutrients, especially Ca2+ and Mg2+, the plant and soil often compete with one another for nutrient uptake, which reduces the amount of K that alfalfa can absorb [137]. High K concentrations could be beneficial to these types of soils because the K+ would then be able to compete with other nutrients and become available for alfalfa to absorb. In soils used for perennial crop production, calcium sulfate application promotes K availability in the soil by assisting in K+ desorption into the subsoil and maintaining a desired pH level [8,166].
Potassium fertilization before alfalfa stand establishment provides an opportunity to incorporate the relatively immobile potassium nutrient needed for the life of the stand. Incorporating the potassium into the soil significantly improves its effectiveness. In field trials, incorporating 270 kg ha−1 potash before seeding and topdressing 270 kg ha−1 potassium in the next year was more effective (about 335 kg ha−1 yield surplus) than topdressing 540 kg ha−1 potash for each of the two years [167].
In the case of low potassium levels in soil, it is recommended to apply potash before seeding, and topdressing annually after the first or third cutting [167]. Broadcast applications of P and K fertilizer work well since fine roots are abundant near the soil surface. Split applications of K after the first and last harvests enhance productivity and avoid luxury consumption of K [168]. However, on soils with excessively high potassium content topdressing is less recommended, as research has shown that the potassium content of the plant tissue can rise to over 4% [167], increasing the risk of milk fever by compromising calcium homeostasis [45] and may lead to boron deficiency, too.
As the soil contact area is reduced when K fertilizer is applied to soil that is high in K-fixing clay minerals, the best results are obtained when K is sprayed in bands as opposed to a broadcast pattern. Significant amounts of soil K can be lost through leaching in coarse-textured or organic soils, especially in areas with high precipitation or under intense irrigation systems [169]. About 35% of the applied K may be leached with crops on tilled ground; losses may be significantly higher on bare soil. Rather than allowing soil potassium levels to rise, the focus should be on split or yearly applications [115].
On soils that require a lot of K, broadcasting is the most widely used method of administering K for growing crops like alfalfa [36,110]. Fertigation, also known as nutrigation, facilitates the movement of K into the soil, enabling alfalfa to absorb it more efficiently and grow more robustly. Changing factors, including soil aeration, temperature, moisture, and positional availability of applied K, and tillage techniques significantly affect the availability of K in the soil [137,170]. When tillage is increased to a depth of approximately 12 to 15 cm, the K need will increase by roughly 50%, depending on the availability of soil K deeper in the tilled layer. Minimal tillage and no-till systems can reduce K availability because of reduced aeration, higher compaction, lower temperature, and limited K availability on the soil surface [137,170,171]. Furthermore, tillage residues may lower soil temperature, lessen evaporation, and enhance moisture transport, all of which may influence the way K+ diffuses in the soil [110,153].
The long-term effects of imbalanced fertilization without potassium (K) on soil quality must be assessed. Heuschele et al. [31] on soil with very high antecedent soil K levels found that after six years with no K fertilization, nutrient removal rates did not result in a perceptible reduction of soil K, and yields were comparable to K fertilized alfalfa. At moderate and high K fertilization rates, soil K did not change over that time period either. The results of a 27-year field study with three treatments—control without fertilization (CK), mineral N and P fertilizer (NP), and mineral N, P, and K fertilizer (NPK)—revealed that long-term NPK fertilization compared to NP fertilization decreases soil biological quality in K-rich soils [172]. Wang et al. [173], based on 20 years of experimental results, stated that nitrogen, phosphorus, and potassium (NPK) fertilizer lowered soil pH by an annual average of 0.07, while organic fertilizer increased soil pH by about 0.04. Organic and chemical fertilizers both increased total soil organic carbon (SOC). To fully understand the underlying mechanisms by which K influences soil quality in agricultural systems, more research is necessary.
9. Conclusions
Potassium (K) is one of the macronutrients needed for plant growth and development. K nutrition is essential in plant metabolic processes including growth, photosynthesis, and respiration and it has a vital role in increasing N fixation in alfalfa productivity. Optimal K mineral concentration is required during the vegetative stage of the growing phase for uptake, and its high concentration in alfalfa tissue could mitigate the impact of abiotic stresses (drought, salt, and temperature) on alfalfa productivity and enhance yield. However, excessive K supply no longer has a positive effect on the yield and can negatively affect the quality of alfalfa. Lack of K in the soil causes deficiencies in plants, affecting growth indices, development, yield, cell wall formation, and tolerance to abiotic stress. When applying potassium, it is important to determine the amount of nutrient to be applied taking into account the soil potassium supply, the cation exchange capacity, and the K supply of the plant tissue. The availability of potassium in alfalfa enhances several cellular processes, including turgor regulation, stomatal movement, protein synthesis, and charge balance. Several factors including soil moisture, soil aeration, soil temperature and tillage system, and poor fertilizer management practices impact the availability and uptake of potassium, rendering it inaccessible to alfalfa crops. To overcome these factors that inhibit the uptake of K and address the deficiencies in soils, proper strategies such as efficient nutrient management, biostimulant supplements, good genotype selection, and regular soil testing should be employed while increasing soil moisture to around 30%. Better tillage systems and maintaining proper soil aeration could improve K diffusion in the soil to increase K+ availability for uptake, which then promotes root growth and keeps cell membrane stability and osmotic adjustment capability. The goal of future studies should be to better understand the transport, utilization, and absorption mechanisms of potassium in both low and high K environments. Further investigation is needed into the structural function of potassium, its mobility, and the identification and optimization of genes to enhance K uptake and utilization rates, thereby improving alfalfa productivity. However, it is also important to monitor the long-term impact of potassium nutrition or the lack of potassium fertilization on soil potassium levels.
Author Contributions
Conceptualization, E.A.A., A.B.-K., J.C., A.O. and E.K.; writing—original draft preparation, E.A.A., A.B.-K., J.C., A.O. and E.K.; writing—review and editing, E.A.A., J.C. and E.K.; visualization, E.A.A.; supervision, E.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
No new data were created in this study.
Acknowledgments
Supported by the University of Debrecen Program for Scientific Publication.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mantino, A.; Tozzini, C.; Bonari, E.; Mele, M.; Ragaglini, G. Competition for light affects alfalfa biomass production more than its nutritive value in an olive-based alley-cropping system. Forests 2021, 12, 233. [Google Scholar] [CrossRef]
- Bo, P.T.; Dong, Y.; Zhang, R.; Soe Htet, M.N.; Hai, J. Optimization of Alfalfa-Based Mixed Cropping with Winter Wheat and Ryegrass in Terms of Forage Yield and Quality Traits. Plants 2022, 11, 1752. [Google Scholar] [CrossRef] [PubMed]
- Mouttet, R.; Escobar-Gutiérrez, A.; Esquibet, M.; Gentzbittel, L.; Mugniéry, D.; Reignault, P.; Castagnone-Sereno, P. Banning of methyl bromide for seed treatment: Could Ditylenchus dipsaci again become a major threat to alfalfa production in Europe? Pest Manag. Sci. 2014, 70, 1017–1022. [Google Scholar] [CrossRef] [PubMed]
- Hojilla-Evangelista, M.P.; Selling, G.W.; Hatfield, R.; Digman, M. Extraction, composition, and functional properties of dried alfalfa (Medicago sativa L.) leaf protein. J. Sci. Food Agric. 2017, 97, 882–888. [Google Scholar] [CrossRef] [PubMed]
- McDonald, I.; Min, D.; Baral, R. Effect of a fall cut on dry matter yield, nutritive value, and stand persistence of alfalfa. J. Anim. Sci. Technol. 2021, 63, 799. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, A.L.; Sheaffer, C.C.; Tautges, N.E.; Putnam, D.H.; Hunter, M.C. Alfalfa, Wildlife, and the Environment, 2nd ed.; National Alfalfa and Forage Alliance: St. Paul, MN, USA, 2019; p. 34. [Google Scholar]
- Wang, Q.; Li, F.; Zhao, X.; Zhao, W.; Zhang, D.; Zhou, X.; Chen, J. Runoff and nutrient losses in alfalfa (Medicago sativa L.) production with tied-ridge-furrow rainwater harvesting on sloping land. Int. Soil Water Conserv. Res. 2022, 10, 308–323. [Google Scholar] [CrossRef]
- Baidoo, M. Alfalfa Response to Phosphorus and Potassium Fertility in Relation to Calcium and Magnesium. Int. J. Agric. Res. Environ. Sci. 2024, 5, 1–7. [Google Scholar]
- Kong, M.; Kang, J.; Han, C.L.; Gu, Y.J.; Siddique, K.H.; Li, F.M. Nitrogen, phosphorus, and potassium resorption responses of alfalfa to increasing soil water and P availability in a semi-arid environment. Agronomy 2020, 10, 310. [Google Scholar] [CrossRef]
- Chamachar, M.M.; Fazeli, M.R.; Salimi, M.; Samadi, N. Growth promoting activity, anti-biofilm effect, and down regulation of papC and rcsA genes expression by Medicago sativa (alfalfa) extract. Food Biosci. 2022, 50, 102182. [Google Scholar] [CrossRef]
- Kushnir, R.; Andersons, Z. Productivity of Alfalfa of Various Slopes for Seeds, Depending on Bio-and Biotic Factors. In Proceedings of the International Competition of Student Scientific Works Black See Science 2022, Odesa, Ukraine, 28 February 2022. [Google Scholar]
- Johnson, R.; Vishwakarma, K.; Hossen, M.S.; Kumar, V.; Shackira, A.M.; Puthur, J.T.; Hasanuzzaman, M. Potassium in plants: Growth regulation, signaling, and environmental stress tolerance. Plant Physiol. Biochem. 2022, 172, 56–69. [Google Scholar] [CrossRef]
- Rawat, J.; Pandey, N.; Saxena, J. Role of potassium in plant photosynthesis, transport, growth and yield. In Role of Potassium in Abiotic Stress, 1st ed.; Iqbal, N., Umar, S., Eds.; Springer: Singapore, 2022; pp. 1–14. [Google Scholar]
- Hageman, A.; Van Volkenburgh, E. Sink strength maintenance underlies drought tolerance in common bean. Plants 2021, 10, 489. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The critical role of potassium in plant stress response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.B.; Nahar, K.; Hossain, M.S.; Mahmud, J.A.; Hossen, M.S.; Fujita, M. Potassium: A vital regulator of plant responses and tolerance to abiotic stresses. Agronomy 2018, 8, 31. [Google Scholar] [CrossRef]
- Pandey, G.K.; Mahiwal, S. Role of Potassium in Plants, 1st ed.; Springer Briefs in Plant Science; Springer: Cham, Switzerland, 2020; p. 81. [Google Scholar]
- Berg, W.K.; Cunningham, S.M.; Brouder, S.M.; Joern, B.C.; Johnson, K.D.; Santini, J.; Volenec, J.J. Influence of phosphorus and potassium on alfalfa yield and yield components. Crop Sci. 2005, 45, 297–304. [Google Scholar] [CrossRef]
- Elgharably, A.; Benes, S. Alfalfa biomass yield and nitrogen fixation in response to applied mineral nitrogen under saline soil conditions. J. Soil Sci. Plant Nutr. 2021, 21, 744–755. [Google Scholar] [CrossRef]
- Warncke, D.; Dahl, J.; Jacobs, L. Nutrient Recommendations for Field Crops in Michigan; Extension Bulletin E2904; Michigan State University: East Lansing, MI, USA, 2009; p. 36. [Google Scholar]
- Berg, W.K.; Brouder, S.M.; Cunningham, S.M.; Volenec, J.J. Potassium and Phosphorus Fertilizer Impacts on Alfalfa Taproot Carbon and Nitrogen Reserve Accumulation and Use During Fall Acclimation and Initial Growth in Spring. Front. Plant Sci. 2021, 12, 715936. [Google Scholar] [CrossRef]
- Islam, M.A.; Baidoo, M.M. Effect of Potassium on Growth and Physiology of Alfalfa. In Handbook of Plant and Crop Physiology, 4th ed.; Pessarakli, M., Ed.; CRC Press: Boca Raton, FL, USA, 2021; Chapter 3; pp. 29–39. [Google Scholar]
- Meyer, R.D.; Mathews, M.C. Potassium fertilization and how it effects yield and quality of alfalfa. In Proceedings of the California Alfalfa Symposium Proceedings, Modesto, CA, USA, 7–8 December 1995. [Google Scholar]
- Lloveras, J.; Ferran, J.; Boixadera, J.; Bonet, J. Potassium fertilization effects on alfalfa in a Mediterranean climate. Agron. J. 2001, 93, 139–143. [Google Scholar] [CrossRef]
- Kitchen, N.R.; Buchholz, D.D.; Nelson, C.J. Potassium fertilizer and potato leafhopper effects on alfalfa growth. Agron. J. 1990, 82, 1069–1074. [Google Scholar] [CrossRef]
- Elouear, Z.; Bouhamed, F.; Boujelben, N.; Bouzid, J. Application of sheep manure and potassium fertilizer to contaminated soil and its effect on zinc, cadmium and lead accumulation by alfalfa plants. Sustain. Environ. Res. 2016, 26, 131–135. [Google Scholar] [CrossRef]
- Ayars, J.E.; Shouse, P.; Lesch, S.M. In situ use of groundwater by alfalfa. Agric. Water Manag. 2009, 96, 1579–1586. [Google Scholar] [CrossRef]
- Yost, M.A.; Russelle, M.P.; Coulter, J.A.; Bolstad, P.V. Alfalfa Stand Length and Subsequent Crop Patterns in the Upper Midwestern United States. Agron. J. 2014, 106, 1697–1708. [Google Scholar] [CrossRef]
- Madeira, F.; Clemente-Orta, G.; Alomar, O.; Batuecas, I.; Sossai, S.; Albajes, R. Land use alters the abundance of herbivore and predatory insects on crops: The case of alfalfa. J. Pest Sci. 2022, 95, 473–491. [Google Scholar] [CrossRef]
- Eckberg, J.O.; Wells, S.S.; Jungers, J.M.; Lamb, J.F.; Sheaffer, C.C. Alfalfa forage yield, milk yield, and nutritive value under intensive cutting. Agrosyst. Geosci. Environ. 2022, 5, e20246. [Google Scholar] [CrossRef]
- Heuschele, D.J.; Gamble, J.; Vetsch, J.A.; Shaeffer, C.C.; Coulter, J.A.; Kaiser, D.E.; Samac, D.A. Influence of potassium fertilization on alfalfa leaf and stem yield, forage quality, nutrient removal, and plant health. Agrosyst. Geosci. Environ. 2023, 6, e20346. [Google Scholar] [CrossRef]
- Kavadia, A.; Omirou, M.; Fasoula, D.; Ioannides, I.M. The importance of microbial inoculants in a climate-changing agriculture in eastern Mediterranean region. Atmosphere 2020, 11, 1136. [Google Scholar] [CrossRef]
- Islam, M.A.; Baidoo, M.M. Productivity and Profitability of Alfalfa in Response to Phosphorus and Potassium in Association with Calcium and Magnesium and Harvest Time. In Proceedings of the 2022 World Alfalfa Congress, San Diego, CA, USA, 14–17 November 2022. [Google Scholar]
- Milić, D.; Katanski, S.; Milošević, B.; Živanov, D. Variety selection in intensive alfalfa cutting management. Ratar. I Povrt. Field Veg. Crops Res. 2019, 56, 20–25. [Google Scholar] [CrossRef]
- Reddy, P.P. Sustainable Intensification—An Overview. In Sustainable Intensification of Crop Production, 1st ed.; Reddy, P.P., Ed.; Springer: Singapore, 2016; p. 405. [Google Scholar]
- Lissbrant, S.; Berg, W.K.; Volenec, J.; Brouder, S.; Joern, B.; Cunningham, S.; Johnson, K. Phosphorus and potassium fertilization of alfalfa. Purdue Univ. Ext. Bull. 2009, AY-331-W, 1–6. [Google Scholar]
- Putnam, D.; Orloff, S.; Ackerly, T. Agronomic practices and forage quality. In Proceedings of the 2000 National Alfalfa Symposium, Las Vegas, NV, USA, 10–12 December 2000. [Google Scholar]
- Ramos-Ulate, C.M.; Pérez-Álvarez, S.; Guerrero-Morales, S.; Palacios-Monarrez, A. Biofertilization and nanotechnology in alfalfa (Medicago sativa L.) as alternatives for a sustainable crop. Charact. Appl. Nanomater. 2022, 5, 111–118. [Google Scholar] [CrossRef]
- Lanyon, L.E.; Griffith, W.K. Nutrition and fertilizer use. Alfalfa Alfalfa Improv. 1988, 29, 333–372. [Google Scholar]
- Jungers, J.M.; Kaiser, D.E.; Lamb, J.F.; Lamb, J.A.; Noland, R.L.; Samac, D.A.; Sheaffer, C.C. Potassium fertilization affects alfalfa forage yield, nutritive value, root traits, and persistence. Agron. J. 2019, 111, 2843–2852. [Google Scholar] [CrossRef]
- NRC (National Research Council). Mineral Tolerance of Animals; The National Academies Press: Washington, DC, USA, 2005. [Google Scholar]
- Weiss, W.P. Mineral tolerances of animals. In Proceedings of the Tri-State Dairy Nutrition Conference, Fort Wayne, IN, USA, 22–23 April 2008. [Google Scholar]
- Khiaosa-Ard, R.; Ottoboni, M.; Verstringe, S.; Gruber, T.; Hartinger, T.; Humer, E.; Zebeli, Q. Magnesium in dairy cattle nutrition: A meta-analysis on magnesium absorption in dairy cattle and assessment of simple solubility tests to predict magnesium availability from supplemental sources. J. Dairy Sci. 2023, 106, 8758–8773. [Google Scholar] [CrossRef]
- Dabbir, B.K.R.; Rajavolu, R.S. New Trends in the Treatment of Hypokalemia in Cows. In Latest Scientific Findings in Ruminant Nutrition—Research for Practical Implementation [Working Title], 1st ed.; Babinszky, L., Ed.; IntechOpen: London, UK, 2024. [Google Scholar] [CrossRef]
- Kronqvist, C.; Emanuelson, U.; Tråvén, M.; Spörndly, R.; Holtenius, K. Relationship between incidence of milk fever and feeding of minerals during the last 3 weeks of gestation. Animal 2012, 6, 1316–1321. [Google Scholar] [CrossRef] [PubMed]
- Ritter, R.J.; Boling, J.A.; Gay, N. Labile magnesium reserves in beef cows subjected to different prepasture supplementation regimens. J. Anim. Sci. 1984, 59, 197–203. [Google Scholar] [CrossRef]
- Rérat, M.; Philipp, A.; Hess, H.D.; Liesegang, A. Effect of different potassium levels in hay on acid–base status and mineral balance in periparturient dairy cows. J. Dairy Sci. 2009, 92, 6123–6133. [Google Scholar] [CrossRef]
- James, D.W.; Tindall, T.A.; Hurst, C.J.; Hussein, A.N. Alfalfa cultivar responses to phosphorus and potassium deficiency: Biomass. J. Plant Nutr. 1995, 18, 2431–2445. [Google Scholar] [CrossRef]
- Berg, W.K.; Cunningham, S.M.; Brouder, S.M.; Joern, B.C.; Johnson, K.D.; Santini, J.B.; Volenec, J.J. The long-term impact of phosphorus and potassium fertilization on alfalfa yield and yield components. Crop Sci. 2007, 47, 2198–2209. [Google Scholar] [CrossRef]
- Daliparthy, J.; Barker, A.V.; Mondal, S.S. Potassium fractions with other nutrients in crops: A review focusing on the tropics. J. Plant Nutr. 1994, 17, 1859–1886. [Google Scholar] [CrossRef]
- Malhi, S.S. Relative response of forage and seed yield of alfalfa to sulfur, phosphorus, and potassium fertilization. J. Plant Nutr. 2011, 34, 888–908. [Google Scholar] [CrossRef]
- Pourebrahimi, F.M.; Savoy, H.; Atotey, N.; Yin, X. Effect of potassium application rate and timing on alfalfa yield and potassium concentration and removal in Tennessee. Agron. Res. 2023, 21, 183–192. [Google Scholar]
- Thinguldstad, B.; Tucker, J.J.; Baxter, L.L.; Segers, J.R.; Hancock, D.W.; Stewart, R.L. Alfalfa response to low potassium under different harvest regimes in Coastal Plains. Agrosyst. Geosci. Environ. 2020, 3, e20029. [Google Scholar] [CrossRef]
- Marani, S.; Madani, H.; Sharifabad, H.H.; Manesh, G.A.; Shirzadi, M.H. Investigation of phosphorus and potassium biological fertilizers on alfalfa (Medicago sativa L.) physiological traits at different ages of the field. Legume Res. Int. J. 2023, 46, 741–745. [Google Scholar] [CrossRef]
- Razmjoo, K.; Henderlong, P.R. Effect of potassium, sulfur, boron, and molybdenum fertilization on alfalfa production and herbage macronutrient contents. J. Plant Nutr. 1997, 20, 1681–1696. [Google Scholar] [CrossRef]
- Wakeel, A.; Ishfaq, M. Potassium in Plants. In Potash Use and Dynamics in Agriculture, 1st ed.; Wakeel, A., Ishfaq, M., Eds.; Springer Nature: Singapore, 2022; pp. 19–27. [Google Scholar]
- Kumari, S.; Chhillar, H.; Chopra, P.; Khanna, R.R.; Khan, M.I.R. Potassium: A track to develop salinity tolerant plants. Plant Physiol. Biochem. 2021, 167, 1011–1023. [Google Scholar] [CrossRef]
- Wakeel, A. Potassium–sodium interactions in soil and plant under saline-sodic conditions. J. Plant Nutr. Soil Sci. 2013, 176, 344–354. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Ashraf, M. Nanoparticles potentially mediate salt stress tolerance in plants. Plant Physiol. Biochem. 2021, 160, 257–268. [Google Scholar] [CrossRef]
- El-Sharkawy, M.S.; El-Beshsbeshy, T.R.; Mahmoud, E.K.; Abdelkader, N.I.; Al-Shal, R.M.; Missaoui, A.M. Response of alfalfa under salt stress to the application of potassium sulfate nanoparticles. Am. J. Plant Sci. 2017, 8, 1751–1773. [Google Scholar] [CrossRef]
- Sheoran, P.; Goel, S.; Boora, R.; Kumari, S.; Yashveer, S.; Grewal, S. Biogenic synthesis of potassium nanoparticles and their evaluation as a growth promoter in wheat. Plant Gene 2021, 27, 100310. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.S.M.A.; Fujita, D.B.S.M.A.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. In Sustainable Agriculture, 1st ed.; Lichtfouse, E., Navarrete, M., Debaeke, P., Véronique, S., Alberola, C., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 153–188. [Google Scholar]
- Mostofa, M.G.; Rahman, M.M.; Ghosh, T.K.; Kabir, A.H.; Abdelrahman, M.; Khan, M.A.R.; Tran, L.S.P. Potassium in plant physiological adaptation to abiotic stresses. Plant Physiol. Biochem. 2022, 186, 279–289. [Google Scholar] [CrossRef]
- Fayez, K.A.; Bazaid, S.A. Improving drought and salinity tolerance in barley by application of salicylic acid and potassium nitrate. J. Saudi Soc. Agric. Sci. 2014, 13, 45–55. [Google Scholar] [CrossRef]
- Gupta, S.; Schillaci, M.; Walker, R.; Smith, P.M.; Watt, M.; Roessner, U. Alleviation of salinity stress in plants by endophytic plant-fungal symbiosis: Current knowledge, perspectives and future directions. Plant Soil 2021, 461, 219–244. [Google Scholar] [CrossRef]
- Kerchev, P.I.; Van Breusegem, F. Improving oxidative stress resilience in plants. Plant J. 2022, 109, 359–372. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Tomar, N.S.; Tittal, M.; Argal, S.; Agarwal, R. Plant growth under water/salt stress: ROS production; antioxidants and significance of added potassium under such conditions. Physiol. Mol. Biol. Plants 2017, 23, 731–744. [Google Scholar] [CrossRef]
- Živčák, M.; Repkova, J.; Olšovská, K.; Brestič, M. Osmotic adjustment in winter wheat varieties and its importance as a mechanism of drought tolerance. Cereal Res. Commun. 2009, 37, 569–572. [Google Scholar]
- Sanders, G.J.; Arndt, S.K. Osmotic adjustment under drought conditions. In Plant Responses to Drought Stress, 1st ed.; Aroca, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 199–229. [Google Scholar]
- Khalil, R.; Elsayed, N.; Khan, T.A.; Yusuf, M. Potassium: A Potent Modulator of Plant Responses under Changing Environment. In Role of Potassium in Abiotic Stress, 1st ed.; Iqbal, N., Umar, S., Eds.; Springer: Singapore, 2022; pp. 221–247. [Google Scholar]
- Amede, T.; Schubert, S.; Stahr, K. Mechanisms of drought resistance in grain legumes I: Osmotic adjustment. SINET Ethiop. J. Sci. 2003, 26, 37–46. [Google Scholar] [CrossRef]
- Carvalho, M.D. Drought stress and reactive oxygen species. Plant Signal Behav. 2008, 3, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Bargaz, A.; Lyamlouli, K.; Chtouki, M.; Zeroual, Y.; Dhiba, D. Soil microbial resources for improving fertilizers efficiency in an integrated plant nutrient management system. Front. Microbiol. 2018, 9, 1606. [Google Scholar] [CrossRef] [PubMed]
- Santos, E.F.; Mateus, N.S.; Rosario, M.O.; Garcez, T.B.; Mazzafera, P.; Lavres, J. Enhancing potassium content in leaves and stems improves drought tolerance of eucalyptus clones. Physiol. Plant. 2021, 172, 552–563. [Google Scholar] [CrossRef]
- Tränkner, M.; Tavakol, E.; Jákli, B. Functioning of potassium and magnesium in photosynthesis, photosynthate translocation and photoprotection. Physiol. Plant. 2018, 163, 414–431. [Google Scholar] [CrossRef] [PubMed]
- Fageria, N.K.; Moreira, A. The role of mineral nutrition on root growth of crop plants. Adv. Agron. 2011, 110, 251–331. [Google Scholar]
- Egilla, J.N.; Davies, F.T.; Boutton, T.W. Drought stress influences leaf water content, photosynthesis, and water-use efficiency of Hibiscus rosa-sinensis at three potassium concentrations. Photosynthetica 2005, 43, 135–140. [Google Scholar] [CrossRef]
- Nishiyama, R.; Watanabe, Y.; Leyva-Gonzalez, M.A.; Van Ha, C.; Fujita, Y.; Tanaka, M.; Tran, L.S.P. Arabidopsis AHP2, AHP3, and AHP5 histidine phosphotransfer proteins function as redundant negative regulators of drought stress response. Proc. Natl. Acad. Sci. USA 2013, 110, 4840–4845. [Google Scholar] [CrossRef]
- Gupta, S.; Agarwal, V.P.; Gupta, N.K. Efficacy of putrescine and benzyladenine on photosynthesis and productivity in relation to drought tolerance in wheat (Triticum aestivum L.). Physiol. Mol. Biol. Plants 2012, 18, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Waraich, E.A.; Ahmad, R.; Ashraf, M.Y. Role of mineral nutrition in alleviation of drought stress in plants. Aust. J. Crop Sci. 2011, 5, 764–777. [Google Scholar]
- Ul Hassan, M.; Rasool, T.; Iqbal, C.; Arshad, A.; Abrar, M.; Abrar, M.M.; Fahad, S. Linking plants functioning to adaptive responses under heat stress conditions: A mechanistic review. J. Plant Growth Regul. 2021, 41, 2596–2613. [Google Scholar] [CrossRef]
- Trono, D.; Pecchioni, N. Candidate Genes Associated with Abiotic Stress Response in Plants as Tools to Engineer Tolerance to Drought, Salinity and Extreme Temperatures in Wheat: An Overview. Plants 2022, 11, 3358. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Freund, D.M.; Hegeman, A.D.; Cohen, J.D. Metabolic signatures of Arabidopsis thaliana abiotic stress responses elucidate patterns in stress priming, acclimation, and recovery. Stress Biol. 2022, 2, 11. [Google Scholar] [CrossRef]
- Santini, J.; Giannettini, J.; Pailly, O.; Herbette, S.; Ollitrault, P.; Berti, L.; Luro, F. Comparison of photosynthesis and antioxidant performance of several Citrus and Fortunella species (Rutaceae) under natural chilling stress. Trees 2013, 27, 71–83. [Google Scholar] [CrossRef]
- Sun, M.; Zhang, Y.; Xia, Q.; Wang, X.; Zhang, Q.; Liu, T.; Zhang, Y. Effects of potash fertilizer types and rates on alfalfa root crown saccharides content and cold resistance under low temperature stress. Agric. Res. Arid Areas 2022, 40, 62–70. [Google Scholar]
- Zhang, Q.; Zhang, Y.; Sun, M.; Xia, Q.; Wang, X.; Liu, T.; Du, X. Effects of Potassium Fertilizer on Alfalfa Antioxidant Enzyme Activity Under Low Temperature Stress. J. Agric. Sci. Technol. 2023, 25, 186–195. [Google Scholar]
- Kumar, P.; Kumar, T.; Singh, S.; Tuteja, N.; Prasad, R.; Singh, J. Potassium: A key modulator for cell homeostasis. J. Biotechnol. 2020, 324, 198–210. [Google Scholar] [CrossRef]
- Kant, S.; Kafkafi, U.; Pasricha, N.; Bansal, S. Potassium and abiotic stresses in plants. Potassium for sustainable crop production. Potash Res. Inst. India Gurgaon 2002, 233, 251. [Google Scholar]
- Russelle, M.P. “Production plus” with potassium. Minnesota Pesticide Recommendations and Applied Research Reports. 1987, pp. 13–26. Available online: https://hdl.handle.net/11299/177465 (accessed on 12 August 2024).
- Wakeel, A.; Magen, H. Potash Use for Sustainable Crop Production in Pakistan: A Review. Int. J. Agric. Biol. 2017, 19, 381. [Google Scholar]
- Verslues, P.E.; Agarwal, M.; Katiyar-Agarwal, S.; Zhu, J.; Zhu, J.K. Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status. Plant J. 2006, 45, 523–539. [Google Scholar] [CrossRef]
- Karimi, R. Potassium-induced freezing tolerance is associated with endogenous abscisic acid, polyamines and soluble sugars changes in grapevine. Sci. Hortic. 2017, 215, 184–194. [Google Scholar] [CrossRef]
- Rogowska, A.; Szakiel, A. The role of sterols in plant response to abiotic stress. Phytochem. Rev. 2020, 19, 1525–1538. [Google Scholar] [CrossRef]
- Chen, G.; Peng, L.; Li, C.; Tu, Y.; Lan, Y.; Wu, C.Q.; Zhang, Q.; Yang, H.; Li, T. Effects of the potassium application rate on lipid synthesis and eating quality of two rice cultivars. J. Integr. Agric. 2023, 22, 2025–2040. [Google Scholar] [CrossRef]
- Rawat, N.; Singla-Pareek, S.L.; Pareek, A. Membrane dynamics during individual and combined abiotic stresses in plants and tools to study the same. Physiol. Plant. 2021, 171, 653–676. [Google Scholar] [CrossRef] [PubMed]
- Golizadeh, F.; Kumleh, H.H. Physiological Responses and Expression Changes of Fatty Acid Metabolism–Related Genes in Wheat (Triticum aestivum) Under Cold Stress. Plant Mol. Biol. Rep. 2019, 37, 224–236. [Google Scholar] [CrossRef]
- Sakamoto, T.; Murata, N. Regulation of the desaturation of fatty acids and its role in tolerance to cold and salt stress. Curr. Opin. Microbiol. 2002, 5, 206–210. [Google Scholar] [CrossRef]
- Adhikari, L.; Baral, R.; Paudel, D.; Min, D.; Makaju, S.O.; Poudel, H.P.; Acharya, J.P.; Missaoui, A.M. Cold stress in plants: Strategies to improve cold tolerance in forage species. Plant Stress 2022, 4, 100081. [Google Scholar] [CrossRef]
- Nadeem, F.; Hanif, M.A.; Majeed, M.I.; Mushtaq, Z. Role of macronutrients and micronutrients in the growth and development of plants and prevention of deleterious plant diseases-a comprehensive review. Int. J. Chem. Biochem. 2018, 13, 31–52. [Google Scholar]
- Kumar, P.; Sharma, M.K. Nutrient Deficiencies of Field Crops: Guide to Diagnosis and Management; Illustrated, Ed.; CABI: Wallingford, UK, 2013; p. 378. [Google Scholar]
- Buchelt, A.C.; Teixeira, G.C.M.; Oliveira, K.S.; Rocha, A.M.S.; de Mello Prado, R.; Caione, G. Silicon contribution via nutrient solution in forage plants to mitigate nitrogen, potassium, calcium, magnesium, and sulfur deficiency. J. Soil Sci. Plant Nutr. 2020, 20, 1532–1548. [Google Scholar] [CrossRef]
- Hosseinifard, M.; Stefaniak, S.; Ghorbani Javid, M.; Soltani, E.; Wojtyla, Ł.; Garnczarska, M. Contribution of exogenous proline to abiotic stresses tolerance in plants: A review. Int. J. Mol. Sci. 2022, 23, 5186. [Google Scholar] [CrossRef]
- Tariq, A.; Zeng, F.; Graciano, C.; Ullah, A.; Sadia, S.; Ahmed, Z.; Murtaza, G.; Ismoilov, K.; Zhang, Z. Regulation of Metabolites by Nutrients in Plants. In Plant Ionomics: Sensing, Signaling, and Regulation, 1st ed.; Singh, V.P., Siddiqui, M.H., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2023; pp. 1–18. [Google Scholar]
- Raj, M.; Lal, K.; Kumar, R.; Ranjan, A. Scenario of Potassium Mining and Management in Indian Agriculture: A Review. Balance 2019, 10, 5–976. [Google Scholar]
- Sun, J.; Zhao, J.; Zhang, T.; Yu, L.; Jin, K. Effects of a Furrow-Bed Seeding System on Stand Establishment, Soil Bacterial Diversity, and the Yield and Quality of Alfalfa Under Saline Condition. Front. Plant Sci. 2022, 13, 919912. [Google Scholar] [CrossRef]
- Sayed, M.R.; Alshallash, K.S.; Safhi, F.A.; Alatawi, A.; ALshamrani, S.M.; Dessoky, E.S.; Sultan, F.M. Genetic diversity, analysis of some agro-morphological and quality traits and utilization of plant resources of alfalfa. Genes 2022, 13, 1521. [Google Scholar] [CrossRef]
- Kumar, G.; Kumari, R.; Shambhavi, S.; Kumar, S.; Kumari, P.; Padbhushan, R. Eight-year Continuous Tillage Practice Impacts Soil Properties and Forms of Potassium under Maize-based Cropping Systems in Inceptisols of Eastern India. Commun. Soil Sci. Plant Anal. 2022, 53, 602–621. [Google Scholar] [CrossRef]
- Andrews, E.M.; Kassama, S.; Smith, E.E.; Brown, P.H.; Khalsa, S.D.S. A review of potassium-rich crop residues used as organic matter amendments in tree crop agroecosystems. Agriculture 2021, 11, 580. [Google Scholar] [CrossRef]
- Jackson, M.L. Chemical composition of soils. In Chemistry of the Soil, 2nd ed.; Bear, F.E., Ed.; Reinhold Pub. Corp: New York, NY, USA, 1964. [Google Scholar]
- Bertsch, P.M.; Thomas, G.W. Potassium status of temperate region soils. In Potassium in Agriculture, 1st ed.; Munson, R.D., Ed.; American Society of Agronomy: Madison, WI, USA, 1985; pp. 129–162. [Google Scholar]
- Rasmussen, P.E.; Collins, H.P. Long-term impacts of tillage, fertilizer, and crop residue on soil organic matter in temperate semiarid regions. Adv. Agron. 1991, 45, 93–134. [Google Scholar]
- Qambrani, N.A.; Rahman, M.M.; Won, S.; Shim, S.; Ra, C. Biochar properties and eco-friendly applications for climate change mitigation, waste management, and wastewater treatment: A review. Renew. Sustain. Energy Rev. 2017, 79, 255–273. [Google Scholar] [CrossRef]
- Simonsson, M.; Hillier, S.; Öborn, I. Changes in clay minerals and potassium fixation capacity as a result of release and fixation of potassium in long-term field experiments. Geoderma 2009, 151, 109–120. [Google Scholar] [CrossRef]
- Bhardwaj, A.K.; Rajwar, D.; Nagaraja, M.S. Soil fertility problems and management. In Managing Salt-Affected Soils for Sustainable Agriculture, 1st ed.; Minhas, P.S., Yadav, R.K., Sharma, P.C., Eds.; Directorate of Knowledge Management in Agriculture, ICAR: New Delhi, India, 2021; pp. 386–407. [Google Scholar]
- Padbhushan, R.; Kumar, A.; Kumar, U.; Kumar, A.; Kumari, R.; Kohli, A. Novel Potassium Management Strategies for Improvement of Soil Health. In Soil Management for Sustainable Agriculture, 1st ed.; Mandal, N., Dey, A., Rakshit, R., Eds.; Apple Academic Press: New York, NY, USA, 2022; pp. 355–377. [Google Scholar]
- Marschner, P. (Ed.) Marschner's Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: Cambridge, MA, USA, 2012; p. 649. [Google Scholar]
- Bernardi, A.C.C.; Rassini, J.B.; Mendonça, F.C.; Ferreira, R.P. Alfalfa dry matter yield, nutritional status and economic analysis of potassium fertilizer doses and frequency. Int. J. Agron. Plant Prod. 2013, 4, 389–398. [Google Scholar]
- Anindita, S.; Sleutel, S.; Vandenberghe, D.; De Grave, J.; Vandenhende, V.; Finke, P. Land use impacts on weathering, soil properties, and carbon storage in wet Andosols, Indonesia. Geoderma 2022, 423, 115963. [Google Scholar] [CrossRef]
- Lalitha, M.; Dhakshinamoorthy, M. Forms of soil potassium-A review. Agric. Rev. 2014, 35, 64–68. [Google Scholar] [CrossRef]
- Kraur, H. Forms of potassium in soil and their relationship with soil properties-A review. Int. J. Curr. Microbiol. App. Sci. 2019, 8, 1580–1586. [Google Scholar] [CrossRef]
- Almeida, T.F.; Carvalho, J.K.; Reid, E.; Martins, A.P.; Bissani, C.A.; Bortoluzzi, E.C.; Brunetto, G.; Anghinoni, I.; Carvalho, P.C.F.; Tiecher, T. Forms and balance of soil potassium from a long-term integrated crop-livestock system in a subtropical Oxisol. Soil Tillage Res. 2021, 207, 104864. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Li, Y.; Li, L.; Tang, M.; Hu, W.; Ai, S. Speciation of heavy metals in soils and their immobilization at micro-scale interfaces among diverse soil components. Sci. Total Environ. 2022, 825, 153862. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, M.J.; Tiller, K.G.; Naidu, R.; Stevens, D.P. The behaviour and environmental impact of contaminants in fertilizers. Soil Res. 1996, 34, 1–54. [Google Scholar] [CrossRef]
- Sparks, D.L. Potassium Dynamics in Soils. In Advances in Soil Science, 1st ed.; Stewart, B.A., Ed.; Springer: New York, NY, USA, 1987; Volume 6, pp. 1–63. [Google Scholar]
- Tarun Kumar, B.; Jayananda, M.; Nasipuri, P.; Guitreau, M.; Aadhiseshan, K.R.; Rao, S.B.M.; Thomas, T.T.; Satyanarayanan, M. Tectono-Thermal History of the Neoarchean Balehonnur Shear Zone, Western Dharwar Craton (Southern India). Lithosphere 2022, 4167477. [Google Scholar] [CrossRef]
- Oeser, R.A.; von Blanckenburg, F. Do degree and rate of silicate weathering depend on plant productivity? Biogeosciences 2020, 17, 4883–4917. [Google Scholar] [CrossRef]
- Dotaniya, M.L.; Meena, V.D.; Basak, B.B.; Meena, R.S. Potassium uptake by crops as well as microorganisms. In Potassium Solubilizing Microorganisms for Sustainable Agriculture; 1st ed., Meena, V.S., Maurya, B.M., Verma, J.P., Meena, R.S., Eds.; Springer: New Delhi, India, 2016; pp. 267–280. [Google Scholar]
- Rasel, M.; Tahjib-Ul-Arif, M.; Hossain, M.A.; Hassan, L.; Farzana, S.; Brestic, M. Screening of salt-tolerant rice landraces by seedling stage phenotyping and dissecting biochemical determinants of tolerance mechanism. J. Plant Growth Regul. 2021, 40, 1853–1868. [Google Scholar] [CrossRef]
- White, P.J. Long-distance transport in the xylem and phloem. In Marschner's Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Academic Press: Cambridge, MA, USA, 2012; pp. 49–70. [Google Scholar]
- Kathpalia, R.; Bhatla, S.C. Plant mineral nutrition. In Plant Physiology, Development and Metabolism, 1st ed.; Bhatla, S.C., Lal, M.A., Eds.; Springer: Singapore, 2018; pp. 37–81. [Google Scholar]
- Heldt, H.W.; Piechulla, B. Plant Biochemistry, 5th ed.; Academic Press: Cambridge, MA, USA, 2021; p. 609. [Google Scholar]
- Griffiths, M.; York, L.M. Targeting root ion uptake kinetics to increase plant productivity and nutrient use efficiency. Plant Physiol. 2020, 182, 1854–1868. [Google Scholar] [CrossRef]
- Hogan, J.A.; Valverde-Barrantes, O.J.; Tang, W.; Ding, Q.; Xu, H.; Baraloto, C. Evidence of elemental homeostasis in fine root and leaf tissues of saplings across a fertility gradient in tropical montane forest in Hainan, China. Plant Soil 2021, 460, 625–646. [Google Scholar] [CrossRef]
- Etesami, H.; Alikhani, H.A. Silicon improves the effect of phosphate-solubilizing bacterium and arbuscular mycorrhizal fungus on phosphorus concentration of salinity-stressed alfalfa (Medicago sativa L.). Rhizosphere 2022, 24, 100619. [Google Scholar]
- Hosseini-Nasr, F.; Etesami, H.; Alikhani, H.A. Silicon Improves Plant Growth-Promoting Effect of Nodule Non-Rhizobial Bacterium on Nitrogen Concentration of Alfalfa Under Salinity Stress. J. Soil Sci. Plant Nutr. 2023, 23, 496–513. [Google Scholar] [CrossRef]
- Clover, M.W.; Mallarino, A.P. Corn and soybean tissue potassium content responses to potassium fertilization and relationships with grain yield. Soil Sci. Soc. Am. J. 2013, 77, 630–642. [Google Scholar] [CrossRef]
- Tan, C.; Cao, X.; Yuan, S.; Wang, W.; Feng, Y.; Qiao, B. Effects of long-term conservation tillage on soil nutrients in sloping fields in regions characterized by water and wind erosion. Sci. Rep. 2015, 5, 17592. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Mohamed, I.; Ali, M.; Abbas, M.H.; Shah, G.M.; Chen, F. Potassium distribution in root and non-root zones of two cotton genotypes and its accumulation in their organs as affected by drought and potassium stress conditions. J. Plant Nutr. Soil Sci. 2019, 182, 72–81. [Google Scholar] [CrossRef]
- Wang, Y.; Mao, Z.; Bakker, M.R.; Kim, J.H.; Brancheriau, L.; Buatois, B.; Stokes, A. Linking conifer root growth and production to soil temperature and carbon supply in temperate forests. Plant Soil 2018, 426, 33–50. [Google Scholar] [CrossRef]
- Calleja-Cabrera, J.; Boter, M.; Oñate-Sánchez, L.; Pernas, M. Root growth adaptation to climate change in crops. Front. Plant Sci. 2020, 11, 544. [Google Scholar] [CrossRef]
- Clarke, S.J.; Lamont, K.J.; Pan, H.Y.; Barry, L.A.; Hall, A.; Rogiers, S.Y. Spring root-zone temperature regulates root growth, nutrient uptake and shoot growth dynamics in grapevines. Aust. J. Grape Wine Res. 2015, 21, 479–489. [Google Scholar] [CrossRef]
- Abuarab, M.E.; El-Mogy, M.M.; Hassan, A.M.; Abdeldaym, E.A.; Abdelkader, N.H.; BI El-Sawy, M. The effects of root aeration and different soil conditioners on the nutritional values, yield, and water productivity of potato in clay loam soil. Agronomy 2019, 9, 418. [Google Scholar] [CrossRef]
- Liu, K.; Harrison, M.T.; Shabala, S.; Meinke, H.; Ahmed, I.; Zhang, Y.; Zhou, M. The state of the art in modeling waterlogging impacts on plants: What do we know and what do we need to know. Earth's Future 2020, 8, e2020EF001801. [Google Scholar] [CrossRef]
- Freschet, G.T.; Roumet, C.; Comas, L.H.; Weemstra, M.; Bengough, A.G.; Rewald, B.; Stokes, A. Root traits as drivers of plant and ecosystem functioning: Current understanding, pitfalls and future research needs. New Phytol. 2021, 232, 1123–1158. [Google Scholar] [CrossRef]
- Li, Y.; Niu, W.; Cao, X.; Wang, J.; Zhang, M.; Duan, X.; Zhang, Z. Effect of soil aeration on root morphology and photosynthetic characteristics of potted tomato plants (Solanum lycopersicum) at different NaCl salinity levels. BMC Plant Biol. 2019, 19, 331. [Google Scholar] [CrossRef] [PubMed]
- Cambi, M.; Certini, G.; Neri, F.; Marchi, E. The impact of heavy traffic on forest soils: A review. For. Ecol. Manag. 2015, 338, 124–138. [Google Scholar] [CrossRef]
- Shah, A.N.; Tanveer, M.; Shahzad, B.; Yang, G.; Fahad, S.; Ali, S.; Souliyanonh, B. Soil compaction effects on soil health and cropproductivity: An overview. Environ. Sci. Pollut. Res. 2017, 24, 10056–10067. [Google Scholar] [CrossRef]
- Wolkowski, R.P. Soil management and K availability. New Horiz. Soil Sci. 2000, 1, 1–6. [Google Scholar]
- Jahan, I.; Shopan, J.; Rahman, M.M.; Sarkar, A.; Baset, M.A.; Zhang, Z.; Hasan, M.K. Long-Term Traditional Fertilization Alters Tea Garden Soil Properties and Tea Leaf Quality in Bangladesh. Agronomy 2022, 12, 2128. [Google Scholar] [CrossRef]
- Ng, J.F.; Ahmed, O.H.; Jalloh, M.B.; Omar, L.; Kwan, Y.M.; Musah, A.A.; Poong, K.H. Soil nutrient retention and pH buffering capacity are enhanced by calciprill and sodium silicate. Agronomy 2022, 12, 219. [Google Scholar] [CrossRef]
- Khan, H.R.; Elahi, S.F.; Hussain, M.S.; Adachi, T. Soil characteristics and behavior of potassium under various moisture regimes. Soil Sci. Plant Nutr. 1994, 40, 243–254. [Google Scholar] [CrossRef]
- Nehela, Y.; Mazrou, Y.S.; Alshaal, T.; Rady, A.M.; El-Sherif, A.M.; Omara, A.E.D.; Hafez, E.M. The integrated amendment of sodic-saline soils using biochar and plant growth-promoting rhizobacteria enhances maize (Zea mays L.) resilience to water salinity. Plants 2021, 10, 1960. [Google Scholar] [CrossRef] [PubMed]
- Mouhamad, R.; Alsaede, A.; Iqbal, M. Behavior of potassium in soil: A mini review. Chem. Int. 2016, 2, 58–69. [Google Scholar]
- Kumari, N.; Mohan, C. Basics of clay minerals and their characteristic properties. In Clay and Clay Minerals, 1st ed.; Morari Do Nascimento, G., Ed.; Intechopen: London, UK, 2021; Volume 24, pp. 15–88. [Google Scholar]
- Bilias, F.; Kotsangeli, E.; Ipsilantis, I.; Barbayiannis, N. Cation Exchange Resins for Predicting Available K on K-Deficient Soils: Extraction Capacity among Different Soil K Pools and First Insights on the Contribution of K Solubilized by Rhizosphere Microbes. Land 2022, 11, 2146. [Google Scholar] [CrossRef]
- Florence, A.; Ransom, M.; Mengel, D. Potassium fixation by oxidized and reduced forms of phyllosilicates. Soil Sci. Soc. Am. J. 2017, 81, 1247–1255. [Google Scholar] [CrossRef]
- Agegnehu, G.; Amede, T.; Erkossa, T.; Yirga, C.; Henry, C.; Tyler, R.; Sileshi, G.W. Extent and management of acid soils for sustainable crop production system in the tropical agroecosystems: A review. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2021, 71, 852–869. [Google Scholar] [CrossRef]
- Trinchant, J.C.; Boscari, A.; Spennato, G.; Van de Sype, G.; Le Rudulier, D. Proline betaine accumulation and metabolism in alfalfa plants under sodium chloride stress. Exploring its compartmentalization in nodules. Plant Physiol. 2004, 135, 1583–1594. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wei, Y.; Yin, Y.; Zhu, W.; Bai, X.; Zhou, Y. Characteristics of Soil Physicochemical Properties and Microbial Community of Mulberry (Morus alba L.) and Alfalfa (Medicago sativa L.) Intercropping System in Northwest Liaoning. Microorganisms 2023, 11, 114. [Google Scholar] [CrossRef]
- Bhattarai, S.; Biswas, D.; Fu, Y.B.; Biligetu, B. Morphological, physiological, and genetic responses to salt stress in alfalfa: A Review. Agronomy 2020, 10, 577. [Google Scholar] [CrossRef]
- Das, D.; Dwivedi, B.S.; Datta, S.P.; Datta, S.C.; Meena, M.C.; Datta, S.C. Intensive cropping with varying nutrient management options alters potassium dynamics in soil. Indian J. Fertil. 2020, 16, 690–704. [Google Scholar]
- Swoboda, P.; Döring, T.F.; Hamer, M. Remineralizing soils? The agricultural usage of silicate rock powders: A review. Sci. Total Environ. 2022, 807, 150976. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Su, R.; Chang, C.; Cheng, X.; Peng, Q.; Lambers, H.; He, H. Effects of oxytetracycline on plant growth, phosphorus uptake, and carboxylates in the rhizosheath of alfalfa. Plant Soil 2021, 461, 501–515. [Google Scholar] [CrossRef]
- Naorem, A.; Jayaraman, S.; Dang, Y.P.; Dalal, R.C.; Sinha, N.K.; Rao, C.S.; Patra, A.K. Soil Constraints in an Arid Environment—Challenges, Prospects, and Implications. Agronomy 2023, 13, 220. [Google Scholar] [CrossRef]
- Setu, H. Effect of phosphorus and potassium fertilizers application on soil chemical characteristics and their accumulation in potato plant tissues. Appl. Environ. Soil Sci. 2022, 1, 5342170. [Google Scholar] [CrossRef]
- Landaverde, A. Floating Treatment Wetlands for Brackish Waters: Plant Selection and Nutrient Uptake Potential. Master’s Thesis, Clemson University, Clemson, SC, USA, May 2022; p. 3736. [Google Scholar]
- Kelling, K.A. Alfalfa Fertilization; University of Wisconsin Extension: Sparta, WI, USA, 2000; UWEX A2448. [Google Scholar]
- Undersander, D.; Cosgrove, D.; Cullen, E.; Grau, C.; Rice, M.E.; Renz, M.; Sheaffer, C.; Shewmaker, G.; Sulc, M. Alfalfa Management Guide; American Society of Agronomy, Crop Science Society of America, Soil Science Society of America: Madison, WI, USA, 2015; p. 60. [Google Scholar]
- Naorem, A.; Jayaraman, S.; Dalal, R.C.; Patra, A.; Rao, C.S.; Lal, R. Soil Inorganic Carbon as a Potential Sink in Carbon Storage in Dryland Soils—A Review. Agriculture 2022, 12, 1256. [Google Scholar] [CrossRef]
- Pintarič, S.; Suhadolc, M.; Eler, K. Straw Management and Slurry Application Affect the Soil Microbial Community Composition and Its Activity. Agronomy 2022, 12, 2781. [Google Scholar] [CrossRef]
- Cárceles Rodríguez, B.; Durán-Zuazo, V.H.; Soriano Rodríguez, M.; García-Tejero, I.F.; Gálvez Ruiz, B.; Cuadros Tavira, S. Conservation Agriculture as a Sustainable System for Soil Health: A Review. Soil Syst. 2022, 6, 87. [Google Scholar] [CrossRef]
- Chen, Q.; Xin, Y.; Liu, Z. Long-Term Fertilization with Potassium Modifies Soil Biological Quality in K-Rich Soils. Agronomy 2020, 10, 771. [Google Scholar] [CrossRef]
- Wang, H.; Xu, J.; Liu, X.; Zhang, D.; Li, L.; Li, W.; Sheng, L. Effects of long-term application of organic fertilizer on improving organic matter content and retarding acidity in red soil from China. Soil Tillage Res. 2019, 195, 104382. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).