Uptake, Translocation, and Metabolism of Pydiflumetofen in Hydroponic Cucumber and Tomato Planting Systems
Abstract
:1. Introduction
2. Material and Methods
2.1. Reagents and Materials
2.2. Plant Cultivation and Exposure Experiments
2.3. Sample Extraction
2.4. UPLC-MS/MS and HPLC-Q-TOF Analyses
2.5. Method Validation
2.6. Data Calculation and Analysis
3. Results and Discussion
3.1. QA/QC Validation
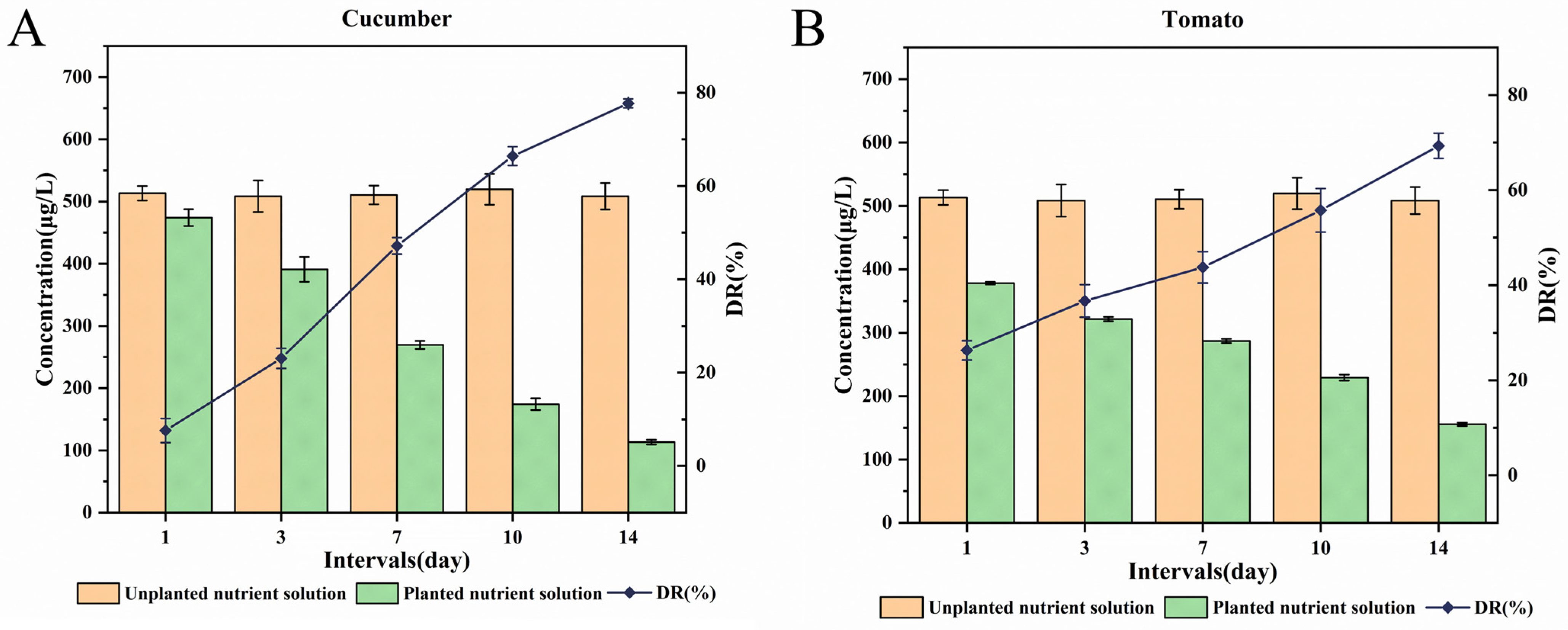
3.2. Dissipation of PYD in Different Nutrient Solutions
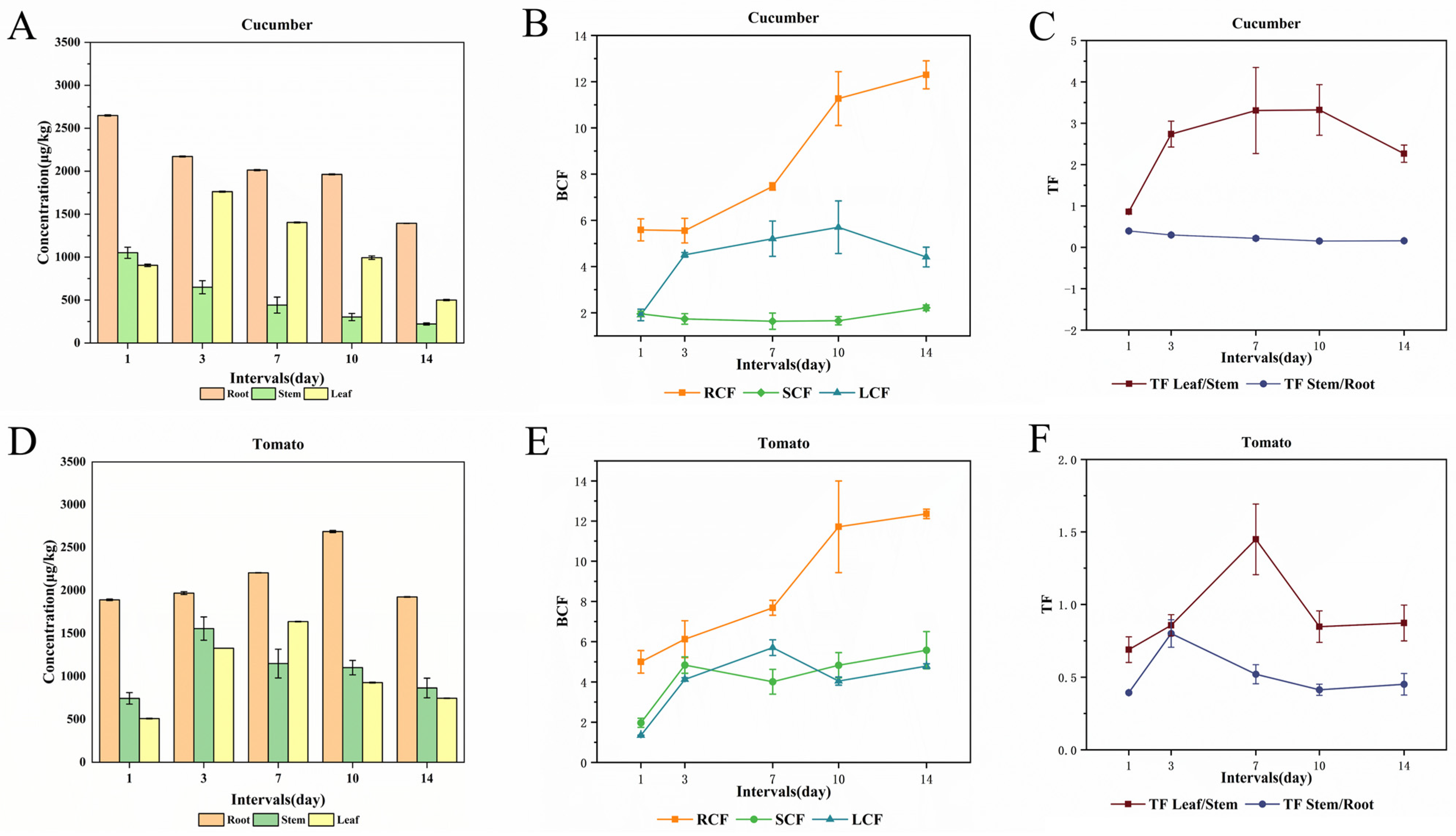
3.3. Uptake and Translocation of PYD within Plants
3.3.1. Cucumber System
3.3.2. Tomato System
3.4. Factors Affecting PYD Absorption and Translocation
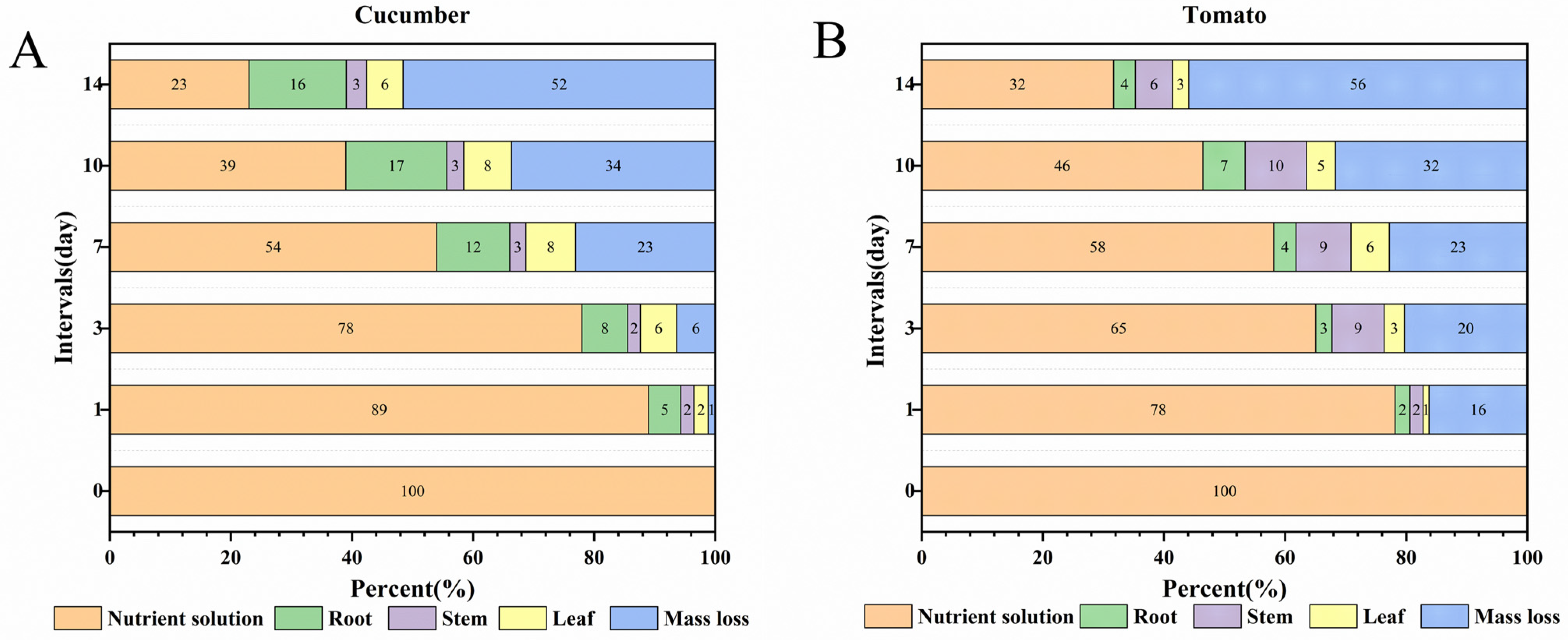
3.5. Distribution of PYD in the Plant–Water Systems
3.5.1. Cucumber–Water System
3.5.2. Tomato-Water System
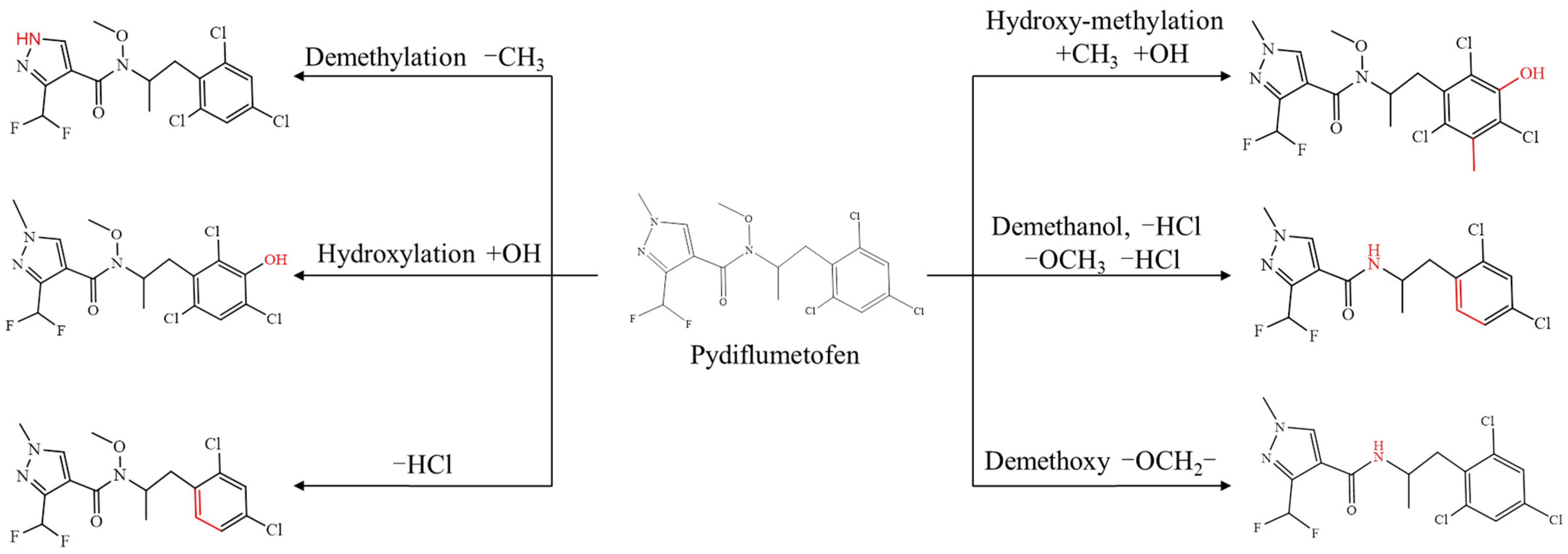
3.6. Identification of Metabolites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tang, F.H.M.; Lenzen, M.; McBratney, A.; Maggi, F. Risk of pesticide pollution at the global scale. Nat. Geosci. 2021, 14, 206–210. [Google Scholar] [CrossRef]
- Li, S.; Li, X.; Zhang, H.; Wang, Z.; Xu, H. The research progress in and perspective of potential fungicides: Succinate dehydrogenase inhibitors. Bioorganic Med. Chem. 2021, 50, 116476. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, Z.; Jing, J.; Bao, F.; Wu, L.; Du, Y.; Zhang, H. Integrating environmental carry capacity based on pesticide risk assessment in soil management: A case study for China. J. Hazard. Mater. 2023, 460, 132341. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Song, J.W.; Jeong, S.H.; Jung, J.H.; Seo, J.S.; Kim, J.H. Dissipation of Four Typical Insecticides on Strawberries and Effects of Different Household Washing Methods. Foods 2023, 12, 1248. [Google Scholar] [CrossRef] [PubMed]
- Abril, C.; Santos, J.L.; Martin, J.; Aparicio, I.; Alonso, E. Uptake and translocation of multiresidue industrial and household contaminants in radish grown under controlled conditions. Chemosphere 2021, 268, 128823. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yao, F.; Liu, Y.W.; Chang, H.Q.; Li, Z.J.; Xue, J.M. Uptake and translocation of organic pollutants in plants: A review. J. Integr. Agric. 2017, 16, 1659–1668. [Google Scholar] [CrossRef]
- Pullagurala, V.L.R.; Rawat, S.; Adisa, I.O.; Hernandez-Viezcas, J.A.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Plant uptake and translocation of contaminants of emerging concern in soil. Sci. Total Environ. 2018, 636, 1585–1596. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Luo, F.; Zhang, X.; Wang, X.; Sun, H.; Lou, Z.; Chen, Z. Uptake, translocation, and metabolism of anthracene in tea plants. Sci. Total Environ. 2022, 821, 152905. [Google Scholar] [CrossRef]
- Ju, C.; Li, X.; He, S.H.; Shi, L.H.; Yu, S.M.; Wang, F.Y.; Xu, S.J.; Cao, D.T.; Fang, H.; Yu, Y.L. Root Uptake of Imidacloprid and Propiconazole Is Affected by Root Composition and Soil Characteristics. J. Agric. Food Chem. 2020, 68, 15381–15389. [Google Scholar] [CrossRef]
- Wang, F.; Li, X.; Yu, S.; He, S.; Cao, D.; Yao, S.; Yu, Y. Chemical factors affecting uptake and translocation of six pesticides in soil by maize (Zea mays L.). J. Hazard. Mater. 2021, 405, 124269. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, X.; Yang, R.; Yang, L.; Sun, B.; Zhu, L. Uptake kinetics, accumulation, and long-distance transport of organophosphate esters in plants: Impacts of chemical and plant properties. Environ. Sci. Technol. 2019, 53, 4940–4947. [Google Scholar] [CrossRef] [PubMed]
- Ju, C.; Zhang, H.; Yao, S.; Dong, S.; Cao, D.; Wang, F.; Yu, Y. Uptake, translocation, and subcellular distribution of azoxystrobin in wheat plant (Triticum aestivum L.). J. Agric. Food Chem. 2019, 67, 6691–6699. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Zhang, S.; Shao, S.; Zheng, R.; Yu, Z.; Ye, Q. Translocation and metabolism of the chiral neonicotinoid cycloxaprid in oilseed rape (Brassica napus L.). J. Hazard. Mater. 2021, 426, 128125. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Xing, Y.; Jing, J.; Yu, P.; He, M.; Zhang, J.; Zhao, E. Insight into the uptake, accumulation, and metabolism of the fungicide phenamacril in lettuce (Lactuca sativa L.) and radish (Raphanus sativus L.). Environ. Pollut. 2022, 304, 119240. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Ou, X.; Tang, J.; Shi, T.; Ma, X.; Wang, Y.; Wu, X.; Li, Q.X.; Hua, R. Uptake, distribution and translocation of imidacloprid-loaded fluorescence double hollow shell mesoporous silica nanoparticles and metabolism of imidacloprid in pakchoi. Sci. Total Environ. 2021, 787, 147578. [Google Scholar] [CrossRef]
- Wang, Z.; Li, R.; Zhang, J.; Liu, S.; He, Z.; Wang, M. Evaluation of exploitive potential for higher bioactivity and lower residue risk enantiomer of chiral fungicide pydiflumetofen. Pest Manag. Sci. 2021, 77, 3419–3426. [Google Scholar] [CrossRef] [PubMed]
- Bui TA, T.; Stridh, H.; Molin, M. Influence of weather conditions on the quality of ‘Ingrid Marie’ apples and their susceptibility to grey mould infection. J. Agric. Food Res. 2021, 3, 100104. [Google Scholar] [CrossRef]
- Deng, H.; Qian, Y. Research Development of New-type Bactericide-pydiflumetofen. Zhejiang Chem. Ind. 2017, 48, 31–33. [Google Scholar]
- Hou, Y.P.; Mao, X.W.; Wang, J.X.; Zhan, S.W.; Zhou, M.G. Sensitivity of Fusarium asiaticum to a novel succinate dehydrogenase inhibitor fungicide pydiflumetofen. Crop Prot. 2017, 96, 237–244. [Google Scholar] [CrossRef]
- Moosavi, B.; Berry, E.A.; Zhu, X.L.; Yang, W.C.; Yang, G.F. The assembly of succinate dehydrogenase: A key enzyme in bioenergetics. Cell. Mol. Life Sci. 2019, 76, 4023–4042. [Google Scholar] [CrossRef]
- Bian, C.; Wang, L.; Cui, Z.; Dong, Z.; Shi, X.; Li, Y.; Li, B. Adsorption-desorption and transport behavior of pydiflumetofen in eight different types of soil. Ecotoxicol. Environ. Saf. 2022, 234, 113378. [Google Scholar] [CrossRef] [PubMed]
- Bian, C.; Luo, J.; Gao, M.; Shi, X.; Li, Y.; Li, B.; Tang, L. Pydiflumetofen in paddy field environments: Its dissipation dynamics and dietary risk. Microchem. J. 2021, 170, 106709. [Google Scholar] [CrossRef]
- Di, S.; Cang, T.; Liu, Z.; Xie, Y.; Zhao, H.; Qi, P.; Wang, Z.; Xu, H.; Wang, X. Comprehensive evaluation of chiral pydiflumetofen from the perspective of reducing environmental risks. Sci. Total Environ. 2022, 826, 154033. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, Y.; Tan, Y.; Li, R.; Zhou, L.; He, Z.; Wang, M. Enantioselective uptake, translocation, and biotransformation of pydiflumetofen in wheat (Triticum aestivum L.): Insights from chiral profiling and molecular simulation. Environ. Int. 2023, 179, 108139. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, S.; Zhao, X.; Tian, B.; Sun, X.; Zhang, J.; Wang, M. Enantioseparation and stereoselective dissipation of the novel chiral fungicide pydiflumetofen by ultra-high-performance liquid chromatography tandem mass spectrometry. Ecotoxicol. Environ. Saf. 2021, 207, 111221. [Google Scholar] [CrossRef]
- Wang, Z.; Tan, Y.; Li, Y.; Duan, J.; Wu, Q.; Li, R.; Wang, M. Comprehensive study of pydiflumetofen in Danio rerio: Enantioselective insight into the toxic mechanism and fate. Environ. Int. 2022, 167, 107406. [Google Scholar] [CrossRef]
- Fang, N.; Zhao, X.; Li, Y.; Luo, Y.; Wang, X.; He, H.; Zhang, C.; Jiang, J. Uptake, translocation and subcellular distribution of broflanilide, afidopyropen, and flupyradifurone in mustard (Brassica juncea). J. Hazard. Mater. 2023, 452, 131381. [Google Scholar] [CrossRef]
- Sijm, D.; Kraaij, R.; Belfroid, A. Bioavailability in soil or sediment: Exposure of different organisms and approaches to study it. Environ. Pollut. 2000, 108, 113–119. [Google Scholar] [CrossRef]
- Li, Y.; Sallach, J.B.; Zhang, W.; Boyd, S.A.; Li, H. Insight into the distribution of pharmaceuticals in soil-water-plant systems. Water Res. 2019, 152, 38–46. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Y.; Dong, F.; Sallach, J.B.; Wu, X.; Liu, X.; Li, Y. Uptake kinetics and accumulation of pesticides in wheat (Triticum aestivum L.): Impact of chemical and plant properties. Environ. Pollut. 2021, 275, 116637. [Google Scholar] [CrossRef]
- Schroll, R.; Bierling, B.; Cao, G.; Dörfler, U.; Lahaniati, M.; Langenbach, T.; Winkler, R. Uptake pathways of organic chemicals from soil by agricultural plants. Chemosphere 1994, 28, 297–303. [Google Scholar] [CrossRef]
- Kaur, H.; Kaur, R.; Singh, S.; Jagota, N.; Sharma, A. Chapter 4—Pesticide biology in plants: Plant uptake, translocation, and accumulation. In Pesticides in the Environment; Sharma, A., Kumar, V., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 67–86. [Google Scholar] [CrossRef]
- Ge, J.; Cui, K.; Yan, H.; Li, Y.; Chai, Y.; Liu, X.; Cheng, J.; Yu, X. Uptake and translocation of imidacloprid, thiamethoxam and difenoconazole in rice plants. Environ. Pollut. 2017, 226, 479–485. [Google Scholar] [CrossRef]
- Wang, W.; Wan, Q.; Li, Y.; Xu, W.; Yu, X. Uptake, translocation and subcellular distribution of pesticides in Chinese cabbage (Brassica rapa var. chinensis). Ecotoxicol. Environ. Saf. 2019, 183, 109488. [Google Scholar] [CrossRef]
- Lv, T.; Zhang, Y.; Casas, M.E.; Carvalho, P.N.; Arias, C.A.; Bester, K.; Brix, H. Phytoremediation of imazalil and tebuconazole by four emergent wetland plant species in hydroponic medium. Chemosphere 2016, 148, 459–466. [Google Scholar] [CrossRef]
- Lyu, T.; Zhang, L.; Xu, X.; Arias, C.A.; Brix, H.; Carvalho, P.N. Removal of the pesticide tebuconazole in constructed wetlands: Design comparison, influencing factors and modelling. Environ. Pollut. 2018, 233, 71–80. [Google Scholar] [CrossRef]
- Morant, M.; Bak, S.; Møller, B.L.; Werck-Reichhart, D. Plant cytochromes P450: Tools for pharmacology, plant protection and phytoremediation. Curr. Opin. Biotechnol. 2003, 14, 151–162. [Google Scholar] [CrossRef]
- Chai, T.; Zhao, H.; Yang, S.; Wang, M.; Qiu, J. Different transformation of carbosulfan to its higher toxic metabolites in pakchoi (Brassica campestris ssp.) and cucumber (Cucumissativus L.) after field application. Int. J. Environ. Anal. Chem. 2015, 95, 1124–1133. [Google Scholar] [CrossRef]
- Tao, Y.; Jia, C.; Jing, J.; Zhao, M.; Yu, P.; He, M.; Zhao, E. Uptake, translocation, and biotransformation of neonicotinoid imidaclothiz in hydroponic vegetables: Implications for potential intake risks. J. Agric. Food Chem. 2021, 69, 4064–4073. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, Y.; Wang, F.; Zhang, M.; Li, L.; Zhao, E. Uptake, Translocation, and Metabolism of Pydiflumetofen in Hydroponic Cucumber and Tomato Planting Systems. Agronomy 2024, 14, 1809. https://doi.org/10.3390/agronomy14081809
Xing Y, Wang F, Zhang M, Li L, Zhao E. Uptake, Translocation, and Metabolism of Pydiflumetofen in Hydroponic Cucumber and Tomato Planting Systems. Agronomy. 2024; 14(8):1809. https://doi.org/10.3390/agronomy14081809
Chicago/Turabian StyleXing, Yinghui, Fuyun Wang, Miaomiao Zhang, Li Li, and Ercheng Zhao. 2024. "Uptake, Translocation, and Metabolism of Pydiflumetofen in Hydroponic Cucumber and Tomato Planting Systems" Agronomy 14, no. 8: 1809. https://doi.org/10.3390/agronomy14081809





