Abstract
Salt stress is a critical abiotic factor that adversely affects plant growth and productivity by impairing photosynthesis. This study explores the impact of exogenous ascorbic acid (AsA) on the photosynthetic performance of tomato seedlings (Solanum lycopersicum L. cv. Ligeer 87-5) under salt stress. Hydroponic experiments were conducted in a solar greenhouse, where tomato seedlings were subjected to the following five treatments: Control, NaCl, NaCl + AsA, NaCl + lycorine (LYC), and NaCl + LYC + AsA. Our findings demonstrate that salt stress significantly reduced chlorophyll and carotenoid contents, levels of chlorophyll synthesis precursors (5-aminolevulinic acid (ALA), porphobilinogen (PBG), uroporphyrinogen III (Urogen III), protoporphyrin IX (Proto IX), magnesium protoporphyrin IX (Mg-Proto IX), protochlorophyllide (Pchl)), and essential elements (Mg, Fe, Mn, Zn, Mo, and P) in both roots and leaves. These reductions led to a substantial decline in net photosynthetic rate (Pn) and compromised photosystem II (PSII). In contrast, exogenous AsA application significantly enhanced the content of photosynthetic pigment precursors and essential elements, improved stomatal aperture and gas exchange efficiency, and boosted the photosynthetic performance of tomato seedlings under salt stress. Furthermore, AsA treatment mitigated the negative effects of salt stress by protecting PSII, increased light energy utilization efficiency, and alleviated both stomatal and non-stomatal limitations. The application of the AsA synthesis inhibitor LYC exacerbated the detrimental effects of salt stress, further reducing chlorophyll content and photosynthetic efficiency. In conclusion, exogenous AsA plays a vital role in enhancing the photosynthetic performance and stress tolerance of tomato seedlings under salt stress by stabilizing chlorophyll biosynthesis, facilitating essential element absorption, and optimizing stomatal function. This study provides a new approach and feasible measures for improving tomato resistance and yield, which is significant for enhancing crop productivity, managing saline soils, and promoting sustainable agricultural practices.
1. Introduction
Soil salinization poses a severe threat to the limited soil resources that are essential for human survival []. Presently, approximately 7% of the world’s land and 33% of its arable land are affected by salinization []. Soil salinization is a major issue in global land degradation, significantly endangering food security. In recent years, changes in global climatic conditions and increased drought have accelerated the rate of salinization []. Consequently, soil salinization has become a primary environmental challenge to the sustainable and high-quality development of global agriculture []. In China, the rapid expansion of vegetable cultivation areas, coupled with environmental degradation and improper irrigation and fertilization practices, has led to significant soil salt accumulation. Tomatoes are particularly sensitive to salt stress []. Research indicates that salt stress consistently inhibits tomato seed germination and prolongs germination time []. It reduces the net photosynthetic rate and water use efficiency, significantly increases reactive oxygen species (ROS) content, and disrupts cellular redox balance, thereby adversely affecting tomato plant growth []. Moreover, salt stress markedly decreases fruit length, fruit diameter, yield, and fruit dry matter content, consequently diminishing fruit quality. Under severe salt stress, transplanted tomato plants may fail to survive, leading to substantial economic losses [,]. Therefore, elucidating the mechanisms of tomato responses to salt stress and the regulatory mechanisms of salt stress resistance is crucial for breeding salt-tolerant tomato varieties, identifying salt-tolerant cultivation practices, and achieving efficient and sustainable development in agriculture.
Plant growth is regulated by a series of complex molecular, physiological, and biochemical processes. Photosynthesis is crucial for all green plants, playing a significant role in their growth and development. Through photosynthesis, plants convert light energy into usable chemical energy, which is then utilized in various metabolic processes. However, salt stress can disrupt photosynthesis by altering chloroplast ultrastructure, reducing concentrations of photosynthetic pigments, destabilizing thylakoid membrane proteins, and affecting the activities of enzymes involved in photosynthesis [,]. Photosynthetic pigments, located in the thylakoid membranes of chloroplast grana, include chlorophyll (Chl), reaction center pigments, and accessory pigments, which are essential for light absorption, energy transfer, and primary photochemical reactions. Salt stress often leads to a reduction in the content of these pigments and damages the thylakoid membrane structure, thereby decreasing photosynthetic capacity []. Chlorophyll biosynthesis is a tightly regulated dynamic process. Disruptions in chlorophyll metabolism can directly affect physiological processes like photosynthesis, cause programmed cell death, and hinder plant growth and development []. The synthesis pathway of chlorophyll precursors is highly susceptible to stress conditions. For instance, high temperatures lead to a decrease in aminolevulinic acid (ALA) and porphobilinogen (PBG) content in lettuce []. Similarly, low temperatures significantly reduce the levels of ALA, protoporphyrin IX (Proto IX), Mg-protoporphyrin IX (Mg-Proto IX), and protochlorophyllide (Pchl) in pepper leaves []. Salt stress inhibits the synthesis of ALA in sunflower, resulting in a notable reduction in chlorophyll content []. Furthermore, nitrate stress markedly decreases the Mg-Proto IX and Proto IX content in cucumber leaves []. These findings underscore the critical role of chlorophyll in photosynthesis and plant growth, leading to significant impacts on plant growth and productivity.
The use of exogenous plant growth regulators to enhance plant growth and stress resistance is an effective strategy. Ascorbic acid (AsA) is an essential antioxidant in plants that is capable of directly scavenging reactive oxygen species (ROS) such as 1O2, O2•− and ·OH. It also participates in the regeneration of vitamin E and the ascorbate peroxidase (APX) reaction, playing a central role in the plant’s antioxidant defense mechanisms []. Exogenous application of AsA can promote plant growth and yield under both normal and stress conditions, resulting in higher economic benefits []. Studies indicate that exogenous AsA is highly effective in safeguarding lipids and proteins against oxidative damage caused by salt or drought stress [,]. Under salt stress, AsA significantly increases chlorophyll (Chl a and Chl b) and carotenoid content, maintains ion homeostasis, reduces excessive ROS accumulation, and mitigates salt-induced damage in pea plants []. AsA can also act as an electron donor for photosystems II and I, serving as a major limiting factor for photosynthesis [,,].
Previous research has demonstrated that exogenous AsA boosts endogenous AsA levels by modulating the activities of enzymes responsible for AsA synthesis and metabolism, maintaining ion homeostasis, mitigating ion toxicity, and alleviating osmotic stress through the regulation of proline metabolism []. AsA also activates cyclic electron flow (CEF) and non-photochemical quenching (NPQ) mechanisms, dissipating excess excitation energy, stabilizing PSII and PSI supercomplexes, and enhancing carbon and nitrogen metabolism while boosting ROS scavenging systems to mitigate oxidative damage caused by photoinhibition [,]. Although it has been hypothesized that AsA participates in chlorophyll synthesis, direct evidence is lacking. Hydroponic systems provide a controlled environment that allows for precise manipulation of nutrient and salt concentrations, making them ideal for studying plant responses to salinity stress []. This method offers several advantages, including the ability to maintain consistent growth conditions, minimize soil-borne diseases, and monitor root development closely. By using hydroponic systems, researchers can accurately assess the effects of salinity on plant physiology and biochemistry, leading to a better understanding of the mechanisms underlying salt tolerance []. Our study hypothesizes that exogenous AsA can enhance chlorophyll biosynthesis in tomato seedlings under salt stress, thereby improving overall photosynthetic performance and salt tolerance. Consequently, this study utilized processing tomato as the experimental material, using hydroponics to simulate a saline environment to examine the effects of exogenous AsA on growth, photosynthetic pigment content, chlorophyll precursor levels, essential element content, photosynthetic characteristics, and stomatal changes under salt stress. The results demonstrated that the application of exogenous AsA significantly increased the content of chlorophyll precursors and essential elements, thereby promoting chlorophyll synthesis, protecting PSII from damage, improving light energy utilization, enhancing photosynthetic performance, and ultimately promoting the growth and salt tolerance of processing tomato seedlings.
2. Materials and Methods
2.1. Plant Materials, Growth Conditions and Treatments
The processing tomato cultivar ‘Ligeer 87-5’ was selected for hydroponic experiments conducted in a solar greenhouse with natural growing conditions at Kaili University, Guizhou, China. Seeds were initially germinated on moist filter paper in darkness at 28 °C for 2 days. After germination, the seeds were sown in plug trays containing a 1:1 (v/v) mixture of peat and vermiculite. Once the seedlings developed two true leaves, they were transplanted into 12-L plastic containers, each holding five seedlings. The containers were filled with 10 L of oxygenated, full-strength Hoagland nutrient solution (pH 6.2).
After a pre-culture period of 7 days, the seedlings were subjected to five distinct treatments. Sodium chloride (NaCl) was added to the nutrient solutions, and ascorbic acid (AsA) or lycorine (LYC) were sprayed onto the leaves. The treatments were as follows: (1) Control (no added NaCl, no sprayed AsA or LYC); (2) 100 mmol L−1 NaCl (NaCl treatment); (3) 100 mmol L−1 NaCl + 0.5 mmol L−1 AsA (NaCl + AsA treatment); (4) 100 mmol L−1 NaCl + 0.25 mmol L−1 LYC (NaCl + LYC treatment); and (5) 100 mmol L−1 NaCl + 0.25 mmol L−1 LYC + 0.5 mmol L−1 AsA (NaCl + LYC + AsA treatment). AsA and LYC were sourced from Sigma-Aldrich (St. Louis, MO, USA) and YuanYe (Shanghai yuanye Bio-Technology Co. Ltd., Shanghai, China), respectively. The concentrations, application volumes, and methods for AsA and LYC treatments were based on our previous research []. The experimental setup was a randomized complete block design with three replications per treatment, each replication consisting of three containers, with each container holding five seedlings. Nutrient solutions were changed every 3 days. Leaf samples from tomato seedlings were collected on the 6th day post-treatment.
2.2. Measurement of Growth Parameters
Relative growth rates (RGRs) were determined to evaluate the growth performance of the tomato seedlings under different treatments. The RGR was calculated based on the increase in biomass over a specified period. The relative growth rate was calculated using the following formula:
where W1 and W2 are the initial and final dry weights, respectively, and t1 and t2 are the initial and final times, respectively. The natural logarithm (ln) of the weights was used to linearize the growth curve, making it possible to calculate the growth rate over the treatment period accurately.
2.3. Measurement of Photosynthetic Pigment Content
The content of photosynthetic pigments, including chlorophyll a (Chl a), chlorophyll b (Chl b), and carotenoids (Cars), was determined using a spectrophotometric method. Fresh tomato leaves (0.5 g) were ground in 10 mL of 80% (v/v) acetone, followed by centrifugation at 12,000× g for 15 min at 4 °C. The resulting supernatant was then subjected to absorbance measurements at 663 nm, 645 nm, and 470 nm using a UV-visible spectrophotometer (UV -1800, Shimadzu Corporation, Kyoto, Japan).
The concentrations of Chl a, Chl b, and Car were calculated using the following equations:
Chl a = 12.7·A663 − 2.69·A645
Chl b = 22.9·A645 − 4.68·A663
Car = (1000·A470 − 1.90·Chl a − 63.14·Chl b)/214
The pigment content was expressed as mg per gram of fresh weight (mg·g−1 FW).
2.4. Determination of Chlorophyll Precursor Content
Protoporphyrin IX (Proto IX), Mg-protoporphyrin IX (Mg-Proto IX), and protochlorophyllide (Pchl) levels were determined following the methodology outlined by Hodgins []. Uroporphyrinogen III (Urogen III) and porphobilinogen (PBG) concentrations were determined according to Bogorad’s method []. The content of 5-aminolevulinic acid (ALA) was quantified using the procedure described by Richard [].
2.5. Determination of Elemental Content in Leaves and Roots
To determine the elemental content (Mg, Fe, Mn, Zn, Mo, P) in tomato leaves and roots, samples were dried at 70 °C until reaching a constant weight in an oven. The dried samples were then finely ground using a mortar and pestle. Subsequently, 0.5 g of the powdered sample underwent digestion with a mixture of concentrated nitric acid (HNO3) and perchloric acid (HClO4) using a microwave digestion system for analysis. After digestion, the solution was filtered and diluted to a known volume with deionized water. The elemental concentrations of magnesium (Mg), iron (Fe), manganese (Mn), zinc (Zn), molybdenum (Mo), and phosphorus (P) in the digested samples were analyzed using an 720 ICP-OES device (Agilent technologies, CA, USA). Calibration standards for each element were prepared from stock solutions and used to create a calibration curve for quantification. The elemental content was expressed as mg per g of dry weight (mg·g−1 DW).
2.6. Determination of Photosynthetic Parameters
Photosynthetic parameters, including net photosynthetic rate (Pn), stomatal conductance (Gs), intercellular CO2 concentration (Ci), and transpiration rate (Tr), were assessed using a portable photosynthetic system (Li-6400, Lincoln, Nebraska, USA). Measurements were taken from 9:00 to 13:00 h under the following conditions: the Photosynthetically Active Radiation (PAR) was 1500 µmol·m⁻2·s⁻1, the CO2 concentration was 380~390 μmol·mol−1, and the temperature 28 °C~32 °C. The stomatal limitation value (Ls) was calculated according to the method described by Berry []. The apparent light energy utilization efficiency (LUEapp) was calculated based on the approach described by Long []. The apparent mesophyll conductance (AMC) was determined following the methodology outlined by Chen Xu []. The intrinsic water use efficiency (WUEi) was calculated as per the method described by Ashraf []. The transient water use efficiency (WUEt) was calculated following the method described by Nijs [].
2.7. Calculation of Fluorescence Parameters
The relative limitation of photosynthetic function (LPFD) and chlorophyll fluorescence decay rate (Rfd) were calculated as follows:
where qp is the photochemical quenching, is the maximum fluorescence yield in the light-adapted state, and Fs is the steady-state fluorescence yield.
where Fm is the maximum fluorescence yield in the dark-adapted state and Fs is the steady-state fluorescence yield.
These parameters were calculated according to the methods described by Bartha et al. [].
2.8. Determination of Stomatal Density and Aperture
Stomatal density and aperture were measured using a fluorescence microscope (Carl Zeiss Co., Oberkochen, Germany) (5 × 40 magnification). The number of stomata per field of view was observed, and the length and width of each stomatal aperture were determined using the microscope’s ZEN 2 (blue edition, v2.0, Zeiss, Oberkochen, Germany). Stomatal aperture was calculated using the formula:
where A is half of the stomatal length and B is half of the stomatal width, as described by Inamullah et al. [] and Wise et al. [].
Stomatal Aperture = π × A × B
2.9. Statistical Analysis
All data are presented as mean ± standard deviation (SD) from four independent replicates. Statistical analyses were conducted using SPSS software (version 22.0, IBM Corp., Armonk, NY, USA). Differences among treatments were assessed using one-way analysis of variance (ANOVA), followed by Duncan’s multiple range test. Statistical significance was defined as p < 0.05.
3. Results
3.1. Effects on Growth Traits
Exposure to 100 mM NaCl stress inhibited the growth of tomato seedlings, resulting in significantly reduced relative growth rates in both shoots and roots compared to the Control (Figure 1). However, the addition of exogenous AsA mitigated the negative impact of NaCl stress on shoot and root relative growth rates, resulting in increases of 43.9% and 89.8%, respectively (Figure 1). In contrast, treatment with lycorine (NaCl + LYC treatment) exacerbated the inhibitory effects of NaCl stress on tomato seedling growth. Conversely, the combined treatment (NaCl + LYC + AsA) effectively reversed these adverse effects compared to the NL treatment (Figure 1).
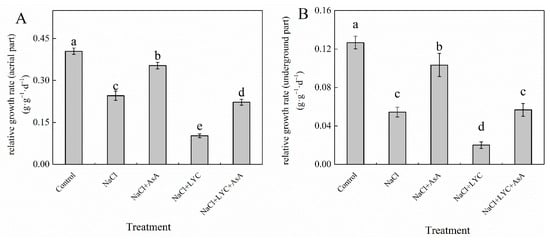
Figure 1.
Relative growth rates of the aerial parts (A) and relative growth rates of the underground parts (B) in tomato seedlings subjected to various treatments: Control (no additional treatments), NaCl (100 mM NaCl), NaCl + AsA (100 mM NaCl + 0.5 mM ascorbic acid), NaCl + LYC (100 mM NaCl + 0.25 mM lycorine), and NaCl + LYC + AsA (100 mM NaCl + 0.25 mM LYC + 0.5 mM AsA). Each treatment was replicated four times. Error bars represent standard deviation (SD). Significant differences among treatments (p < 0.05) are indicated by different letters. Sampling was conducted on the sixth day after treatment application.
3.2. Effects on Chlorophyll Content
Exposure to salt stress (100 mM NaCl) significantly decreased chlorophyll (Chl) and carotenoid (Car) content in tomato seedling leaves, while markedly increasing the Chla/b ratio compared to the Control (Figure 2). Conversely, treatment with exogenous AsA mitigated the effects of NaCl stress on Chl, Car, and Chl a/b. Specifically, AsA increased Chl a, Chl b, total Chl (Chl a + Chl b), and Car levels by 46.2%, 143.8%, 65.4%, and 70.7%, respectively, and reduced the Chla/b ratio by 41.2% (Figure 2). In contrast, treatment with LYC significantly decreased the Chl a, Chl b, total Chl, and Car content in tomato leaves, with no significant effect on the Chl a/b ratio compared to NaCl treatment. Notably, the treatment of NaCl + LYCA effectively reversed the effects observed in the NaCl + LYC treatment (Figure 2).
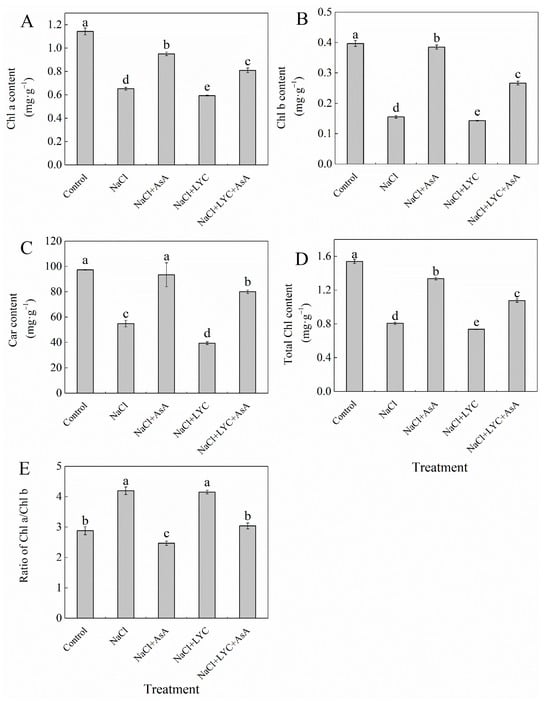
Figure 2.
Chl a content (A), Chl b content (B), Car content (C), total Chl content (D) and ratio of Chl a/Chl b (E) in tomato seedlings subjected to various treatments: Control (no additional treatments), NaCl (100 mM NaCl), NaCl + AsA (100 mM NaCl + 0.5 mM ascorbic acid), NaCl + LYC (100 mM NaCl + 0.25 mM lycorine), and NaCl + LYC + AsA (100 mM NaCl + 0.25 mM LYC + 0.5 mM AsA). Each treatment was replicated four times. Error bars represent standard deviation (SD). Significant differences among treatments (p < 0.05) are indicated by different letters. Sampling was conducted on the sixth day after treatment application.
3.3. Effects on Key Enzyme Activities in Chlorophyll Synthesis Precursors
NaCl stress significantly decreased the levels of 5-aminolevulinic acid (ALA), porphobilinogen (PBG), uroporphyrinogen III (Urogen III), protoporphyrin IX (Proto IX), magnesium-protoporphyrin IX (Mg-Proto IX), and protochlorophyllide (Pchl) by 18.0%, 19.1%, 19.7%, 19.2%, 14.8%, and 31.7%, respectively, compared to the Control (Figure 3). However, the application of exogenous AsA alleviated the negative impacts of NaCl stress on chlorophyll synthesis precursors in tomato seedlings, significantly increasing their levels by 27.9%, 14.9%, 32.4%, 30.4%, 28.7%, and 53.5%, respectively (Figure 3). In contrast, treatment with LYC exacerbated the reduction in chlorophyll precursors under NaCl stress. The treatment with NaCl + LYC + AsA significantly improved this condition compared to the NaCl + LYC treatment (Figure 3).
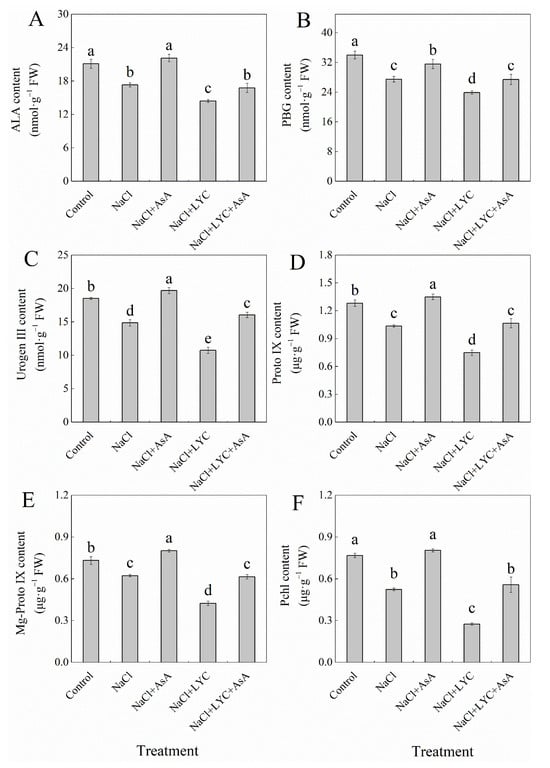
Figure 3.
ALA content (A), PBG content (B), Urogen III content (C), Proto IX content (D), Mg-Proto IX content, (E) and Pchl content (F) in tomato seedlings subjected to various treatments: Control (no additional treatments), NaCl (100 mM NaCl), NaCl + AsA (100 mM NaCl + 0.5 mM ascorbic acid), NaCl + LYC (100 mM NaCl + 0.25 mM lycorine), and NaCl + LYC + AsA (100 mM NaCl + 0.25 mM LYC + 0.5 mM AsA). Each treatment was replicated four times. Error bars represent standard deviation (SD). Significant differences among treatments (p < 0.05) are indicated by different letters. Sampling was conducted on the sixth day after treatment application.
3.4. Effects on Elemental Content
NaCl stress significantly reduced the contents of magnesium (Mg), iron (Fe), manganese (Mn), zinc (Zn), molybdenum (Mo), and phosphorus (P) in both the leaves and roots of tomato seedlings compared to the Control (Figure 4). However, the addition of exogenous AsA under salt stress significantly increased the levels of these elements in the leaves and roots by 13.7% and 16.3% (Mg), 29.1% and 46.0% (Fe), 18.1% and 29.2% (Mn), 15.6% and 22.6% (Zn), 17.9% and 5.7% (Mo), and 21.9% and 18.3% (P), respectively (Figure 4). Conversely, the application of the AsA inhibitor LYC led to a significant decrease in the elemental content in leaves and roots, as follows: Mg decreased by 35.2% and 18.1%, Fe by 26.9% and 23.2%, Mn by 52.5% and 10.0%, Zn by 24.7% and 10.7%, Mo by 11.7% and 11.1%, and P by 37.8% and 17.1%, respectively. This negative effect was significantly reversed by the subsequent application of AsA (NaCl + LYC + AsA treatment) (Figure 4).
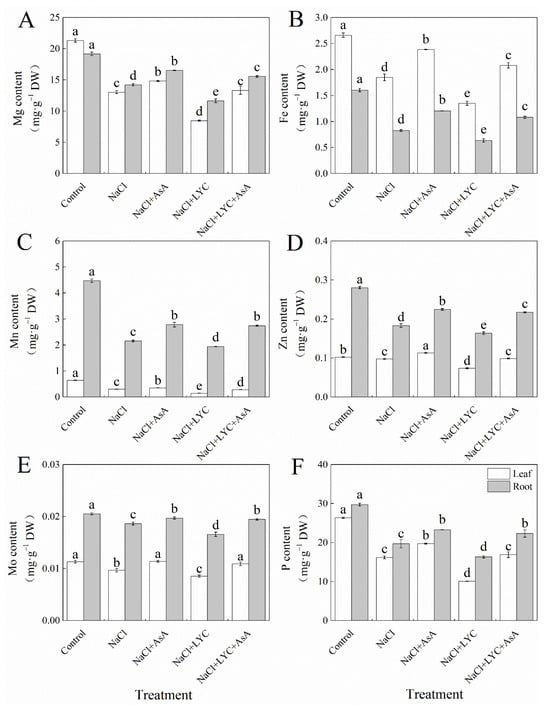
Figure 4.
Mg content (A), Fe content (B), Mn content (C), Zn content (D), Mo content (E) and P content (F) in tomato seedlings subjected to various treatments: Control (no additional treatments), NaCl (100 mM NaCl), NaCl + AsA (100 mM NaCl + 0.5 mM ascorbic acid), NaCl + LYC (100 mM NaCl + 0.25 mM lycorine), and NaCl + LYC + AsA (100 mM NaCl + 0.25 mM LYC + 0.5 mM AsA). Each treatment was replicated four times. Error bars represent standard deviation (SD). Significant differences among treatments (p < 0.05) are indicated by different letters. Sampling was conducted on the sixth day after treatment application.
3.5. Effect on the Leaf Gas Exchange Parameters
NaCl treatment significantly decreased the net photosynthetic rate (Pn), stomatal conductance (Gs), and intercellular CO2 concentration (Ci) in the leaves of tomato seedlings while significantly increasing the stomatal limitation value (Ls) compared to the Control (Figure 5). However, the application of exogenous AsA reversed the effects of NaCl stress on Pn, Gs, Ci and Ls (Figure 5). The LYC treatment significantly reduced Pn, Gs and Ci under salt stress by 26.8%, 46.7%, and 6.3%, respectively, while significantly increasing Ls by 25.6%. Compared to the NL treatment, the addition of AsA (NaCl + LYC + AsA treatment) significantly improved these parameters (Figure 5).
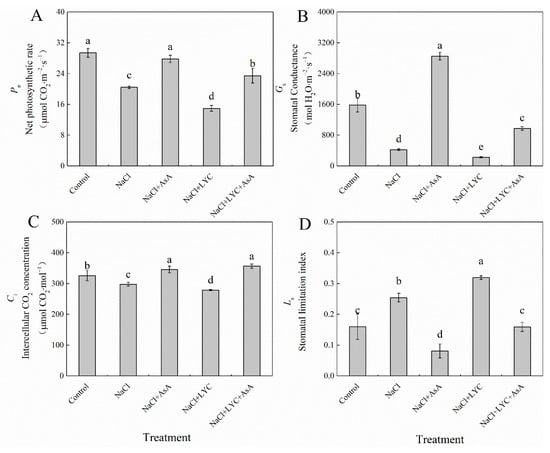
Figure 5.
Pn (A), Gs (B), Ci (C) and Ls (D) in tomato seedlings subjected to various treatments: Control (no additional treatments), NaCl (100 mM NaCl), NaCl + AsA (100 mM NaCl + 0.5 mM ascorbic acid), NaCl + LYC (100 mM NaCl + 0.25 mM lycorine), and NaCl + LYC + AsA (100 mM NaCl + 0.25 mM LYC + 0.5 mM AsA). Each treatment was replicated four times. Error bars represent standard deviation (SD). Significant differences among treatments (p < 0.05) are indicated by different letters. Sampling was conducted on the sixth day after treatment application.
NaCl treatment resulted in a marked increase in intrinsic water use efficiency (WUEi) by 157.6% compared to the Control while significantly reducing the apparent light energy utilization efficiency (LUEapp) and apparent mesophyll conductance (AMC) by 30.4% and 24.0%, respectively, with no change in the transient water use efficiency (WUEt) (Figure 6). However, when treated with AsA (NaCl + AsA treatment), the WUEi and WUEt decreased notably by 79.9% and 27.6%, respectively, while the LUEapp and AMC increased by 36.1% and 17.2%, respectively, compared to NaCl treatment alone. The application of LYC under salt stress conditions (NaCl + LYC treatment) significantly decreased the WUEt, LUEapp, and AMC while increasing the WUEi. Notably, the NaCl + LYC + AsA treatment significantly reversed these changes compared to the NL treatment (Figure 6).
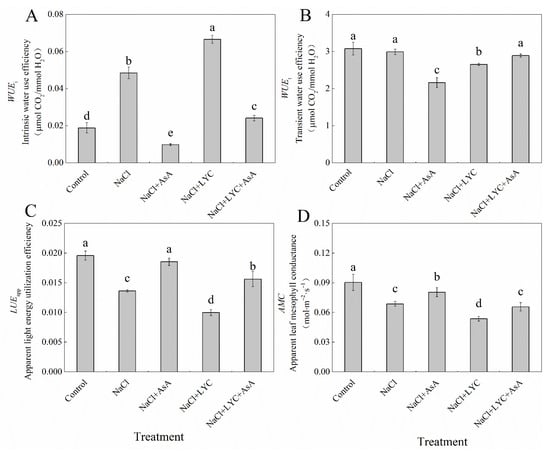
Figure 6.
WUEi (A), WUEt (B), LUEapp (C) and AMC (D) in tomato seedlings subjected to various treatments: Control (no additional treatments), NaCl (100 mM NaCl), NaCl + AsA (100 mM NaCl + 0.5 mM ascorbic acid), NaCl + LYC (100 mM NaCl + 0.25 mM lycorine), and NaCl + LYC + AsA (100 mM NaCl + 0.25 mM LYC + 0.5 mM AsA). Each treatment was replicated four times. Error bars represent standard deviation (SD). Significant differences among treatments (p < 0.05) are indicated by different letters. Sampling was conducted on the sixth day after treatment application.
3.6. Effects on Photosynthetic Function Relative Limitation Value and Chlorophyll Fluorescence Decay Rate
NaCl treatment significantly increased the photosynthetic function relative limitation value (LPFD) and decreased the chlorophyll fluorescence decay rate (Rfd) in tomato seedling leaves compared to the Control (Figure 7). The addition of exogenous AsA under salt stress conditions reduced the LPFD by 22.8% and increased the Rfd by 7.6%. Conversely, LYC treatment under salt stress conditions significantly elevated the LPFD and further decreased the Rfd. However, these adverse effects were substantially reversed with the application of AsA (NaCl + LYC + AsA treatment) (Figure 7).
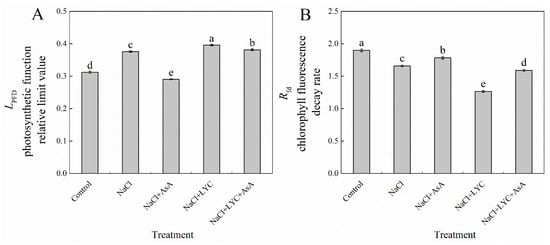
Figure 7.
LPFD (A) and Rfd (B) in tomato seedlings subjected to various treatments: Control (no additional treatments), NaCl (100 mM NaCl), NaCl + AsA (100 mM NaCl + 0.5 mM ascorbic acid), NaCl + LYC (100 mM NaCl + 0.25 mM lycorine), and NaCl + LYC + AsA (100 mM NaCl + 0.25 mM LYC + 0.5 mM AsA). Each treatment was replicated four times. Error bars represent standard deviation (SD). Significant differences among treatments (p < 0.05) are indicated by different letters. Sampling was conducted on the sixth day after treatment application.
3.7. Effects on Stomatal Aperture and Number
NaCl treatment significantly decreased stomatal aperture by 72.2% and increased the stomatal number by 73.3% compared to the Control (Figure 8). The application of exogenous AsA (NaCl + AsA treatment) under salt stress significantly enhanced stomatal aperture by 148.3% and reduced the stomatal number compared to NaCl treatment alone. In contrast, LYC treatment under salt stress conditions significantly decreased stomatal aperture without significantly affecting the stomatal number. However, the application of LYC followed by AsA (NaCl + LYC + AsA treatment) significantly increased stomatal aperture and reduced the stomatal number compared to the NaCl + LYC treatment (Figure 8).
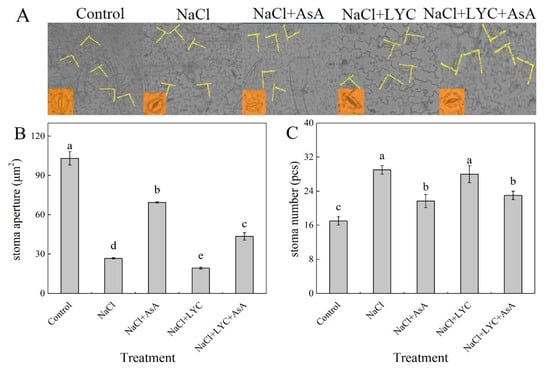
Figure 8.
Photo of stomatal aperture (A), stomatal aperture (B) and the stomatal number (C) in tomato seedlings subjected to various treatments: Control (no additional treatments), NaCl (100 mM NaCl), NaCl + AsA (100 mM NaCl + 0.5 mM ascorbic acid), NaCl + LYC (100 mM NaCl + 0.25 mM lycorine), and NaCl + LYC + AsA (100 mM NaCl + 0.25 mM LYC + 0.5 mM AsA). Each treatment was replicated four times. Error bars represent standard deviation (SD). Significant differences among treatments (p < 0.05) are indicated by different letters. Sampling was conducted on the sixth day after treatment application.
3.8. Correlation Analysis
The results of the correlation analysis indicate that there are significant positive or negative correlations among certain physiological and morphological parameters in response to salt stress (Figure 9). For instance, the relative growth rate of the shoot exhibits a significant negative correlation with the photosynthetic function relative limitation value (LPFD). Similarly, the intercellular CO2 concentration shows a significant positive correlation with the molybdenum (Mo) content in leaves. This suggests that, during the response to salt stress, some parameters may exhibit similar or opposite responses to the stress. However, it is crucial to understand that a significant linear correlation between two parameters does not indicate that they are interchangeable.
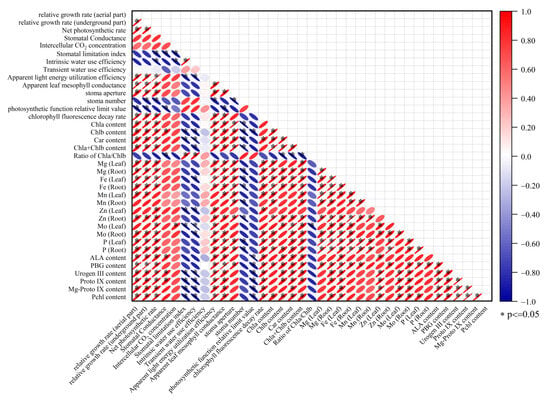
Figure 9.
Linear regression coefficients among 37 salt response parameters.
3.9. Role of AsA in Mitigating Salt Stress
Salt stress reduces the uptake of essential elements (Mg, Fe, Mn, Zn, Mo, P) in leaves and roots, leading to a decrease in key chlorophyll synthesis precursors such as ALA, PBG, Urogen III, Proto IX, Mg-Proto IX, and Pchl. This reduction results in decreased levels of Chla and Chlb, negatively affecting chloroplast function, reducing photosynthesis, and inhibiting plant growth. In contrast, the application of exogenous AsA alleviates these negative effects. AsA promotes the synthesis of chlorophyll precursors, increases chlorophyll content, and enhances the uptake of essential elements, thereby improving plant growth and enhancing stress resistance.
4. Discussion
Photosynthesis, the foundation of plant growth and development, is highly susceptible to salt stress. Numerous studies have demonstrated that salt stress inhibits plant photosynthesis, resulting in stunted growth, and our findings align with these results (Figure 1). Chlorophyll is the primary molecule involved in the energy conversion processes of photosynthesis, playing a critical role in the absorption, transmission, and distribution of light energy, which is essential for plant growth and development [,]. The chlorophyll content in plants depends on the dynamic balance between its synthesis and degradation. Abiotic stress often leads to a reduction in chlorophyll content, severely impacting the photosynthetic capacity of leaves. This reduction is likely due to stress conditions inhibiting chlorophyll synthesis while accelerating its degradation [,]. The biosynthesis pathway of chlorophyll is as follows: glutamate → 5-aminolevulinic acid (ALA) → porphobilinogen (PBG) → uroporphyrinogen III (Urogen III) → protoporphyrin IX (Proto IX) → magnesium protoporphyrin IX (Mg-Proto IX) → protochlorophyllide (Pchl) → chlorophyll a → chlorophyll b. Any disruption at any step in this pathway can impair chlorophyll synthesis, leading to a decrease in its content [].
This study demonstrates that salt stress reduces chlorophyll and carotenoid contents in tomato seedlings’ leaves (Figure 2), similar to findings in pepper [] and rice []. Additionally, salt stress decreased the levels of chlorophyll synthesis precursors (ALA, PBG, Urogen III, Proto IX, Mg-Proto IX, and Pchl) and the contents of essential elements such as Mg, Fe, Mn, Zn, Mo, and P in roots and leaves (Figure 3 and Figure 4). Mg is a fundamental component of chlorophyll pigments in chloroplast light-harvesting complexes, with 15–35% of the absorbed Mg being fixed in chlorophyll pigments. Mg also serves as a cofactor for more than 300 enzymes that are essential in both chlorophyll biosynthesis and photosynthetic CO2 fixation [,]. Fe, although not a component of chlorophyll, is necessary for chlorophyll synthesis. Iron deficiency affects not only chlorophyll synthesis but also the synthesis of chloroplast membranes, chlorophyll–protein complexes, reaction centers, and associated electron carriers []. Mn is essential for maintaining chloroplast structure and is involved in water photolysis and electron transport, decomposing H2O to release O2 and electrons, which are then transferred to photosystem II []. Zn participates in CO2 hydration and the biosynthesis of photosynthetic pigments []. Mo deficiency impacts the conversion of ALA to Urogen III in winter wheat leaves, hindering chlorophyll synthesis and reducing its content. Mo application significantly improves net photosynthetic rate and stomatal limitation in winter wheat under low-temperature stress and reduces stomatal conductance, intercellular CO2 concentration, and transpiration rate []. P is essential for the metabolism of ATP, NADPH, nucleic acids, and phospholipids, all of which play crucial roles in plant growth, production, signal transduction, and photosynthesis. Phosphorus deficiency reduces the content of photosynthetic pigments such as carotenoids and chlorophyll [,]. Carotenoids in photosynthetic pigments not only capture light energy as accessory pigments but also quench reactive oxygen species, preventing damage to photosynthetic organs []. Previous studies have demonstrated that exogenous AsA can enhance ionic homeostasis in tomato plants under salt stress []. Our findings further revealed that the application of exogenous AsA significantly increased the levels of chlorophyll synthesis precursors (ALA, PBG, Urogen III, Proto IX, Mg-Proto IX, and Pchl) as well as essential elements (Mg, Fe, Mn, Zn, Mo, and P) in both roots and leaves under salt stress conditions (Figure 3 and Figure 4). This suggests that AsA plays a crucial role in stabilizing chlorophyll biosynthesis by facilitating the absorption and utilization of these vital elements. Conversely, when the AsA synthesis inhibitor lycorine (LYC) was applied, the detrimental effects of salt stress were exacerbated, leading to further reductions in chlorophyll content, chlorophyll precursors, and essential elements (Figure 2, Figure 3 and Figure 4). This indicates that inhibiting AsA synthesis compromises the stability of chlorophyll synthesis, reduces the capacity for light energy absorption and conversion, and diminishes the ability to quench reactive oxygen species.
The reduction in photosynthesis under salt stress in plants involves both stomatal and non-stomatal limitations in addition to changes in photosynthetic pigment content []. This study found that NaCl treatment significantly reduced the Pn of tomato seedlings, accompanied by simultaneous decreases in Gs and Ci and an increase in Ls (Figure 5). This indicates that stomatal factors are the main cause of the reduced photosynthetic rate. Stomata are crucial channels for carbon and water exchange between crops and the external environment, effectively controlling key physiological processes such as photosynthesis, respiration, and transpiration, ultimately impacting plant yield []. Gas exchange processes are regulated by stomatal density, aperture size, and spatial distribution patterns []. Stomatal differentiation and development are influenced not only by genetic factors but also by environmental factors such as temperature, water, and salinity [,]. Intrinsic water-use efficiency (iWUE), which assesses the ratio of CO2 assimilation to stomatal conductance [], is impacted by variations in stomatal opening. Changes in stomatal conductance can affect both the photosynthetic rate and WUE []. Salt stress reduces stomatal aperture, hindering CO2 molecules from entering the leaf interior, thus lowering the CO2 concentration at photosynthetic reaction sites and consequently reducing the net photosynthetic rate of leaves []. AsA, as a signaling molecule in plant stress responses, can regulate stomatal movement and signaling in guard cells through its redox state []. This study found that exogenous AsA increased gas exchange efficiency and thereby improved the Pn of tomato seedlings’ leaves under salt stress by enlarging stomatal aperture and reducing stomatal density (Figure 8). However, when exogenous AsA synthesis inhibitor LYC was applied, the Pn, Gs, Ci and WUEt of tomato seedlings’ leaves significantly decreased while the Ls and WUEi significantly increased, indicating that LYC exacerbated the damage to the photosynthetic performance of tomato seedlings under salt stress over time. Conversely, exogenous AsA increased the Pn, Gs and Ci, and reduced the Ls and WUEi under both NaCl and NL treatments (Figure 5 and Figure 6), suggesting that one of the key factors by which exogenous AsA improves the photosynthetic performance of tomato seedlings under NaCl stress is by alleviating stomatal limitations induced by salt stress.
AMC is commonly used to estimate RuBPCase activity []. In this experiment, AMC was significantly reduced in seedlings treated with NaCl, indicating a significant inhibition of CO2 assimilation in the dark reaction. NL treatment further reduced AMC, while exogenous AsA inhibited the decrease in AMC under both NaCl and NL treatments, indicating that AsA can enhance the utilization of light energy in tomato seedlings’ leaves, as evidenced by a significant increase in LUEapp (Figure 6). The LPFD mainly reflects the extent of limitation on photosynthetic capacity under stress conditions, while the Rfd is related to the potential CO2 assimilation activity of leaves and is used to reflect the photosynthetic potential of plant leaves. The results of this experiment showed that the LPFD significantly increased and Rfd significantly decreased in tomato seedlings under NaCl treatment. The application of LYC further promoted the increase in LPFD and the decrease in Rfd (Figure 7), indicating that NaCl stress not only damaged PSII but also severely inhibited photosynthetic carbon assimilation and its output and operation, exacerbating damage to the photosynthetic apparatus. Conversely, AsA effectively mitigated the damage to PSII in leaves under both NaCl and NL treatments.
5. Conclusions
This study demonstrates that salt stress significantly reduces the chlorophyll and carotenoid content, photosynthetic pigment precursors, and essential elements in tomato seedlings, ultimately inhibiting photosynthesis (Figure 10). However, the application of exogenous AsA significantly increased the levels of photosynthetic pigment precursors and essential elements, enhanced stomatal aperture and gas exchange efficiency, and improved photosynthetic performance under salt stress. Additionally, AsA alleviated both stomatal and non-stomatal limitations, protected PSII, and increased light energy utilization efficiency. Therefore, AsA plays a crucial role in mitigating the negative effects of salt stress on the photosynthesis of tomato seedlings. However, this study primarily focuses on the short-term effects of exogenous AsA under salt stress. Further research is needed to explore the regulatory mechanisms of AsA under medium- and long-term salt stress conditions, as well as its impact on tomato yield.
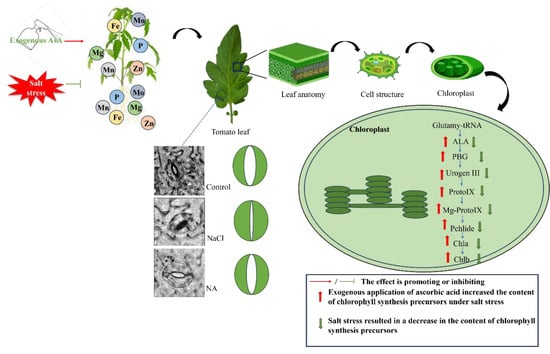
Figure 10.
Mechanism by which exogenous AsA mitigates salt stress effects in tomato seedlings (original).
Author Contributions
This research was a collaborative effort among all authors. H.L. and X.C. conceived and coordinated the study. X.C. designed the methodologies and experiments, conducted the laboratory work, analyzed the data, interpreted the results, and authored the manuscript. Y.J. and Y.C. assisted with the determination of chlorophyll precursors and elemental content and analyzed the data. X.L. assisted with the determination of photosynthetic parameters. Q.Y. assisted with the determination of stomatal density and aperture. J.X. assisted with the determination of photosynthetic pigment content. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Growth of Young Scientific and Technological Talents of Guizhou Educational Commission (Qian Jiaoji [2024]232), the Specialized Fund for the Doctoral of Kaili University (grant No. BS20240218), the Guizhou Provincial Basic Research Program (Natural Science) (No. Qian Ke He Ji Chu2018[1413]) and the Key Laboratory of the Department of Education of Guizhou Province (No. Qianjiaoji [2022] 053).
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Wang, Z.; Hong, Y.; Zhu, G.; Li, Y.; Niu, Q.; Yao, J.; Hua, K.; Bai, J.; Zhu, Y.; Shi, H.; et al. Loss of salt tolerance during tomato domestication conferred by variation in a Na(+)/K(+) transporter. EMBO J. 2020, 39, e103256. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhang, H.; Song, C.; Zhu, J.K.; Shabala, S. Mechanisms of plant responses and adaptation to soil salinity. Innovation 2020, 1, 100017. [Google Scholar] [CrossRef] [PubMed]
- Chele, K.H.; Tinte, M.M.; Piater, L.A.; Dubery, I.A.; Tugizimana, F. Soil Salinity, a Serious Environmental Issue and Plant Responses: A Metabolomics Perspective. Metabolites 2021, 11, 724. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, R.; Sarkar, B.; Jat, H.S.; Sharma, P.C.; Bolan, N.S. Soil salinity under climate change: Challenges for sustainable agriculture and food security. J. Environ. Manag. 2021, 280, 111736. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.; Takeda, S.; Nick, P. Life and death under salt stress: Same players, different timing? J. Exp. Bot. 2024, 65, 2963–2979. [Google Scholar] [CrossRef] [PubMed]
- Tanveer, K.; Gilani, S.; Hussain, Z.; Ishaq, R.; Adeel, M.; Ilyas, N. Effect of salt stress on tomato plant and the role of calcium. J. Plant Nutr. 2019, 43, 28–35. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, F.; He, Z.; Liu, Y.; Chen, Z.; Ottosen, C.-O.; Mittler, R.; Wu, Z.; Zhou, R. Higher Intensity of Salt Stress Accompanied by Heat Inhibits Stomatal Conductance and Induces ROS Accumulation in Tomato Plants. Antioxidants 2024, 13, 448. [Google Scholar] [CrossRef] [PubMed]
- Gharsallah, C.; Fakhfakh, H.; Grubb, D.; Gorsane, F. Effect of salt stress on ion concentration, proline content, antioxidant enzyme activities and gene expression in tomato cultivars. AoB Plants 2016, 8, plw055. [Google Scholar] [CrossRef] [PubMed]
- Naeem, M.; Basit, A.; Ahmad, I.; Mohamed, H.I.; Wasila, H. Effect of Salicylic Acid and Salinity Stress on the Performance of Tomato Plants. Gesunde Pflanz. 2020, 72, 393–402. [Google Scholar] [CrossRef]
- Sudhir, P.; Murthy, S.D. Effects of salt stress on basic processes of photosynthesis. Photosynthetica 2004, 42, 481–486. [Google Scholar] [CrossRef]
- Zahra, N.; Al Hinai, M.S.; Hafeez, M.B.; Rehman, A.; Wahid, A.; Siddique, K.H.; Farooq, M. Regulation of photosynthesis under salt stress and associated tolerance mechanisms. Plant Physiol. Biochem. 2022, 178, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Taïbi, K.; Taïbi, F.; Abderrahim, L.A.; Ennajah, A.; Belkhodja, M.; Mulet, J.M. Effect of salt stress on growth, chlorophyll content, lipid peroxidation and antioxidant defence systems in Phaseolus vulgaris L. S. Afr. J. Bot. 2016, 105, 306–312. [Google Scholar] [CrossRef]
- Geng, R.; Pang, X.; Li, X.; Shi, S.; Hedtke, B.; Grimm, B.; Bock, R.; Huang, J.; Zhou, W. PROGRAMMED CELL DEATH8 interacts with tetrapyrrole biosynthesis enzymes and ClpC1 to maintain homeostasis of tetrapyrrole metabolites in Arabidopsis. New Phytol. 2023, 238, 2545–2560. [Google Scholar] [CrossRef]
- Yang, X.; Han, Y.; Hao, J.; Qin, X.; Liu, C.; Fan, S. Exogenous spermidine enhances the photosynthesis and ultrastructure of lettuce seedlings under high-temperature stress. Sci. Hortic. 2022, 291, 110570. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Z.; Luo, S.; Li, J.; Zhang, J.; Li, L.; Xie, J. 5-Aminolevulinic acid and hydrogen sulphide alleviate chilling stress in pepper (Capsicum annuum L.) seedlings by enhancing chlorophyll synthesis pathway. Plant Physiol. Biochem. 2021, 167, 567–576. [Google Scholar] [CrossRef]
- Santos, C.V. Regulation of chlorophyll biosynthesis and degradation by salt stress in sunflower leaves. Sci. Hortic. 2004, 103, 93–99. [Google Scholar] [CrossRef]
- Gou, T.; Yang, L.; Hu, W.; Chen, X.; Zhu, Y.; Guo, J.; Gong, H. Silicon improves the growth of cucumber under excess nitrate stress by enhancing nitrogen assimilation and chlorophyll synthesis. Plant Physiol. Biochem. 2020, 152, 53–61. [Google Scholar] [CrossRef]
- Kamran, A.; Mushtaq, M.; Arif, M.; Rashid, S. Role of biostimulants (ascorbic acid and fulvic acid) to synergize Rhizobium activity in pea (Pisum sativum L. var. Meteor). Plant Physiol. Biochem. 2023, 196, 668–682. [Google Scholar] [CrossRef]
- Celi GE, A.; Gratão, P.L.; Lanza MG, D.B.; Dos Reis, A.R. Physiological and biochemical roles of ascorbic acid on mitigation of abiotic stresses in plants. Plant Physiol. Biochem. 2023, 202, 107970. [Google Scholar] [CrossRef] [PubMed]
- Ghahremani, Z.; Mikaealzadeh, M.; Barzegar, T. ME Ranjbar. Foliar Application of Ascorbic Acid and Gamma Aminobutyric Acid Can Improve Important Properties of Deficit Irrigated Cucumber Plants (Cucumis sativus cv. Us). Gesunde Pflanz. 2021, 73, 77–84. [Google Scholar] [CrossRef]
- Kakan, X.; Yu, Y.; Li, S.; Li, X.; Wang, J. Ascorbic Acid Modulation by ABI4 Transcriptional Repression of VTC2 in the Salt Tolerance of Arabidopsis. BMC Plant Biol 2021, 21, 112. [Google Scholar] [CrossRef]
- Kanwal, R.; Maqsood, M.F.; Shahbaz, M.; Naz, N.; Zulfiqar, U.; Muhammad, F.A.; Jamil, M.; Khalid, F.; Qasim, A.; Muhammad, A.S.; et al. Exogenous ascorbic acid as a potent regulator of antioxidants, osmo-protectants, and lipid peroxidation in pea under salt stress. BMC Plant Biol. 2024, 24, 1–16. [Google Scholar] [CrossRef]
- Mano, J.; Hideg, É.; Asada, K. Ascorbate in Thylakoid Lumen Functions as an Alternative Electron Donor to Photosystem II and Photosystem I. Arch. Biochem. Biophys. 2004, 429, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, B.N. Role of Ascorbic Acid in Photosynthesis. Biochem. Mosc. 2014, 79, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Alegre, M.L.; Steelheart, C.; Baldet, P.; Rothan, C.; Just, D.; Okabe, Y.; Ezura, H.; Smirnoff, N.; Gergoff Grozeff, G.E.; Bartoli, C.G. Deficiency of GDP-l-Galactose Phosphorylase, an Enzyme Required for Ascorbic Acid Synthesis, Reduces Tomato Fruit Yield. Planta 2020, 251, 54. [Google Scholar] [CrossRef]
- Chen, X.; Han, H.; Cong, Y.; Li, X.; Zhang, W.; Cui, J.; Xu, W.; Pang, S.; Liu, H. Ascorbic Acid Improves Tomato Salt Tolerance by Regulating Ion Homeostasis and Proline Synthesis. Plants 2024, 13, 1672. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Han, H.; Cong, Y.; Li, X.; Zhang, W.; Wan, W.; Cui, J.; Xu, W.; Diao, M.; Liu, H. The Protective Effect of Exogenous Ascorbic Acid on Photosystem Inhibition of Tomato Seedlings Induced by Salt Stress. Plants 2023, 12, 1379. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhou, Y.; Cong, Y.; Zhu, P.; Xing, J.; Cui, J.; Xu, W.; Shi, Q.; Diao, M.; Liu, H.-Y. Ascorbic Acid-Induced Photosynthetic Adaptability of Processing Tomatoes to Salt Stress Probed by Fast OJIP Fluorescence Rise. Front. Plant Sci 2021, 12, 594400. [Google Scholar] [CrossRef]
- Moncada, A.; Vetrano, F.; Miceli, A. Alleviation of Salt Stress by Plant Growth-Promoting Bacteria in Hydroponic Leaf Lettuce. Agronomy 2020, 10, 1523. [Google Scholar] [CrossRef]
- Torres Mendonça, A.J.; Silva, A.A.R.D.; Lima, G.S.D.; Soares, L.A.D.A.; Nunes Oliveira, V.K.; Gheyi, H.R.; Lacerda, C.F.D.; Azevedo, C.A.V.D.; Lima, V.L.A.D.; Fernandes, P.D. Salicylic Acid Modulates Okra Tolerance to Salt Stress in Hydroponic System. Agriculture 2022, 12, 1687. [Google Scholar] [CrossRef]
- Hodgins, R.R.; Huystee, R. Rapid simultaneous estimation of protoporphyrin and Mg-porphyrins in higher plants. J. Plant Physiol. 1986, 125, 311–323. [Google Scholar] [CrossRef]
- Bogorad, L. Porphyrin synthesis. Methods Enzym. 1962, 5, 885–895. [Google Scholar] [CrossRef]
- Richard, A.T. Biochemical Spectroscopy; Adam Hilger Ltd.: London, UK, 1975; pp. 327–330. [Google Scholar]
- Wellburn, A.R.; Lichtenthaler, H. Formulae and Program to Determine Total Carotenoids and Chlorophylls A and B of Leaf Extracts in Different Solvents. Springer Neth. 1984, 2, 9–12. [Google Scholar] [CrossRef]
- Berry, J.; Bjorkman, O. Photosynthetic Response and Adaptation to Temperature in Higher Plants. Annu. Rev. Plant Physiol. 1980, 31, 491–543. [Google Scholar] [CrossRef]
- Ashraf, M. Relationships between leaf gas exchange characteristics and growth of differently adapted populations of Blue panicgrass (Panicum antidotale Retz.) under salinity or waterlogging. Plant Sci. 2003, 165, 69–75. [Google Scholar] [CrossRef]
- Nijs, I.; Ferris, R.; Blum, H.; Hendrey, G.; Impens, I. Stomatal regulation in a changing climate: A field study using Free Air Temperature Increase (FATI) and Free Air CO2 Enrichment (FACE). Plant Cell Environ. 1997, 20, 1041–1050. [Google Scholar] [CrossRef]
- Long, S.P.; Baker, N.R.; Raines, C.A. Analysing the responses of photosynthetic CO2 assimilation to long-term elevation of atmospheric CO2 concentration. CO2 Biosph. 1993, 14, 33–46. [Google Scholar] [CrossRef]
- Inamullah Isoda, A. Adaptive Responses of Soybean and Cotton to Water Stress: I. Transpiration Changes in Relation to Stomatal Area and Stomatal Conductance. Plant Prod. Sci. 2005, 8, 16–26. [Google Scholar] [CrossRef]
- Wise, R.R.; Sassenrath-Cole, G.F.; Percy, R.G. A Comparison of Leaf Anatomy in Field-grown Gossypium hirsutum and G. barbadense. Ann. Bot. 2000, 86, 731–738. [Google Scholar] [CrossRef]
- Perera-Castro, A.V.; Brito, P.; González-Rodríguez, A.M. Changes in thermic limits and acclimation assessment for an alpine plant by chlorophyll fluorescence analysis: Fv/Fmvs. Rfd. Photosynthetica 2018, 56, 527–536. [Google Scholar] [CrossRef]
- Yang, Y.; Nan, R.; Mi, T.; Song, Y.; Shi, F.; Liu, X.; Wang, Y.; Sun, F.; Xi, Y.; Zhang, C. Rapid and Nondestructive Evaluation of Wheat Chlorophyll under Drought Stress Using Hyperspectral Imaging. Int. J. Mol. Sci 2023, 24, 5825. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Shree, B.; Khurana, P. Myo-inositol phosphate synthase improves heat stress tolerance by ethylene-mediated modulation of chlorophyll content and photosynthetic efficiency. Protoplasma 2023, 260, 1097–1107. [Google Scholar] [CrossRef] [PubMed]
- Azeem, A.; Wu, Y.; Xing, D.; Javed, Q.; Ullah, I. Photosynthetic response of two okra cultivars under salt stress and re-watering. J. Plant Interact. 2017, 12, 67–77. [Google Scholar] [CrossRef]
- Terletskaya, N.; Zobova, N.; Stupko, V.; Shuyskaya, E. Growth and photosynthetic reactions of different species of wheat seedlings under drought and salt stress. Period. Biol. 2017, 119, 37–45. [Google Scholar] [CrossRef]
- Porra, R.J. Recent progress in porphyrin and chlorophyll biosynthesis. Photochem. Photobiol. 1997, 65, 492–516. [Google Scholar] [CrossRef]
- Hand, M.J.; Taffouo, V.D.; Nouck, A.E.; Nyemene, K.P.; Tonfack, B.; Meguekam, T.L.; Youmbi, E. Effects of salt stress on plant growth, nutrient partitioning, chlorophyll content, leaf relative water content, accumulation of osmolytes and antioxidant compounds in pepper (Capsicum annuum L.) cultivars. Not. Bot. Horti Agrobot. 2017, 45, 481–490. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, H.; Feng, N.; Zheng, D.; Ma, G.; Feng, S.; Huang, A. Physiological and transcriptome analysis reveals that prohexadione-calcium promotes rice seedling’s development under salt stress by regulating antioxidant processes and photosynthesis. PLoS ONE 2023, 18, e0286505. [Google Scholar] [CrossRef] [PubMed]
- Gerendás, J.; Führs, H. The significance of magnesium for crop quality. Plant Soil 2013, 368, 101–128. [Google Scholar] [CrossRef]
- Santra, B.; Singh, K.; Dawson, J. Effect of zinc and magnesium on growth and yield of maize (Zea mays L.). Int. J. Res. Agron. 2024, 7, 76–78. [Google Scholar] [CrossRef]
- Spiller, S.; Terry, N. Limiting factors in photosynthesis: II. Iron stress diminishes photochemical capacity by reducing the number of photosynthetic units. Plant Physiol. 1980, 65, 121–125. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Plant Physiology, 5th ed.; Sinauer Associates Inc.: Sunderland, MA, USA, 2010. [Google Scholar]
- Broadley, M.R.; White, P.J.; Hammond, J.P.; Zelko, I.; Lux, A. Zinc in plants. New Phytol. 2007, 173, 677–702. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Hu, C.X.; Wang, Y.H. Effects of molybdenum on the precur-sors of chlorophyll biosynthesis in winter wheat cultivars under low temperature. Sci. Agric. Sin. 2006, 39, 702–708. [Google Scholar]
- Hong, L.; Fei, W.; Wang, Y.; Lin, R.; Wang, Z.; Chuanzao, M. Molecular mechanisms and genetic improvement of low-phosphorus tolerance in rice. Plant Cell Environ. 2023, 46, 1104–1119. [Google Scholar] [CrossRef]
- Niu, J.; Chen, Z.; Guo, Z.; Xu, N.; Sui, X.; Roy, M.; Kareem, H.A.; Hassan, M.U.; Cui, J.; Wang, Q. Exogenous melatonin promotes the growth of alfalfa (Medicago sativa L.) under NaCl stress through multiple pathways. Ecotoxicol. Environ. Saf. 2022, 242, 113938. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-H.; Deng, Y.-J.; Wang, Y.-H.; Lou, Y.-R.; He, L.-F.; Liu, H.; Li, T.; Yan, Z.-M.; Zhuang, J.; Xiong, A.-S. Changes in Carotenoid Concentration and Expression of Carotenoid Biosynthesis Genes in Daucus carota Taproots in Response to Increased Salinity. Horticulturae 2022, 8, 650. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Sharkey, T.D. Stomatal Conductance and Photosynthesis. Annu. Rev. Plant Physiol. 1982, 33, 317–345. [Google Scholar] [CrossRef]
- Zheng, Y.; Xu, M.; Hou, R.; Shen, R.; Qiu, S.; Ouyang, Z. Effects of experimental warming on stomatal traits in leaves of maize (Zea may L.). Ecol. Evol. 2013, 3, 3095–3111. [Google Scholar] [CrossRef]
- Larkin, J.C.; Marks, M.D.; Nadeau, J.; Sack, F. Epidermal cell fate and patterning in leaves. Plant Cell 1997, 9, 1109–1120. [Google Scholar] [CrossRef] [PubMed]
- Gan, Y.; Zhou, L.; Shen, Z.J.; Shen, Z.X.; Zhang, Y.Q.; Wang, G.X. Stomatal clustering, a new marker for environmental perception and adaptation in terrestrial plants. Bot. Stud. 2010, 51, 325–336. [Google Scholar] [CrossRef]
- Xu, Z.Z.; Zhou, G.S. Effects of water stress and high nocturnal temperature on photosynthesis and nitrogen level of a perennial grass Leymus chinensis. Plant Soil 2005, 269, 131–139. [Google Scholar] [CrossRef]
- Gentilesca, T.; Battipaglia, G.; Borghetti, M.; Colangelo, M.; Altieri, S.; Ferrara, A.M.; Ripullone, F. Evaluating growth and intrinsic water-use efficiency in hardwood and conifer mixed plantations. Trees 2021, 35, 1329–1340. [Google Scholar] [CrossRef]
- Hou, J.; Liu, X.; Zhang, J.; Wei, Z.; Ma, Y.; Wan, H.; Liu, F. Combined application of biochar and partial root-zone drying irrigation improves water relations and water use efficiency of cotton plants under salt stress. Agric. Water Manag. 2023, 290, 108584. [Google Scholar] [CrossRef]
- Chu, X.; Wang, C.; Chen, X.; Lu, W.; Li, H.; Wang, X.; Guo, X. The Cotton WRKY Gene GhWRKY41 Positively Regulates Salt and Drought Stress Tolerance in Transgenic Nicotiana benthamiana. PLoS ONE 2015, 10, e0143022. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Gallie, D.R. The Ascorbic Acid Redox State Controls Guard Cell Signaling and Stomatal Movement. Plant Cell 2004, 16, 1143–1162. [Google Scholar] [CrossRef] [PubMed]
- Loreto, F.; Centritto, M.; Chartzoulakis, K. Photosynthetic limitations in olive cultivars with different sensitivity to salt stress. Plant Cell Environ. 2003, 26, 595–601. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).