Zinc Seed Priming Alleviates Salinity Stress and Enhances Sorghum Growth by Regulating Antioxidant Activities, Nutrient Homeostasis, and Osmolyte Synthesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Details
2.2. Germination and Growth Parameters
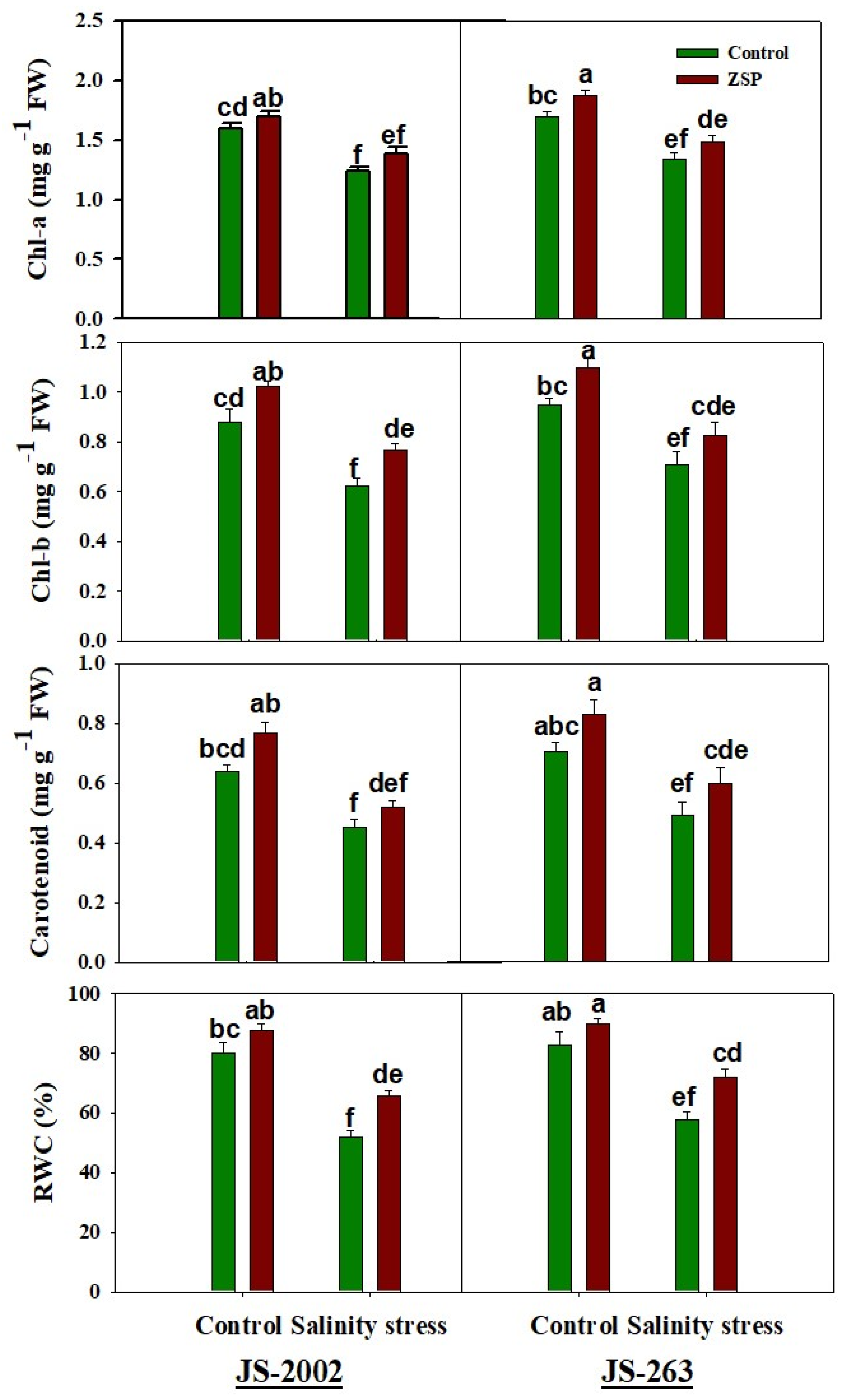
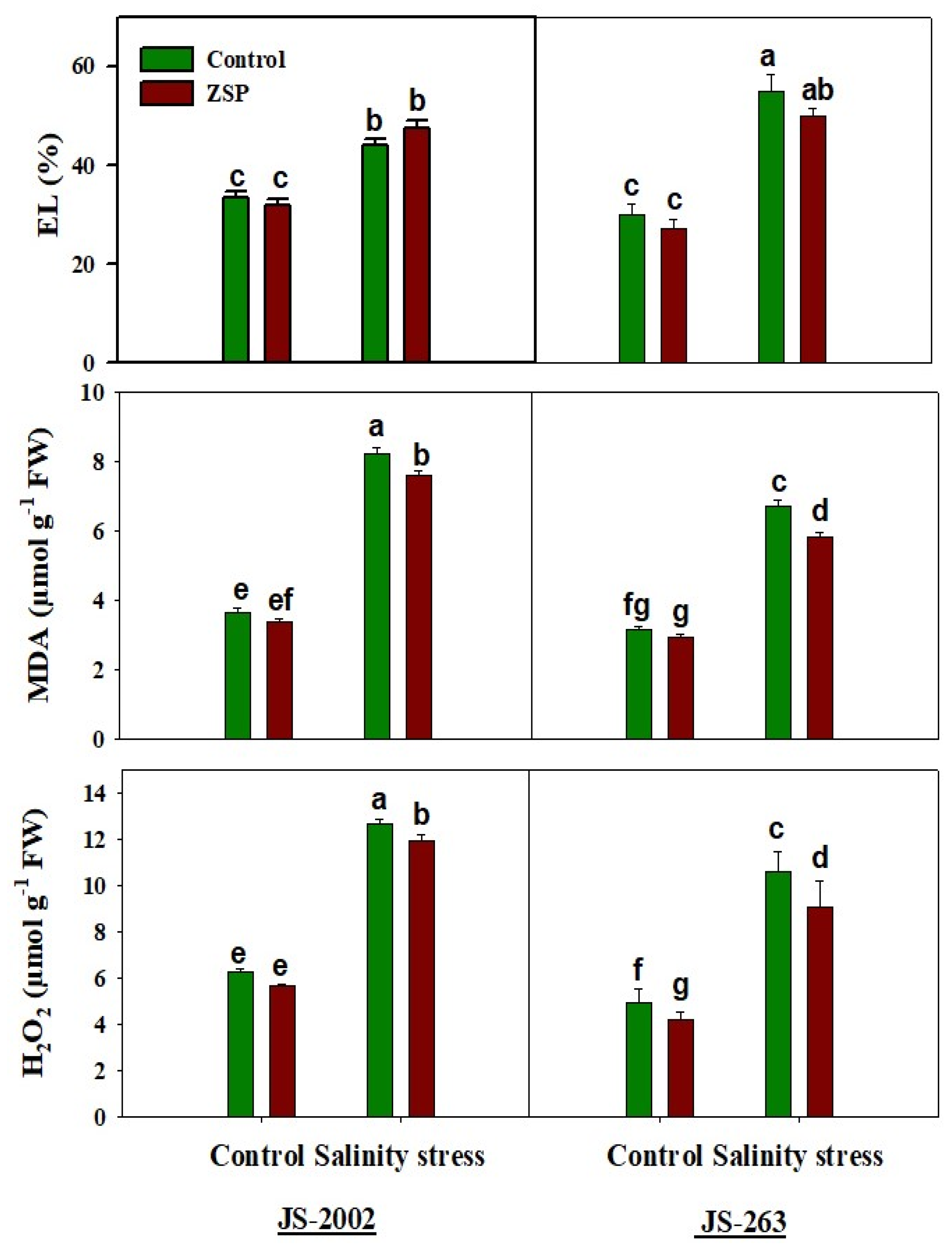
2.3. Relative Water Contents, Oxidative Stress Markers, and Photosynthetic Pigments
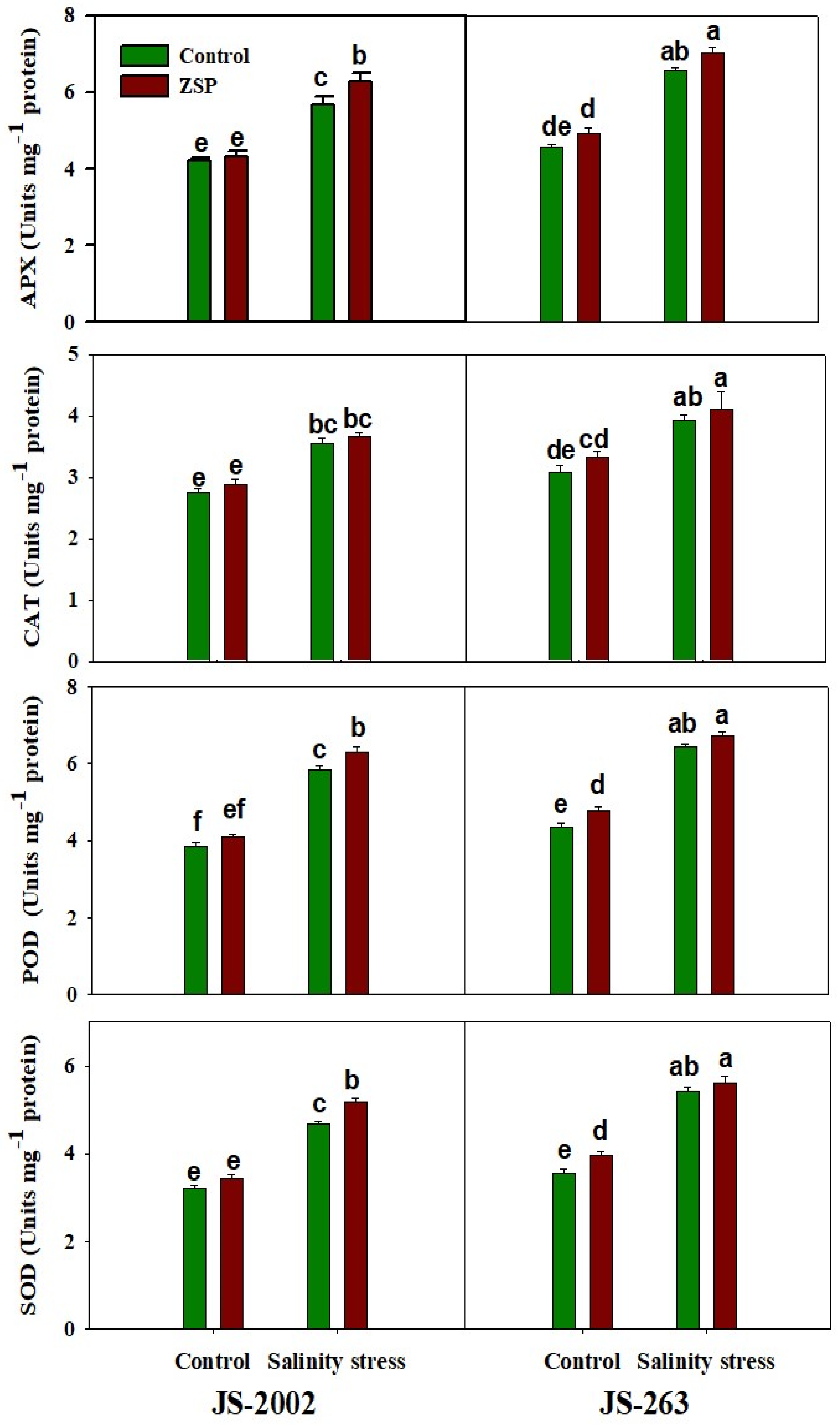
2.4. Antioxidant Enzymes
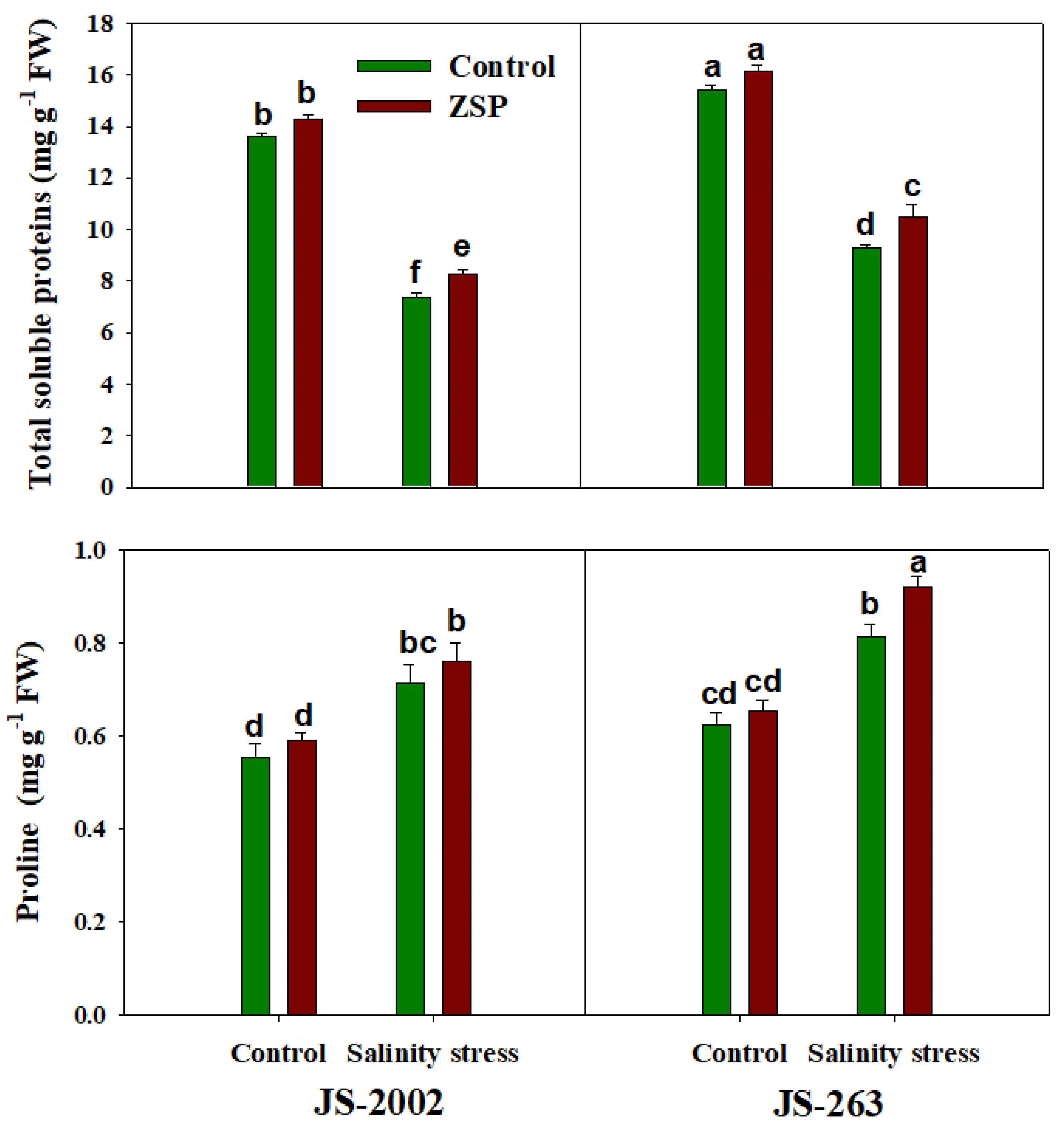
2.5. Determination of Potential Osmolytes and Ions Concentration
2.6. Data Analysis
3. Results
3.1. Germination and Growth Traits
3.2. Photosynthetic Pigments
3.3. Oxidative Stress Markers and Potential Osmolyte
3.4. Antioxidant Activities
3.5. Nutrient Accumulation in Plants
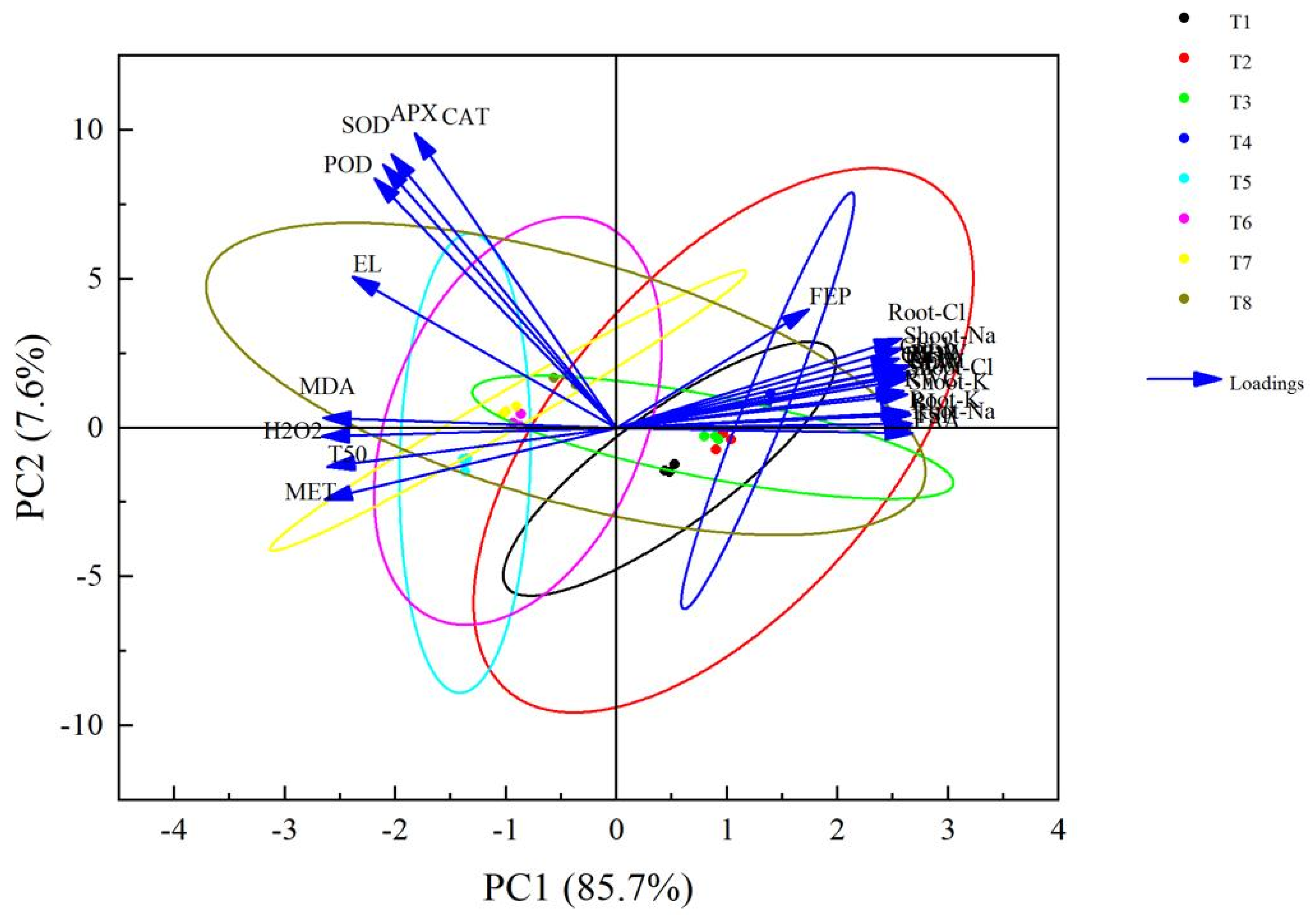
3.6. Principal Component Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Hualpa-Ramirez, E.; Carrasco-Lozano, E.C.; Madrid-Espinoza, J.; Tejos, R.; Ruiz-Lara, S.; Stange, C.; Norambuena, L. Stress salinity in plants: New strategies to cope with in the foreseeable scenario. Plant Physiol. Biochem. 2024, 208, 108507. [Google Scholar] [CrossRef]
- Ingrao, C.; Strippoli, R.; Lagioia, G.; Huisingh, D. Water scarcity in agriculture: An overview of causes, impacts and approaches for reducing the risks. Heliyon 2023, 9, e18507. [Google Scholar] [CrossRef] [PubMed]
- Kopecká, R.; Kameniarová, M.; Černý, M.; Brzobohatý, B.; Novák, J. Abiotic stress in crop production. Int. J. Mol. Sci. 2023, 24, 6603. [Google Scholar] [CrossRef]
- Atta, K.; Mondal, S.; Gorai, S.; Singh, A.P.; Kumari, A.; Ghosh, T.; Roy, A.; Hembram, S.; Gaikwad, D.J.; Mondal, S. Impacts of salinity stress on crop plants: Improving salt tolerance through genetic and molecular dissection. Front. Plant Sci. 2023, 14, 1241736. [Google Scholar] [CrossRef] [PubMed]
- Okur, B.; Örçen, N. Soil salinization and climate change. In Climate Change and Soil Interactions; Elsevier: Amsterdam, The Netherlands, 2020; pp. 331–350. [Google Scholar]
- Arora, S. Nutrient management strategies for enhancing use efficiency and crop growth in salt affected soils. In Proceedings of the Souvenir, National Seminar on “Recent Developments in Nutrient Management Strategies for Sustainable Agriculture: The Indian Context, Bhagalpur, India, 18–19 June 2022. [Google Scholar]
- Liaqa, K.; Shakeel, A.; Khalid, M.N.; Amjad, I.; Saeed, A. Assessment of tomato accessions for various seedling attributes under NaCl salt stress. Int. J. Agric. Biosci. 2023, 12, 116–121. [Google Scholar]
- Khan, I.; Muhammad, A.; Chattha, M.U.; Skalicky, M.; Bilal Chattha, M.; Ahsin Ayub, M.; Rizwan Anwar, M.; Soufan, W.; Hassan, M.U.; Rahman, M.A. Mitigation of salinity-induced oxidative damage, growth, and yield reduction in fine rice by sugarcane press mud application. Front. Plant Sci. 2022, 13, 840900. [Google Scholar] [CrossRef]
- Mondal, S.; Chakraborty, K. Brassicaceae plants response and tolerance to salinity. In The Plant Family Brassicaceae: Biology and Physiological Responses to Environmental Stresses; Springer: Berlin/Heidelberg, Germany, 2020; pp. 203–228. [Google Scholar]
- Zahra, N.; Al Hinai, M.S.; Hafeez, M.B.; Rehman, A.; Wahid, A.; Siddique, K.H.; Farooq, M. Regulation of photosynthesis under salt stress and associated tolerance mechanisms. Plant Physiol. Biochem. 2022, 178, 55–69. [Google Scholar] [CrossRef]
- Seifikalhor, M.; Aliniaeifard, S.; Shomali, A.; Azad, N.; Hassani, B.; Lastochkina, O.; Li, T. Calcium signaling and salt tolerance are diversely entwined in plants. Plant Signal. Behav. 2019, 14, 1665455. [Google Scholar] [CrossRef]
- Yan, S.; Chong, P.; Zhao, M. Effect of salt stress on the photosynthetic characteristics and endogenous hormones, and: A comprehensive evaluation of salt tolerance in Reaumuria soongorica seedlings. Plant Signal. Behav. 2022, 17, 2031782. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; He, P.; Guo, R.; Huang, K.; Huang, X. Effects of salt stress on root morphology, carbon and nitrogen metabolism, and yield of Tartary buckwheat. Sci. Rep. 2023, 13, 12483. [Google Scholar] [CrossRef] [PubMed]
- Blumwald, E. Sodium transport and salt tolerance in plants. Curr. Opin. Cell Biol. 2000, 12, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Kim, W.-Y.; Yun, D.-J. A New Insight of Salt Stress Signalingin Plant. Mol. Cell 2016, 39, 447–459. [Google Scholar] [CrossRef]
- Shumilina, J.; Kusnetsova, A.; Tsarev, A.; Janse van Rensburg, H.C.; Medvedev, S.; Demidchik, V.; Van den Ende, W.; Frolov, A. Glycation of plant proteins: Regulatory roles and interplay with sugar signalling? Int. J. Mol. Sci. 2019, 20, 2366. [Google Scholar] [CrossRef]
- Fatemi, H.; Carvajal, M.; Rios, J.J. Foliar application of Zn alleviates salt stress symptoms of pak choi plants by activating water relations and glucosinolate synthesis. Agronomy 2020, 10, 1528. [Google Scholar] [CrossRef]
- Siddiqui, H.; Singh, P.; Arif, Y.; Sami, F.; Naaz, R.; Hayat, S. Role of micronutrients in providing abiotic stress tolerance. In Microbial Biofertilizers and Micronutrient Availability: The Role of Zinc in Agriculture and Human Health; Springer: Berlin/Heidelberg, Germany, 2022; pp. 115–136. [Google Scholar]
- Umair Hassan, M.; Aamer, M.; Umer Chattha, M.; Haiying, T.; Shahzad, B.; Barbanti, L.; Nawaz, M.; Rasheed, A.; Afzal, A.; Liu, Y. The critical role of zinc in plants facing the drought stress. Agriculture 2020, 10, 396. [Google Scholar] [CrossRef]
- Shao, J.; Tang, W.; Huang, K.; Ding, C.; Wang, H.; Zhang, W.; Li, R.; Aamer, M.; Hassan, M.U.; Elnour, R.O. How does zinc improve salinity tolerance? Mechanisms and future prospects. Plants 2023, 12, 3207. [Google Scholar] [CrossRef]
- Cakmak, I.; Brown, P.; Colmenero-Flores, J.M.; Husted, S.; Kutman, B.Y.; Nikolic, M.; Rengel, Z.; Schmidt, S.B.; Zhao, F.-J. Micronutrients. In Marschner’s Mineral Nutrition of Plants; Elsevier: Amsterdam, The Netherlands, 2023; pp. 283–385. [Google Scholar]
- Swaefy, H.M.; Abdallh, A.M. Mitigation of salinity stress in fenugreek plants using zinc oxide nanoparticles and zinc sulfate. J. Plant Prod. 2021, 12, 1367–1374. [Google Scholar] [CrossRef]
- Riyazuddin, R.; Verma, R.; Singh, K.; Nisha, N.; Keisham, M.; Bhati, K.K.; Kim, S.T.; Gupta, R. Ethylene: A master regulator of salinity stress tolerance in plants. Biomolecules 2020, 10, 959. [Google Scholar] [CrossRef]
- Shah, T.; Latif, S.; Saeed, F.; Ali, I.; Ullah, S.; Alsahli, A.A.; Jan, S.; Ahmad, P. Seed priming with titanium dioxide nanoparticles enhances seed vigor, leaf water status, and antioxidant enzyme activities in maize (Zea mays L.) under salinity stress. J. King Saud Univ. Sci. 2021, 33, 101207. [Google Scholar] [CrossRef]
- Tlahig, S.; Bellani, L.; Karmous, I.; Barbieri, F.; Loumerem, M.; Muccifora, S. Response to salinity in legume species: An insight on the effects of salt stress during seed germination and seedling growth. Chem. Biodiver. 2021, 18, e2000917. [Google Scholar] [CrossRef] [PubMed]
- Hezaveh, T.A.; Pourakbar, L.; Rahmani, F.; Alipour, H. Interactive effects of salinity and ZnO nanoparticles on physiological and molecular parameters of rapeseed (Brassica napus L.). Commun. Soil Sci. Plant Anal. 2019, 50, 698–715. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Ali, B.; Adrees, M.; Arshad, M.; Hussain, A.; ur Rehman, M.Z.; Waris, A.A. Zinc and iron oxide nanoparticles improved the plant growth and reduced the oxidative stress and cadmium concentration in wheat. Chemosphere 2019, 214, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Alyemeni, M.N.; Al-Huqail, A.A.; Alqahtani, M.A.; Wijaya, L.; Ashraf, M.; Kaya, C.; Bajguz, A. Zinc oxide nanoparticles application alleviates arsenic (As) toxicity in soybean plants by restricting the uptake of as and modulating key biochemical attributes, antioxidant enzymes, ascorbate-glutathione cycle and glyoxalase system. Plants 2020, 9, 825. [Google Scholar] [CrossRef]
- El-Badri, A.M.; Batool, M.; Mohamed, I.A.; Khatab, A.; Sherif, A.; Wang, Z.; Salah, A.; Nishawy, E.; Ayaad, M.; Kuai, J. Modulation of salinity impact on early seedling stage via nano-priming application of zinc oxide on rapeseed (Brassica napus L.). Plant Physiol. Biochem. 2021, 166, 376–392. [Google Scholar] [CrossRef]
- Banerjee, A.; Singh, A.; Sudarshan, M.; Roychoudhury, A. Silicon nanoparticle-pulsing mitigates fluoride stress in rice by fine-tuning the ionomic and metabolomic balance and refining agronomic traits. Chemosphere 2021, 262, 127826. [Google Scholar] [CrossRef]
- Dobrikova, A.; Apostolova, E.; Hanć, A.; Yotsova, E.; Borisova, P.; Sperdouli, I.; Adamakis, I.-D.S.; Moustakas, M. Tolerance mechanisms of the aromatic and medicinal plant Salvia sclarea L. to excess zinc. Plants 2021, 10, 194. [Google Scholar] [CrossRef] [PubMed]
- Marsalis, M.; Angadi, S.; Contreras-Govea, F. Dry matter yield and nutritive value of corn, forage sorghum, and BMR forage sorghum at different plant populations and nitrogen rates. Field Crops Res. 2010, 116, 52–57. [Google Scholar] [CrossRef]
- Iqbal, M.A. Agronomic management strategies elevate forage sorghum yield: A Review. J. Adv. Bot. Zoo. 2015, 3, 1–6. [Google Scholar]
- Krishnamurthy, L.; Serraj, R.; Hash, C.T.; Dakheel, A.J.; Reddy, B.V. Screening sorghum genotypes for salinity tolerant biomass production. Euphytica 2007, 156, 15–24. [Google Scholar] [CrossRef]
- Rajabi Dehnavi, A.; Zahedi, M.; Ludwiczak, A.; Cardenas Perez, S.; Piernik, A. Effect of salinity on seed germination and seedling development of sorghum (Sorghum bicolor (L.) Moench) genotypes. Agronomy 2020, 10, 859. [Google Scholar] [CrossRef]
- Hassan, M.U.; Chattha, M.U.; Khan, I.; Nawaz, M.; Ullah, M.A.; Aamer, M. influence of different levels of salinity stress on germination and growth attributes of sorghum cultivars. J. Adv. Bot. Zool. 2017, 5, 1–3. [Google Scholar]
- Atar, B.; Uygur, V.; Sukuşu, E. Effects of priming with copper, zinc and phosphorus on seed and seedling composition in wheat and barley. Turk. J. Agric. Nat. Sci. 2020, 7, 104–111. [Google Scholar] [CrossRef]
- Ellis, R.; Roberts, E. The quantification of ageing and survival in orthodox seeds. Seed Sci. Technol. 1981, 9. [Google Scholar]
- Mostofa, M.G.; Fujita, M. Salicylic acid alleviates copper toxicity in rice (Oryza sativa L.) seedlings by up-regulating antioxidative and glyoxalase systems. Ecotoxicology 2013, 22, 959–973. [Google Scholar] [CrossRef]
- Rao, K.M.; Sresty, T. Antioxidative parameters in the seedlings of pigeonpea (Cajanus cajan (L.) Millspaugh) in response to Zn and Ni stresses. Plant Sci. 2000, 157, 113–128. [Google Scholar]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. In Methods in Enzymology; Academic Press: New York, NY, USA, 1987; Volume 148, pp. 350–382. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Aebi, H. [13] Catalase in vitro. In Methods in Enzymology; Academic Press: New York, NY, USA, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Chance, B.; Maehly, A. Assay of catalase and peroxidase in. In Methods of Enzymology; Colowick, S.P., Kaplar, N.O., Eds.; Academic Press: New York, NY, USA, 1955; Volume 2, p. 764. [Google Scholar]
- Zhang, X. The measurement and mechanism of lipid peroxidation and SOD, POD and CAT activities in biological system. Res. Methodol. Crop Hysiol. 1991, 15, 208–211. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Steel, R.G.; Torrie, J.H.; Dickey, D.A. Principles and Procedures of Statistics: A Biometrical Approach; McGraw-Hill Kogakusha: Tokyo, Japan, 1997. [Google Scholar]
- Lv, B.S.; Li, X.W.; Ma, H.Y.; Sun, Y.; Wei, L.X.; Jiang, C.J.; Liang, Z.W. Differences in growth and physiology of rice in response to different saline-alkaline stress factors. Agron. J. 2013, 105, 1119–1128. [Google Scholar] [CrossRef]
- AbdElgawad, H.; Zinta, G.; Hegab, M.M.; Pandey, R.; Asard, H.; Abuelsoud, W. High salinity induces different oxidative stress and antioxidant responses in maize seedlings organs. Front. Plant Sci. 2016, 7, 276. [Google Scholar] [CrossRef] [PubMed]
- Fatma, M.; Iqbal, N.; Gautam, H.; Sehar, Z.; Sofo, A.; D’Ippolito, I.; Khan, N.A. Ethylene and sulfur coordinately modulate the antioxidant system and ABA accumulation in mustard plants under salt stress. Plants 2021, 10, 180. [Google Scholar] [CrossRef] [PubMed]
- Zulfiqar, F.; Ashraf, M. Nanoparticles potentially mediate salt stress tolerance in plants. Plant Physiol. Biochem. 2021, 160, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Zhou, Y.; Ma, C.; Feng, Y.; Hao, Y.; Rui, Y.; Wu, W.; Gui, X.; Han, Y.; Wang, Y. Jointed toxicity of TiO2 NPs and Cd to rice seedlings: NPs alleviated Cd toxicity and Cd promoted NPs uptake. Plant Physiol. Biochem. 2017, 110, 82–93. [Google Scholar] [CrossRef]
- Faizan, M.; Bhat, J.A.; Hessini, K.; Yu, F.; Ahmad, P. Zinc oxide nanoparticles alleviates the adverse effects of cadmium stress on Oryza sativa via modulation of the photosynthesis and antioxidant defense system. Ecotoxicol. Environ. Saf. 2021, 220, 112401. [Google Scholar] [CrossRef] [PubMed]
- Farouk, S.; Al-Amri, S.M. Exogenous zinc forms counteract NaCl-induced damage by regulating the antioxidant system, osmotic adjustment substances, and ions in canola (Brassica napus L. cv. Pactol) plants. J. Soil Sci. Plant Nutr. 2019, 19, 887–899. [Google Scholar] [CrossRef]
- Guo, Q.; Liu, L.; Barkla, B.J. Membrane lipid remodeling in response to salinity. Int. J. Mol. Sci. 2019, 20, 4264. [Google Scholar] [CrossRef]
- Almeida, D.M.; Oliveira, M.M.; Saibo, N.J. Regulation of Na+ and K+ homeostasis in plants: Towards improved salt stress tolerance in crop plants. Genet. Mol. Biol. 2017, 40, 326–345. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Al-Selwey, W.A.; Ibrahim, A.A.; Shady, M.; Alsadon, A.A. Foliar applications of ZnO and SiO2 nanoparticles mitigate water deficit and enhance potato yield and quality traits. Agronomy 2023, 13, 466. [Google Scholar] [CrossRef]
- Cakmak, I. Enrichment of cereal grains with zinc: Agronomic or genetic biofortification? Plant Soil 2008, 302, 1–17. [Google Scholar] [CrossRef]
- Yang, Z.; Li, J.L.; Liu, L.N.; Xie, Q.; Sui, N. Photosynthetic regulation under salt stress and salt-tolerance mechanism of sweet sorghum. Front. Plant Sci. 2020, 10, 1722. [Google Scholar] [CrossRef] [PubMed]
- Latef, A.; Alhmad, M.; Sallam, M. Comparative study on the physiological response of two wheat cultivars to salinity stress. Assiut. Univ. J. Bot 2014, 43, 55–69. [Google Scholar]
- Sturikova, H.; Krystofova, O.; Huska, D.; Adam, V. Zinc, zinc nanoparticles and plants. J. Hazard. Mater. 2018, 349, 101–110. [Google Scholar] [CrossRef]
- Venkatachalam, P.; Priyanka, N.; Manikandan, K.; Ganeshbabu, I.; Indiraarulselvi, P.; Geetha, N.; Muralikrishna, K.; Bhattacharya, R.; Tiwari, M.; Sharma, N. Enhanced plant growth promoting role of phycomolecules coated zinc oxide nanoparticles with P supplementation in cotton (Gossypium hirsutum L.). Plant Physiol. Biochem. 2017, 110, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Alabdallah, N.M.; Alzahrani, H.S. Impact of ZnO nanoparticles on growth of cowpea and okra plants under salt stress conditions. Biosci. Biotechnol. Res. Asia 2020, 17, 329–340. [Google Scholar] [CrossRef]
- Salama, D.M.; Osman, S.A.; Abd El-Aziz, M.; Abd Elwahed, M.S.; Shaaban, E. Effect of zinc oxide nanoparticles on the growth, genomic DNA, production and the quality of common dry bean (Phaseolus vulgaris). Biocatal. Agric. Biotechnol. 2019, 18, 101083. [Google Scholar] [CrossRef]
- Hussein, M.; Embiale, A.; Husen, A.; Aref, I.M.; Iqbal, M. Salinity-induced modulation of plant growth and photosynthetic parameters in faba bean (Vicia faba) cultivars. Pak. J. Bot. 2017, 49, 867–877. [Google Scholar]
- Mushtaq, N.U.; Alghamdi, K.M.; Saleem, S.; Tahir, I.; Bahieldin, A.; Henrissat, B.; Alghamdi, M.K.; Rehman, R.U.; Hakeem, K.R. Exogenous zinc mitigates salinity stress by stimulating proline metabolism in proso millet (Panicum miliaceum L.). Front. Plant Sci. 2023, 14, 1053869. [Google Scholar] [CrossRef]
- Beyaz, R.; Kır, H. Physio-biochemical analyses in seedlings of sorghum-sudangrass hybrids that are grown under salt stress under in vitro conditions. Turk. J. Biochem. 2020, 45, 177–184. [Google Scholar] [CrossRef]
- Beyaz, R. Comparison of biochemical responses of common vetch (Vicia sativa L.) seedling organs to salinity. Leg. Res. Intern. J. 2021, 44, 641–645. [Google Scholar] [CrossRef]
- El-Esawi, M.A.; Alaraidh, I.A.; Alsahli, A.A.; Alzahrani, S.M.; Ali, H.M.; Alayafi, A.A.; Ahmad, M. Serratia liquefaciens KM4 improves salt stress tolerance in maize by regulating redox potential, ion homeostasis, leaf gas exchange and stress-related gene expression. Int. J. Mol. Sci. 2018, 19, 3310. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Ali, S.; ur Rehman, M.Z.; Adrees, M.; Arshad, M.; Qayyum, M.F.; Ali, L.; Hussain, A.; Chatha, S.A.S.; Imran, M. Alleviation of cadmium accumulation in maize (Zea mays L.) by foliar spray of zinc oxide nanoparticles and biochar to contaminated soil. Environ. Pollut. 2019, 248, 358–367. [Google Scholar] [CrossRef]
- Rameshraddy; Pavithra, G.; Rajashekar Reddy, B.; Salimath, M.; Geetha, K.; Shankar, A. Zinc oxide nano particles increases Zn uptake, translocation in rice with positive effect on growth, yield and moisture stress tolerance. Ind. J. Plant Physiol. 2017, 22, 287–294. [Google Scholar]
- Cakmak, I. Tansley Review No. 111 Possible roles of zinc in protecting plant cells from damage by reactive oxygen species. New Phytol. 2000, 146, 185–205. [Google Scholar] [CrossRef]
- Wani, A.S.; Ahmad, A.; Hayat, S.; Fariduddin, Q. Salt-induced modulation in growth, photosynthesis and antioxidant system in two varieties of Brassica juncea. Saudi J. Biol. Sci. 2013, 20, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Amini, S.; Ghadiri, H.; Chen, C.; Marschner, P. Salt-affected soils, reclamation, carbon dynamics, and biochar: A review. J. Soils Sediments 2016, 16, 939–953. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Saddiq, M.S.; Afzal, I.; Iqbal, S.; Hafeez, M.B.; Raza, A. Low leaf sodium content improves the grain yield and physiological performance of wheat genotypes in saline-sodic soil. Pesqui. Agropecu. Trop. 2021, 51, e67663. [Google Scholar] [CrossRef]
- Heikal, Y.M.; El-Esawi, M.A.; El-Ballat, E.M.; Abdel-Aziz, H.M. Applications of nanoparticles for mitigating salinity and drought stress in plants: An overview on the physiological, biochemical and molecular genetic aspects. N. Z. J. Crop Hortic. Sci. 2023, 51, 297–327. [Google Scholar] [CrossRef]
- Dey, A.; Somaiah, S. Green synthesis and characterization of zinc oxide nanoparticles using leaf extract of Thryallis glauca (Cav.) Kuntze and their role as antioxidant and antibacterial. Microsc. Res. Tech. 2022, 85, 2835–2847. [Google Scholar] [CrossRef]
| Treatment | Cultivars | Priming | T50 (days) | MET (days) | FEP (%) | RL (cm) | SL (cm) | RFW (g) | RDW (g) | SFW (g) | SDW (g) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CK | JS-2002 | NP | 3.18 de ± 0.18 | 4.28 c ± 0.21 | 87 | 18.02 c ± 0.98 | 27.80 bc ± 1.15 | 6.55 bc ± 0.09 | 4.44 de ± 0.14 | 14.99 b ± 0.78 | 6.91 c ± 0.29 |
| CK | JS-2002 | ZSP | 2.95 ef ± 0.22 | 3.84 e ± 0.18 | 93 | 22.03 a ± 1.23 | 31.43 ab ± 0.92 | 7.19 ab ± 0.19 | 4.93 b ± 0.21 | 16.42 a ± 1.12 | 7.57 b ± 0.41 |
| CK | JS-263 | NP | 3.11 ef ± 0.09 | 4.05 d ± 0.33 | 93 | 20.31 b ± 1.76 | 29.10 bc ± 2.23 | 6.89 b ± 0.065 | 4.75 bc ± 0.34 | 15.36 b ± 0.87 | 7.20 c ± 0.49 |
| CK | JS-263 | ZSP | 2.89 f ± 0.21 | 3.59 f ± 0.22 | 100 | 23.39 a ± 2.82 | 34.73 a ± 0.79 | 7.71 a ± 0.41 | 5.29 a ± 0.33 | 17.31 a ± 0.56 | 8.34 a ± 0.55 |
| SS | JS-2002 | NP | 3.89 a ± 0.14 | 4.99 a ± 0.17 | 73 | 12.81 f ± 2.22 | 17.73 e ± 0.98 | 5.09 f ± 0.45 | 3.50 g ± 0.19 | 10.66 f ± 0.55 | 5.19 f ± 0.32 |
| SS | JS-2002 | ZSP | 3.59 bc ± 0.19 | 4.64 b ± 0.29 | 87 | 14.43 de ± 1.14 | 21.87 de ± 1.01 | 5.81 de ± 0.52 | 3.91 ef ± 0.14 | 12.66 d ± 0.48 | 5.98 d ± 0.29 |
| SS | JS-263 | NP | 3.75 ab ± 0.26 | 4.77 b ± 0.32 | 80 | 13.67 ef ± 1.76 | 20.49 e ± 0.67 | 5.37 ef ± 0.13 | 3.77 fg ± 0.29 | 11.72 e ± 0.78 | 5.64 e ± 0.24 |
| SS | JS-263 | ZSP | 3.35 cd ± 0.17 | 4.43 c ± 0.18 | 87 | 15.34 d ± 1.92 | 25.22 cd ± 1.17 | 6.16 cd ± 0.21 | 4.17 de ± 0.32 | 13.84 b ± 0.82 | 6.24 d ± 0.41 |
| Treatment | Cultivars | Priming | Root-Na | Shoot-Na | Root-Cl | Shoot-Cl | Root-K | Shoot-K |
|---|---|---|---|---|---|---|---|---|
| CK | JS-2002 | NP | 5.95 c ± 0.78 | 3.66 cd ± 0.26 | 6.80 cd ± 0.41 | 5.66 c ± 0.44 | 9.70 d ± 0.82 | 7.55 c ± 0.44 |
| CK | JS-2002 | ZSP | 6.12 bc ± 0.82 | 3.89 bc ± 0.29 | 7.02 bc ± 0.73 | 5.96 bc ± 0.19 | 10.18 bc ± 0.66 | 7.85 bc ± 0.62 |
| CK | JS-263 | NP | 6.35 ab ± 0.56 | 4.09 ab ± 0.33 | 7.24 ab ± 0.39 | 6.18 ab ± 0.28 | 10.76 ab ± 0.78 | 8.08 ab ± 0.54 |
| CK | JS-263 | ZSP | 6.56 a ± 0.43 | 4.30 a ± 0.41 | 7.45 a ± 0.48 | 6.29 a ± 0.35 | 10.95 a ± 0.81 | 8.26 a ± 0.39 |
| SS | JS-2002 | NP | 4.72 e ± 0.66 | 2.89 g ± 0.25 | 5.28 f ± 0.67 | 4.25 e ± 0.56 | 7.22 f ± 0.59 | 5.95 e ± 0.33 |
| SS | JS-2002 | ZSP | 4.93 e ± 0.44 | 3.13 fg ± 0.22 | 5.86 e ± 0.44 | 4.53 e ± 0.61 | 7.82 ef ± 0.67 | 6.22 e ± 0.49 |
| SS | JS-263 | NP | 5.04 de ± 0.29 | 3.28 ef ± 0.41 | 6.15 e ± 0.56 | 4.98 d ± 0.49 | 8.23 de ± 0.49 | 6.73 d ± 0.39 |
| SS | JS-263 | ZSP | 5.29 d ± 0.33 | 3.51 de ± 0.20 | 6.50 d ± 0.88 | 5.24 d ± 0.32 | 8.59 d ± 0.55 | 6.95 d ± 0.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Umair Hassan, M.; Chattha, M.U.; Khan, I.; Khan, T.A.; Nawaz, M.; Tang, H.; Noor, M.A.; Asseri, T.A.Y.; Hashem, M.; Guoqin, H. Zinc Seed Priming Alleviates Salinity Stress and Enhances Sorghum Growth by Regulating Antioxidant Activities, Nutrient Homeostasis, and Osmolyte Synthesis. Agronomy 2024, 14, 1815. https://doi.org/10.3390/agronomy14081815
Umair Hassan M, Chattha MU, Khan I, Khan TA, Nawaz M, Tang H, Noor MA, Asseri TAY, Hashem M, Guoqin H. Zinc Seed Priming Alleviates Salinity Stress and Enhances Sorghum Growth by Regulating Antioxidant Activities, Nutrient Homeostasis, and Osmolyte Synthesis. Agronomy. 2024; 14(8):1815. https://doi.org/10.3390/agronomy14081815
Chicago/Turabian StyleUmair Hassan, Muhammad, Muhammad Umer Chattha, Imran Khan, Tahir Abbas Khan, Mohsin Nawaz, Haiying Tang, Mehmood Ali Noor, Tahani A. Y. Asseri, Mohamed Hashem, and Huang Guoqin. 2024. "Zinc Seed Priming Alleviates Salinity Stress and Enhances Sorghum Growth by Regulating Antioxidant Activities, Nutrient Homeostasis, and Osmolyte Synthesis" Agronomy 14, no. 8: 1815. https://doi.org/10.3390/agronomy14081815
APA StyleUmair Hassan, M., Chattha, M. U., Khan, I., Khan, T. A., Nawaz, M., Tang, H., Noor, M. A., Asseri, T. A. Y., Hashem, M., & Guoqin, H. (2024). Zinc Seed Priming Alleviates Salinity Stress and Enhances Sorghum Growth by Regulating Antioxidant Activities, Nutrient Homeostasis, and Osmolyte Synthesis. Agronomy, 14(8), 1815. https://doi.org/10.3390/agronomy14081815









