Heavy Metals in the Cultivated Soils of Central and Western Serbia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites and Sample Collection
2.2. Soil Sampling and Chemical Analysis
2.3. Statistical Analysis
3. Results
3.1. Chemical Properties and Levels of Heavy Metals in Soils
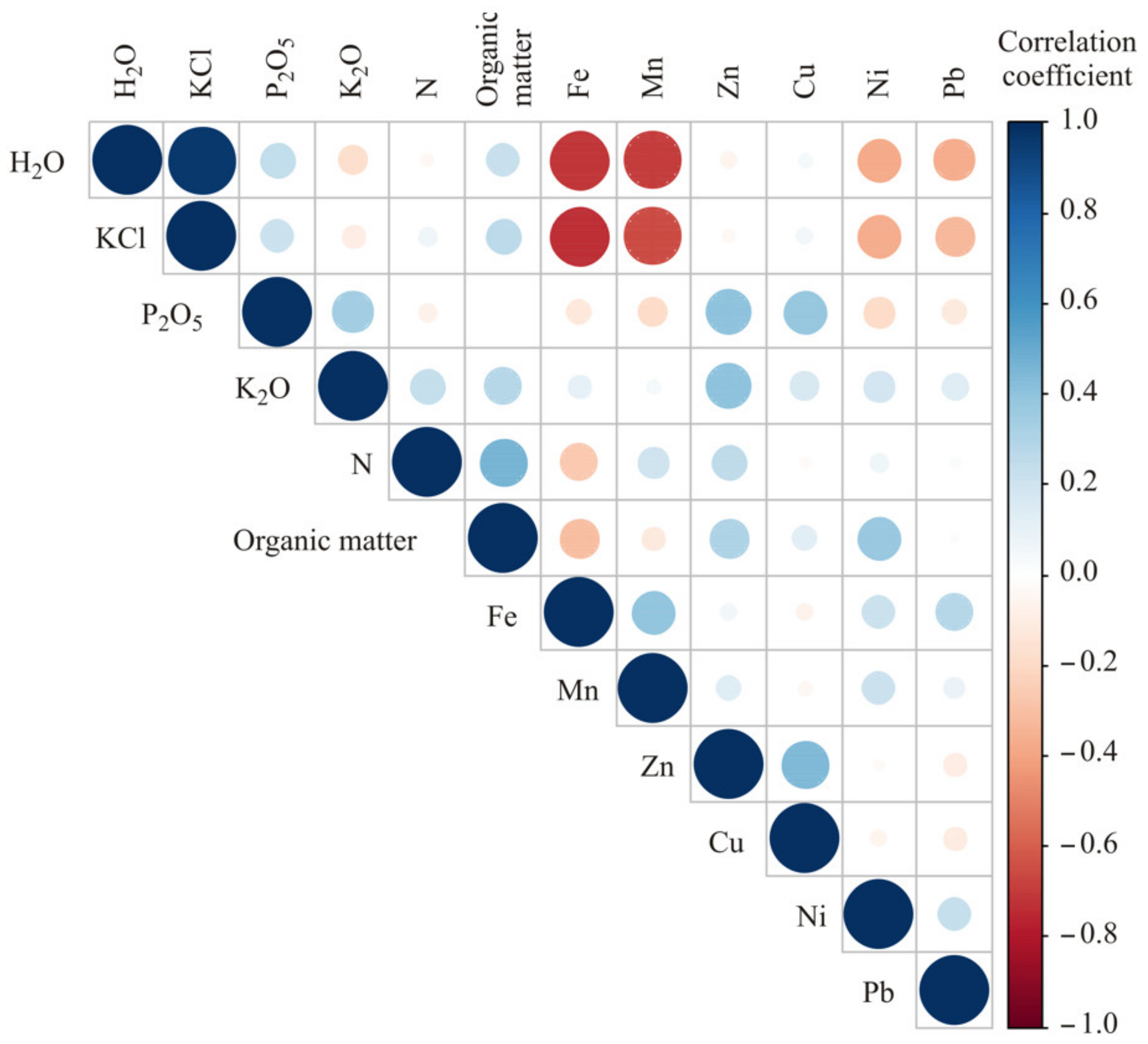
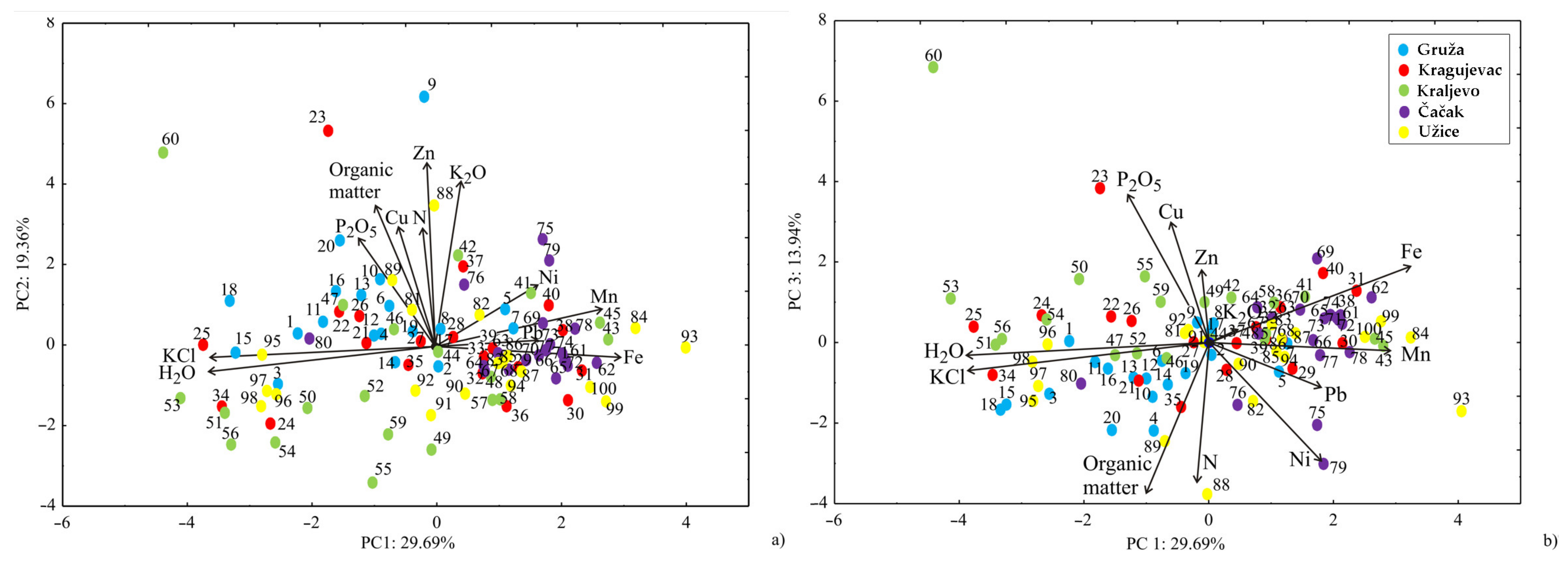
3.2. Chemometric Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pavlović, P.; Kostić, N.; Karadžić, B.; Mitrović, M. The soils of Serbia. In World Soils Book Series; Hartemink, A.E., Ed.; Springer Science + Business Media: Dordrecht, The Netherlands, 2017; ISBN 978-94-017-8659-1. [Google Scholar]
- Kabata-Pendias, A. Soil–plant transfer of trace elements—An environmental issue. Geoderma 2014, 122, 143–149. [Google Scholar] [CrossRef]
- Čakmak, D.; Perović, V.; Antić-Mladenović, S.; Kresović, M.; Saljnikov, E.; Mitrović, M.; Pavlović, P. Contamination, risk, and source apportionment of potentially toxic microelements in river sediments and soil after extreme flooding in the Kolubara River catchment in Western Serbia. J. Soils Sediments 2018, 18, 1981–1993. [Google Scholar] [CrossRef]
- Herrero, A.; Gutiérrez-Cánovas, C.; Vigiak, O.; Lutz, S.; Kumar, R.; Gampe, D.; Sabater, S. Multiple stressor effects on biological quality elements in the Ebro River: Present diagnosis and predicted responses. Sci. Total Environ. 2018, 630, 1608–1618. [Google Scholar] [CrossRef] [PubMed]
- Menzies, N.W.; Donn, M.J.; Kopittke, P.M. Evaluation of extractants for estimation of the phytoavailable trece metals in soils. Environ. Pollut. 2007, 145, 121–130. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Ahmed, F.S.; Kumar, S.P.; Rozbu, R.M.; Chowdhury, T.A.; Nuzhat, A.; Rafa, N.; Mahlia, T.M.I.; Ong, C.H.; Mofijur, M. Heavy metal toxicity, sources, and remediation techniques for contaminated water and soil. Environ. Technol. Innov. 2022, 25, 102114. [Google Scholar] [CrossRef]
- Kicińska, A.; Pomykala, R.; Izquierdo-Diaz, M. Changes in soil pH and mobility of heavy metals in contaminated soils. Eur. J. Soil Sci. 2022, 71, e13203. [Google Scholar] [CrossRef]
- Rule, H.J. Trace metal cation adsorption in soils: Selective chemical extractions and biological availability. Stud. Surf. Sci. Catal. 1999, 120, 319–349. [Google Scholar] [CrossRef]
- Lepp, N.W. Effect of heavy metal pollution on plants. Volume 1: Effects of trace metals on plant function. Appl. Sci. 1981, 145, 100–101. [Google Scholar]
- Pikuła, D.; Stępień, W. Effect of the Degree of Soil Contamination with Heavy Metals on Their Mobility in the Soil Profile in a Microplot Experiment. Agronomy 2021, 11, 878. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front Pharmacol. 2021, 13, 643972. [Google Scholar] [CrossRef]
- Harja, M.; Ciocinta, R.C.; Ondrasek, G.; Bucur, D.; Dirja, M. Accumulation of Heavy Metal Ions from Urban Soil in Spontaneous Flora. Water 2023, 15, 768. [Google Scholar] [CrossRef]
- Pandey, V.C.; Singh, N. Aromatic plants versus arsenic hazards in soils. J. Geochem. Explor. 2015, 157, 77–80. [Google Scholar] [CrossRef]
- Wu, B.; Li, J.; Kuang, H.; Shangguan, Y.; Chen, J. Mercapto-based palygorskite modified soil micro-biology and reduced the uptake of heavy metals by Salvia miltiorrhiza in cadmium and lead co-contaminated soil. J. Environ. Manag. 2023, 345, 118859. [Google Scholar] [CrossRef]
- Rashid, A.; Schutte, B.J.; Ulery, A.; Deyholos, M.K.; Sanogo, S.; Lehnhoff, E.A.; Beck, L. Heavy Metal Contamination in Agricultural Soil: Environmental Pollutants Affecting Crop Health. Agronomy 2023, 13, 1521. [Google Scholar] [CrossRef]
- Ghuge, A.S.; Nikalje, C.G.; Kadam, S.U.; Suprasanna, P.; Hong, C.J. Comprehensive mechanisms of heavy metal toxicity in plants, detoxification, and remediation. J. Hazard. Mater. 2023, 450, 131039. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Hu, B.; Chen, B.; Jiang, Z. Inductively coupled plasma optical emission spectrometry for rare earth elements analysis. Phys. Sci. Rev. 2017, 2, 1–37. [Google Scholar] [CrossRef]
- Wei, L.Y.; Li, Z.H.; Sun, J.T.; Zhu, L.Z. Pollution characteristics and health risk assessment of phthalate esters in agricultural soil and vegetables in the Yangtze River Delta of China. Sci. Total Environ. 2020, 726, 137978. [Google Scholar] [CrossRef] [PubMed]
- Turan, O.; Ozdemir, H.; Demir, G. Deposition of heavy metals on coniferous tree leaves and soils near heavy urban traffic. Front. Life Sci. Relat. Technol. 2022, 1, 35–41. Available online: https://dergipark.org.tr/tr/download/article-file/1242667 (accessed on 10 July 2024).
- Shi, T.; Zhang, J.; Shen, W.; Wang, J.; Li, X. Machine learning can identify the sources of heavy metals in agricultural soil: A case study in northern Guangdong Province, China. Ecotoxicol. Environ. Saf. 2022, 245, 114107. [Google Scholar] [CrossRef]
- Palansooriya, N.K.; Shaheen, M.S.; Chen, S.S.; Tsang, C.W.D.; Hashimoto, Y.; Hou, D.; Bolan, S.N.; Rinklebe, J.; Ok, S.Y. Soil amendments for immobilization of potentially toxic elements in contaminated soils: A critical review. Environ. Int. 2020, 134, 105046. [Google Scholar] [CrossRef]
- Zamulina, V.I.; Gorovtsov, V.A.; Minkina, M.T.; Mandzhieva, S.S.; Bauer, V.T.; Burachevskaya, V.M. The influence of long-term Zn and Cu contamination in Spolic Technosols on water-soluble organic matter and soil biological activity. Ecotoxicol. Environ. Saf. 2021, 208, 111471. [Google Scholar] [CrossRef] [PubMed]
- Alloway, B.J. (Ed.) Heavy metals in soils. In Trace Metals and Metalloids in Soils and Their Bioavailability; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Cui, X.; Liang, J.; Lu, W.; Chen, H.; Liu, F.; Lin, G.; Xu, F.; Luo, Y.; Lin, G. Stronger ecosystem carbon sequestration potential of mangrove wetlands with respect to terrestrial forests in subtropical China. Agric. For. Meteorol. 2018, 249, 71–80. [Google Scholar] [CrossRef]
- Liu, X.; Shi, H.; Bai, Z.; Zhou, W.; Liu, K.; Wang, M.; He, Y. Heavy metal concentrations of soils near the large opencast coal mine pits in China. Chemosphere 2020, 244, 125360. [Google Scholar] [CrossRef]
- Vestin, L.K.J.; Nambu, K.; van Hees, A.W.A.; Bylund, D.; Lundström, U.S. The influence of alkaline and non-alkaline parent material on soil chemistry. Geoderma 2006, 135, 97–106. [Google Scholar] [CrossRef]
- Djalovic, I.; Jockovic, Đ.; Dugalic, G.; Bekavac, G.; Purar, B.; Seremesic, S.; Jockovic, M. Soil acidity and mobile aluminum status in pseudogley soils in the Čačak–Kraljevo Basin. J. Serb. Chem. Soc. 2012, 77, 833–843. [Google Scholar] [CrossRef]
- Debreczeni, K.; Kismányoky, T. Acidification of soils in long-term field experiments. Commun. Soil Sci. Plant Anal. 2005, 36, 321–329. [Google Scholar] [CrossRef]
- Adriano, D.C. Trace Elements in the Terrestrial Environments: Biogeochemistry Bioavailability, and Risks of Metals, 2nd ed.; Springer: New York, NY, USA, 2021; p. 867. [Google Scholar] [CrossRef]
- Carrillo-González, R.; Šimůnek, J.; Sauvé, S.; Adriano, D. Mechanisms and Pathways of Trace Element Mobility in Soils. Adv. Agron. 2006, 91, 111–178. [Google Scholar] [CrossRef]
- Caporale, G.A.; Violante, A. Chemical Processes Affecting the Mobility of Heavy Metals and Metalloids in Soil Environments. Curr. Pollut. Rep. 2016, 2, 15–27. [Google Scholar] [CrossRef]
- Djalovic, I.; Maksimovic, I.; Kastori, R.; Jelic, M. Mechanisms of Adaptation of Small Grains to Soil Acidity. Proc. Nat. Sci. Matica Srp. Novi Sad 2010, 118, 107–120. [Google Scholar] [CrossRef]
- Mrvić, V.; Kostić-Kravljanac, L.J.; Čakmak, D.; Sikirić, B.; Brebanović, B.; Perović, V.; Nikoloski, M. Pedogeochemical mapping and background limit of trace elements in soils of Branicevo Province (Serbia). J. Geochem. Explor. 2011, 109, 18–25. [Google Scholar] [CrossRef]
- Cerdan, O.; Govers, G.; Le Bissonnais, Y.; Oost, K.; Poesen, J.; Saby, N.; Gobin, A.; Vacca, A.; Quinton, J.; Auerswald, K.; et al. Rates and spatial variations of soil erosion in Europe: A study based on erosion plot data. Geomorphology 2010, 122, 167–177. [Google Scholar] [CrossRef]
- Panagos, P.; Hiederer, R.; Van Liedekerke, M.; Bampa, F. Estimating soil organic carbon in Europe based on data collected through an European network. Ecol. Indic. 2013, 24, 439–450. [Google Scholar] [CrossRef]
- Saint-Laurent, D.; Beaulac-Gervais, V.; Berthelot, J.S. Comparison of soil organic carbon and total nitrogen contents in inundated and non-inundated zones in southern Québec, Canada. Catena 2014, 113, 1–8. [Google Scholar] [CrossRef]
- Wan, Y.; Liu, J.; Zhuang, Z.; Wang, Q.; Li, H. Heavy Metals in Agricultural Soils: Sources, Influencing Factors, and Remediation Strategies. Toxics 2024, 12, 63. [Google Scholar] [CrossRef]
- Noulas, C.; Tziouvalekas, M.; Karyotis, T. Zinc in soils, water and food crops. J. Trace Elem. Med. Biol. 2018, 49, 252–260. [Google Scholar] [CrossRef]
- Chlopecka, A. Assessment of form of Cd, Zn and Pb in contaminated calcareous and gleyed soils in southwest Poland. Sci. Total Environ. 1996, 188, 253–262. [Google Scholar] [CrossRef]
- Avadhani, D.N.; Mahesh, H.M.; Karunakara, N.; Narayana, Y.; Somashekarappa, H.M.; Siddappa, K. Distribution and behaviour of natural radionuclides in soil samples of Goa on the southwest coast of India. In The Natural Radiation Environment VII; McLaughlin, J.P., Simopoulos, S.E., Steinhausler, F., Eds.; Elsevier Ltd.: Oxford, UK, 2005. [Google Scholar]
- Ozden, B.; Uzgur, A.; Esetlili, T.; Esetlili, B.C.; Kurucu, Y. Assessment of the effects of physical-chemical parameters on 210Po and 210Pb concentrations in cultivated and uncultivated soil from different areas. Geoderma 2013, 192, 7–11. [Google Scholar] [CrossRef]
- Madrid, L.; D’ıaz-Barrientos, E.; Reinoso, R.; Madrid, F. Metals in urban soils of Sevilla: Seasonal changes and relations with other soil components and plant contents. Eur. J. Soil Sci. 2004, 55, 209–217. [Google Scholar] [CrossRef]
- Maldonado, V.M.; Rubio Arias, H.O.; Quintana, R.; Saucedo, R.A.; Gutierrez, M.; Ortega, J.A.; Nevarez, G.V. Heavy Metal Content in Soils under Different Wastewater Irrigation Patterns in Chihuahua, Mexico. Int. J. Environ. Res. Public Health 2008, 5, 441–449. [Google Scholar] [CrossRef]
- Edo, I.G.; Samuel, O.P.; Oloni, O.G.; Ezekiel, O.G.; Ikpekoro, O.V.; Obasohan, P.; Ongulu, J.; Otunuya, F.C.; Opiti, R.A.; Ajakaye, S.R.; et al. Environmental persistence, bioaccumulation, and ecotoxicology of heavy metals. Chem. Ecol. 2024, 40, 322–349. [Google Scholar] [CrossRef]
- Angon, B.P.; Islam, S.M.; Shreejana, K.; Das, A.; Anjum, N.; Poudel, A.; Suchi, A.S. Sources, effects and present perspectives of heavy metals contamination: Soil, plants and human food chain. Heliyon 2024, 10, e28357. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djalovic, I.; Prasad, P.V.V.; Pezo, L.; Janić Hajnal, E.; Saulic, M.; Dugalić, M.; Kolarić, L. Heavy Metals in the Cultivated Soils of Central and Western Serbia. Agronomy 2024, 14, 1836. https://doi.org/10.3390/agronomy14081836
Djalovic I, Prasad PVV, Pezo L, Janić Hajnal E, Saulic M, Dugalić M, Kolarić L. Heavy Metals in the Cultivated Soils of Central and Western Serbia. Agronomy. 2024; 14(8):1836. https://doi.org/10.3390/agronomy14081836
Chicago/Turabian StyleDjalovic, Ivica, P. V. Vara Prasad, Lato Pezo, Elizabet Janić Hajnal, Markola Saulic, Marijana Dugalić, and Ljubiša Kolarić. 2024. "Heavy Metals in the Cultivated Soils of Central and Western Serbia" Agronomy 14, no. 8: 1836. https://doi.org/10.3390/agronomy14081836
APA StyleDjalovic, I., Prasad, P. V. V., Pezo, L., Janić Hajnal, E., Saulic, M., Dugalić, M., & Kolarić, L. (2024). Heavy Metals in the Cultivated Soils of Central and Western Serbia. Agronomy, 14(8), 1836. https://doi.org/10.3390/agronomy14081836








