Soil and Plant Nitrogen Management Indices Related to Within-Field Spatial Variability
Abstract
:1. Introduction
2. Materials and Methods
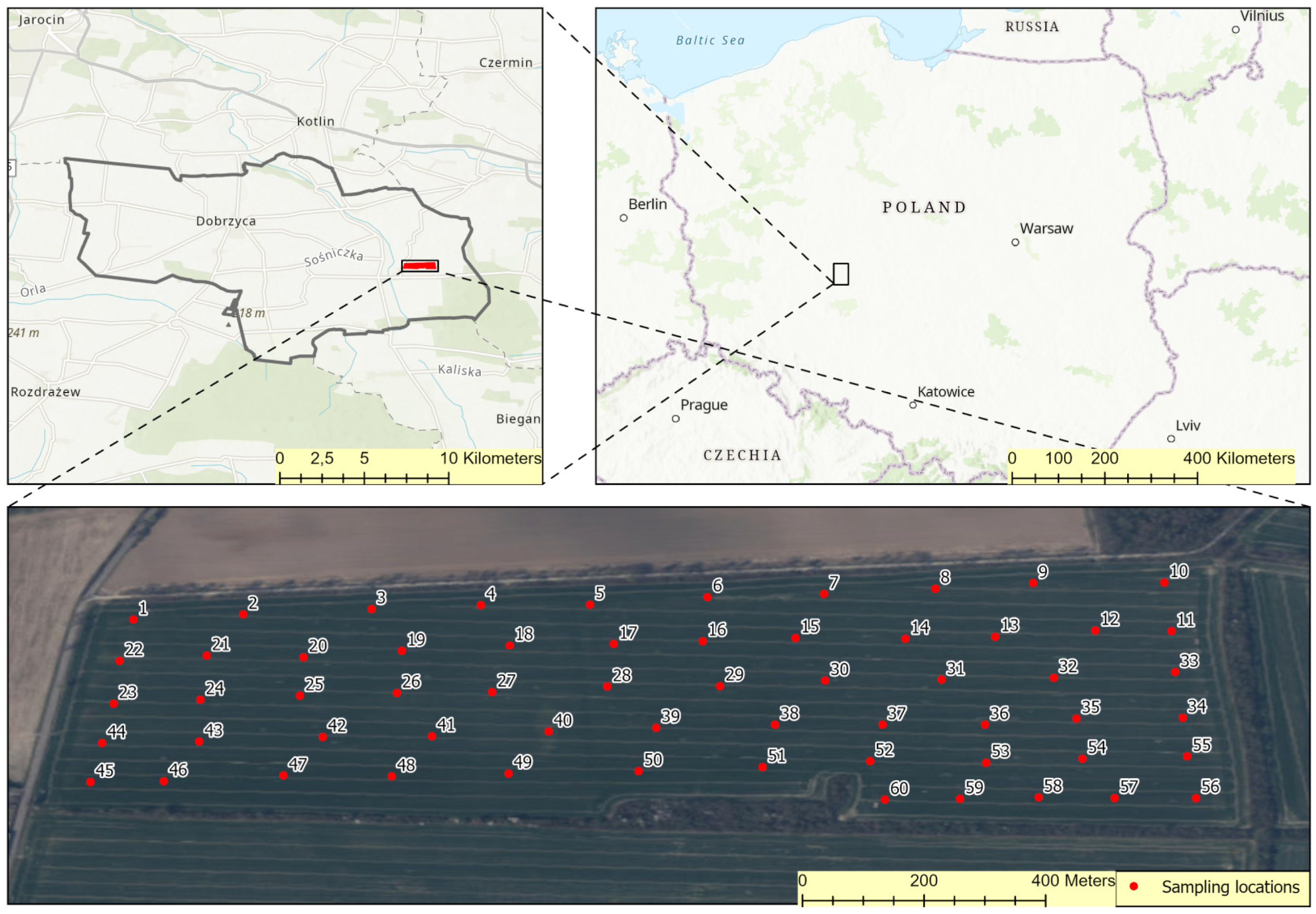
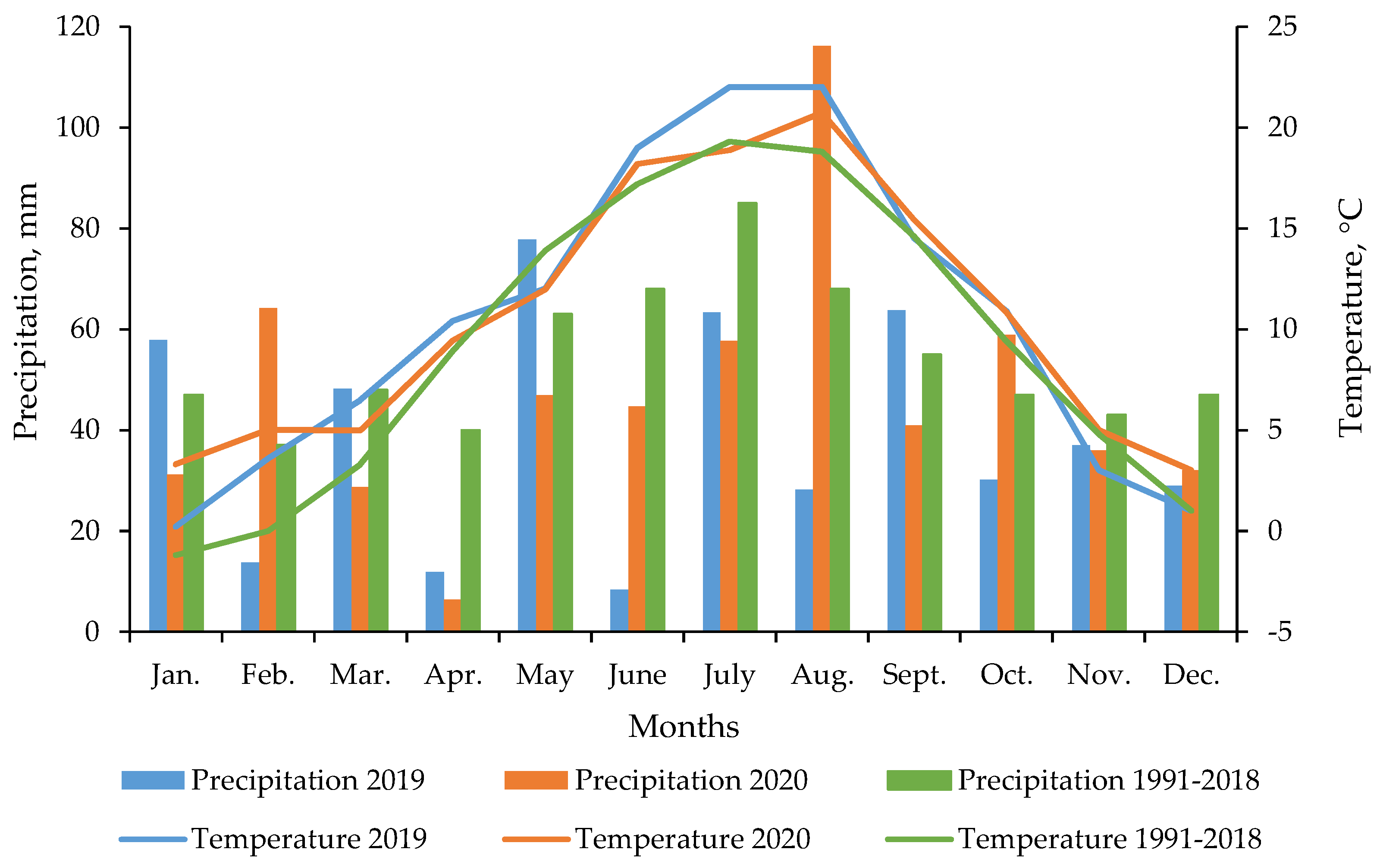
2.1. Field Location and Site Description
2.2. Soil Sampling
2.3. Agrotechnics Practices
2.4. Plant Sampling
2.5. Soil Samples Analysis
2.6. Plant Samples Analysis
2.7. Plant Nitrogen Management Indices
- Total N accumulation,
- 2.
- N harvest index,
- 3.
- Unit N uptake,
- 4.
- Unit N productivity,
- 5.
- Partial factor productivity of N input,
- 6.
- N fertilizers balance,
- 7.
- Efficiency of N fertilization,
- 8.
- N input from soil and fertilizers,
- 9.
- N input balance,
- 10.
- N mineralized during the growing season,
- 11.
- Total N input,
- 12.
- N input efficiency,
- 13.
- Total N input efficiency,
2.8. Statistical and Spatial Variation Analyses
3. Results
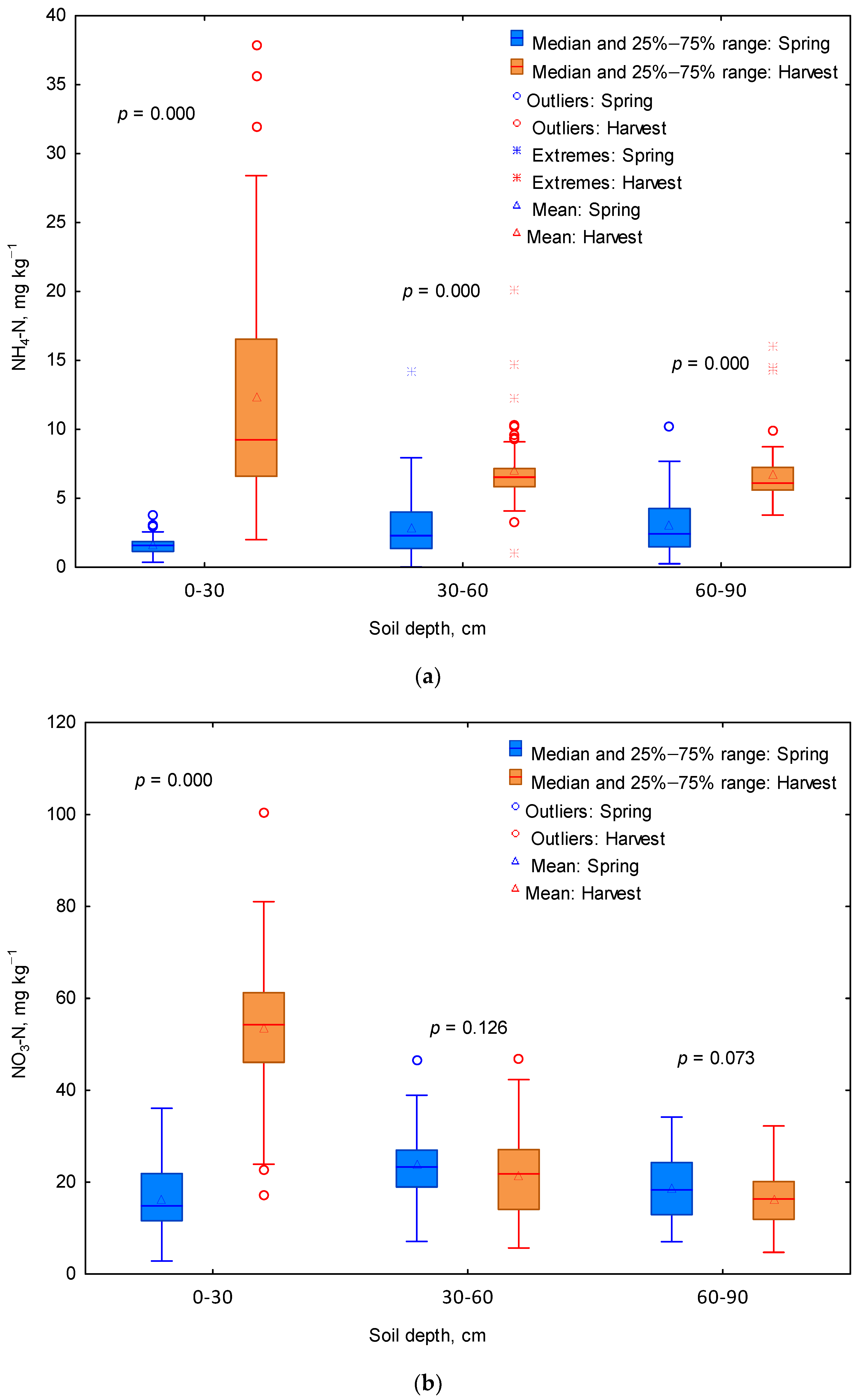
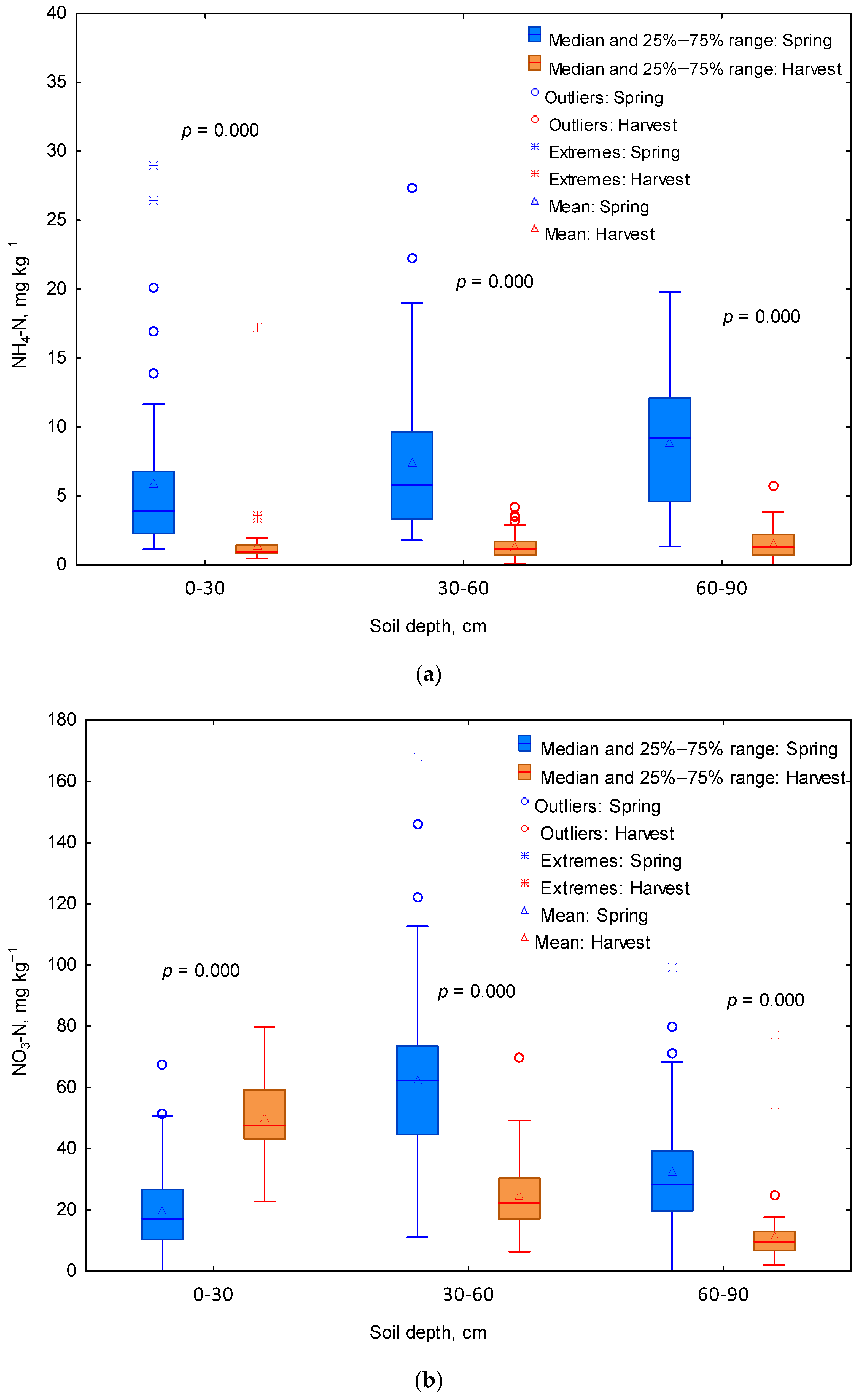
3.1. Mineral N Content of the Soil
3.2. Crop Yields and Indices of N Management
3.3. Soil N Management Indices
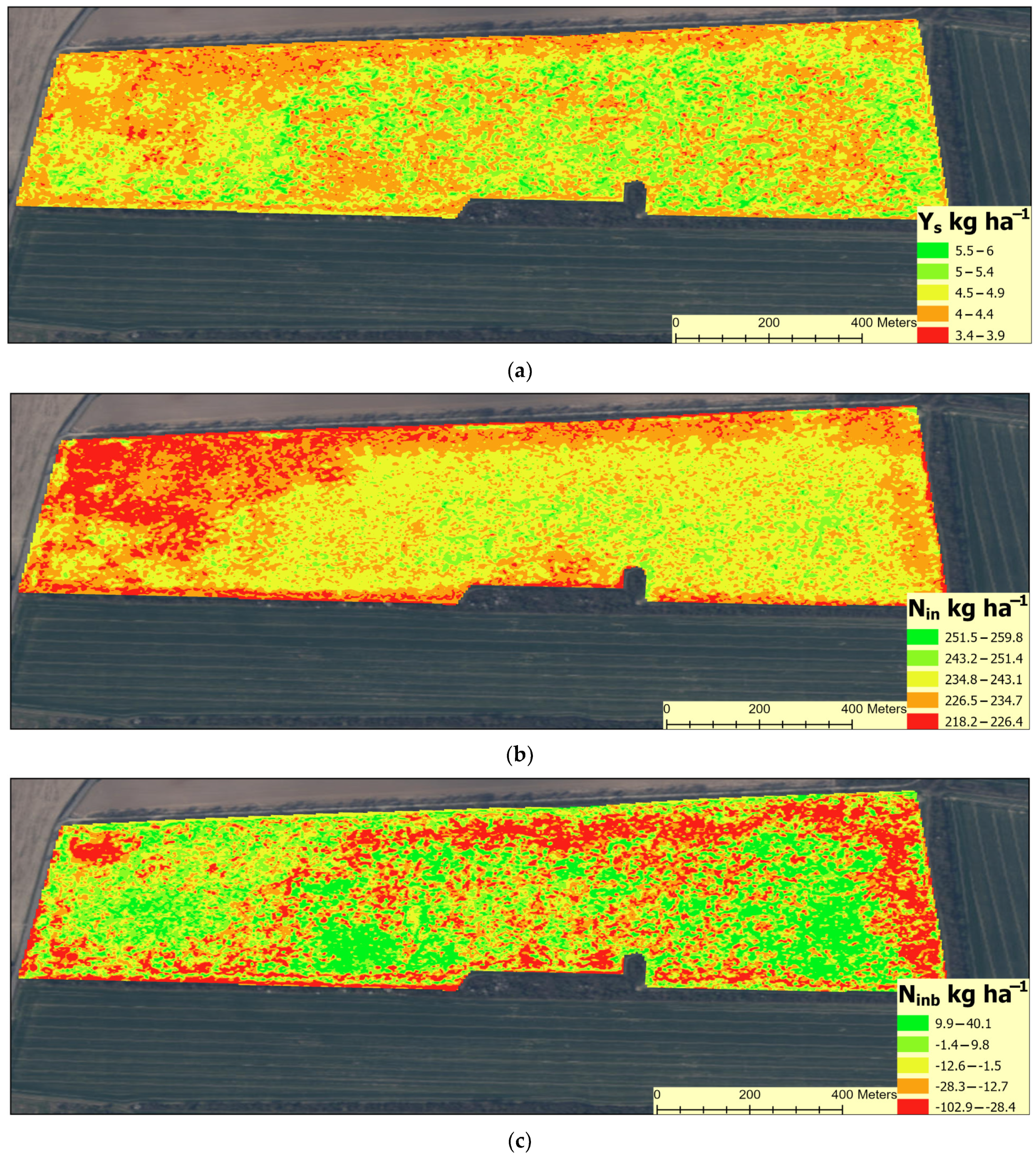
3.4. Spatial Variability of Crop Yield and N Management Indices
4. Discussion
4.1. Soil Mineral N Content
4.2. Plant Indices of N Management
4.3. Soil Indices of N Management
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Evans, J.R. Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 1989, 78, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Chen, Y. The physiological response of photosynthesis to nitrogen deficiency. Plant Physiol. Biochem. 2021, 158, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Noor, H.; Ding, P.; Ren, A.; Sun, M.; Gao, Z. Effects of Nitrogen Fertilizer on Photosynthetic Characteristics and Yield. Agronomy 2023, 13, 1550. [Google Scholar] [CrossRef]
- Burton, A.; Häner, L.L.; Schaad, N.; Strebel, S.; Vuille-dit-Bille, N.; de Figueiredo Bongiovani, P.; Holzkämper, A.; Pellet, D.; Herrera, J.M. Evaluating nitrogen fertilization strategies to optimize yield and grain nitrogen content in top winter wheat varieties across Switzerland. Field Crops Res. 2024, 307, 109251. [Google Scholar] [CrossRef]
- FAOSTAT. Food and Agriculture Organization of the United Nations (FAO). Available online: https://www.fao.org/home/en (accessed on 18 April 2024).
- Hubert, B.; Rosegrant, M.; van Boekel, M.A.J.S.; Ortiz, R. The Future of Food: Scenarios for 2050. Crop Sci. 2010, 50 (Suppl. S1), S-33–S-50. [Google Scholar] [CrossRef]
- Mălinaş, A.; Vidican, R.; Rotar, I.; Mălinaş, C.; Moldovan, C.M.; Proorocu, M. Current Status and Future Prospective for Nitrogen Use Efficiency in Wheat (Triticum aestivum L.). Plants 2022, 11, 217. [Google Scholar] [CrossRef]
- Sapkota, T.B.; Bijay-Singh; Takele, R. Chapter Five—Improving nitrogen use efficiency and reducing nitrogen surplus through best fertilizer nitrogen management in cereal production: The case of India and China. Adv. Agron. 2023, 178, 233–294. [Google Scholar] [CrossRef]
- Mogollón, J.M.; Lassaletta, L.; Beusen, A.H.W.; van Grinsven, H.J.M.; Westhoek, H.; Bouwman, A.F. Assessing future reactive nitrogen inputs into global croplands based on the shared socioeconomic pathways. Environ. Res. Lett. 2018, 13, 044008. [Google Scholar] [CrossRef]
- Congreves, K.A.; Otchere, O.; Ferland, D.; Farzadfar, S.; Williams, S.; Arcand, M.M. Nitrogen Use Efficiency Definitions of Today and Tomorrow. Front. Plant Sci. 2021, 12, 637108. [Google Scholar] [CrossRef]
- Cassman, K.G.; Dobermann, A.; Walters, D.T. Agroecosystems, nitrogen-use, and nitrogen management. Ambio 2002, 31, 132–140. [Google Scholar] [CrossRef]
- You, L.; Ros, G.H.; Chen, Y.; Shao, Q.; Young, M.D.; Zhang, F.; de Vries, W. Global mean nitrogen recovery efficiency in croplands can be enhanced by optimal nutrient, crop and soil management practices. Nat. Commun. 2023, 14, 5747. [Google Scholar] [CrossRef] [PubMed]
- EU Nitrogen Expert Panel. Nitrogen Use Efficiency (NUE)—An Indicator for the Utilization of Nitrogen in Agriculture and Food Systems; Wageningen University: Wageningen, The Netherlands, 2015. [Google Scholar]
- Lassaletta, L.; Billen, G.; Grizzetti, B.; Anglade, J.; Garnier, J. 50 year trends in nitrogen use efficiency of world cropping systems: The relationship between yield and nitrogen input to cropland. Environ. Res. Lett. 2014, 9, 105011. [Google Scholar] [CrossRef]
- Martínez-Dalmau, J.; Berbel, J.; Ordóñez-Fernández, R. Nitrogen Fertilization. A Review of the Risks Associated with the Inefficiency of Its Use and Policy Responses. Sustainability 2021, 13, 5625. [Google Scholar] [CrossRef]
- Jwaideh, M.A.; Sutanudjaja, E.H.; Dalin, C. Global impacts of nitrogen and phosphorus fertiliser use for major crops on aquatic biodiversity. Int. J. Life Cycle Assess. 2022, 27, 1058–1080. [Google Scholar] [CrossRef]
- Grennfelt, P.; Hultberg, H. Effects of nitrogen deposition on the acidification of terrestrial and aquatic ecosystems. Water Air Soil Pollut. 1986, 30, 945–963. [Google Scholar] [CrossRef]
- Chai, R.; Ye, X.; Ma, C.; Wang, Q.; Tu, R.; Zhang, L.; Gao, H. Greenhouse gas emissions from synthetic nitrogen manufacture and fertilization for main upland crops in China. Carbon Balance Manag. 2019, 30, 20. [Google Scholar] [CrossRef] [PubMed]
- Hirel, B.; Tétu, T.; Lea, P.J.; Dubois, F. Improving Nitrogen Use Efficiency in Crops for Sustainable Agriculture. Sustainability 2011, 3, 1452–1485. [Google Scholar] [CrossRef]
- Barłóg, P.; Grzebisz, W.; Łukowiak, R. Fertilizers and Fertilization Strategies Mitigating Soil Factors Constraining Efficiency of Nitrogen in Plant Production. Plants 2022, 11, 1855. [Google Scholar] [CrossRef]
- Johnston, A.M.; Bruulsema, T.W. 4R Nutrient Stewardship for Improved Nutrient Use Efficiency. “SYMPHOS 2013”, 2nd International Symposium on Innovation and Technology in the Phosphate Industry. Procedia Eng. 2014, 83, 365–370. [Google Scholar] [CrossRef]
- Olfs, H.-W.; Blankenau, K.; Brentrup, F.; Jasper, J.; Link, A.; Lammel, J. Soil-and plant-based nitrogen-fertilizer recommendations in arable farming. J. Plant Nutr. Soil Sci. 2005, 168, 414–431. [Google Scholar] [CrossRef]
- Oyebiyi, F.B.; Aula, L.; Omara, P.; Nambi, E.; Dhillon, J.S.; Raun, W.R. Maize (Zea mays L.) Grain Yield Response to Methods of Nitrogen Fertilization. Commun. Soil Sci. Plant 2019, 50, 2694–2700. [Google Scholar] [CrossRef]
- Haberle, J.; Kroulík, M.; Svoboda, P.; Lipavský, J.; Krejčová, J.; Cerhanová, D. The spatial variability of mineral nitrogen content in topsoil and subsoil. Plant Soil Environ. 2004, 50, 425–433. [Google Scholar] [CrossRef]
- Cao, Q.; Cui, Z.; Chen, X.; Khosla, R.; Dao, T.H.; Miao, Y. Quantifying spatial variability of indigenous nitrogen supply for precision nitrogen management in small scale farming. Precis. Agric. 2012, 13, 45–61. [Google Scholar] [CrossRef]
- Radočaj, D.; Jurišić, M.; Gašparović, M. The Role of Remote Sensing Data and Methods in a Modern Approach to Fertilization in Precision Agriculture. Remote Sens. 2022, 14, 778. [Google Scholar] [CrossRef]
- Weiss, M.; Jacob, F.; Duveiller, G. Remote sensing for agricultural applications: A metareview. Remote Sens. Environ. 2020, 236, 111402. [Google Scholar] [CrossRef]
- Córdova, C.; Barrera, J.A.; Magna, C. Spatial variation in nitrogen mineralization as a guide for variable application of nitrogen fertilizer to cereal crops. Nutr. Cycl. Agroecosyst. 2018, 110, 83–88. [Google Scholar] [CrossRef]
- Łukowiak, R.; Grzebisz, W.; Ceglarek, J.; Podolski, A.; Kaźmierowski, C.; Piekarczyk, J. Spatial Variability of Yield and Nitrogen Indicators—A Crop Rotation Approach. Agronomy 2020, 10, 1959. [Google Scholar] [CrossRef]
- Luce, M.S.; Whalen, J.K.; Ziadi, N.; Zebarth, B.J. Nitrogen dynamics and indices to predict soil nitrogen supply in humid temperate soils. Adv. Agron. 2011, 112, 55–102. [Google Scholar]
- Barłóg, P.; Łukowiak, R.; Grzebisz, W. Predicting the content of soil mineral nitrogen based on the content of calcium chloride-extractable nutrients. J. Plant Nutr. Soil Sci. 2017, 180, 624–635. [Google Scholar] [CrossRef]
- Fan, J.; McConkey, B.; Wang, H.; Janzen, H. Root distribution by depth for temperate agricultural crops. Field Crops Res. 2016, 189, 68–74. [Google Scholar] [CrossRef]
- Malagoli, P.; Lainé, P.; Le Deunff, E.; Rossato, L.; Ney, B.; Ourry, A. Modeling nitrogen uptake in oilseed rape cv capitol during a growth cycle using influx kinetics of root nitrate transport systems and field experimental data. Plant Physiol. 2004, 134, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Stettmer, M.; Maidl, F.-X.; Schwarzensteiner, J.; Hülsbergen, K.-J.; Bernhardt, H. Analysis of Nitrogen Uptake in Winter Wheat Using Sensor and Satellite Data for Site-Specific Fertilization. Agronomy 2022, 12, 1455. [Google Scholar] [CrossRef]
- Tilling, A.K.; O’Leary, G.J.; Ferwerda, J.G.; Jones, S.D.; Fitzgerald, G.J.; Rodriguez, D.; Belford, R. Remote sensing of nitrogen and water stress in wheat. Field Crops Res. 2007, 104, 77–85. [Google Scholar] [CrossRef]
- World Reference Base for Soil Resources 2014. Word Soil Resources Reports, 106; Food and Agriculture Organization 885 of the United Nations: Rome, Italy, 2015; Available online: http://www.fao.org/3/a-i3794e.pdf (accessed on 19 March 2024).
- Kęsik, K.; Jadczyszyn, T.; Lipiński, W.; Jurga, B. Adaptation of the Mehlich 3 procedure for routine determination of phosphorus, potassium and magnesium in soil. Przemysł Chem. 2015, 94, 973–976. (In Polish) [Google Scholar]
- Mehlich, A. Mehlich 3 soil test extractant: A modification of Mehlich 2 extractant. Commun. Soil Sci. Plant Anal. 1984, 15, 1409–1416. [Google Scholar] [CrossRef]
- Houba, V.J.G.; Temminghoff, E.J.M.; Gaikhorst, G.A.; van Vark, W. Soil analysis procedures using 0.01 M calcium chloride as extraction reagents. Commun. Soil Sci. Plant Anal. 2000, 31, 1299–1396. [Google Scholar] [CrossRef]
- Grzebisz, W.; Łukowiak, R.; Sassenrath, G.F. Virtual nitrogen as a tool for assessment of nitrogen management at the field scale: A crop rotation approach. Field Crops Res. 2018, 218, 182–194. [Google Scholar] [CrossRef]
- Wilding, L.P.; Dress, L.R. Spatial variability and pedology. In Pedogenesis and Soil Taxonomy; Wilding, L.P., Smeck, N., Hall, G.F., Eds.; Elsevier: Wageningen, The Netherlands, 1983; pp. 83–116. [Google Scholar]
- Jones, T.A. Skewness and kurtosis as criteria of normality in observed frequency distributions. J. Sediment. Res. 1969, 39, 1622–1627. [Google Scholar] [CrossRef]
- Robertson, G.P.; Groffman, P.M. Nitrogen transformations. In Soil Microbiology, Ecology and Biochemistry, 4th ed.; Paul, E.A., Ed.; Academic Press: Burlington, MA, USA, 2015; pp. 421–446. [Google Scholar] [CrossRef]
- Baxter, S.J.; Oliver, M.A.; Gaunt, J. A Geostatistical Analysis of the Spatial Variation of Soil Mineral Nitrogen and Potentially Available Nitrogen Within an Arable Field. Precis. Agric. 2023, 4, 213–226. [Google Scholar] [CrossRef]
- Długosz, J.; Piotrowska-Długosz, A. Spatial variability of soil nitrogen forms and the activity of N-cycle enzymes. Plant Soil Environ. 2016, 62, 502–507. [Google Scholar] [CrossRef]
- Schuster, J.; Mittermayer, M.; Maidl, F.X.; Nätscher, L.; Hülsbergen, K.-J. Spatial variability of soil properties, nitrogen balance and nitrate leaching using digital methods on heterogeneous arable fields in southern Germany. Precis. Agric. 2023, 24, 647–676. [Google Scholar] [CrossRef]
- Montemurro, F. Different Nitrogen Fertilization Sources, Soil Tillage, and Crop Rotations in Winter Wheat: Effect on Yield, Quality, and Nitrogen Utilization. J. Plant Nutr. 2009, 32, 1–18. [Google Scholar] [CrossRef]
- Giles, M.; Morley, N.; Baggs, E.M.; Daniell, T.J. Soil nitrate reducing processes—Drivers, mechanisms for spatial variation, and significance for nitrous oxide production. Front. Microbiol. 2012, 3, 417. [Google Scholar] [CrossRef]
- Riley, W.J.; Ortiz-Monasterio, I.; Matson, P.A. Nitrogen leaching and soil nitrate, nitrite, and ammonium levels under irrigated wheat in Northern Mexico. Nutr. Cycl. Agroecosyst. 2001, 61, 223–236. [Google Scholar] [CrossRef]
- Nieder, R.; Benbi, D.K.; Scherer, H.W. Fixation and defixation of ammonium in soils: A review. Biol. Fertil. Soils 2011, 47, 1–14. [Google Scholar] [CrossRef]
- De la Luz Mora, M.; Cartes, P.; Núñez, P.; Salazar, M.; Demanet, R. Movement of NO3−-N and NH4+-N in an Andisol and its influence on ryegrass production in a short term study. J. Soil Sci. Plant. Nutr. 2007, 7, 46–63. [Google Scholar]
- Wu, H.; Zhang, Z.; Hu, C.; Liu, D.; Qiao, Y.; Xiao, Z.; Wu, Y. Short-Term Straw Return Combined with Nitrogen Fertilizer Alters the Soil Nitrogen Supply in Rice–Rapeseed Planting Systems. Agronomy 2024, 14, 1226. [Google Scholar] [CrossRef]
- Zhang, J.B.; Zhu, T.B.; Cai, Z.C.; Quin, S.W.; Müller, C. Effects of long-term repeated mineral and organic fertilizer applications on soil nitrogen transformations. Eur. J. Soil Sci. 2012, 63, 75–85. [Google Scholar] [CrossRef]
- Haberle, J.; Kusá, H.; Svoboda, P.; Klír, J. The Changes of Soil Mineral Nitrogen Observed on Farms between Autumn and Spring and Modelled with a Simple Leaching Equation. Soil Water Res. 2009, 4, 159–167. [Google Scholar] [CrossRef]
- Diacono, M.; Rubino, P.; Montemurro, F. Precision nitrogen management of wheat. A review. Agron. Sustain. Dev. 2012, 33, 219–241. [Google Scholar] [CrossRef]
- Washmon, C.N.; Solie, J.B.; Raun, W.R.; Itenfisu, D.D. Within field variability in wheat grain yields over nine years in Oklahoma. J. Plant Nutr. 2002, 25, 2655–2662. [Google Scholar] [CrossRef]
- McBratney, A.B.; Mendonça Santos, M.L.; Minasny, B. On digital soil mapping. Geoderma 2023, 117, 3–52. [Google Scholar] [CrossRef]
- Mzuku, M.; Khosla, R.; Reich, R.; Inman, D.; Smith, F.; MacDonald, L. Spatial variability of measured soil properties across site-specific management zones. Soil Sci. Soc. Am. J. 2005, 69, 1572–1579. [Google Scholar] [CrossRef]
- Hausherr Lüder, R.-M.; Qin, R.; Richner, W.; Stamp, P.; Streit, B.; Noulas, C. Effect of Tillage Systems on Spatial Variation in Soil Chemical Properties and Winter Wheat (Triticum aestivum L.) Performance in Small Fields. Agronomy 2019, 9, 182. [Google Scholar] [CrossRef]
- Irmak, A.; Batchelor, W.D.; Jones, J.W.; Irmak, S.; Paz, J.O.; Beck, H.W.; Egeh, M. Relationship between plant available soil water and yield for explaining soybean yield variability. Appl. Eng. Agric. 2002, 18, 471–482. [Google Scholar] [CrossRef]
- Wong, M.T.F.; Asseng, S. Determining the causes of spatial and temporal variability of wheat yields at sub-field scale using a new method of upscaling a crop model. Plant Soil 2006, 283, 203–215. [Google Scholar] [CrossRef]
- Maestrini, B.; Basso, B. Drivers of within-field spatial and temporal variability of crop yield across the US Midwest. Sci. Rep. 2018, 8, 14833. [Google Scholar] [CrossRef] [PubMed]
- Amirahmadi, E.; Ghorbani, M.; Moudrý, J.; Bernas, J.; Mukosha, C.E.; Hoang, T.N. Environmental Assessment of Dryland and Irrigated Winter Wheat Cultivation under Compost Fertilization Strategies. Plants 2024, 13, 509. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, D.R.; Biggar, J.W.; Erh, K.T. Spatial variability of field-measured soil-water properties. Hilgardia 1973, 42, 215–259. [Google Scholar] [CrossRef]
- Perez-Quezada, J.F.; Pettygrove, G.S.; Plant, R.E. Spatial temporal analysis of yield and soil factors in two four-crop rotation fields in the Sacramento Valley, California. Agron. J. 2003, 95, 676–687. [Google Scholar]
- Sadras, V.O.; Villalobos, F.J.; Orgaz, F.; Fereres, E. Effects of water stress on crop production. In Principles of Agronomy for Sustainable Agriculture; Villalobos, F.J., Fereres, E., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 189–204. [Google Scholar] [CrossRef]
- Vaněk, V.; Balík, J.; Šilha, J.; Černý, J. Spatial variability of total soil nitrogen and sulphur content at two conventionally managed fields. Plant Soil Environ. 2008, 54, 413–419. [Google Scholar] [CrossRef]
- Xue, L.H.; Cao, W.X.; Luo, W.H.; Dai, T.B.; Zhu, Y. Monitoring leaf nitrogen status in rice with canopy spectral reflectance. Agron. J. 2004, 96, 135–142. [Google Scholar] [CrossRef]
- Feng, X.; Li, Y.; Zhao, Y.; Chen, J. Spatial Variability Analysis of Wheat Nitrogen Yield Response: A Case Study of Henan Province, China. Agronomy 2023, 13, 1796. [Google Scholar] [CrossRef]
- Neugschwandtner, R.W.; Bernhuber, A.; Kammlander, S.; Wagentristl, H.; Klimek-Kopyra, A.; Lošák, T.; Bernas, J.; Koppensteiner, L.J.; Zholamanov, K.K.; Ghorbani, M.; et al. Effect of Two Seeding Rates on Nitrogen Yield and Nitrogen Fixation of Winter and Spring Faba Bean. Plants 2023, 12, 1711. [Google Scholar] [CrossRef]
- Song, X.; Yang, G.; Yang, C.; Wang, J.; Cui, B. Spatial Variability Analysis of Within-Field Winter Wheat Nitrogen and Grain Quality Using Canopy Fluorescence Sensor Measurements. Remote Sens. 2017, 9, 237. [Google Scholar] [CrossRef]
- Clarke, D.E.; Stockdale, E.A.; Hannam, J.A.; Marchant, B.P.; Hallett, S.H. Spatial-temporal variability in nitrogen use efficiency: Insights from a long-term experiment and crop simulation modeling to support site specific nitrogen management. Eur. J. Agron. 2024, 158, 127224. [Google Scholar] [CrossRef]
- Faber, A.; Jarosz, Z.; Jadczyszyn, T. Nitrogen use efficiency of winter wheat on farms in Poland. Pol. J. Agron. 2016, 26, 21–25. [Google Scholar]
- Mittermayer, M.; Gilg, A.; Maidl, F.-X.; Nätscher, L.; Hülsbergen, K.-J. Site-specific nitrogen balances based on spatially variable soil and plant properties. Precis. Agric. 2021, 22, 1416–1436. [Google Scholar] [CrossRef]
- Grzebisz, W.; Szczepaniak, W.; Grześ, S. Sources of nutrients for high-yielding winter oilseed rape (Brassica napus L.) during post-flowering growth. Agronomy 2020, 10, 626. [Google Scholar] [CrossRef]
- Barłóg, P.; Grzebisz, W. Effect of timing and nitrogen fertilizers application on yielding of winter oilseed rape (Brassica napus L.). II. Nitrogen uptake dynamics and fertilizer efficiency. J. Agron. Crop Sci. 2004, 190, 314–323. [Google Scholar] [CrossRef]
- Łukowiak, R.; Grzebisz, W. Effect of Site Specific Nitrogen Management on Seed Nitrogen—A Driving Factor of Winter Oilseed Rape (Brassica napus L.) Yield. Agronomy 2020, 10, 1364. [Google Scholar] [CrossRef]
- Dalgaard, T.; Bienkowski, J.F.; Bleeker, A.; Dragosits, U.; Drouet, J.L.; Durand, P.; Frumau, A.; Hutchings, N.J.; Kedziora, A.; Magliulo, V.; et al. Farm nitrogen balances in six European landscapes as an indicator for nitrogen losses and basis for improved management. Biogeosciences 2012, 9, 5303–5321. [Google Scholar] [CrossRef]
- Chen, S.; Du, T.; Wang, S.; Parsons, D.; Wu, D.; Guo, X.; Li, D. Evaluation and simulation of spatial variability of soil property effects on deep percolation and nitrate leaching within a large-scale field in arid Northwest China. Sci. Total Environ. 2020, 732, 139324. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | Soil Depth | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0–30 cm | 30–60 cm | 60–90 cm | |||||||
| Mean | SD | CV, % | Mean | SD | CV, % | Mean | SD | CV, % | |
| Particle size fraction | |||||||||
| Sand 1, % | 74.5 | 2.0 | 2.7 | 66.5 | 4.0 | 6.1 | 58.7 | 2.9 | 4.9 |
| Coarse silt, % | 6.7 | 1.1 | 16.1 | 7.2 | 1.6 | 22.3 | 7.3 | 0.9 | 11.9 |
| Fine silt, % | 16.1 | 2.1 | 12.8 | 12.3 | 2.2 | 18.1 | 10.2 | 11.1 | 11.3 |
| Clay, % | 2.8 | 1.3 | 46.0 | 14.0 | 5.0 | 35.6 | 24.1 | 2.5 | 10.5 |
| Agrochemical properties | |||||||||
| pH 2 | 5.2 | 0.5 | 9.6 | 5.5 | 0.6 | 10.9 | 5.9 | 1.1 | 18.6 |
| TSC 3, g kg−1 | 12.42 | 12.35 | 21.45 | 5.65 | 5.47 | 9.82 | 3.68 | 3.54 | 6.39 |
| P 4, mg kg−1 | 106.4 | 26.4 | 24.8 | 60.1 | 40.5 | 67.3 | 25.3 | 9.4 | 37.2 |
| K 4, mg kg−1 | 258.2 | 55.0 | 21.3 | 107.2 | 48.6 | 45.3 | 147.8 | 40.2 | 27.2 |
| Mg 4, mg kg−1 | 251.3 | 42.4 | 16.9 | 282.5 | 64.7 | 22.9 | 304.6 | 71.1 | 23.3 |
| Ca 4, mg kg−1 | 742.2 | 275.2 | 37.1 | 803.4 | 413.6 | 51.5 | 1147.7 | 511.2 | 44.5 |
| Season/Crop | Agrotechnical Practices | |||||
|---|---|---|---|---|---|---|
| Liming | NPK Complex Fertilizer | Sowing Seeds | Mg, S Application | Spring N Application | Harvest | |
| 2018/2019 winter oilseed rape | 28 July 2018 (1.0 t ha−1 of CaCO3) | 16 August 2018 (18:26:75 kg ha−1 of N, P, and K) | 16 August 2018 | 21 February 2019 (15 and 20 kg ha−1 of Mg and S, respectively) | 1 March 2019 12 March 2019 (102 + 68 kg N ha−1) | 1 August 2019 |
| 2019/2020 winter wheat | 13 September 2019 (18:26:75 kg ha−1 of N, P, and K) | 12 October 2019 | 6 March 2020 7 April 2020 10 May 2020 (34 + 34 + 34 kg N ha−1) | 21 July 2020 | ||
| Sampling Date | Mean | SD | CV, % | Median | Min. | Max. | Skewness | Kurtosis | K–S Test, d |
|---|---|---|---|---|---|---|---|---|---|
| 2019, winter rape | |||||||||
| Spring | 66.3 | 15.5 | 23.4 | 64.8 | 42.9 | 110.2 | 0.76 | 0.48 | 0.087 |
| Harvest | 117.2 *** | 22.6 | 19.3 | 114.8 | 66.1 | 185.3 | 0.38 | 0.73 | 0.081 |
| 2020, winter wheat | |||||||||
| Spring | 137.3 | 43.6 | 31.7 | 142.0 | 51.2 | 241.0 | −0.29 | −0.31 | 0.118 |
| Harvest | 90.3 *** | 25.1 | 27.8 | 89.3 | 49.0 | 195.4 | 1.58 | 4.92 | 0.109 |
| Variables | Mean | SD | CV, % | Median | Min | Max | Skewness | Kurtosis | K–S Test, d |
|---|---|---|---|---|---|---|---|---|---|
| Ys, t ha−1 | 4.52 | 1.06 | 23.5 | 4.26 | 2.52 | 6.71 | 0.37 | −0.63 | 0.106 |
| Yr, t ha−1 | 10.38 | 3.34 | 32.2 | 9.74 | 6.43 | 24.62 | 0.31 | 0.61 | 0.141 |
| Ns, kg ha−1 | 151.0 | 34.4 | 22.8 | 146.1 | 90.0 | 232.8 | 0.49 | −0.43 | 0.073 |
| Nr, kg ha−1 | 90.7 | 33.4 | 36.9 | 84.1 | 49.1 | 206.4 | 1.50 | 2.07 | 0.176 * |
| Nt, kg ha−1 | 241.7 | 64.5 | 26.7 | 231.6 | 152.8 | 424.4 | 0.99 | 0.55 | 0.097 |
| NHI, % | 63.1 | 4.54 | 7.2 | 63.6 | 51.4 | 74.8 | −0.15 | 0.07 | 0.085 |
| UNU, kg t−1 | 53.5 | 6.1 | 11.3 | 53.2 | 41.6 | 71.7 | 0.34 | 0.22 | 0.071 |
| UNP, kg kg−1 | 29.9 | 1.7 | 5.8 | 29.9 | 25.9 | 34.0 | 0.13 | −0.29 | 0.062 |
| PFP, kg kg−1 | 19.2 | 4.4 | 23.1 | 19.0 | 11.3 | 29.2 | 0.18 | −0.52 | 0.050 |
| Variables | Mean | SD | CV, % | Median | Min | Max | Skewness | Kurtosis | K–S Test, d |
|---|---|---|---|---|---|---|---|---|---|
| Ys, t ha−1 | 7.78 | 1.12 | 14.4 | 7.87 | 5.34 | 10.89 | 0.19 | 0.02 | 0.068 |
| Yr, t ha−1 | 7.95 | 1.08 | 13.6 | 8.09 | 5.86 | 11.18 | 0.21 | 0.60 | 0.087 |
| Ns, kg ha−1 | 151.7 | 29.9 | 19.7 | 151.8 | 93.8 | 225.5 | 0.30 | −0.41 | 0.077 |
| Nr, kg ha−1 | 61.3 | 9.5 | 15.5 | 60.9 | 42.1 | 85.2 | 0.27 | −0.20 | 0.082 |
| Nt, kg ha−1 | 213.0 | 36.2 | 17.0 | 214.9 | 142.5 | 310.7 | 0.31 | −0.10 | 0.073 |
| NHI, % | 70.9 | 3.5 | 4.9 | 71.6 | 63.4 | 76.3 | −0.24 | −0.91 | 0.085 |
| UNU, kg t−1 | 27.4 | 2.9 | 10.7 | 27.5 | 21.5 | 33.5 | 0.03 | −0.64 | 0.065 |
| UNP, kg kg−1 | 37.1 | 4.3 | 11.7 | 36.4 | 29.8 | 49.1 | 0.57 | −0.04 | 0.087 |
| PFP, kg kg−1 | 33.1 | 5.3 | 15.9 | 33.5 | 24.9 | 48.9 | 0.50 | 0.06 | 0.094 |
| Variables | Mean | SD | CV, % | Median | Min | Max | Skewness | Kurtosis | K–S Test, d |
|---|---|---|---|---|---|---|---|---|---|
| Nb, kg ha−1 | 19.0 | 34.4 | 181.2 | 23.9 | −62.8 | 80.0 | −0.49 | −0.43 | 0.073 |
| NEb, % | 88.8 | 20.3 | 22.8 | 86.0 | 52.9 | 136.9 | 0.49 | −0.43 | 0.073 |
| Nin, kg ha−1 | 236.3 | 15.5 | 6.5 | 234.8 | 212.9 | 280.2 | 0.76 | 0.48 | 0.087 |
| Ninb, kg ha−1 | −5.4 | 63.8 | 1171.9 | −1.0 | −178.7 | 112.7 | −0.57 | 0.10 | 0.098 |
| Ngain, kg ha−1 | 122.7 | 70.3 | 57.3 | 118.2 | −29.6 | 334.1 | 0.50 | 0.48 | 0.112 |
| NinT, kg ha−1 | 359.0 | 70.8 | 19.7 | 345.4 | 244.5 | 579.8 | 0.92 | 0.78 | 0.141 |
| NEin, % | 64.0 | 14.2 | 22.1 | 63.1 | 38.1 | 97.5 | 0.19 | −0.49 | 0.052 |
| NEinT, % | 42.0 | 4.2 | 9.9 | 42.0 | 31.0 | 52.3 | 0.08 | 0.26 | 0.048 |
| Variables | Mean | SD | CV, % | Median | Min | Max | Skewness | Kurtosis | K–S Test, d |
|---|---|---|---|---|---|---|---|---|---|
| Nb, kg ha−1 | −49.7 | 29.9 | 60.2 | −49.8 | −123.5 | 8.2 | −0.30 | −0.41 | 0.078 |
| NEb, % | 148.7 | 29.3 | 19.7 | 148.8 | 92.0 | 221.1 | 0.30 | −0.41 | 0.078 |
| Nin, kg ha−1 | 239.3 | 43.6 | 18.2 | 244.0 | 153.2 | 343.0 | −0.29 | −0.31 | 0.118 |
| Ninb, kg ha−1 | 26.3 | 39.4 | 149.9 | 24.9 | −75.1 | 131.7 | 0.11 | 0.27 | 0.083 |
| Ngain, kg ha−1 | 64.0 | 49.9 | 78.0 | 62.3 | −73.6 | 239.6 | 0.34 | 2.07 | 0.066 |
| NinT, kg ha−1 | 303.3 | 48.4 | 16.0 | 301.8 | 226.1 | 473.4 | 0.76 | 1.08 | 0.082 |
| NEin, % | 64.4 | 11.8 | 18.3 | 62.9 | 38.2 | 95.7 | 0.26 | −0.04 | 0.072 |
| NEinT, % | 49.9 | 5.0 | 10.1 | 49.7 | 36.6 | 60.8 | −0.08 | 0.04 | 0.068 |
| Variables | R2 | Band Importance | |||
|---|---|---|---|---|---|
| B1 | B2 | B3 | B4 | ||
| 2019, winter oilseed rape | |||||
| Ys | 0.6719 | 0.4225 | 0.3095 | 0.2680 | 0.0000 |
| Nin | 0.5455 | 0.0123 | 0.4353 | 0.3133 | 0.2391 |
| Ninb | 0.6070 | 0.0000 | 0.6118 | 0.3882 | 0.0000 |
| 2020, winter wheat | |||||
| Yg | 0.7117 | 0.2998 | 0.0034 | 0.2232 | 0.4736 |
| Nin | 0.6222 | 0.0000 | 0.8878 | 0.0000 | 0.1122 |
| Ninb | 0.6943 | 0.0000 | 0.2511 | 0.0000 | 0.7489 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łukowiak, R.; Barłóg, P.; Ceglarek, J. Soil and Plant Nitrogen Management Indices Related to Within-Field Spatial Variability. Agronomy 2024, 14, 1845. https://doi.org/10.3390/agronomy14081845
Łukowiak R, Barłóg P, Ceglarek J. Soil and Plant Nitrogen Management Indices Related to Within-Field Spatial Variability. Agronomy. 2024; 14(8):1845. https://doi.org/10.3390/agronomy14081845
Chicago/Turabian StyleŁukowiak, Remigiusz, Przemysław Barłóg, and Jakub Ceglarek. 2024. "Soil and Plant Nitrogen Management Indices Related to Within-Field Spatial Variability" Agronomy 14, no. 8: 1845. https://doi.org/10.3390/agronomy14081845





