Improvement of Yield and Quality Properties of Radish by the Organic Fertilizer Application Combined with the Reduction of Chemical Fertilizer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site Description
2.2. Experimental Materials
2.2.1. Radish Variety
2.2.2. Fertilizer
2.3. Experimental Design
2.4. Measurement Indices and Methods
2.4.1. Measurement of Plant Growth Parameters
2.4.2. Measurement of Radish Quality Indices
2.4.3. Yield Determination
2.4.4. Determination of Physical and Chemical Properties of Soil
2.4.5. Soil DNA Extraction and 16s rDNA Sequencing
2.4.6. Soil Microbial Community Analysis
2.4.7. Statistical Analysis
3. Results
3.1. Effect of Chemical Fertilizer Reduction with Organic Fertilizer Application on Agronomic Traits of Radish
3.2. The Effect of Chemical Fertilizer Reduction with Organic Fertilizer on the Nutrient Content and Radish Quality
3.3. Effect of Chemical Fertilizer Reduction and Organic Fertilizer Application on Radish Yield
3.4. Effect of Chemical Fertilizer Reduction with Organic Fertilizer Application on Soil Nutrient Content
3.5. Effect of Chemical Fertilizer Reduction with Organic Fertilizer Application on Soil Enzyme Activity
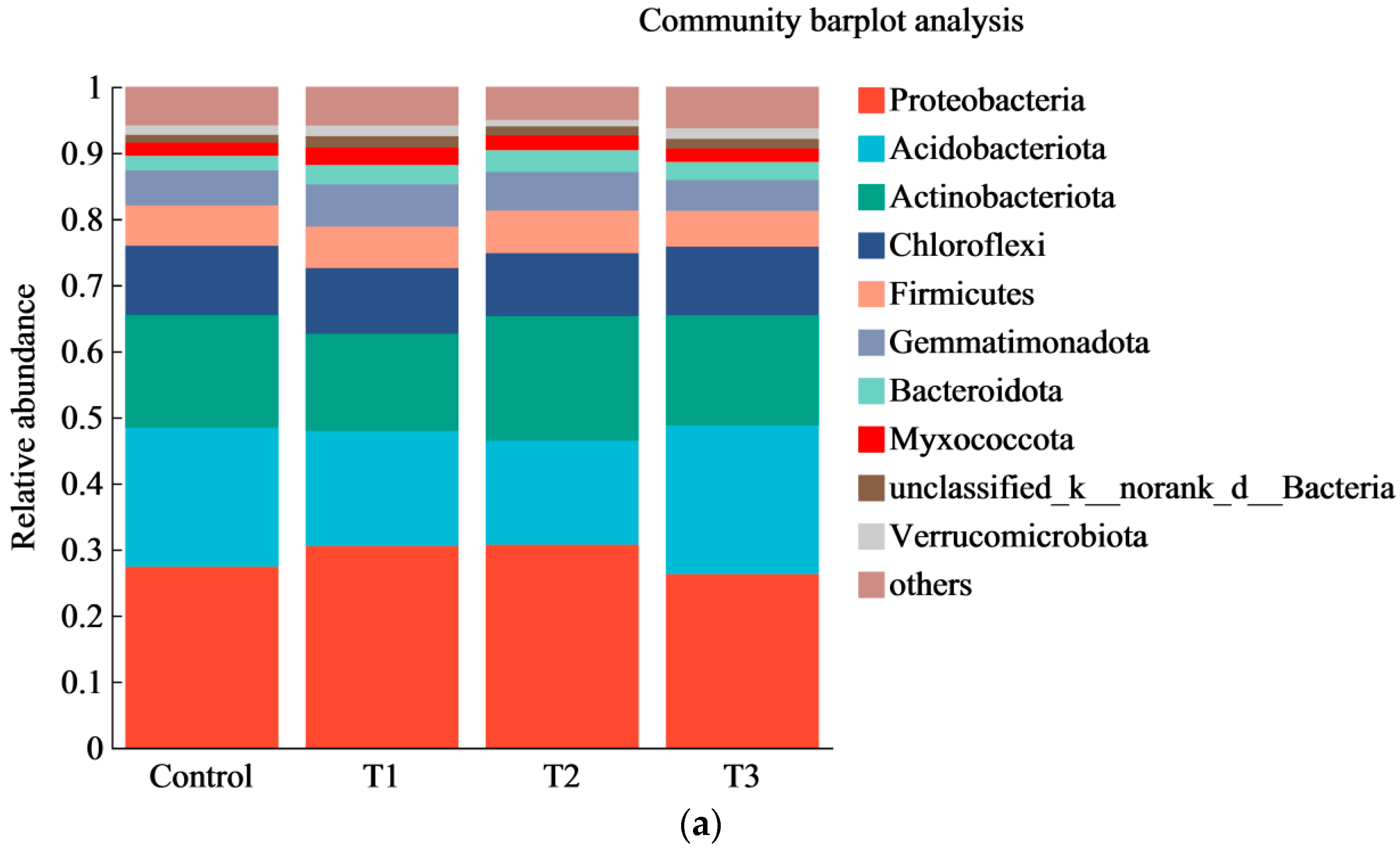
3.6. Effect of Chemical Fertilizer Reduction Combined with Organic Fertilizer Application on the Soil Bacterial Community
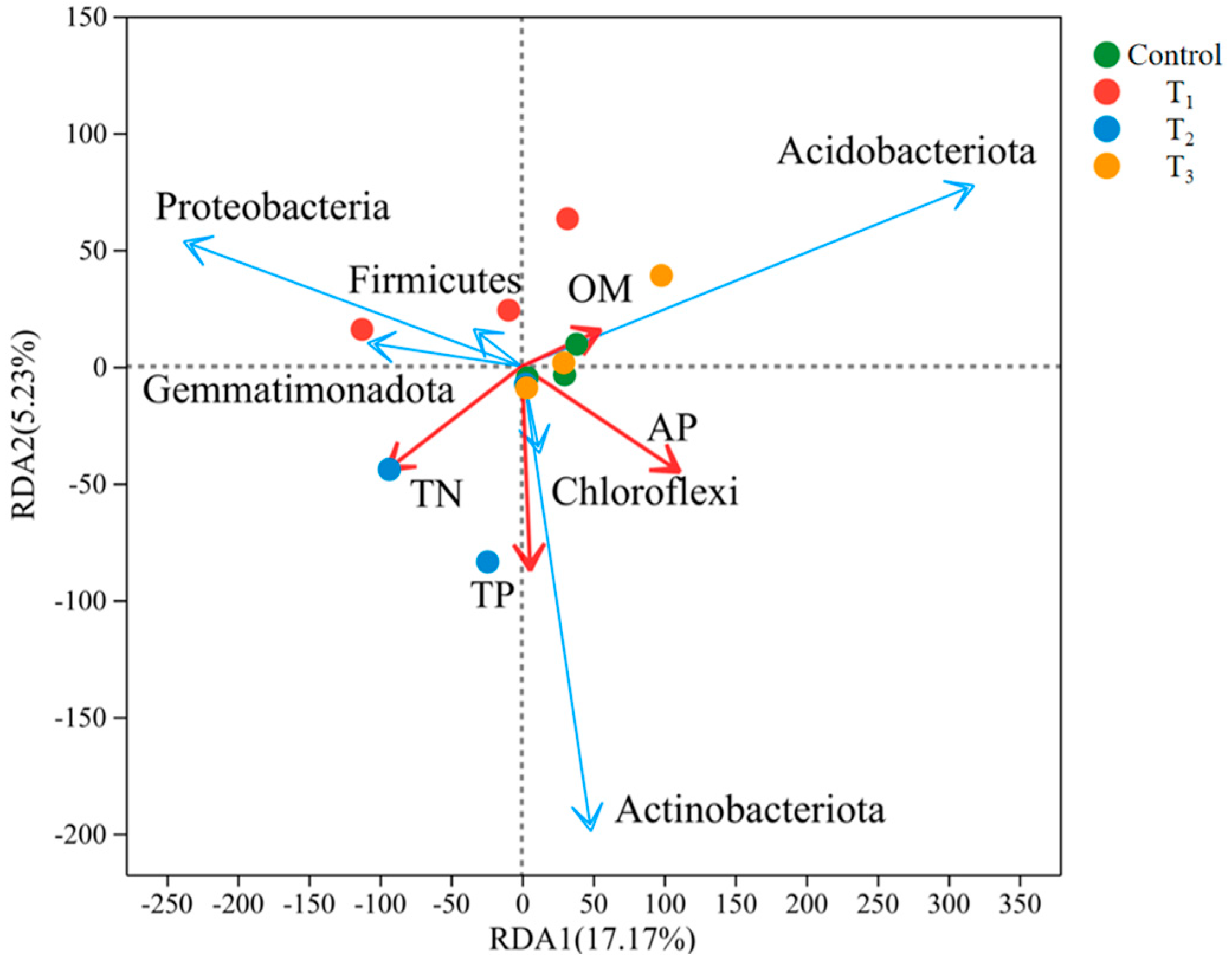
3.7. RDA Analysis of Soil Physicochemical Properties and Environmental Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, L.; Zhang, X.; Zhou, Z.; Lu, C.; Pan, X.; Deng, X. Comparison of extraction methods for insoluble dietary fiber from radish and their effects on biscuit digestion. Agric. Prod. Process. 2020, 14, 38–41. [Google Scholar]
- Shan, N.; Chuan, L.; Liu, J.; Zheng, H.; Zhao, T. Application effects of fertilizer recommendation by Nutrient Expert System on radish. J. Appl. Ecol. 2020, 31, 3719–3728. [Google Scholar]
- Shan, S.; Wei, Z.; Cheng, W.; Du, D.; Zheng, D.; Ma, G. Biofertilizer based on halotolerant microorganisms promotes the growth of rice plants and alleviates the effects of saline stress. Front. Microbiol. 2023, 14, 1165631. [Google Scholar]
- Du, G. Effects of different fertilization treatments on soil microbial communities in the Huang-Huai-Hai region. Ph.D. Dissertation, Graduate School of the Chinese Academy of Agricultural Sciences, Beijing, China, 2012. [Google Scholar]
- Li, D.; Wu, Z. Ecological environmental effects of chemical fertilizers. J. Appl. Ecol. 2008. (In Chinese) [Google Scholar]
- Sun, Z.; Li, X. Technical efficiency of chemical fertilizer use and its influencing factors in China’s rice production. Sustainability 2021, 13, 1155. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Sun, Z.; Xue, C.; Ma, W. Comparative study on yield and nutrient utilization efficiency of radish under different nutrient management measures. J. Hebei Agric. Univ. 2017, 40, 25–30. [Google Scholar]
- Fei, L.; Pan, Y.; Ma, H.; Guo, R.; Wang, M.; Ling, N.; Shen, Q.; Guo, S. Optimal organic-inorganic fertilization increases rice yield through source-sink balance during grain filling. Field Crops Res. 2024, 308, 109285. [Google Scholar]
- Xu, X.; Bi, R.; Song, M.; Dong, Y.; Jiao, Y.; Wang, B.; Xiong, Z. Organic substitutions enhanced soil carbon stabilization and reduced carbon footprint in a vegetable farm. Soil Tillage Res. 2024, 236, 105955. [Google Scholar]
- Wu, X.; Zhang, T.; Zhao, J.; Wang, L.; Yang, D.; Li, G.; Xiu, W. Variation of soil bacterial and fungal communities from fluvo-aquic soil under chemical fertilizer reduction combined with organic materials in North China Plain. J. Soil Sci. Plant Nutr. 2021, 21, 349–363. [Google Scholar]
- Shi, X.; Zhao, Y.; Xu, M.; Ma, L.; Adams, J.M.; Shi, Y. Insights into plant–microbe interactions in the rhizosphere to promote sustainable agriculture in the new crops era. New Crops 2024, 1, 100004. [Google Scholar]
- Zhang, P.; Li, J.; Wang, D. Effects of organic fertilizer on physiological characteristics, soil nutrients, and microorganisms of spring wheat. Jiangsu Agric. Sci. 2018, 46, 66–72. [Google Scholar]
- Niu, X.; Ju, X. Organic fertilizer resources and utilization in China. J. Plant Nutr. Fertil. 2017, 23, 1462–1479. [Google Scholar]
- Liu, X.; Wang, S.; Li, L.; Wang, W.; Mu, J. Changes in main agronomic traits during the fleshy root swelling process of radish. Shandong Agric. Sci. 2010, 4, 31–33. [Google Scholar]
- Ma, Y.; Shen, S.; Wan, C.; Wang, S.; Yang, F.; Zhang, K.; Gao, W. Organic fertilizer substitution over six years improves the productivity of garlic, bacterial diversity, and microbial communities network complexity. Appl. Soil Ecol. 2023, 182, 104718. [Google Scholar]
- Kakar, K.; Xuan, T.D.; Noori, Z.; Aryan, S.; Gulab, G. Effects of organic and inorganic fertilizer application on growth, yield, and grain quality of rice. Agriculture 2020, 10, 544. [Google Scholar] [CrossRef]
- Nafiu, Y.; Liu, Y.; Zhang, S.; Huang, J.; Han, T.; Du, J.; Muhammad, N.K.; Nano, A.D.; Lv, Z.; Hou, H.; et al. Phosphorus use efficiency, uptake and apparent balance response to substituting long-term chemical fertilizer with organic fertilizer in a double-rice cropping system. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Singh, V.K.; Kumar, R.; Kumar, N. Determining the Effect of Organic and Inorganic Fertilizers on Growth and Yield in Wheat (Triticum aestivum L.). Int. J. Plant Soil Sci. 2024, 36, 549–555. [Google Scholar]
- Hu, M.; Andrew, J.W.; Shen, W.; Zhong, Z.; Qiu, C.; Lin, X. Effects of organic fertilizers produced using different techniques on rice grain yield and ammonia volatilization in double-cropping rice fields. Pedosphere 2024, 34, 110–120. [Google Scholar]
- Çokkızgın, A.; Girgel, Ü.; Kara, Z.; Çölkesen, M.; Saltalı, K.; Yürürdurmaz, C. The effect of organic fertilizers on the yield components of corn plant, protein and starch content of grain. Harran Tarım Ve Gıda Bilim. Derg. 2022, 26, 133–142. [Google Scholar]
- Wang, T.; Wang, J.; Jia, Y.; Wang, Y.; Bao, W.; Zhang, G. Study on quality and volatile substances of different radish varieties. J. Food Saf. Qual. 2023, 14, 23. [Google Scholar]
- Gamba, M.; Asllanaj, E.; Raguindin, P.F.; Glisic, M.; Franco, O.H.; Minder, B.; Bussler, W.; Metzger, B.; Kern, H.; Muka, T. Nutritional and phytochemical characterization of radish (Raphanus sativus): A systematic review. Trends Food Sci. Technol. 2021, 113, 205–218. [Google Scholar]
- Zhao, G.; Zhang, L.; Zuo, Q.; Wang, X.; Dou, T.; Bian, X. Effects of potassium nutrition on nutrient uptake and yield of radish in alpine cold region. North. Hortic. 2010, 14, 5–8. [Google Scholar]
- Zhang, X.; Guan, Y.; Wu, L.; Wang, K.; Qin, A.; Zhang, Q. Radish mechanization precision sowing cultivation technology. North. Hortic. 2021, 15, 174–177. [Google Scholar]
- Zhao, Y.; Dai, Y.; Cui, X.; Zhang, W.; Ma, N. Determination of soluble protein content in Radix AuControllandiae by Coomassie brilliant blue G-250 staining method. J. Yunnan Minzu Univ. Nat. Sci. Ed. 2006, 15, 235–237. [Google Scholar]
- Leng, F.; Sun, S.; Jing, Y.; Wang, F.; Wei, Q.; Wang, X.; Zhu, X. A rapid and sensitive method for determination of trace amounts of glucose by anthrone-sulfuric acid method. Bulg. Chem. Commun. 2016, 48, 109–113. [Google Scholar]
- Xiao, Y.; Lei, E.; Guan, X.; Guan, X. Comparison of two methods for determining reduced vitamin C in vegetables. Hubei Agric. Sci. 2011, 50, 1035–1037. [Google Scholar]
- Hua, B.; Qiu, Y.; Duan, Y.; Cui, N.; Zhang, X.; Shen, D.; Li, X. Analysis and evaluation of glucosinolate content in radish (Raphanus sativus L.) germplasm resources. J. Plant Genet. Resour. 2013, 14, 1038–1044. [Google Scholar]
- Lu, R. Soil Agricultural Chemical Analysis Methods; China Agricultural Science and Technology Press: Beijing, China, 2000. [Google Scholar]
- Guo, H.; Yao, J.; Cai, M.; Qian, Y.; Guo, Y.; Richnow, H.H.; Blake, R.E.; Doni, S.; Ceccanti, B. Effects of petroleum contamination on soil microbial numbers, metabolic activity and urease activity. Chemosphere 2012, 87, 1273–1280. [Google Scholar]
- Gao, M.; Song, W.; Zhou, Q.; Ma, X.; Chen, X. Interactive effect of oxytetracycline and lead on soil enzymatic activity and microbial biomass. Environ. Toxicol. Pharmacol. 2013, 36, 667–674. [Google Scholar]
- Yang, L.; Zeng, Q.; Li, H.; Yan, J. Determination of soil catalase activity by ultraviolet spectrophotometry. Soil Bull. 2011, 42, 207–210. [Google Scholar]
- Cao, H.; Sun, H.; Yang, H.; Sun, B.; Zhao, Q. Research progress on soil enzyme activity and its indication of soil quality. J. Appl. Environ. Biol. 2003, 9, 105–109. [Google Scholar]
- Liu, C.; Zhao, D.; Ma, W.; Guo, Y.; Wang, A.; Wang, Q.; Lee, D.J. Denitrification and sulfide removal from saline wastewater by salt-tolerant bacteria. Appl. Microbiol. Biotechnol. 2016, 100, 1421–1426. [Google Scholar] [PubMed]
- Lan, Z.; Zhang, Y.; Liang, R.; Wang, Z.; Sun, J.; Lu, X.; Wang, Y. Comprehensive comparison of integrated fixed-film activated sludge (IFAS) and AAO activated sludge methods: Influence of different operational parameters. Chemosphere 2024, 357, 142068. [Google Scholar] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Mago, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Callahan, B.J.; Mcmurdie, P.J.; Rosen, M.J. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Xu, L. Effects of organic-inorganic combined application on yield, quality, and soil fertility of Chinese cabbage under plastic greenhouse cultivation. Jiangsu Agric. Sci. 2021, 49, 109–114. [Google Scholar]
- Zhao, Y.; Cui, J.; Xie, H.; Pei, H.; Gao, J. Effects of different organic fertilizers on the growth, development, and yield of sand-cultured tomatoes. Guizhou Agric. Sci. 2017, 45, 69–71. [Google Scholar]
- Shi, Y.; Niu, X.; Chen, B.; Pu, S.; Ma, H.; Li, P.; Ma, X. Chemical fertilizer reduction combined with organic fertilizer affects the soil microbial community and diversity and yield of cotton. Front. Microbiol. 2023, 14, 1295722. [Google Scholar]
- Zhang, Y.; Fan, X.; Xu, G.; Yang, Y.; Li, Y.; Sun, Y. Effects of different nitrogen and organic fertilizer combinations on garlic yield and quality. Jiangsu Agric. Sci. 2019, 47, 114–117. [Google Scholar]
- Tang, G.; Zhou, X.; Tian, C.; Peng, H.; Zhang, Y.; Rong, X. Effects of organic and inorganic nitrogen fertilizer combinations on vegetable yield, quality, and economic benefits. Chin. J. Ecol. 2017, 36, 1292–1299. [Google Scholar]
- Qiu, W.; Yao, X.; Lu, H.; Guo, J.; Lu, D. Effects of different fertilization treatments on yield, quality, and nitrogen uptake efficiency of spring-sown sweet corn. J. Yangzhou Univ. Agric. Life Sci. Ed. 2023, 6, 26–35. [Google Scholar] [CrossRef]
- Zhou, J.; Tian, W.; Zhao, S.; Xu, D.; Han, W.; Chen, X. Effects of optimized fertilization on yield, quality, and nutrient utilization efficiency of radish-cabbage rotation system in Emei Mountain region. Hubei Agric. Sci. 2023, 62, 21. [Google Scholar]
- Wang, X.; Zhang, P.; Zhang, J.; Ji, X.; Zhang, X.; Liang, J. Regulation of fertilization patterns on root system morphology construction and yield formation of soybean in loess plateau area. Soybean Sci. 2021, 40, 813–820. [Google Scholar]
- Zhang, T.; Liu, Y.; Zhu, G.; Zhang, C.; Zhang, Y.; Yao, Q.; Sun, Z. Effects of localized application of chemical fertilizer combined with cow manure on vegetable yield and soil fertility. Chin. J. Soil Fertil. 2021, 1, 161–168. [Google Scholar]
- Wang, N.; Nan, H.; Feng, K. Effects of reduced chemical fertilizer application combined with organic fertilizer on soil microbial biomass, enzyme activity, and cotton yield in cotton field. Chin. J. Appl. Ecol. 2020, 31, 173. [Google Scholar]
- Kabir, A.H.; Baki, M.Z.I.; Ahmed, B.; Mostofa, M.G. Current, faltering, and future strategies for advancing microbiome-assisted sustainable agriculture and environmental resilience. New Crops 2024, 1, 100013. [Google Scholar]
- Kou, C.; Song, F.; Li, D.; Xu, H.; Zhang, S.; Yang, W.; Shi, W.; Gao, Z. A necessary considering factor for crop resistance: Precise regulation and effective utilization of beneficial microorganisms. New Crops 2024, 1, 100023. [Google Scholar]
- Wang, X.; Zhu, M.; Yang, F.; Dou, P.; Zhang, J.; Ma, X.; Kong, F. Effects of organic fertilizer combined with reduced nitrogen on soil microbial biomass and enzyme activity in hilly area of Sichuan Province. J. Soil Water Conserv. 2017, 31, 271–276. [Google Scholar]
- Hu, W.; Jiao, Z.; Wu, F.; Liu, Y.; Dong, M.; Ma, X.; Fan, T.; An, L.; Feng, H. Long-term effects of fertilizer on soil enzymatic activity of wheat field soil in Loess Plateau, China. Ecotoxicology 2014, 23, 2069–2080. [Google Scholar]
- Sun, S. Study on the Effects of Microbial Fertilizers and Controlled-Release Chemical Fertilizers on Potato Growth, Yield, and Quality. Ph.D. Dissertation, Inner Mongolia Agricultural University, Hohhot, China, 2019. [Google Scholar]
- Zhou, X.; Liu, P.; Chang, J.; Guo, L.; Zhang, Q.; Ma, Y. Effects of Pig Manure Substitution for Chemical Fertilizers on Tea Yield, Quality, and Soil Fertility. Soil Bull. 2022, 2, 413–420. [Google Scholar] [CrossRef]
- Zhu, L.; Cao, M.; Sang, C.; Chen, R.; Xu, S.; Li, L.; Liu, T. Effects of Biological Organic Fertilizer Substituting Chemical Fertilizer on Soil Fertility and Enzyme Activity in Maize. J. Sichuan Agric. Univ. 2022, 40, 67–72. [Google Scholar]
- Zhou, G.; Wang, X.; Ma, Y.; Wang, X. Effects of Different Biogas Slurry Organic Fertilizer Treatments on Yield, Disease Resistance, and Quality of Autumn Cucumber. J. Hebei Agric. Univ. 2017, 40, 39–44. [Google Scholar]
- Qu, C.; Chen, X.; Han, Z.; Zhang, X.; Huang, C.; Liu, Y. Effects of Applying Biological Organic Fertilizer on Soil Fertility Characteristics and Enzyme Activity at Different Growth Stages of Cucumber. J. Soil Water Conserv. 2017, 6, 279–284. [Google Scholar]
- Li, R.; Tao, R.; Wang, D.; Chu, G. Effects of Reduced Nitrogen Combined with Organic Fertilizer on Soil Biological Properties and Aggregate Characteristics in Drip-Irrigated Cotton Field. J. Appl. Ecol. 2017, 28, 3297–3304. [Google Scholar]
- Shang, L.; Wan, L.; Zhou, X.; Li, S.; Li, X. Effects of organic fertilizer on soil nutrient status, enzyme activity, and bacterial community diversity in Leymus chinensis steppe in Inner Mongolia, China. PLoS ONE 2020, 15, e0240559. [Google Scholar]
- Sun, R.; Guo, X.; Wang, D.; Chu, H. The impact of long-term application of chemical fertilizers and straw returning on soil bacterial community. Microbiol. China 2015, 42, 2049–2057. [Google Scholar]
- Mayerhofer, H.; Mueller-DieControlmann, J. Cloning, expression, purification and preliminary X-ray analysis of the dimerization domain of ethylene response sensor 1 (ERS1) from Arabidopsis thaliana. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2013, 69, 1029–1032. [Google Scholar]
- Fierer, N.; Bradford, M.A.; JaControlson, R.B. Toward an ecological classification of soil bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar]
- Kirchman, D.L. The ecology of Cytophaga–Flavobacteria in aquatic environments. FEMS Microbiol. Ecol. 2002, 39, 91–100. [Google Scholar]
- Singh, U.; Choudhary, A.K.; Sharma, S. A 3-year field study reveals that agri-management practices drive the dynamics of dominant bacterial taxa in the rhizosphere of Cajanus cajan. Symbiosis 2022, 86, 215–227. [Google Scholar]
- Yan, D.; Zhang, T.; Su, J.; Zhao, L.L.; Wang, H.; Fang, X.M.; Zhang, Y.Q.; Liu, H.Y.; Yu, L.Y. Structural variation in the bacterial community associated with airborne particulate matter in Beijing, China, during hazy and nonhazy days. Appl. Environ. Microbiol. 2018, 84, e00004-18. [Google Scholar] [PubMed]
- Ai, C.; Zhang, S.; Zhang, X.; Guo, D.; Zhou, W.; Huang, S. Distinct responses of soil bacterial and fungal communities to changes in fertilization regime and crop rotation. Geoderma 2018, 319, 156–166. [Google Scholar]
- Liang, Z.; Yu, Y.; Ye, Z.; Li, G.; Wang, W.; An, T. Pollution profiles of antibiotic resistance genes associated with airborne opportunistic pathogens from typical area, Pearl River Estuary and their exposure risk to human. Environ. Int. 2020, 143, 105934. [Google Scholar]
- Hartmann, M.; Frey, B.; Mayer, J.; Mäder, P.; Widmer, F. Distinct soil microbial diversity under long-term organic and conventional farming. ISME J. 2015, 9, 1177–1194. [Google Scholar]
- Bi, Q. Microbiological Mechanisms of Soil Phosphorus Availability and Carbon-Nitrogen-Phosphorus Coupling Transformation Affected by Fertilization Modes and Years of Cultivation. Ph.D. Dissertation, Zhejiang University, Hangzhou, China, 2020. [Google Scholar]
- Lee, S.H.; Ka, J.O.; Cho, J.C. Members of the phylum Acidobacteria are dominant and metabolically active in rhizosphere soil. FEMS Microbiol. Lett. 2008, 285, 263–269. [Google Scholar]
- Li, H. Effects of Fertilization Levels on Growth of Super Hybrid Rice and Rhizosphere Soil Characteristics. Master’s Thesis, Hunan University, Changsha, China, 2018. [Google Scholar]
- Yuan, H.; Wu, H.; Ge, T.; Li, K.; Wu, J.; Wang, J. Effects of Long-Term Fertilization on Bacterial and Archaeal Diversity and Community Structure in Rice Field Soil. Chin. J. Appl. Ecol. 2015, 26, 1807–1813. [Google Scholar]
- Yu, B. Effects of Long-Term Different Fertilization Treatments on the Degradation of 13C-Labeled Maize Straw and Associated Microorganisms in Red Soil. Ph.D. Dissertation, Chinese Academy of Agricultural Sciences, Beijing, China, 2017. [Google Scholar]
- Rousk, J.; Bååth, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Knight, R.; Fierer, N. Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 2010, 4, 1340–1351. [Google Scholar]
- Zhang, Y. Effects of Fertilization Gradient on Soil Environment and Maize Growth under Furrow Mulching Cultivation. Ph.D. Dissertation, Northwest A&F University, Xianyang, China, 2020. [Google Scholar]
- Chai, Z. Analysis of Soil Bacterial Community Structure in Different Origins of Ginger and the Effects of Fertilization. Master’s Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, 2021. [Google Scholar]
- Lu, Y.; Liao, Y.; Nie, J.; Zhou, X.; Xie, J.; Yang, Z. Evolution Characteristics of Phosphorus in Long-Term Fertilized Red Paddy Soils and Its Response to Phosphorus Surplus or Deficiency. Acta Pedol. Sin. 2017, 54, 1471–1485. [Google Scholar]

| Property | TN g/kg | HN mg/kg | TP g/kg | AP mg/kg | pH kg | OM % |
|---|---|---|---|---|---|---|
| Original soil sample | 1.22 | 96.0 | 0.016 | 102 | 5.77 | 12.98 |
| Treatments | Organic Fertilizer (kg·ha−1) | Compound Chemical Fertilizer (kg·ha−1) | Reduction of Chemical Fertilizer (%) |
|---|---|---|---|
| Control | 4500 | 375 | 0% |
| T1 | 4500 | 330 | 12% |
| T2 | 4500 | 300 | 20% |
| T3 | 4500 | 270 | 28% |
| Treatments | Maximum Functional Leaf Width (cm) | Root Diameter at Harvest Time (mm) | Long Root Length at Harvest Time (cm) | Leaf Dry Weight (kg plant−1) | Dry Weight of Fleshy Roots (kg plant−1) | Thick Skin (mm) |
|---|---|---|---|---|---|---|
| Control | 15.75 ± 0.75 c | 56.67 ± 4.05 b | 41.50 ± 0.58 c | 0.18 ± 0.19 bc | 0.34 ± 0.01 c | 3.82 ± 0.23 a |
| T1 | 18.50 ± 1.45 b | 56.45 ± 1.56 b | 43.00 ± 0.82 b | 0.22 ± 0.20 a | 0.50 ± 0.01 a | 3.23 ± 0.14 b |
| T2 | 23.25 ± 1.48 a | 62.21 ± 2.74 a | 44.50 ± 0.58 a | 0.18 ± 0.18 b | 0.49 ± 0.01 a | 2.94 ± 0.07 c |
| T3 | 19.08 ± 0.79 b | 56.30 ± 1.17 b | 41.75 ± 0.50 c | 0.15 ± 0.17 c | 0.45 ± 0.01 b | 3.00 ± 0.05 c |
| Treatments | Vitamin C (mg/g. FW) | Soluble Sugar (mg/g. FW) | Sulforaphane (mg/g. FW) | Soluble Solids (%) | Titratable Acid (%) | Cellulose (mg/g. FW) |
|---|---|---|---|---|---|---|
| Control | 56.38 ± 1.14 b | 52.93 ± 0.07 c | 0.06 ± 0.00 c | 3.94 ± 0.06 c | 0.41 ± 0.01 c | 164.33 ± 3.22 b |
| T1 | 61.97 ± 1.75 a | 54.13 ± 0.09 a | 0.08 ± 0.00 ab | 5.04 ± 0.08 a | 0.60 ± 0.01 a | 193.00 ± 5.29 a |
| T2 | 62.37 ± 1.00 a | 54.07 ± 0.08 ab | 0.09 ± 0.00 a | 5.00 ± 0.13 a | 0.59 ± 0.01 a | 147.67 ± 6.66 c |
| T3 | 57.75 ± 1.26 b | 53.63 ± 0.43 b | 0.08 ± 0.00 b | 4.71 ± 0.40 b | 0.54 ± 0.02 b | 132.33 ± 7.23 d |
| Treatments | Per Plant Yield (g) | Equivalent Total Yield (kg h−1) | Yield Increase Rate (%) |
|---|---|---|---|
| Control | 1493.80 ± 67.59 b | 26,189.24 ± 1185.01 b | - |
| T1 | 1586.68 ± 61.23 b | 27,817.67 ± 1073.49 b | 6.22 |
| T2 | 1778.42 ± 99.43 a | 29,572.10 ± 1516.26 a | 12.92 |
| T3 | 1573.58 ± 54.20 b | 27,588.06 ± 950.23 b | 5.34 |
| Treatments | Organic Matter g/kg | TN % | TP % | HN mg/kg | AP mg/kg |
|---|---|---|---|---|---|
| Control | 19.47 ± 0.23 b | 1.04 ± 0.05 b | 0.07 ± 0.00 b | 91.03 ± 0.25 a | 70.27 ± 0.49 a |
| T1 | 19.67 ± 0.25 ab | 1.10 ± 0.04 ab | 0.07 ± 0.00 b | 90.90 ± 3.82 a | 63.60 ± 2.10 b |
| T2 | 19.93 ± 0.25 ab | 1.12 ± 0.02 a | 0.08 ± 0.00 a | 88.27 ± 3.59 a | 68.03 ± 1.10 a |
| T3 | 20.37 ± 0.76 a | 1.05 ± 0.02 b | 0.07 ± 0.00 a | 89.93 ± 2.29 a | 70.43 ± 0.67 a |
| Treatments | S-UE μg/d/g | S-SC mg/d/g | S-AKP μmol/d/g | S−CAT mmol/d/g |
|---|---|---|---|---|
| Control | 455.33 ± 27.50 ab | 4.56 ± 0.35 c | 1.06 ± 0.09 b | 15.00 ± 0.50 b |
| T1 | 416.00 ± 28.00 c | 5.28 ± 0.35 b | 1.26 ± 0.03 a | 14.57 ± 0.35 b |
| T2 | 506.00 ± 40.45 a | 6.58 ± 0.18 a | 1.34 ± 0.12 a | 16.40 ± 0.82 a |
| T3 | 490.67 ± 27.65 a | 6.39 ± 0.19 a | 0.87 ± 0.06 c | 12.97 ± 0.55 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, D.; Lu, Z.; Song, X.; Ahammed, G.J.; Yan, Y.; Chen, S. Improvement of Yield and Quality Properties of Radish by the Organic Fertilizer Application Combined with the Reduction of Chemical Fertilizer. Agronomy 2024, 14, 1847. https://doi.org/10.3390/agronomy14081847
Jin D, Lu Z, Song X, Ahammed GJ, Yan Y, Chen S. Improvement of Yield and Quality Properties of Radish by the Organic Fertilizer Application Combined with the Reduction of Chemical Fertilizer. Agronomy. 2024; 14(8):1847. https://doi.org/10.3390/agronomy14081847
Chicago/Turabian StyleJin, Duo, Zewei Lu, Xiangcan Song, Golam Jalal Ahammed, Yan Yan, and Shuangchen Chen. 2024. "Improvement of Yield and Quality Properties of Radish by the Organic Fertilizer Application Combined with the Reduction of Chemical Fertilizer" Agronomy 14, no. 8: 1847. https://doi.org/10.3390/agronomy14081847






