Genome-Wide Association Study of Yield-Related Traits in a Nested Association Mapping Population Grown in Kazakhstan
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Experimental Design
2.2. Genome-Wide Association Studies and Data Analysis
3. Results
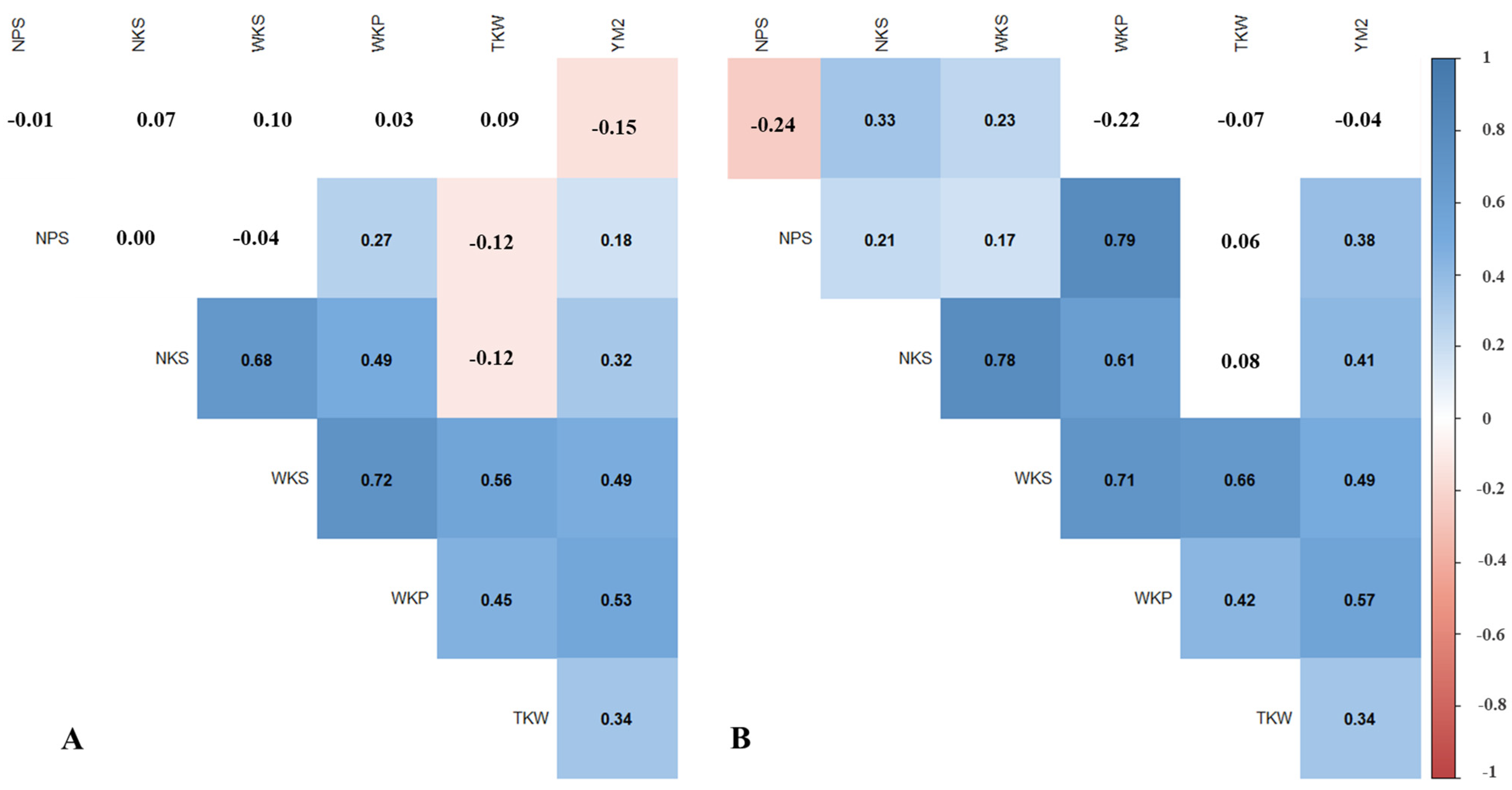
3.1. Phenotypic Variation of NAM Population for Yield-Related Traits
3.2. Assessment of GGE the NAM Population in the Two Studied Regions
3.3. NKS, TKW, and YM2 Assessment of Individual RILs in the Two Locations
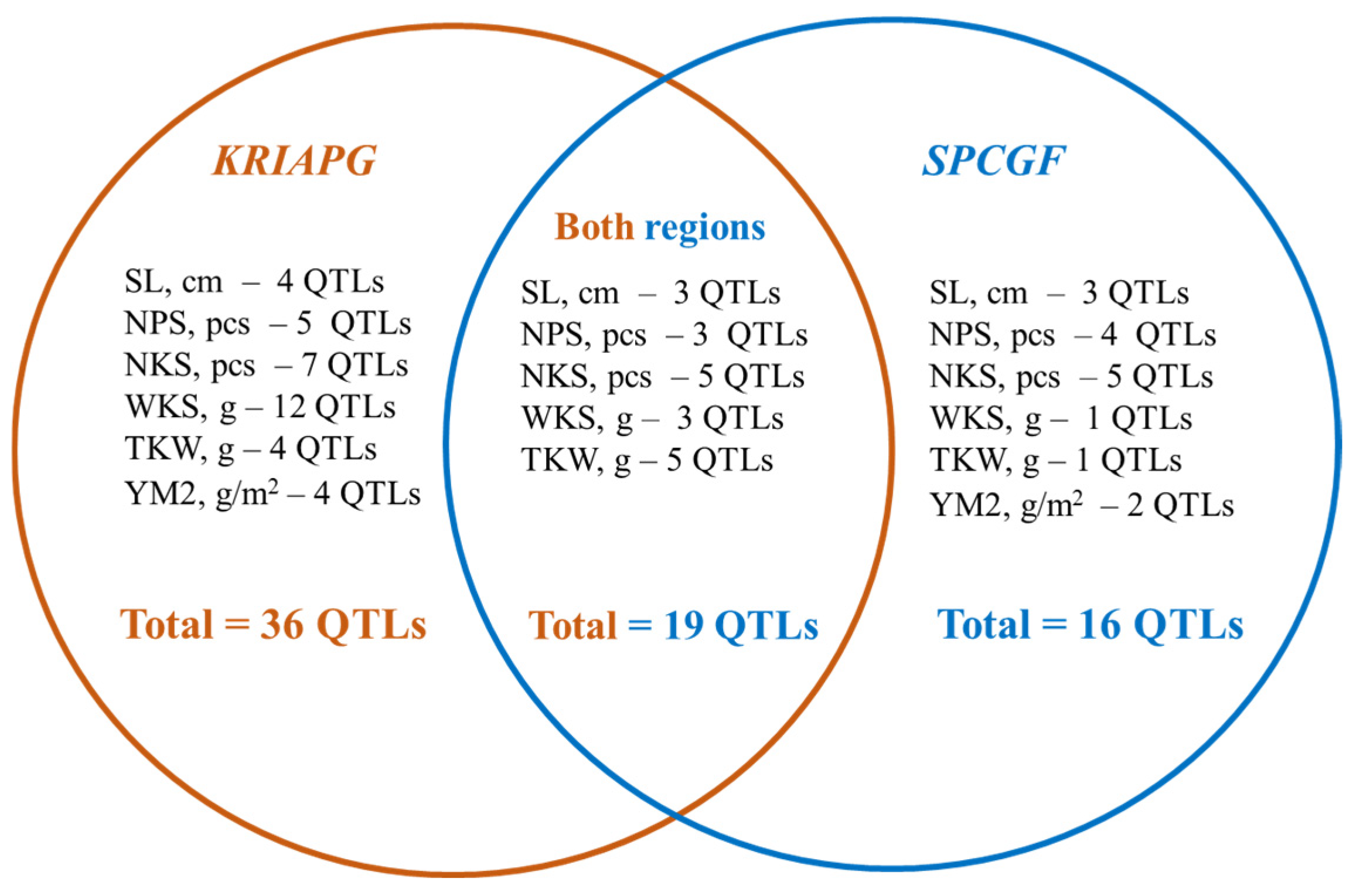
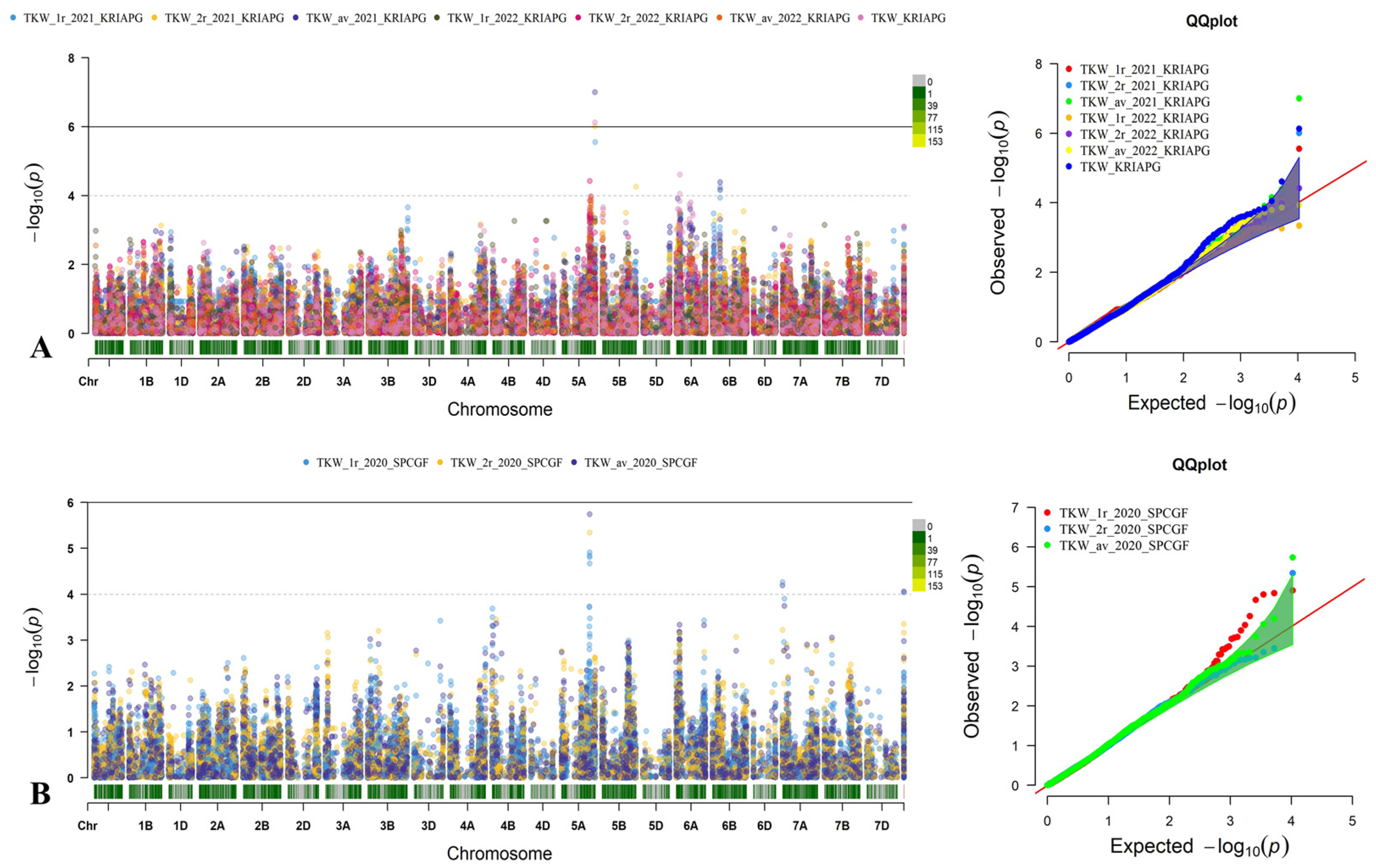
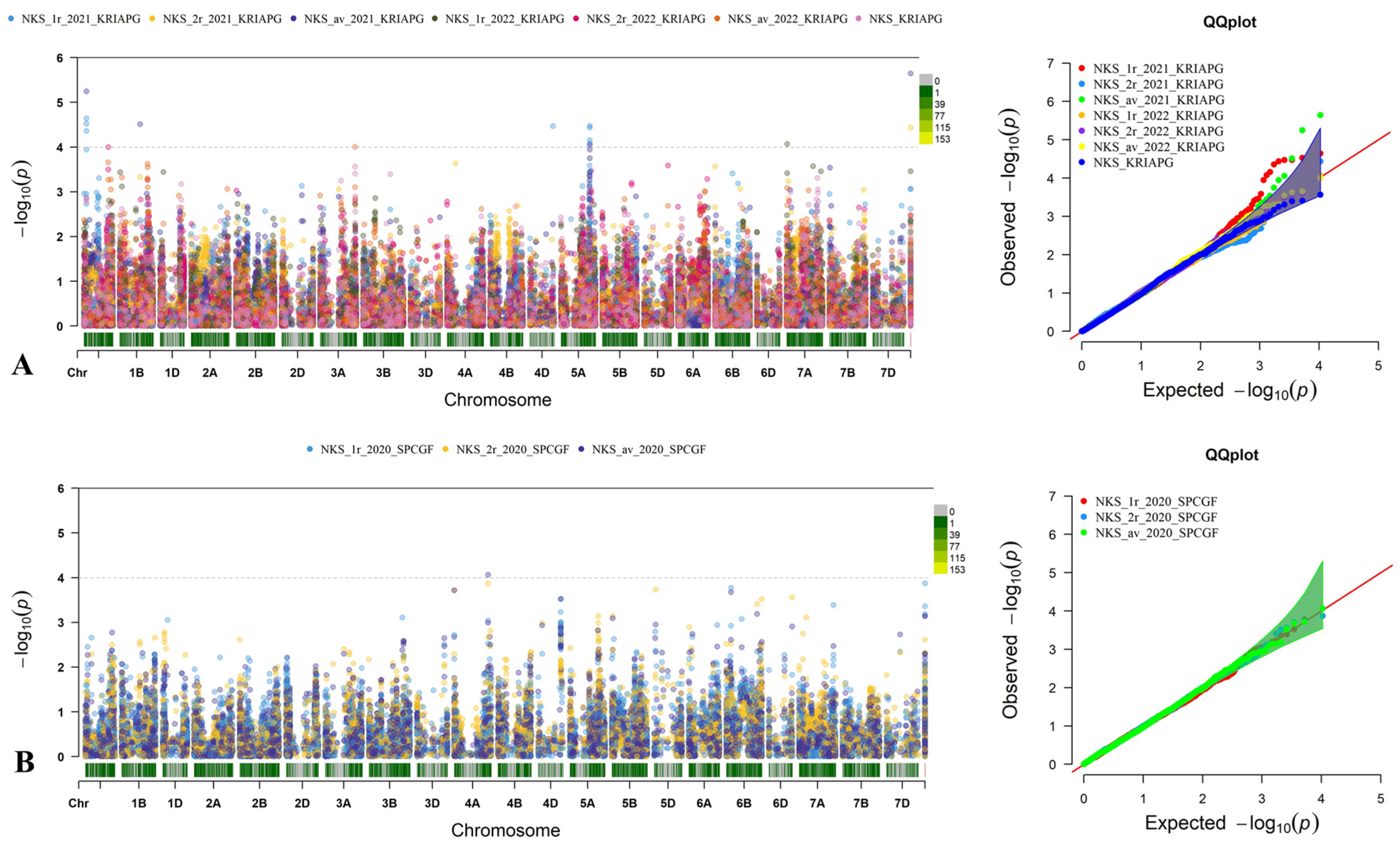
3.4. Identification of Quantitative Trait Loci for Studied Traits Associated with Yield Components
3.5. Putative Candidate Genes and SSR Markers Associated with QTLs
3.6. The Effect of QTLs Associated with NKS and TKW Identified Two Regions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/faostat/en/#home (accessed on 25 April 2024).
- United Nations World Population Prospects 2019: Highlights. Available online: https://population.un.org/wpp/Publications/Files/WPP2019_Highlights.pdf (accessed on 15 April 2024).
- Curtis, B.C.; Rajaram, S.; Gómez Macpherson, H. (Eds.) . Bread Wheat: Improvement and Production; Food and Agriculture Organization of the United Nations: Rome, Italy, 2002; 554p. [Google Scholar]
- Shiferaw, B.; Smale, M.; Braun, H.J.; Duveiller, E.; Reynolds, M.; Muricho, G. Crops that feed the world 10. Past successes and future challenges to the role played by wheat in global food security. Food Secur. 2013, 5, 291–317. [Google Scholar] [CrossRef]
- USDA. Commodity Intelligence Report. Kazakhstan: Better MY 2022/2023 Wheat Prospects Than Last Year. Available online: https://ipad.fas.usda.gov/highlights/2022/08/Kazakhstan/index.pdf (accessed on 20 April 2024).
- Abugalieva, A.; Peña-Bautista, R.J. Grain quality of spring and winter wheat of Kazakhstan. Asian Australas. J. Plant Sci. Biotech. 2010, 4, 87–90. [Google Scholar]
- Abugaliyeva, A.I.; Morgounov, A.I. Genetic Potential of Winter Wheat Grain Quality in Central Asia. Int. J. Environ. Sci. Educ. 2016, 11, 4869–4884. [Google Scholar]
- Amalova, A.; Yermekbayev, K.; Griffiths, S.; Abugalieva, S.; Babkenov, A.; Fedorenko, E.; Abugalieva, A.; Turuspekov, Y. Identification of quantitative trait loci of agronomic traits in bread wheat using a Pamyati Azieva× Paragon mapping population harvested in three regions of Kazakhstan. PeerJ 2022, 10, e14324. [Google Scholar] [PubMed]
- Amalova, A.; Abugalieva, S.; Babkenov, A.; Babkenova, S.; Turuspekov, Y. Genome-wide association study of yield components in spring wheat collection harvested under two water regimes in Northern Kazakhstan. PeerJ 2021, 9, e11857. [Google Scholar] [PubMed]
- Morgounov, A.; Sonder, K.; Abugalieva, A.; Bhadauria, V.; Cuthbert, R.D.; Shamanin, V.; Zelenskiy, Y.; DePauw, R.M. Effect of climate change on spring wheat yields in North America and Eurasia in 1981–2015 and implications for breeding. PLoS ONE 2018, 13, e0204932. [Google Scholar]
- Aidarkhanov, B.; Atabaeva, S.; Tursunov, O. Influence of climate conditions on the productivity of spring wheat in Northern Kazakhstan. J. Agric. Sci. 2020, 25, 789–798. [Google Scholar]
- Nurbekov, A.; Atabayeva, S.; Koishybayev, M. Advances in wheat production and breeding in Kazakhstan. J. Agric. Sci. Technol. 2015, 17, 345–356. [Google Scholar]
- Karatayev, M.; Clarke, M.; Salnikov, V.; Bekseitova, R.; Nizamova, M. Monitoring climate change, drought conditions and wheat production in Eurasia: The case study of Kazakhstan. Heliyon 2022, 8, e08660. [Google Scholar] [CrossRef]
- Sadras, V.O.; Calderini, D.F. (Eds.) Crop Physiology: Applications for Genetic Improvement and Agronomy; Academic Press: Cambridge, MA, USA, 2009; pp. 571–580. [Google Scholar]
- Slafer, G.A.; Savin, R.; Sadras, V.O. Coarse and fine regulation of wheat yield components in response to genotype and environment. Field Crops Res. 2014, 157, 71–83. [Google Scholar]
- Lobell, D.B.; Gourdji, S.M. The influence of climate change on global crop productivity. Plant Physiol. 2012, 160, 1686–1697. [Google Scholar]
- Nicotra, A.B.; Atkin, O.K.; Bonser, S.P.; Davidson, A.M.; Finnegan, E.J.; Mathesius, U.; Poot, P.; Purugganan, M.D.; Richards, C.L.; Valladares, F.; et al. Plant phenotypic plasticity in a changing climate. Trends Plant Sci. 2010, 15, 684–692. [Google Scholar] [CrossRef]
- Reynolds, M.P.; Bonnett, D.; Chapman, S.C.; Furbank, R.T.; Manès, Y.; Mather, D.E.; Parry, M.A.J. Raising yield potential in wheat. J. Exp. Bot. 2009, 62, 439–448. [Google Scholar]
- Li, L.; Bian, Y.; Dong, Y.; Song, J.; Liu, D.; Zeng, J.; Wang, F.; Zhang, Y.; He, Z.; Xia, X.; et al. Identification and validation of stable quantitative trait loci for yield component traits in wheat. Crop J. 2023, 11, 558–563. [Google Scholar] [CrossRef]
- Yi, X.; Ye, Y.; Wang, J.; Li, Z.; Li, J.; Chen, Y.; Ma, J.; Pu, Z.; Peng, Y.; Qi, P.; et al. Identification and validation of two major QTLs for spikelet number per spike in wheat (Triticum aestivum L.). Front. Plant Sci. 2023, 14, 1144486. [Google Scholar] [CrossRef]
- Simmonds, J.; Scott, P.; Brinton, J.; Mestre, T.C.; Bush, M.; Del Blanco, A.; Dubcovsky, J.; Uauy, C. A splice acceptor site mutation in TaGW2-A1 increases thousand grain weight in tetraploid and hexaploid wheat through wider and longer grains. Theor. Appl. Genet. 2016, 129, 1305–1316. [Google Scholar] [CrossRef]
- Chidzanga, C.; Mullan, D.; Roy, S.; Baumann, U.; Garcia, M. Nested association mapping-based GWAS for grain yield and related traits in wheat grown under diverse Australian environments. Theor. Appl. Genet. 2022, 135, 4437–4456. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, Y.; Hakim, M.S.; Al-Yossef, A.; Saleh, M.M.; Shaaban, A.S.A.D.; Kabbaj, H.; Zaim, M.; Kleinerman, C.; Bassi, F.M. Genomic regions involved in the control of 1,000-kernel weight in wild relative-derived populations of durum wheat. Front. Plant Sci. 2023, 14, 1297131. [Google Scholar] [CrossRef]
- Aisawi, K.A.B.; Reynolds, M.P.; Singh, R.P.; Foulkes, M.J. The physiological basis of the genetic progress in yield potential of CIMMYT spring wheat cultivars from 1966 to 2009. Crop Sci. 2015, 55, 1749–1764. [Google Scholar]
- Thanh, P.T.; Vladutu, C.I.; Kianian, S.F.; Thanh, P.T.; Ishii, T.; Nitta, M.; Nasuda, S.; Mori, N. Molecular genetic analysis of domestication traits in emmer wheat. I: Map construction and QTL analysis using an F2 population. Biotechnol. Biotechnol. Equip. 2013, 27, 3627–3637. [Google Scholar] [CrossRef]
- Groos, C.; Robert, N.; Bervas, E.; Charmet, G. Genetic analysis of grain protein-content, grain yield and thousand-kernel weight in bread wheat. Theor. Appl. Genet. 2003, 106, 1032–1040. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Cui, F.; Fan, Z.; Li, J.; Ding, A.; Wang, H. Genetic analysis of important loci in the winter wheat backbone parent Aimengniu. Aust. J. Crop Sci. 2013, 7, 182. [Google Scholar]
- Li, B.; Li, Q.; Mao, X.; Li, A.; Wang, J.; Chang, X.; Hao, C.; Zhang, X.; Jing, R. Two novel AP2/EREBP transcription factor genes TaPARG have pleiotropic functions on plant architecture and yield-related traits in common wheat. Front. Plant Sci. 2016, 7, 11–91. [Google Scholar] [CrossRef]
- Varshney, R.K.; Roorkiwal, M.; Sorrells, M.E. Genomics-guided breeding for crop improvement. Annu. Rev. Plant Biol. 2014, 65, 583–606. [Google Scholar]
- Tshikunde, N.M.; Mashilo, J.; Shimelis, H.; Odindo, A. Agronomic and physiological traits, and associated quantitative trait loci (QTL) affecting yield response in wheat (Triticum aestivum L.): A review. Front. Plant Sci. 2019, 10, 1428. [Google Scholar]
- Tester, M.; Langridge, P. Breeding technologies to increase crop production in a changing world. Science 2010, 327, 818–822. [Google Scholar] [PubMed]
- Zanke, C.D.; Ling, J.; Plieske, J.; Kollers, S.; Ebmeyer, E.; Korzun, V.; Röder, M.S. Analysis of main effect QTL for thousand-grain weight in European winter wheat (Triticum aestivum L.) by means of a high-density SNP map. Theor. Appl. Genet. 2015, 128, 1785–1800. [Google Scholar]
- Guo, Z.; Chen, D.; Alqudah, A.M.; Röder, M.S.; Ganal, M.W.; Schnurbusch, T. Genome-wide association analyses of 54 traits identified multiple loci for the determination of floret fertility in wheat. New Phytol. 2017, 214, 257–270. [Google Scholar]
- Jaiswal, V.; Gahlaut, V.; Meher, P.K.; Mir, R.R.; Jaiswal, J.P.; Rao, A.R.; Balyan, H.S.; Gupta, P.K. Genome Wide Single Locus Single Trait, Multi-Locus and Multi-Trait Association Mapping for Some Important Agronomic Traits in Common Wheat (T. aestivum L.). PLoS ONE 2016, 11, e0159343. [Google Scholar] [CrossRef]
- Sukumaran, S.; Lopes, M.; Dreisigacker, S.; Reynolds, M.P. Genetic analysis of multi-environmental spring wheat trials identifies genomic regions for locus-specific trade-offs for grain weight and grain number. Theor. Appl. Genet. 2015, 128, 1747–1760. [Google Scholar]
- Oyiga, B.C.; Sharma, R.C.; Baum, M.; Ogbonnaya, F.C.; Léon, J.; Ballvora, A. Allelic variations and differential expressions detected at quantitative trait loci for salt stress tolerance in wheat. Plant Cell Environ. 2017, 41, 919–935. [Google Scholar] [CrossRef] [PubMed]
- Quarrie, S.A.; Steed, A.; Calestani, C.; Semikhodskii, A.; Lebreton, C.; Chinoy, C.; Steele, N.; Pljevljakusic, D.; Waterman, E.; Weyen, J.; et al. A high-density genetic map of hexaploid wheat (Triticum aestivum L.) from the cross Chinese spring × SQ1 and its use to compare QTLs for grain yield across a range of environments. Theor Appl Genet. 2005, 110, 865–880. [Google Scholar] [PubMed]
- Turuspekov, Y.; Baibulatova, A.; Yermekbayev, K.; Tokhetova, L.; Chudinov, V.; Sereda, G.; Ganal, M.; Griffiths, S.; Abugalieva, S. GWAS for plant growth stages and yield components in spring wheat (Triticum aestivum L.) harvested in three regions of Kazakhstan. BMC Plant Biol. 2017, 17, 190. [Google Scholar]
- Huang, X.; Han, B. Natural variations and genome-wide association studies in crop plants. Annu. Rev. Plant Biol. 2014, 65, 531–551. [Google Scholar] [CrossRef]
- Yu, J.; Holland, J.B.; McMullen, M.D.; Buckler, E.S. Genetic design and statistical power of nested association mapping in maize. Genetics 2008, 178, 539–551. [Google Scholar]
- Gireesh, C.; Sundaram, R.M.; Anantha, S.M.; Pandey, M.K.; Madhav, M.S.; Rathod, S.; Yathish, K.R.; Senguttuvel, P.; Kayani, B.M.; Ranjith, E.; et al. Nested association mapping (NAM) populations: Present status and future prospects in the genomics era. Crit. Rev. Plant Sci. 2021, 40, 49–67. [Google Scholar] [CrossRef]
- Khan, H.; Krishnappa, G.; Kumar, S.; Mishra, C.N.; Krishna, H.; Devate, N.B.; Rathan, N.; Parkash, O.; Singh, G.P. Genome-wide association study for grain yield and component traits in bread wheat (Triticum aestivum L.). Front. Genet. 2022, 13, 982589. [Google Scholar] [CrossRef]
- Ren, D.; Fang, X.; Jiang, P.; Zhang, G.; Hu, J.; Wang, X.; Meng, Q.; Cui, W.; Lan, S.; Ma, X.; et al. Genetic architecture of nitrogen-deficiency tolerance in wheat seedlings based on a nested association mapping (NAM) population. Front. Plant Sci. 2018, 9, 845. [Google Scholar]
- Sallam, A.H.; Manan, F.; Bajgain, P.; Martin, M.; Szinyei, T.; Conley, E.; Brown-Guedira, G.; Muehlbauer, G.J.; Anderson, J.A.; Steffenson, B.J. Genetic architecture of agronomic and quality traits in a nested association mapping population of spring wheat. Plant Genome 2020, 13, e20051. [Google Scholar] [CrossRef]
- Basavaraddi, P.A.; Savin, R.; Sukumaran, S.; Reynolds, M.P.; Griffiths, S.; Slafer, G.A. Genotypic differences in wheat yield determinants within a NAM population based on elite parents. Eur. J. Agron. 2021, 123, 126223. [Google Scholar] [CrossRef]
- Donaire, G.; Vanzetti, L.S.; Conde, M.B.; Bainotti, C.; Mir, L.; Borrás, L.; Chicaiza, O.; Helguera, M. Dissecting genetic loci of yield, yield components, and protein content in bread wheat nested association mapping population. Euphytica 2023, 219, 65. [Google Scholar] [CrossRef]
- Wingen, L.U.; West, C.; Leverington-Waite, M.; Collier, S.; Orford, S.; Goram, R.; Yang, C.; King, J.; Allen, A.; Burridge, A.; et al. Wheat landrace genome diversity. Genetics 2017, 205, 1657–1676. [Google Scholar]
- Wingen, L.U.; Orford, S.; Goram, R.; Leverington-Waite, M.; Bilham, L.; Patsiou, T.S.; Ambrose, M.; Dicks, J.; Griffiths, S. Establishing the AE Watkins landrace cultivar collection as a resource for systematic gene discovery in bread wheat. Theor. Appl. Genet. 2014, 127, 1831–1842. [Google Scholar]
- Dospekhov, B. Methods of Field Experience; Kolos: Moscow, Russia, 1985. [Google Scholar]
- RStudio Team. RStudio: Integrated Development for R; RStudio. Inc.: Boston, FL, USA, 2015; Available online: http://www.maizegenetics.net/GAPIT (accessed on 20 January 2024).
- Covarrubias-Pazaran, G.; Martini, J.W.; Quinn, M.; Atlin, G. Strengthening public breeding pipelines by emphasizing quantitative genetics principles and open source data management. Front. Plant Sci. 2021, 12, 681624. [Google Scholar] [CrossRef]
- VSN International. The Guide to the Genstat Command Language (Release 20), Part 2 Statistics; VSN International: Hemel Hempstead, UK, 2019. [Google Scholar]
- Allen, A.M.; Barker, G.L.; Berry, S.T.; Coghill, J.A.; Gwilliam, R.; Kirby, S.; Robinson, P.; Brenchley, R.C.; D’Amore, R.; McKenzie, N.; et al. Transcript-specific. single-nucleotide polymorphism discovery and linkage analysis in hexaploid bread wheat (Triticum aestivum L.). Plant Biotechnol. J. 2011, 9, 1086–1099. [Google Scholar]
- Pritchard, J.K.; Stephens, M.; Donnelly., P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software STRUCTURE: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [PubMed]
- Segura, V.; Vilhjálmsson, B.J.; Platt, A.; Korte, A.; Seren, Ü.; Long, Q.; Nordborg, M. An efficient multi-locus mixed-model approach for genome-wide association studies in structured populations. Nat. Genet. 2012, 44, 825–830. [Google Scholar] [PubMed]
- Lipka, A.E.; Tian, F.; Wang, Q.; Peiffer, J.; Li, M.; Bradbury, P.J.; Core, M.; Buckler, E.; Zhang, Z. GAPIT: Genome association and prediction integrated tool. Bioinformatics 2012, 28, 2397–2399. [Google Scholar] [PubMed]
- Yin, L.; Zhang, H.; Tang., Z.; Xu., J.; Yin., D.; Zhang., Z.; Yuan., X.; Zhu., M.; Zhao., S.; Li., X.; et al. rMVP: A memory-efficient, visualization-enhanced, and parallel-accelerated tool for genome-wide association study. Genom. Proteom. Bioinform. 2021, 19, 619–628. [Google Scholar]
- Voorrips, R.E. MapChart: Software for the graphical presentation of linkage maps and QTLs. J. Hered. 2002, 93, 77–78. [Google Scholar]
- Ensembl Plants. Available online: https://plants.ensembl.org/Triticum_aestivum/Info/Index (accessed on 15 February 2024).
- Zhang, J.; Hao, C.; Ren, Q.; Chang, X.; Liu, G.; Jing, R. Association mapping of dynamic developmental plant height in common wheat. Planta 2011, 234, 891–902. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.S.; Li, X.; Yang, Z.J.; Liao, M.L. A new reduced height gene found in the tetraploid semi-dwarf wheat landrace Aiganfanmai. Genet. Mol. Res. 2011, 10, 2349–2357. [Google Scholar] [CrossRef]
- Zhang, B.; Shi, W.; Li, W.; Chang, X.; Jing, R. Efficacy of pyramiding elite alleles for dynamic development of plant height in common wheat. Mol. Breed. 2013, 32, 327–338. [Google Scholar] [CrossRef]
- Shariatipour, N.; Heidari, B.; Tabmasebi, A.; Richards, C. Comparative genomic analysis of quantitative trait loci associated with micronutrient contents, grain quality, and agronomic traits in wheat (Triticum aestivum L.). Front. Plant Sci. 2021, 12, 709–817. [Google Scholar] [CrossRef]
- Amalova, A.; Yessimbekova, M.; Ortaev, A.; Rsaliyev, S.; Griffiths, S.; Burakhoja, A.; Turuspekov, Y.; Abugalieva, S. Association Mapping of quantitative trait loci for agronomic traits in a winter wheat collection grown in Kazakhstan. Agronomy 2023, 13, 2054. [Google Scholar] [CrossRef]
- Würschum, T.; Leiser, W.L.; Langer, S.M.; Tucker, M.R.; Longin, C.F.H. Phenotypic and genetic analysis of spike and kernel characteristics in wheat reveals long-term genetic trends of grain yield components. Theor. Appl. Genet. 2018, 131, 2071–2084. [Google Scholar] [CrossRef]
- Li, Y.; Tang, J.; Liu, W.; Yan, W.; Sun, Y.; Che, J.; Tian, C.; Zhang, H.; Yu, L. The genetic architecture of grain yield in spring wheat based on genome-wide association study. Front. Genet. 2021, 12, 728472. [Google Scholar] [CrossRef]
- Wang, J.S.; Liu, W.H.; Wang, H.; Li, L.H.; Wu, J.; Yang, X.M.; Li, X.; Gao, A. QTL mapping of yield-related traits in the wheat germplasm. Euphytica 2011, 177, 277–292. [Google Scholar] [CrossRef]
- Amalova, A.; Abugalieva, S.; Chudinov, V.; Sereda, G.; Tokhetova, L.; Abdikhalyk, A.; Turuspekov, Y. QTL mapping of agronomic traits in wheat using the UK Avalon× Cadenza reference mapping population grown in Kazakhstan. PeerJ 2021, 9, e10733. [Google Scholar] [CrossRef] [PubMed]
- Abugalieva, S. QTL analysis of productivity and its components in common wheat in the conditions of south-east Kazakhstan. Newsl. Kazakh Agrar. (Res. Results) 2007, 2, 35–40. (In Russian) [Google Scholar]
- Yuan, Q.; Deng, Z.; Peng, T.; Tian, J. QTL-based analysis of heterosis for number of grains per spike in wheat using DH and immortalized F 2 populations. Euphytica 2012, 188, 387–395. [Google Scholar] [CrossRef]
- Cui, F.; Zhao, C.; Li, J.; Ding, A.; Li, X.; Bao, Y.; Li, J.; Ji, J.; Wang, H. Kernel weight per spike: What contributes to it at the individual QTL level? Mol. Breed. 2013, 31, 265–278. [Google Scholar] [CrossRef]
- Cuthbert, J.L.; Somers, D.J.; Brûlé-Babel, A.L.; Brown, P.D.; Crow, G.H. Molecular mapping of quantitative trait loci for yield and yield components in spring wheat (Triticum aestivum L.). Theor. Appl. Genet. 2008, 117, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Bai, G.; Carver, B.F.; Chao, S.; Wang, Z. Single nucleotide polymorphism markers linked to QTL for wheat yield traits. Euphytica 2015, 206, 89–101. [Google Scholar] [CrossRef]
- Jain, M.; Nijhawan, A.; Arora, R.; Agarwal, P.; Ray, S.; Sharma, P.; Kapoor, S.; Tyagi, A.K.; Khurana, J.P. F-box proteins in rice. Genome-wide analysis, classification, temporal and spatial gene expression during panicle and seed development, and regulation by light and abiotic stress. Plant Physiol. 2007, 143, 1467–1483. [Google Scholar] [CrossRef]
- Hong, M.J.; Kim, D.Y.; Kang, S.Y.; Kim, D.S.; Kim, J.B.; Seo, Y.W. Wheat F-box protein recruits proteins and regulates their abundance during wheat spike development. Mol. Biol. Rep. 2012, 39, 9681–9696. [Google Scholar] [CrossRef]
- Wang, L.; Shen, R.; Chen, L.T.; Liu, Y.G. Characterization of a novel DUF1618 gene family in rice. J. Integr. Plant Biol. 2014, 56, 151–158. [Google Scholar] [CrossRef]
- Shewry, P.R.; Halford, N.G. Cereal seed storage proteins: Structures, properties and role in grain utilization. J. Exp. Bot. 2002, 53, 947–958. [Google Scholar] [CrossRef]
- Wei, K.; Li, Y. Functional genomics of the protein kinase superfamily from wheat. Mol. Breed. 2019, 39, 1–23. [Google Scholar] [CrossRef]
- Collard, B.C.Y.; Mackill, D.J. Marker-assisted selection: An approach for precision plant breeding in the twenty-first century. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 557–572. [Google Scholar] [CrossRef]


| Site/Region | KRIAPG (Almaty Region, Southeastern Kazakhstan) | SPCGF (Akmola Region, Northern Kazakhstan) | |
|---|---|---|---|
| Latitude/longitude | 43°21′/76°53′ | 51°40′/71°00′ | |
| Soil type | Light chestnut (humus 2.0–2.5%) | Southern carbonate chernozem (humus 3.6%) | |
| Conditions | Rainfed | Rainfed | |
| Year | 2021 | 2022 | 2020 |
| Annual rainfall, mm | 183 | 250 | 426 |
| Mean temperature, °C | 21.8 | 22.2 | 19.2 |
| Max temperature, °C | 27.4 | 26.5 | 20.7 |
| Min temperature, °C | 12.4 | 16.7 | 17.6 |
| Traits | Factor | Df | Sum Sq | Mean Sq | F-Value | hb2 |
|---|---|---|---|---|---|---|
| SL, cm | Genotype (G) | 271 | 1209.8 | 4.5 | 5.525 | 26.8% |
| Environment (E) | 2 | 2023.0 | 1011.5 | 1251.946 | ||
| G:E | 540 | 627.7 | 1.2 | 1.439 | ||
| Residuals | 813 | 656.9 | 0.8 | |||
| NPS, pcs | Genotype (G) | 271 | 70.86 | 0.26 | 0.734 | 21.4% |
| Environment (E) | 2 | 78.84 | 39.42 | 110.719 | ||
| G:E | 540 | 161.49 | 0.30 | 0.84 | ||
| Residuals | 813 | 289.46 | 0.36 | |||
| NKS, pcs | Genotype (G) | 271 | 34,845 | 129 | 2.808 | 11.8% |
| Environment (E) | 2 | 52,234 | 26,117 | 570.299 | ||
| G:E | 540 | 38,454 | 71 | 1.555 | ||
| Residuals | 813 | 37,231 | 46 | |||
| WKS, g | Genotype (G) | 271 | 47.65 | 0.176 | 2.885 | 23.1% |
| Environment (E) | 2 | 58.40 | 29.198 | 479.062 | ||
| G:E | 540 | 50.63 | 0.094 | 1.538 | ||
| Residuals | 813 | 49.55 | 0.061 | |||
| TKW, g | Genotype (G) | 271 | 22,012 | 81 | 10.003 | 37.8% |
| Environment (E) | 2 | 17,070 | 8535 | 1051.107 | ||
| G:E | 540 | 12,535 | 23 | 2.859 | ||
| Residuals | 813 | 6602 | 8 | |||
| YM2, g/m2 | Genotype (G) | 271 | 8,347,006 | 30,801 | 5.76 | 15.0% |
| Environment (E) | 2 | 28,891,641 | 14,445,821 | 2701.583 | ||
| G:E | 540 | 13,998,647 | 25,923 | 4.848 | ||
| Residuals | 813 | 4,347,248 | 5347 |
| Kazakh Research Institute of Agriculture and Plant Growing (Almaty region) | |||||
| RILs | NKS, pcs | RILs | TKW, g | RILs | YM2, g |
| NAM-049 | 49.33 | NAM-002 | 35.38 | NAM-032 | 388.23 |
| NAM-081 | 50.25 | NAM-045 | 35 | NAM-081 | 374.96 |
| NAM-094 | 49.33 | NAM-069 | 36.13 | NAM-141 | 348.84 |
| NAM-261 | 49.17 | NAM-164 | 38.43 | NAM-163 | 374.72 |
| NAM-266 | 49.92 | NAM-175 | 33.5 | NAM-198 | 384.97 |
| NAM-268 | 49.92 | NAM-197 | 33.5 | NAM-272 | 337.09 |
| NAM-273 | 50.17 | NAM-198 | 36.6 | NAM-273 | 357.77 |
| NAM-284 | 49.67 | NAM-205 | 34.85 | NAM-274 | 389.95 |
| NAM-295 | 49.42 | NAM-207 | 34.78 | NAM-276 | 342.94 |
| NAM-299 | 52.00 | NAM-208 | 33.45 | NAM-290 | 334.07 |
| NAM-307 | 50.92 | NAM-220 | 36.4 | NAM-299 | 400.58 |
| NAM-326 | 51.42 | NAM-308 | 35.05 | NAM-300 | 381.67 |
| Kaz 4 | 35.33 | Kaz 4 | 29.80 | Kaz 4 | 333.66 |
| Min * | 22.46 | Min * | 18.30 | Min * | 47.08 |
| Max * | 52.00 | Max * | 38.43 | Max * | 400.58 |
| Mean ± SE * | 39.25 ± 0.34 | Mean ± SE * | 27.33 ± 0.21 | Mean ± SE * | 226.82 ± 3.81 |
| Alexandr Barayev Scientific-Production Center for Grain Farming (Shortandy region) | |||||
| RILs | NKS, pcs | RILs | TKW, g | RILs | YM2, g |
| NAM-011 | 48.2 | NAM-047 | 43.5 | NAM-168 | 755.4 |
| NAM-065 | 46.1 | NAM-164 | 43.3 | NAM-193 | 758.6 |
| NAM-193 | 44.5 | NAM-197 | 45.5 | NAM-272 | 745.5 |
| NAM-255 | 43.2 | NAM-198 | 42 | NAM-275 | 1069.3 |
| NAM-262 | 44.4 | NAM-205 | 50 | NAM-282 | 834 |
| NAM-266 | 46.4 | NAM-206 | 41.9 | NAM-297 | 770.4 |
| NAM-294 | 48.3 | NAM-207 | 41.8 | NAM-318 | 739.2 |
| NAM-297 | 44.6 | NAM-223 | 42.4 | NAM-321 | 756 |
| NAM-333 | 44 | NAM-286 | 44.7 | NAM-328 | 794.2 |
| NAM-334 | 46 | NAM-303 | 42.7 | NAM-333 | 762 |
| Astana | 27.6 | Astana | 37.32 | Astana | 382.13 |
| Min * | 15.70 | Min * | 11.97 | Min * | 32.73 |
| Max * | 48.25 | Max * | 50.03 | Max * | 1069.32 |
| Mean ± SE * | 31.40 ± 0.35 | Mean ± SE * | 32.91 ± 0.33 | Mean ± SE * | 387.33 ± 9.91 |
| QTL | Chr | Allele | KRIAPG | SPCGF | ||||
|---|---|---|---|---|---|---|---|---|
| NKS, pcs | TKW, g | YM2, g/m2 | NKS, pcs | TKW, g | YM2, g/m2 | |||
| QNKS.ta.NAM.ipbb-1A.1 | 1A | G | −1.36 | 0.03 | −12.38 | −0.23 | 0.32 | 16.91 |
| QNKS.ta.NAM.ipbb-1A.2 | 1A | G | 0.63 | −0.02 | −2.01 | 0.52 | −0.30 | −2.15 |
| QNKS.ta.NAM.ipbb-1B.1 | 1B | A | 1.43 | −0.33 | −3.04 | 0.29 | −0.07 | −4.27 |
| QNKS.ta.NAM.ipbb-1B.2 | 1B | G | −11.07 | −2.88 | −122.07 | −1.92 | 5.09 | 51.48 |
| QNKS.ta.NAM.ipbb-1B.3 | 1B | C | −0.97 | 0.35 | 5.18 | −0.13 | 0.01 | −10.38 |
| QNKS.ta.NAM.ipbb-3A | 3A | A | 1.26 | 0.05 | 5.43 | 0.09 | −0.11 | 5.05 |
| QNKS.ta.NAM.ipbb-4A.1 | 4A | C | −0.32 | 0.04 | 19.55 | −1.04 | 0.10 | −12.74 |
| QNKS.ta.NAM.ipbb-4A.2 | 4A | G | −0.81 | 0.41 | 3.77 | −1.33 | 0.57 | −1.56 |
| QNKS.ta.NAM.ipbb-4D | 4D | G | −2.69 | −0.38 | −131.00 | 3.88 | −0.96 | 91.71 |
| QNKS.ta.NAM.ipbb-5A | 5A | A | 0.26 | 0.55 | 15.58 | −0.68 | 0.48 | −3.43 |
| QNKS.ta.NAM.ipbb-5B | 5B | T | −0.01 | 0.15 | 4.94 | −0.81 | 0.24 | −6.99 |
| QNKS.ta.NAM.ipbb-6A | 6A | A | 0.42 | 0.08 | −9.16 | 0.49 | 0.43 | 1.60 |
| QNKS.ta.NAM.ipbb-6B.1 | 6B | T | −0.04 | 0.01 | −1.69 | −0.87 | −0.07 | −4.40 |
| QNKS.ta.NAM.ipbb-6B.2 | 6B | T | 0.63 | −0.39 | −9.55 | 0.53 | 0.22 | 3.08 |
| QNKS.ta.NAM.ipbb-6B.3 | 6B | G | 0.50 | −0.09 | −0.38 | 1.39 | 0.11 | 1.74 |
| QNKS.ta.NAM.ipbb-7A | 7A | C | −0.59 | 0.01 | −6.40 | −0.27 | 0.10 | −4.73 |
| QNKS.ta.NAM.ipbb-UNK | UNK | C | −0.79 | −0.15 | −1.86 | −0.24 | 0.06 | −5.42 |
| QTL | Chr | Allele | KRIAPG | SPCGF | ||||
|---|---|---|---|---|---|---|---|---|
| NKS, pcs | TKW, g | YM2 g/m2 | NKS, pcs | TKW, g | YM2 g/m2 | |||
| QTKW.ta.NAM.ipbb-4A | 4A | G | 0.05 | −1.10 | −4.86 | 0.32 | −1.33 | −21.06 |
| QTKW.ta.NAM.ipbb-5A.1 | 5A | G | −0.29 | −0.54 | −13.84 | 0.64 | −0.50 | 1.53 |
| QTKW.ta.NAM.ipbb-5A.2 | 5A | C | 0.82 | −0.18 | 2.93 | 0.73 | −0.68 | −3.01 |
| QTKW.ta.NAM.ipbb-6A.1 | 6A | A | 0.63 | −0.79 | 4.43 | 0.27 | −0.93 | −5.20 |
| QTKW.ta.NAM.ipbb-6A.2 | 6A | G | −1.32 | 0.96 | −13.49 | −0.37 | 1.19 | 0.61 |
| QTKW.ta.NAM.ipbb-6A.3 | 6A | A | 0.67 | −0.75 | 4.15 | 0.23 | −0.62 | 5.49 |
| QTKW.ta.NAM.ipbb-6A.4 | 6A | C | −0.82 | 1.07 | −17.28 | −0.49 | 0.61 | −10.99 |
| QTKW.ta.NAM.ipbb-6B.1 | 6B | A | −0.60 | 0.92 | 21.02 | −0.38 | 0.34 | −7.11 |
| QTKW.ta.NAM.ipbb-7A.1 | 7A | C | −0.82 | 0.06 | 11.16 | 0.01 | 0.66 | 14.24 |
| QTKW.ta.NAM.ipbb-UNK | UNK | G | 2.88 | −2.49 | 24.27 | −5.43 | −9.77 | −148.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amalova, A.; Griffiths, S.; Abugalieva, S.; Turuspekov, Y. Genome-Wide Association Study of Yield-Related Traits in a Nested Association Mapping Population Grown in Kazakhstan. Agronomy 2024, 14, 1848. https://doi.org/10.3390/agronomy14081848
Amalova A, Griffiths S, Abugalieva S, Turuspekov Y. Genome-Wide Association Study of Yield-Related Traits in a Nested Association Mapping Population Grown in Kazakhstan. Agronomy. 2024; 14(8):1848. https://doi.org/10.3390/agronomy14081848
Chicago/Turabian StyleAmalova, Akerke, Simon Griffiths, Saule Abugalieva, and Yerlan Turuspekov. 2024. "Genome-Wide Association Study of Yield-Related Traits in a Nested Association Mapping Population Grown in Kazakhstan" Agronomy 14, no. 8: 1848. https://doi.org/10.3390/agronomy14081848
APA StyleAmalova, A., Griffiths, S., Abugalieva, S., & Turuspekov, Y. (2024). Genome-Wide Association Study of Yield-Related Traits in a Nested Association Mapping Population Grown in Kazakhstan. Agronomy, 14(8), 1848. https://doi.org/10.3390/agronomy14081848






