Comparative Physiological and Gene Expression Analyses Provide Insights into Ion Transports and Osmotic Adjustment of Sweet Sorghum under Salt Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Determination of Growth-Related Parameters
2.3. Measurements of Photosynthesis-Related Parameters
2.4. Determination of Malondialdehyde (MDA) Content in Leaf Blades
2.5. Measurement of Tissue Ion Contents
2.6. Determination of Free Proline, Soluble Sugar, and Betaine Contents
2.7. Determination of Leaf Water Potential, Osmotic Potential, and Turgor Pressure
2.8. Real-Time Quantitative PCR Analysis
2.9. Data Analysis
3. Results
3.1. Effects of NaCl Treatments on the Growth of Sweet Sorghum Cultivars
3.2. Effects of NaCl Treatments on the Photosynthesis of Sweet Sorghum Cultivars
3.3. Ion Contents in Tissues of Sweet Sorghum Cultivars under NaCl Treatments
3.4. Effect of NaCl Treatments on the Leaf Free Proline, Soluble Sugar, and Betaine Contents of Sweet Sorghum Cultivars
3.5. Effects of NaCl Treatments on the Leaf OA Capacity of Sweet Sorghum Cultivars
3.6. The Expressions of Genes Related to Na+ and K+ Transport in Sweet Sorghum under NaCl Treatment
3.7. The Expressions of Key Genes Related to Cl− and NO3− Transport in Sweet Sorghum under NaCl Treatment
4. Discussion
4.1. Sweet Sorghum Is a Typical Na+ Exclusion Species That Can Be Used as a Model to Elucidate the Mechanisms of Poaceae Plants to Alleviate Na+ Toxicity
4.2. The Large Accumulation of Cl− in Leaf Sheaths Play a Vital Role in Sweet Sorghum to Cope with Cl− Toxicity
4.3. K+, NO3−, Soluble Sugars, and Betaine Should Be Important for the Osmotic Adjustment of Sweet Sorghum under NaCl Treatments
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar]
- Kobayashi, N.I.; Yamaji, N.; Yamamoto, H.; Okubo, K.; Ueno, H.; Costa, A.; Tanoi, K.; Matsumura, H.; Fujii-Kashino, M.; Horiuchi, T.; et al. OsHKT1;5 mediates Na+ exclusion in the vasculature to protect leaf blades and reproductive tissues from salt toxicity in rice. Plant J. 2007, 91, 657–670. [Google Scholar]
- Zhang, H.; Yu, F.; Xie, P.; Sun, S.; Qiao, X.; Tang, S.; Chen, C.; Yang, S.; Mei, C.; Yang, D.; et al. A Gγ protein regulates alkaline sensitivity in crops. Science 2023, 379, eade8416. [Google Scholar]
- Wang, W.Y.; Liu, Y.Q.; Duan, H.R.; Yin, X.X.; Cui, Y.N.; Chai, W.W.; Song, X.; Flowers, T.J.; Wang, S.M. SsHKT1;1 is coordinated with SsSOS1 and SsNHX1 to regulate Na+ homeostasis in Suaeda salsa under saline conditions. Plant Soil 2020, 449, 117–131. [Google Scholar]
- Huang, X.; Rong, W.; Zhang, X.; Gao, Y.; Zhou, Y.; Su, J.; Luo, H.; Chu, G.; Wang, M. Transcriptome and metabolome analysis reveal the dynamic changes and biosynthesis pathways of alkaloids in Sophora alopecuroides L. under drought stress. Ind. Crop. Prod. 2024, 212, 118365. [Google Scholar]
- Regassa, T.H.; Wortmann, C.S. Sweet sorghum as a bioenergy crop: Literature review. Biomass Bioenerg. 2014, 64, 348–355. [Google Scholar]
- López-Sandin, I.; Zavala-García, F.; Levin, L.; Ruiz, H.A.; Hernández-Luna, C.E.; Gutiérrez-Soto, G. Evaluation of bioethanol production from sweet sorghum variety roger under different tillage and fertilizer treatments. Bioenerg. Res. 2021, 14, 1058–1069. [Google Scholar]
- Cui, J.; Ren, G.; Qiao, H.; Xiang, X.; Huang, H.; Chang, J. Comparative transcriptome analysis of seedling stage of two sorghum cultivars under salt stress. J. Plant Growth Regul. 2018, 37, 986–998. [Google Scholar]
- Yang, Z.; Li, J.L.; Liu, L.N.; Xie, Q.; Sui, N. Photosynthetic regulation under salt stress and salt-tolerance mechanism of sweet sorghum. Front. Plant Sci. 2020, 10, 1722. [Google Scholar]
- Zheng, L.Y.; Guo, X.S.; He, B.; Sun, L.J.; Peng, Y.; Dong, S.S.; Liu, T.F.; Jiang, S.; Ramachandran, S.; Liu, C.M.; et al. Genome-wide patterns of genetic variation in sweet and grain sorghum (Sorghum bicolor). Genome Biol. 2011, 12, R114. [Google Scholar]
- Li, J.; Lei, S.; Gong, H.; Liu, Z.; Zhang, Y.; Ouyang, Z. Field performance of sweet sorghum in salt-affected soils in China: A quantitative synthesis. Environ. Res. 2023, 222, 115362. [Google Scholar] [PubMed]
- Song, Y.; Li, S.; Sui, Y.; Zheng, H.; Han, G.; Sun, X.; Yang, W.; Wang, H.; Zhuang, K.; Kong, F.; et al. SbbHLH85, a bHLH member, modulates resilience to salt stress by regulating root hair growth in sorghum. Theor. Appl. Genet. 2022, 135, 201–216. [Google Scholar] [PubMed]
- Guo, H.; Nie, C.Y.; Li, Z.; Kang, J.; Wang, X.L.; Cui, Y.N. Physiological and transcriptional analyses provide insight into maintaining ion homeostasis of sweet sorghum under salt stress. Int. J. Mol. Sci. 2023, 24, 11045. [Google Scholar] [CrossRef]
- Deinlein, U.; Stephan, A.B.; Horie, T.; Luo, W.; Xu, G.; Schroeder, J.I. Plant salt-tolerance mechanisms. Trends Plant Sci. 2014, 19, 371–379. [Google Scholar]
- Cui, Y.N.; Li, X.T.; Yuan, J.Z.; Wang, F.Z.; Guo, H.; Xia, Z.R.; Wang, S.M.; Ma, Q. Chloride is beneficial for growth of the xerophyte Pugionium cornutum by enhancing osmotic adjustment capacity under salt and drought stresses. J. Exp. Bot. 2020, 71, 4215–4231. [Google Scholar] [PubMed]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar]
- Ďúranová, H.; Šimora, V.; Ďurišová, L.; Olexiková, L.; Kovar, M.; Požgajová, M. Modifications in ultrastructural characteristics and redox status of plants under environmental stress: A review. Plants 2023, 12, 1666. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Bioch. 2010, 48, 909–930. [Google Scholar]
- Horie, T.; Hauser, F.; Schroeder, J.I. HKT transporter-mediated salinity resistance mechanisms in Arabidopsis and monocot crop plants. Trends Plant Sci. 2009, 14, 660–668. [Google Scholar]
- Munns, R.; James, R.A.; Xu, B.; Athman, A.; Conn, S.J.; Jordans, C.; Byrt, C.S.; Hare, R.A.; Tyerman, S.D.; Tester, M.; et al. Wheat grain yield on saline soils is improved by an ancestral Na+ transporter gene. Nat. Biotechnol. 2012, 30, 360–364. [Google Scholar]
- Bao, A.K.; Du, B.Q.; Touil, L.; Kang, P.; Wang, Q.L.; Wang, S.M. Co-expression of tonoplast Cation/H+ antiporter and H+-pyrophosphatase from xerophyte Zygophyllum xanthoxylum improves alfalfa plant growth under salinity, drought and field conditions. Plant Biotechnol. J. 2016, 14, 964–975. [Google Scholar]
- Zhang, M.; Cao, Y.; Wang, Z.; Wang, Z.Q.; Shi, J.; Liang, X.; Song, W.; Chen, Q.; Lai, J.; Jiang, C. A retrotransposon in an HKT1 family sodium transporter causes variation of leaf Na+ exclusion and salt tolerance in maize. New Phytol. 2018, 217, 1161–1176. [Google Scholar] [PubMed]
- Jossier, M.; Kroniewicz, L.; Dalmas, F.; Thiec, D.L.; Ephritikhine, G.; Barbier-Brygoo, H.; Vavasseur, A.; Filleur, S.; Leonhardt, N. The Arabidopsis vacuolar anion transporter, AtCLCc, is involved in the regulation of stomatal movements and contributes to salt tolerance. Plant J. 2010, 64, 563–576. [Google Scholar] [PubMed]
- Nguyen, C.T.; Agorio, A.; Jossier, M.; Depre, S.; Thomine, S.; Filleur, S. Characterization of the chloride channel-like, AtCLCg, involved in chloride tolerance in Arabidopsis thaliana. Plant Cell Physiol. 2016, 57, 764–775. [Google Scholar] [PubMed]
- Wen, Z.Y.; Tyerman, S.D.; Dechorgnat, J.; Ovchinnikova, E.; Dhugga, K.S.; Kaiser, B.N. Maize NPF6 proteins are homologs of Arabidopsis CHL1 that are selective for both nitrate and chloride. Plant Cell 2018, 29, 2581–2596. [Google Scholar]
- Ma, Q.; Hu, J.; Zhou, X.R.; Yuan, H.J.; Kumar, T.; Luan, S.; Wang, S.M. ZxAKT1 is essential for K+ uptake and K+/Na+ homeostasis in the succulent xerophyte Zygophyllum xanthoxylum. Plant J. 2017, 90, 48–60. [Google Scholar] [PubMed]
- Drechsler, N.; Zheng, Y.; Bohner, A.; Nobmann, B.; von Wirén, N.; Kunze, R.; Rausch, C. Nitrate-dependent control of shoot K homeostasis by the nitrate transporter1/peptide transporter family member NPF7.3/NRT1.5 and the stelar K+ outward rectifier SKOR in Arabidopsis. Plant Physiol. 2015, 169, 2832–2847. [Google Scholar] [PubMed]
- Wang, W.; Hu, B.; Li, A.; Chu, C. NRT1.1s in plants: Functions beyond nitrate transport. J. Exp. Bot. 2019, 71, 4373–4379. [Google Scholar]
- Chen, C.Z.; Lv, X.F.; Li, J.Y.; Gong, J.M. Arabidopsis NRT1.5 is another essential component in the regulation of nitrate reallocation and stress tolerance. Plant Physiol. 2012, 159, 1582–1590. [Google Scholar]
- Song, Y.; Li, J.; Sui, Y.; Han, G.; Zhang, Y.; Guo, S.; Sui, N. The sweet sorghum SbWRKY50 is negatively involved in salt response by regulating ion homeostasis. Plant Mol. Biol. 2020, 102, 603–614. [Google Scholar]
- Wei, X.C.; Liu, L.L.; Lu, C.X.; Yuan, F.; Han, G.L.; Wang, B.S. SbCASP4 improves salt exclusion by enhancing the root apoplastic barrier. Planta 2021, 254, 81. [Google Scholar] [PubMed]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar]
- Peever, T.L.; Higgins, V.J. Electrolyte leakage, lipoxygenase, and lipid peroxidation induced in tomato leaf tissue by specific and non specific elicitors from Cladosporium fluvum. Plant Physiol. 1989, 90, 867–875. [Google Scholar] [PubMed]
- Wang, S.M.; Zhang, J.L.; Flowers, T.J. Low-affinity Na+ uptake in the halophyte Suaeda maritima. Plant Physiol. 2007, 145, 559–571. [Google Scholar]
- Li, B.; Byrt, C.; Qiu, J.; Baumann, U.; Hrmova, M.; Evrard, A.; Johnson, A.A.; Birnbaum, K.D.; Mayo, G.M.; Jha, D.; et al. Identification of a stelar-localized transport protein that facilitates root-to-shoot transfer of chloride in Arabidopsis. Plant Physiol. 2016, 170, 1014–1029. [Google Scholar] [PubMed]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar]
- Pe’er, S.; Cohen, Y. Sugar accumulation in tobacco plants systemically protected against blue mold (Peronospora tabacina). Phytoparasitica 1987, 15, 307–315. [Google Scholar]
- Pan, Y.Q.; Guo, H.; Wang, S.M.; Zhao, B.; Zhang, J.L.; Ma, Q.; Yin, H.J.; Bao, A.K. The photosynthesis, Na+/K+ homeostasis and osmotic adjustment of Atriplex canescens in response to salinity. Front. Plant Sci. 2016, 7, 848. [Google Scholar]
- Ma, Q.; Yue, L.J.; Zhang, J.L.; Wu, G.Q.; Bao, A.K.; Wang, S.M. Sodium chloride improves photosynthesis and water status in the succulent xerophyte Zygophyllum xanthoxylum. Tree Physiol. 2012, 32, 4–13. [Google Scholar]
- Ueda, A.; Kanechi, M.; Uno, Y.; Inagaki, N. Photosynthetic limitations of a halophyte sea aster (Aster tripolium L.) under water stress and NaCl stress. J. Plant Res. 2003, 116, 65–70. [Google Scholar]
- Duan, H.R.; Ma, Q.; Zhang, J.L.; Hu, J.; Bao, A.K.; Wei, L.; Wang, Q.; Luan, S.; Wang, S.M. The inward-rectifying K+ channel SsAKT1 is a candidate involved in K+ uptake in the halophyte Suaeda salsa under saline condition. Plant Soil 2015, 395, 173–187. [Google Scholar]
- Teng, X.X.; Cao, W.L.; Lan, H.X.; Tang, H.J.; Bao, Y.M.; Zhang, H.S. OsNHX1, an Na+/H+ antiporter gene, can enhance salt tolerance in rice plant through more effective accumulation of toxic Na+ in leaf mesophyll and bundle sheath cells. Acta Physiol. Plant. 2007, 39, 113–125. [Google Scholar]
- Yang, Y.; Nong, B.; Chen, C.; Wang, J.; Xia, X.; Zhang, Z.; Wei, Y.; Zeng, Y.; Feng, R.; Wu, Y.; et al. OsNPF3.1, a member of the NRT1/PTR family, increases nitrogen use efficiency and biomass production in rice. Crop J. 2023, 11, 108–118. [Google Scholar]
- Garg, N.; Manchanda, G. ROS generation in plants: Boon or bane? Plant Biosyst. 2009, 143, 8–96. [Google Scholar]
- James, R.A.; Davenport, R.J.; Munns, R. Physiological characterization of two genes for Na+ exclusion in durum wheat, Nax1 and Nax2. Plant Physiol. 2016, 142, 1537–1547. [Google Scholar]
- Apse, M.P.; Blumwald, E. Na+ transport in plants. FEBS Lett. 2007, 581, 2247–2254. [Google Scholar]
- McCubbin, T.; Bassil, E.; Zhang, S.; Blumwald, E. Vacuolar Na+/H+ NHX-type antiporters are required for cellular K+ homeostasis, microtubule organization and directional root growth. Plants 2014, 3, 409–426. [Google Scholar] [CrossRef]
- Huang, S.B.; Spielmeyer, W.; Lagudah, E.S.; James, R.A.; Platten, J.D.; Dennis, E.S.; Munns, R. A sodium transporter (HKT7) is a candidate for Nax1, a gene for salt tolerance in durum wheat. Plant Physiol. 2006, 142, 1718–1727. [Google Scholar] [PubMed]
- Wang, B.S.; Zou, Q.; Zhao, K.F. Effect of NaCl stress on ionic contents in different organs of sorghum plants. Acta Agron. Sin. 2000, 26, 845–850. [Google Scholar]
- Hussain, S.; Zhang, R.; Liu, S.; Li, R.; Zhou, Y.; Chen, Y.; Hou, H.; Dai, Q. Transcriptome-wide analysis revealed the potential of the High-Affinity Potassium Transporter (HKT) gene family in rice salinity tolerance via ion homeostasis. Bioengineering 2022, 9, 410. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Tester, M.; Gilliham, M. Chloride on the move. Trends Plant Sci. 2017, 22, 236–248. [Google Scholar] [PubMed]
- Cui, Y.N.; Lin, Z.R.; Cai, M.M.; Liu, R.W.; Wang, S.M.; Ma, Q. PcCLCg is involved in the accumulation of Cl− in shoots for osmotic adjustment and salinity resistance in the Cl−-tolerant xerophyte Pugionium cornutum. Plant Soil 2023, 487, 283–298. [Google Scholar]
- Shabala, S.; Shabala, L. Ion transport and osmotic adjustment in plants and bacteria. Biomol. Concepts 2011, 2, 407–419. [Google Scholar] [PubMed]
- Rubio, F.; Nieves-Cordones, M.; Aleman, F.; Martinez, V. Relative contribution of AtHAK5 and AtAKT1 to K+ uptake in the high-affinity range of concentrations. Physiol. Plant. 2008, 134, 598–608. [Google Scholar] [PubMed]
- Poustini, K.; Siosemardeh, A.; Ranjbar, M. Proline accumulation as a response to salt stress in 30 wheat (Triticum aestivum L.) cultivars differing in salt tolerance. Genet. Resour. Crop Evol. 2007, 54, 925–934. [Google Scholar]


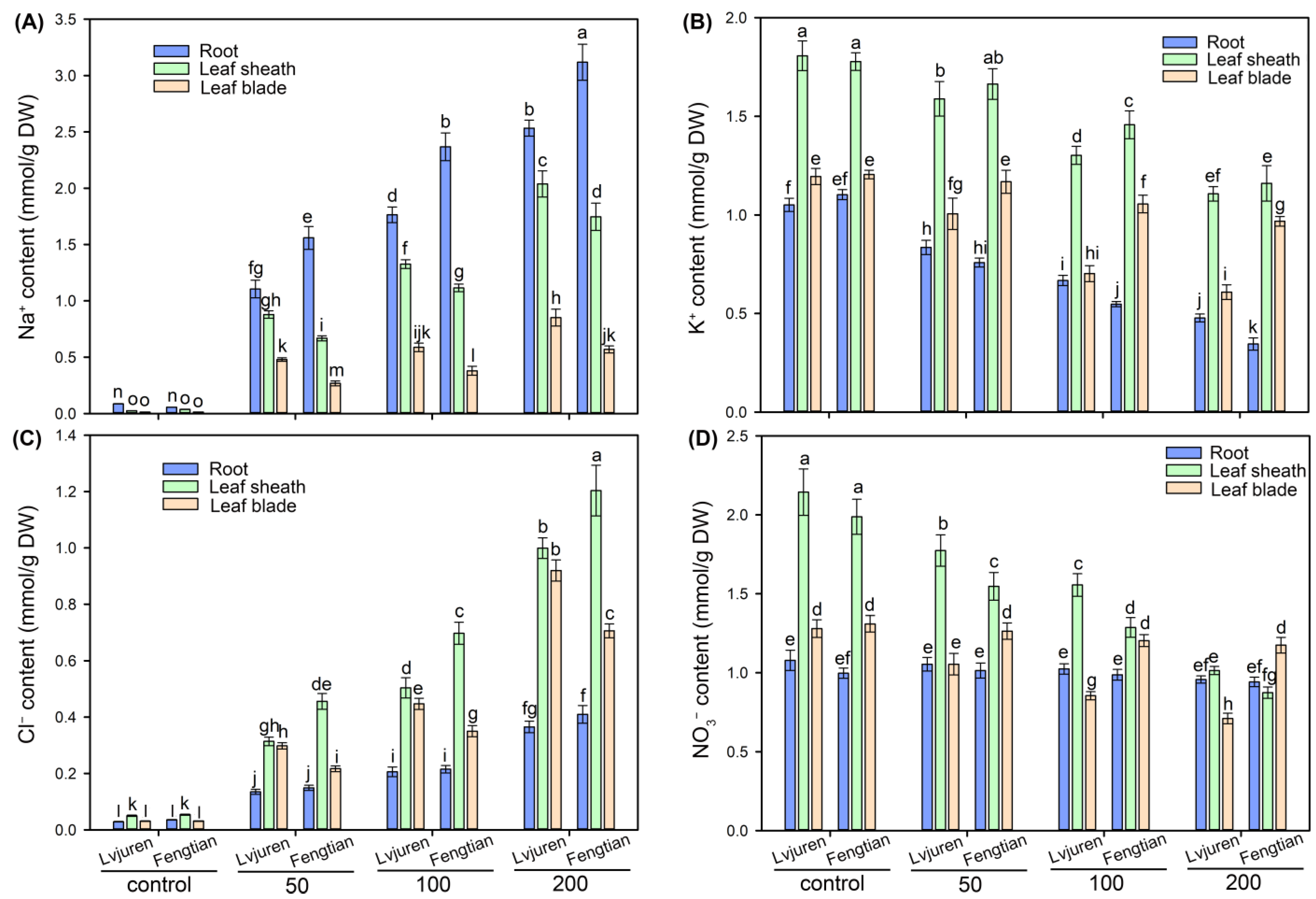

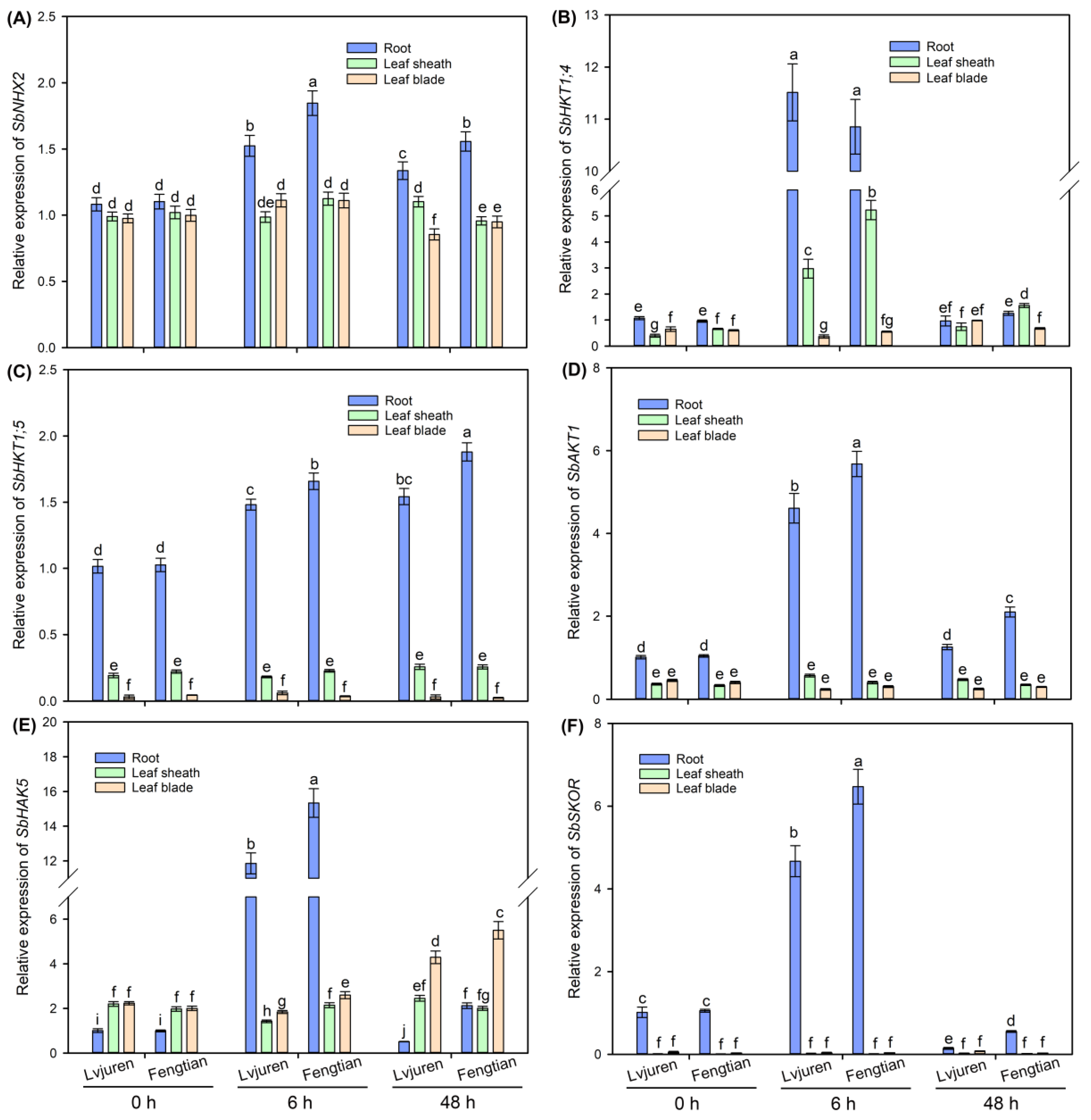
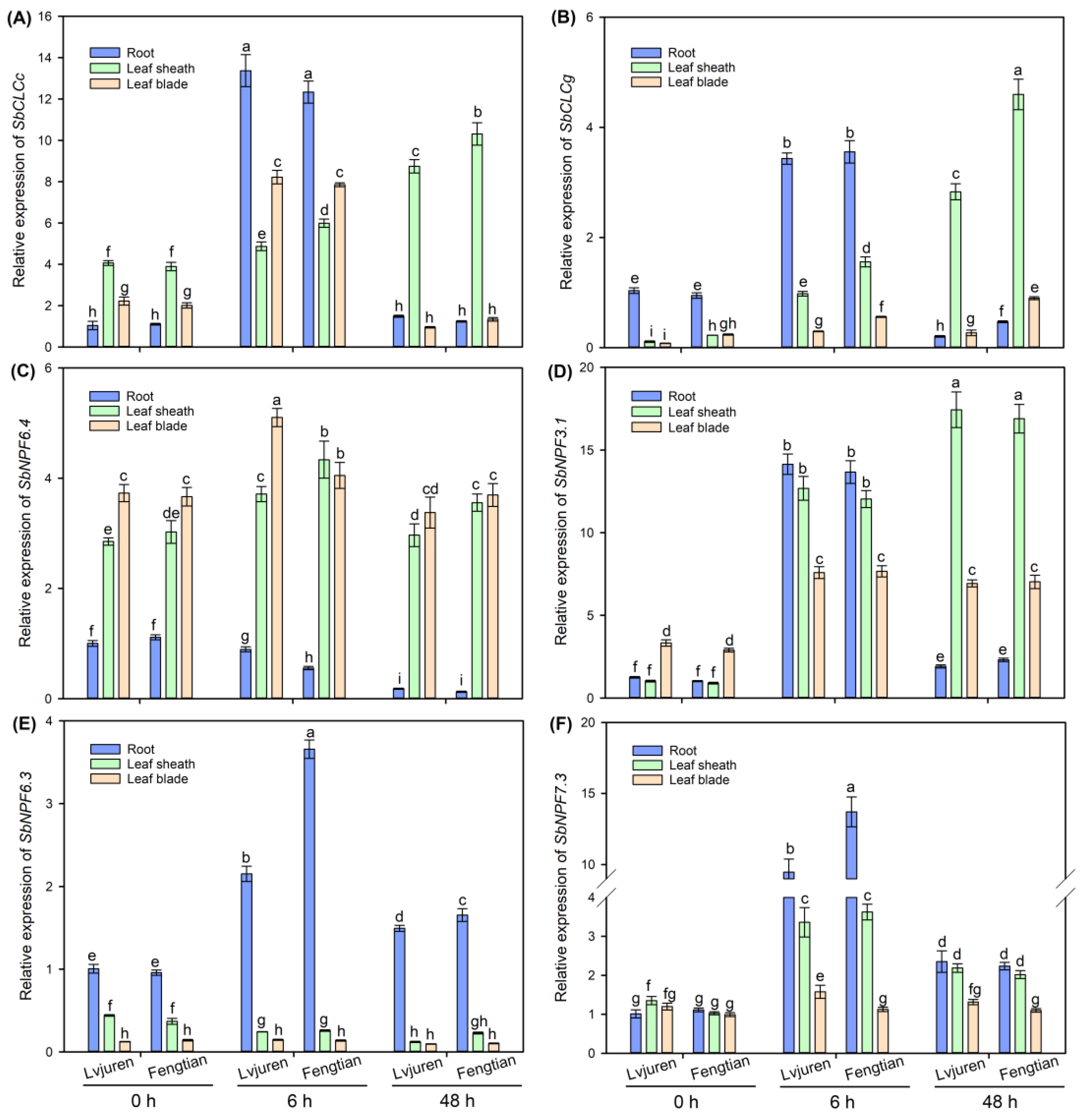
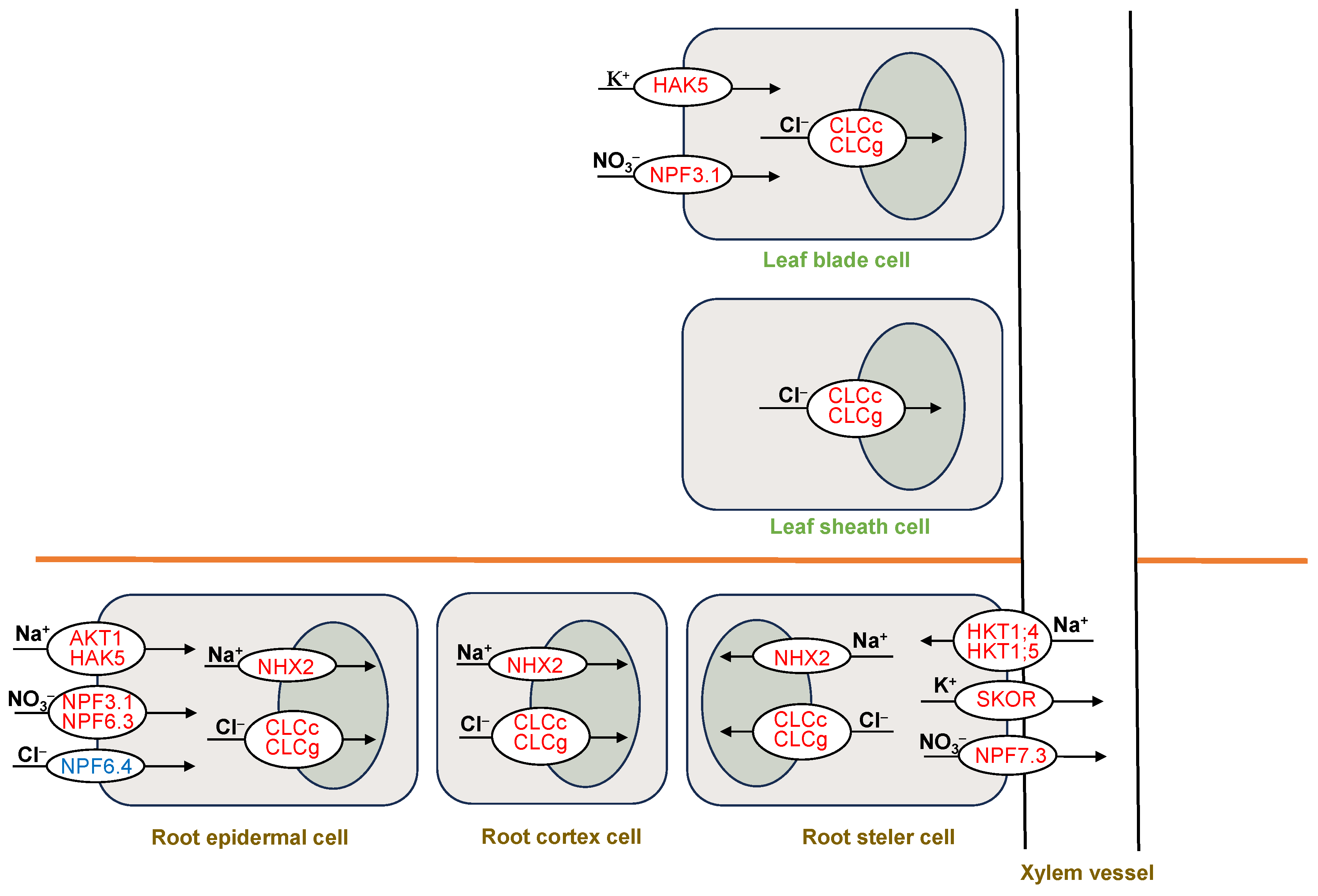
| Cultivars | Treatments | Water Potential (Ψw, MPa) | Osmotic Potential (Ψs, MPa) | Turgor Pressure (Ψt, MPa) |
|---|---|---|---|---|
| Lvjuren | Control | −0.18 ± 0.04 a | −0.63 ± 0.02 a | 0.45 ± 0.01 a |
| 50 | −0.46 ± 0.03 c | −0.75 ± 0.03 b | 0.29 ± 0.03 b | |
| 100 | −0.81 ± 0.05 e | −1.02 ± 0.05 d | 0.21 ± 0.01 c | |
| 200 | −1.12 ± 0.06 g | −1.25 ± 0.05 f | 0.13 ± 0.02 d | |
| Fengtian | Control | −0.19 ± 0.03 a | −0.64 ± 0.01 a | 0.45 ± 0.03 a |
| 50 | −0.39 ± 0.02 b | −0.83 ± 0.03 c | 0.44 ± 0.04 a | |
| 100 | −0.70 ± 0.03 d | −1.18 ± 0.06 e | 0.30 ± 0.01 b | |
| 200 | −0.96 ± 0.04 f | −1.35 ± 0.08 g | 0.29 ± 0.01 b |
| Cultivars | Treatments | K+ | NO3− | Proline | Sugars | Betaine |
|---|---|---|---|---|---|---|
| Lvjuren | Control | 39.44 ± 2.15 a | 42.03 ± 2.38 a | 0.11 ± 0.01 b | 5.33 ± 0.23 c | 6.86 ± 0.38 b |
| 50 | 31.81 ± 1.74 b | 33.88 ± 2.01 b | 0.13 ± 0.01 b | 5.30 ± 0.26 c | 5.86 ± 0.29 c | |
| 100 | 18.01 ± 1.05 d | 21.79 ± 1.00 d | 0.14 ± 0.01 b | 4.61 ± 0.21 d | 5.90 ± 0.30 c | |
| 200 | 15.07 ± 0.69 e | 16.31 ± 0.57 e | 0.18 ± 0.01 a | 4.40 ± 0.21 d | 6.01 ± 0.35 c | |
| Fengtian | Control | 38.63 ± 1.77 a | 42.00 ± 1.90 a | 0.11 ± 0.01 b | 5.16 ± 0.31 c | 6.78 ± 0.37 b |
| 50 | 31.39 ± 1.60 b | 33.90 ± 1.47 b | 0.11 ± 0.01 b | 5.93 ± 0.33 b | 6.28 ± 0.28 bc | |
| 100 | 22.02 ± 1.10 c | 25.00 ± 1.11 c | 0.10 ± 0.01 b | 6.08 ± 0.33 ab | 7.44 ± 0.39 a | |
| 200 | 20.00 ± 0.87 cd | 23.40 ± 1.12 c | 0.12 ± 0.01 b | 6.43 ± 0.5 a | 7.80 ± 0.40 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, J.; Wang, X.-L.; Yan, S.-J.; Guo, H.; Cui, Y.-N. Comparative Physiological and Gene Expression Analyses Provide Insights into Ion Transports and Osmotic Adjustment of Sweet Sorghum under Salt Stress. Agronomy 2024, 14, 1849. https://doi.org/10.3390/agronomy14081849
Kang J, Wang X-L, Yan S-J, Guo H, Cui Y-N. Comparative Physiological and Gene Expression Analyses Provide Insights into Ion Transports and Osmotic Adjustment of Sweet Sorghum under Salt Stress. Agronomy. 2024; 14(8):1849. https://doi.org/10.3390/agronomy14081849
Chicago/Turabian StyleKang, Jie, Xiao-Long Wang, Shi-Jie Yan, Huan Guo, and Yan-Nong Cui. 2024. "Comparative Physiological and Gene Expression Analyses Provide Insights into Ion Transports and Osmotic Adjustment of Sweet Sorghum under Salt Stress" Agronomy 14, no. 8: 1849. https://doi.org/10.3390/agronomy14081849
APA StyleKang, J., Wang, X.-L., Yan, S.-J., Guo, H., & Cui, Y.-N. (2024). Comparative Physiological and Gene Expression Analyses Provide Insights into Ion Transports and Osmotic Adjustment of Sweet Sorghum under Salt Stress. Agronomy, 14(8), 1849. https://doi.org/10.3390/agronomy14081849





