Exogenous Hemin Increases the Yield, Phenolic Compound Content, and Antioxidant Enzyme Activity of Dragon Fruit during the High-Temperature Period
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Design of the Experiment
2.3. Determination of Morphological Indicators of Fruit
2.4. Determination of Peel Colour Index
2.5. Determination of Fruit Nutritional Indicators
2.6. Determination of Enzyme Activities Related to the Antioxidant System of the Fruit Pulp
2.7. Determination of Chlorophyll Content of Dragon Fruit Stems
2.8. Metabolomic Measurements
2.9. Data Analysis
3. Results
3.1. Effects of Hemin on Morphological Traits of Dragon Fruits
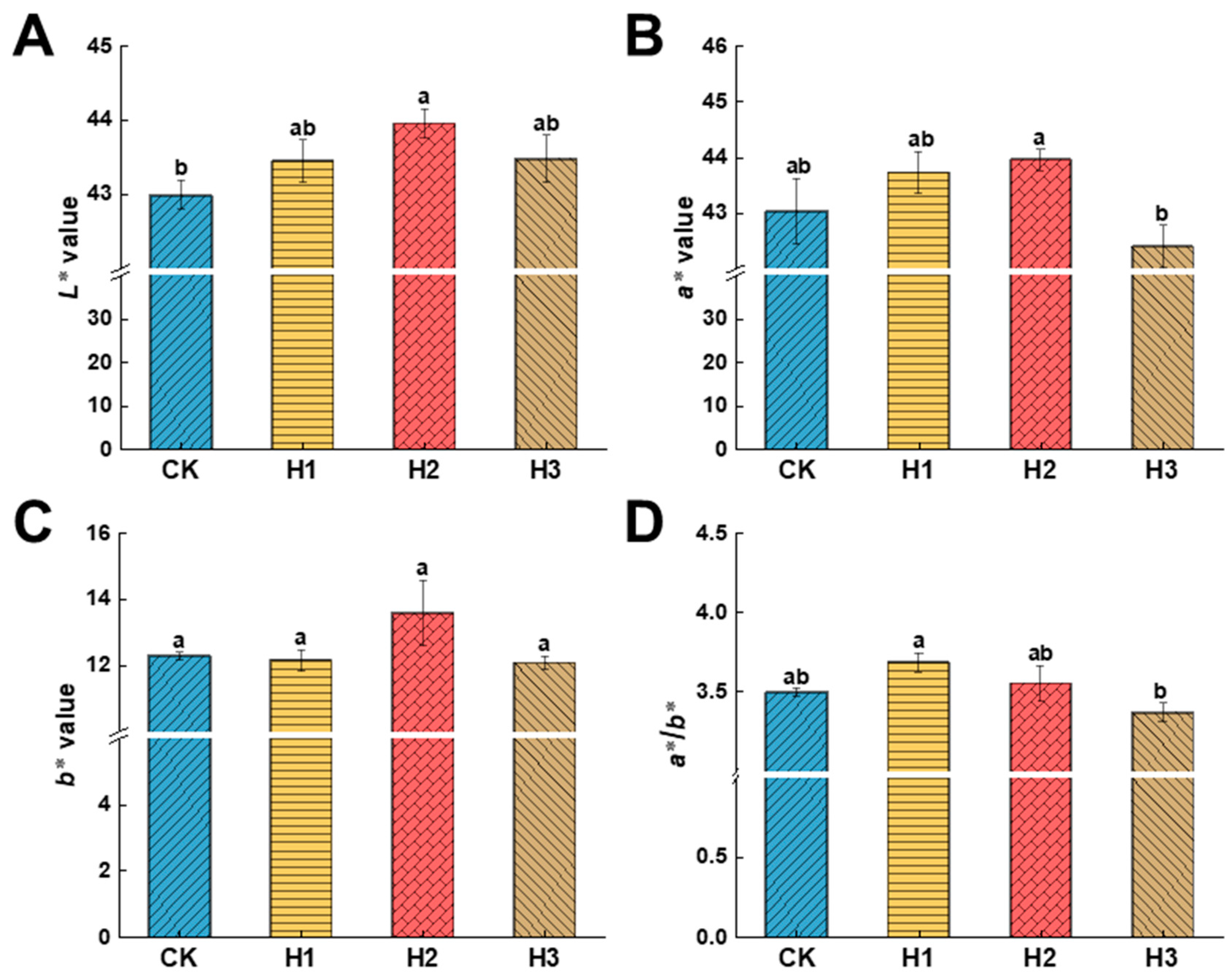
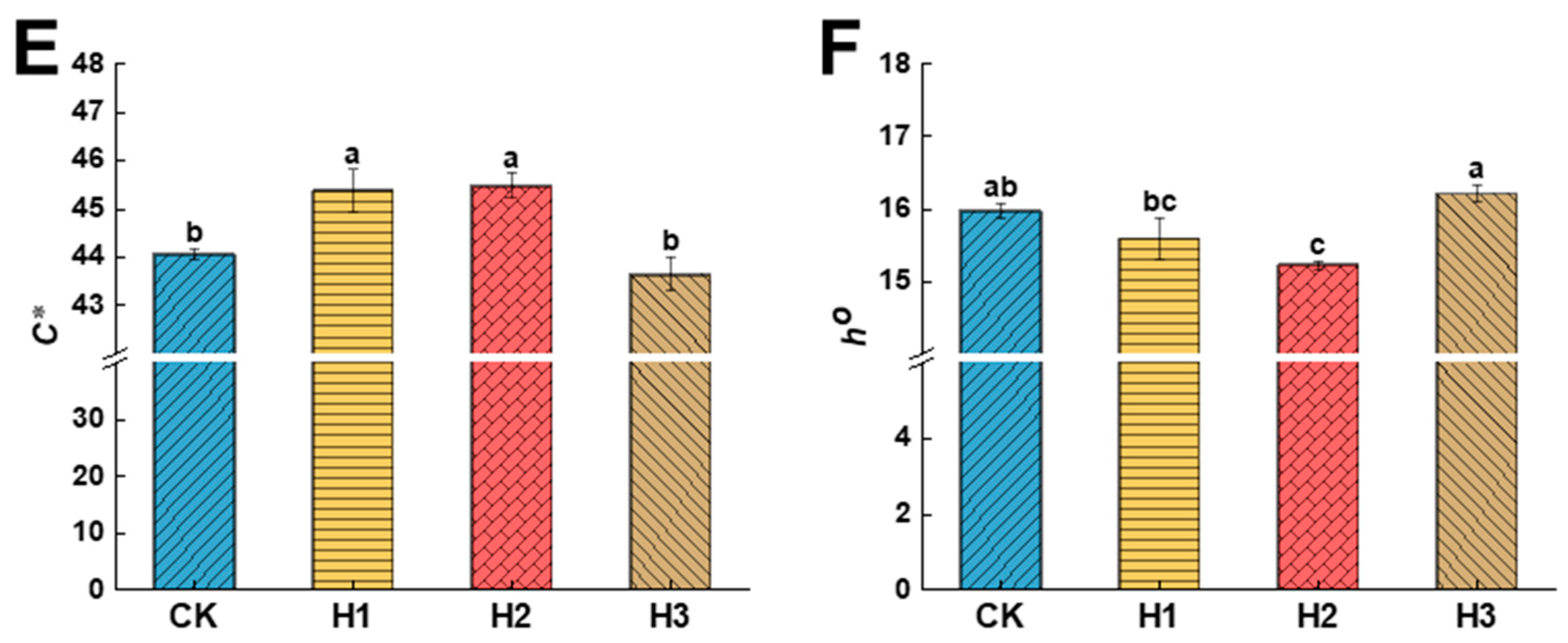
3.2. Effects of Hemin Treatment on the Peel Colour Index
3.3. Effects of Hemin Treatment on Fruit Nutritional Indices
3.3.1. Effect of Hemin Treatment on Soluble Solids, Soluble Protein, Starch and Sucrose
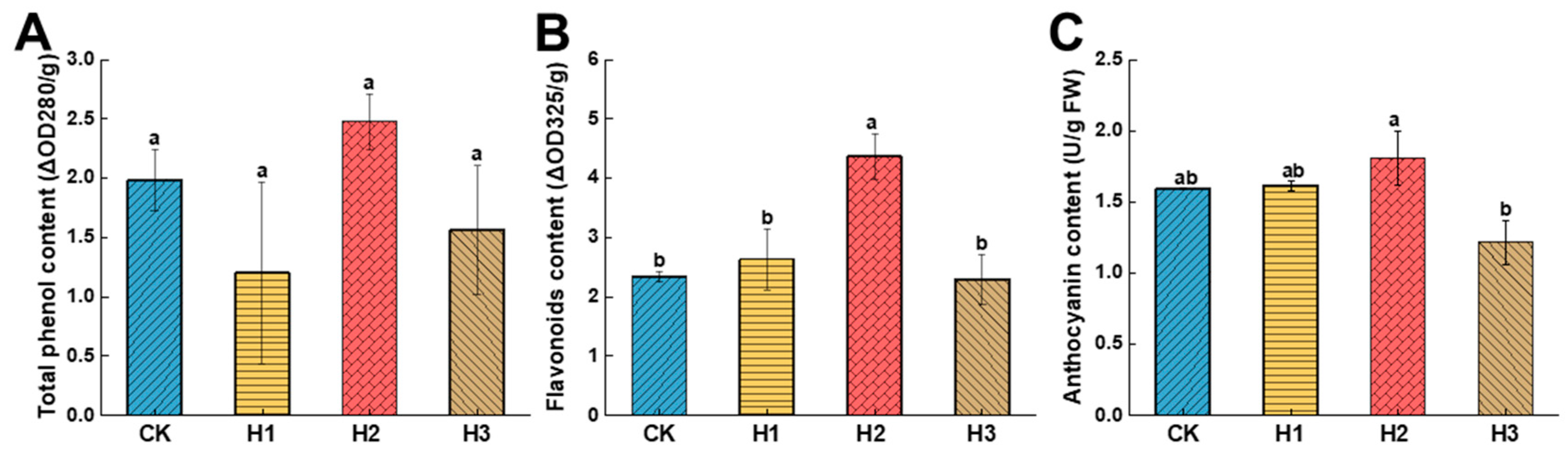
3.3.2. Effects of Hemin Treatment on the Phenolic Content of Dragon Fruit
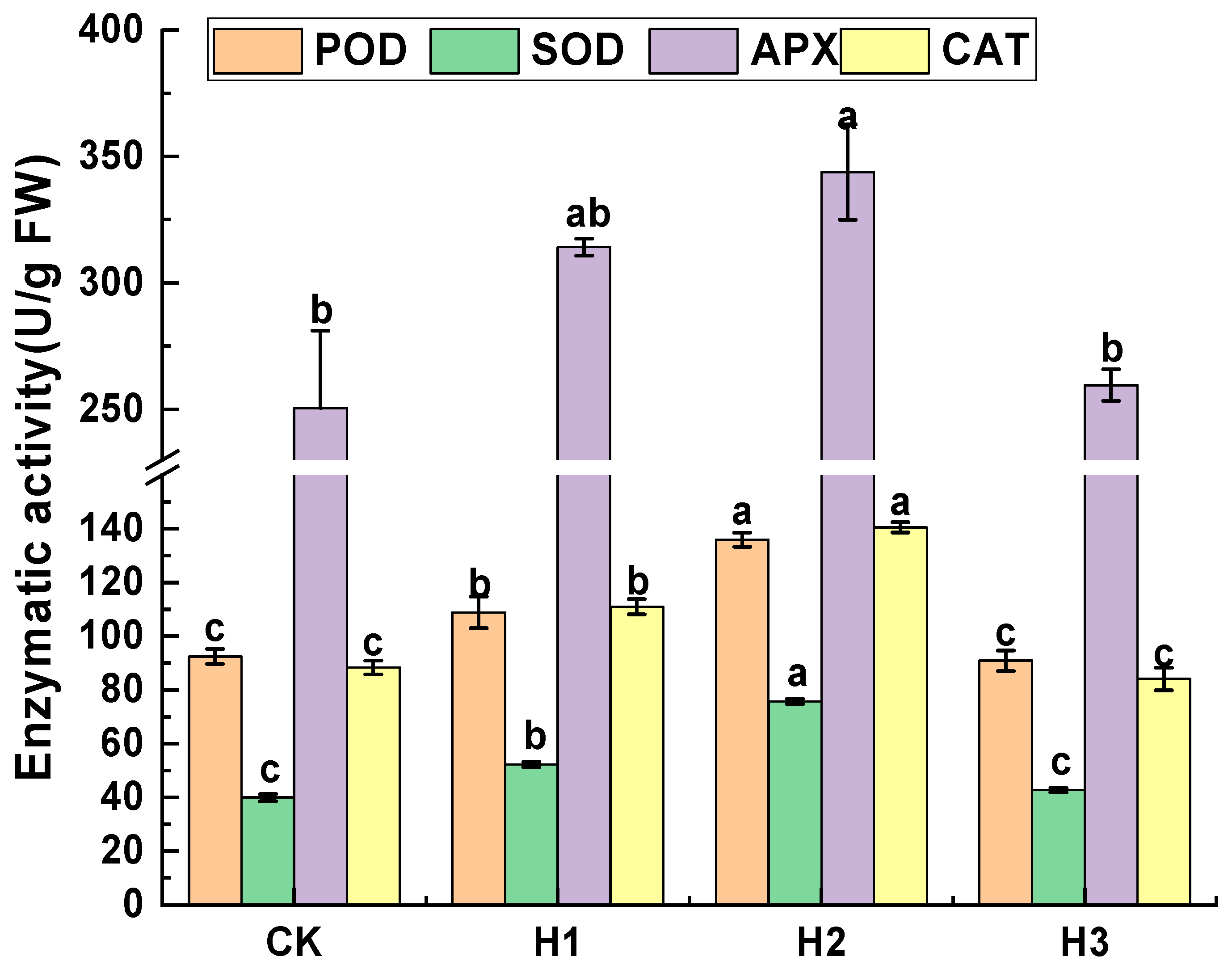
3.4. Effects of Hemin Treatment on Antioxidant Enzyme Activities of the Pulp of Fruit
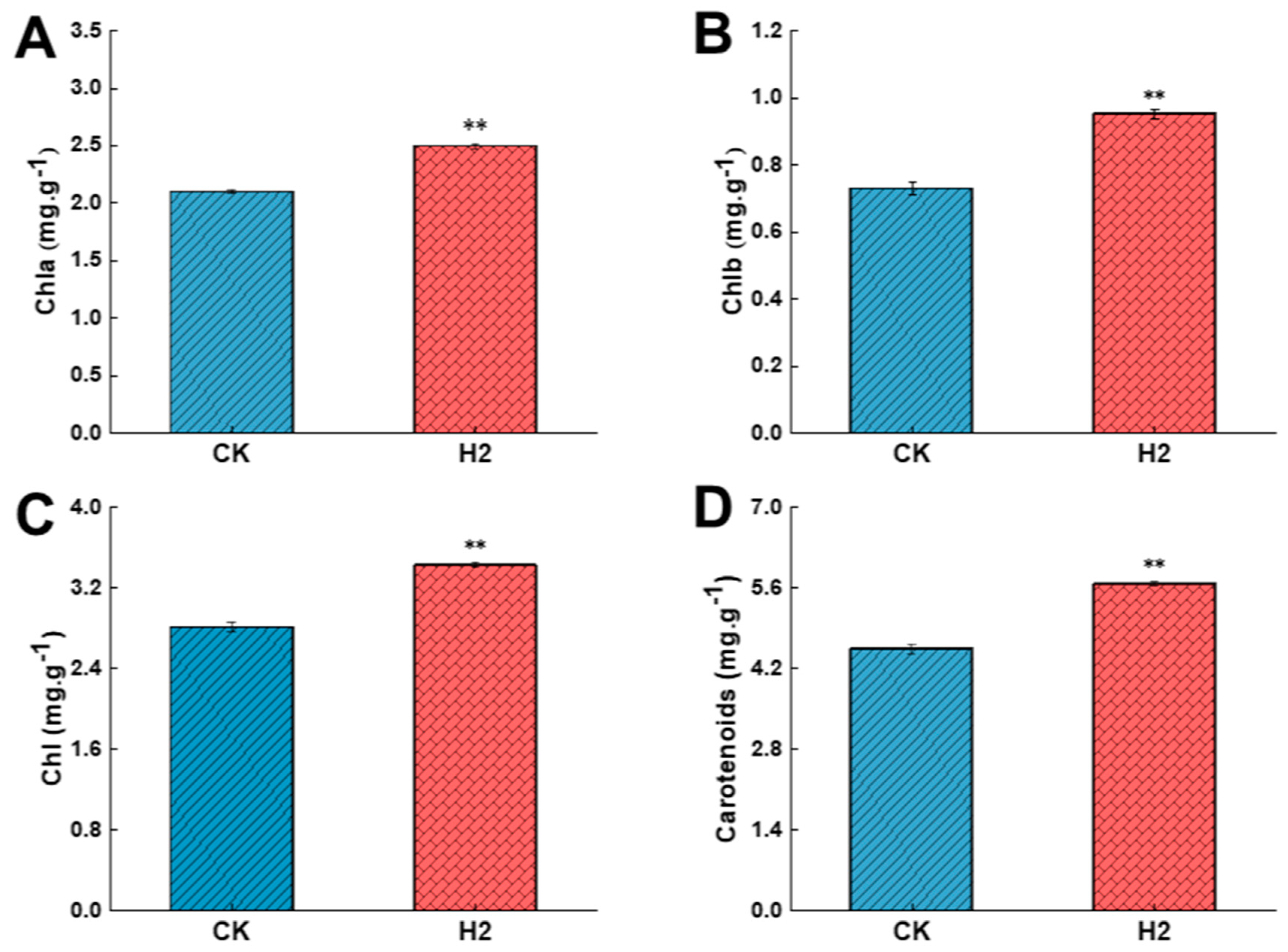
3.5. Effect of Hemin Treatment on Chlorophyll Content of Dragon Fruit Stems
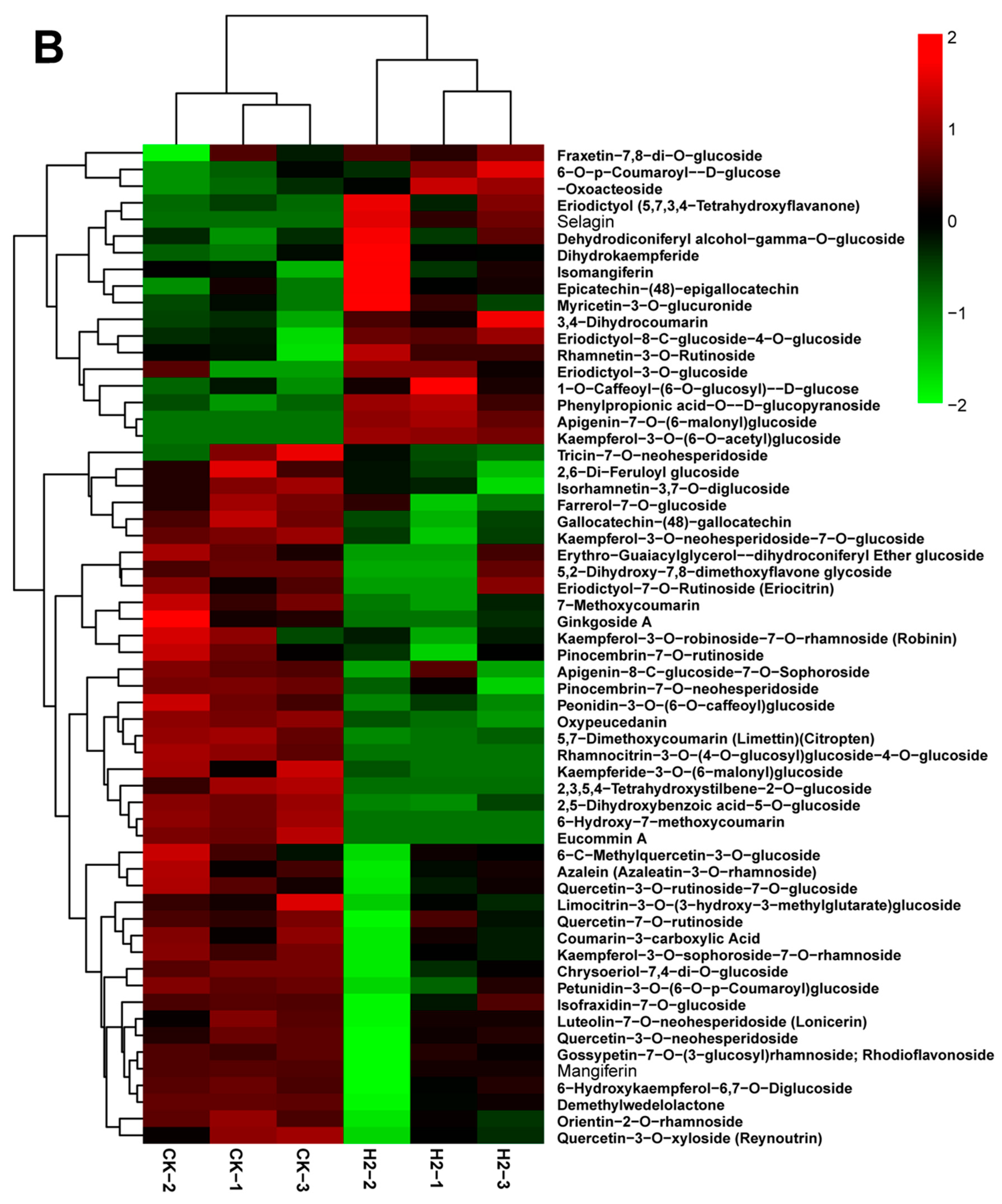
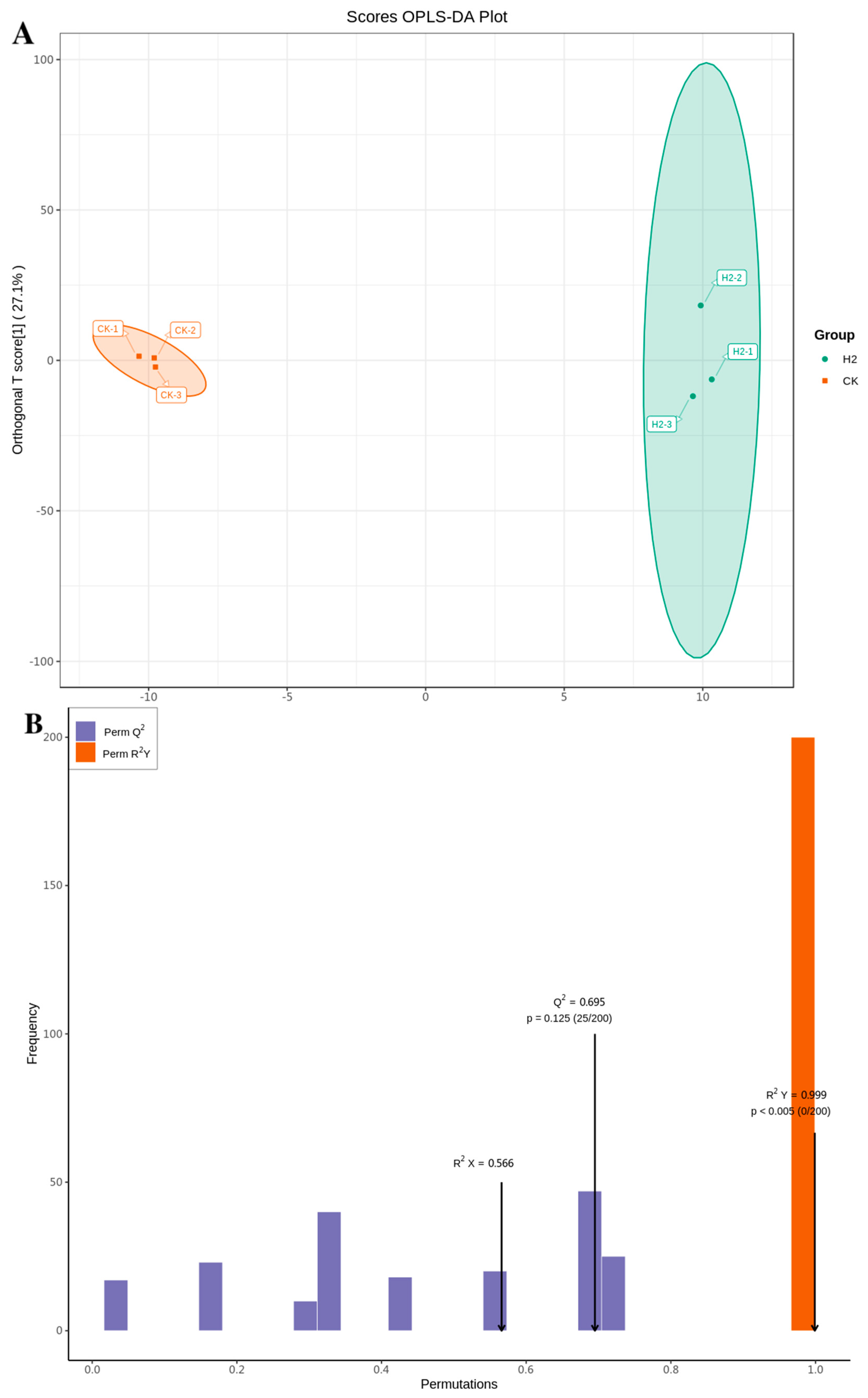
3.6. Analysis of Metabolomic Differences
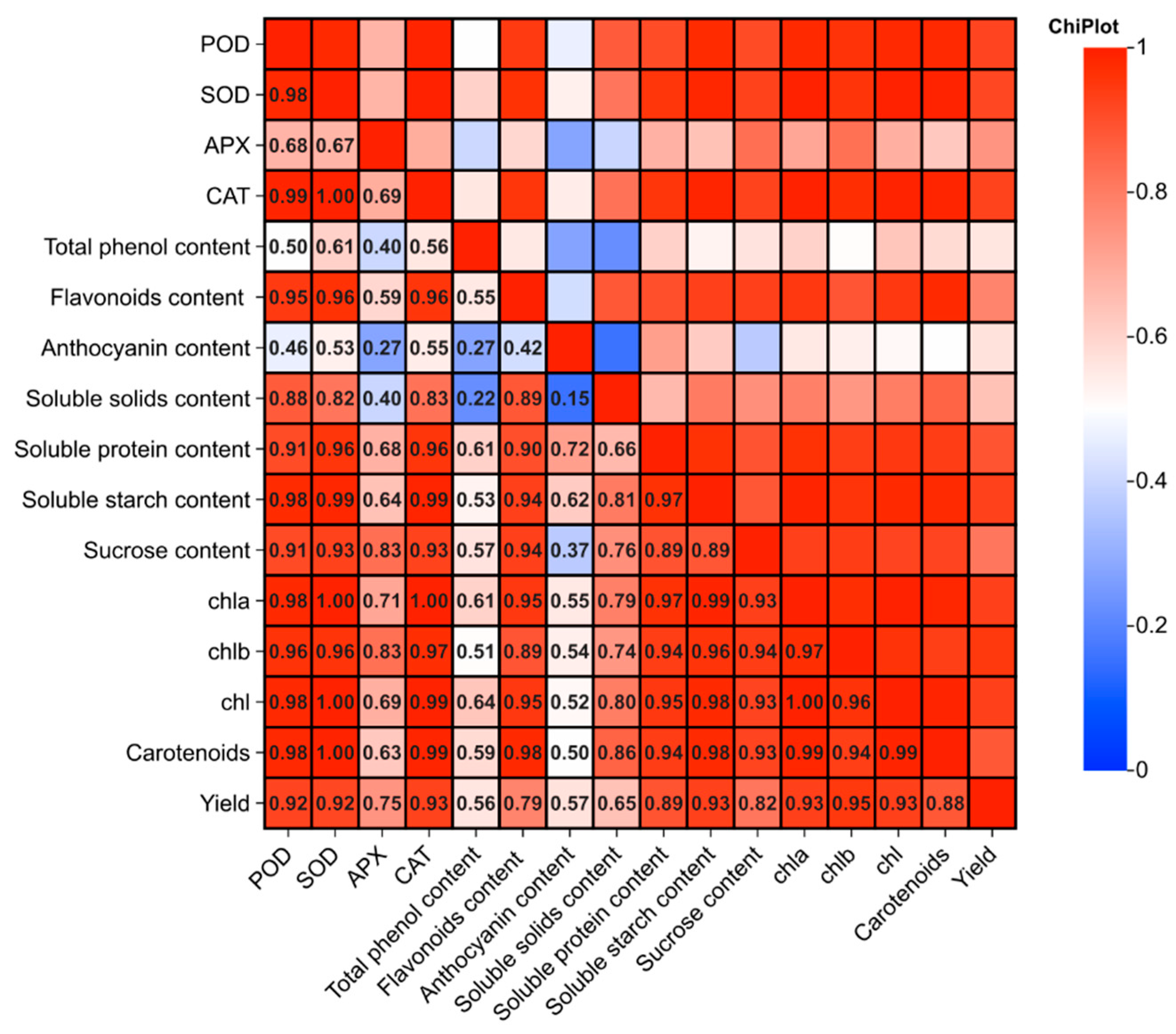
3.7. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shah, K.; Chen, J.; Chen, J.; Qin, Y. Pitaya nutrition, biology, and biotechnology: A review. Int. J. Mol. Sci. 2023, 24, 13986. [Google Scholar] [CrossRef] [PubMed]
- Widyaningsih, A.; Setiyani, O.; Umaroh, U.; Sofro, M.A.U.; Amri, F. Effect of consuming red dragon fruit (hylocereus costaricensis) juice on the levels of hemoglobin and erythrocyte among pregnant women. Belitung Nurs. J. 2017, 3, 255–264. [Google Scholar] [CrossRef]
- Holanda, M.O.; Lira, S.M.; Silva, J.Y.G.D.; Marques, C.G.; Coelho, L.C.; Lima, C.L.S.; Costa, J.T.G.; Silva, G.S.D.; Santos, G.B.M.; Zocolo, G.J.; et al. Intake of pitaya (Hylocereus polyrhizus (f.a.c. Weber) britton & rose) beneficially affects the cholesterolemic profile of dyslipidemic c57bl/6 mice. Food Biosci. 2021, 42, 101181. [Google Scholar]
- Luo, H.; Cai, Y.; Peng, Z.; Liu, T.; Yang, S. Chemical composition and in vitroevaluation of the cytotoxic and antioxidant activities of supercritical carbon dioxide extracts of pitaya (dragon fruit) peel. Chem. Cent. J. 2014, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Tischer, B.; Pangloli, P.; Nieto-Veloza, A.; Reeder, M.; Dia, V.P.; Sarker, U. Bioactive compounds, antioxidant capacity and anti-inflammatory activity of native fruits from brazil. PLoS ONE 2023, 18, e285625. [Google Scholar] [CrossRef]
- Carrillo Salazar, J.A.; Ortiz Hernández, Y.D. Pitahaya (Hylocereus spp.): A short review. Comun. Sci. 2012, 3, 220–237. [Google Scholar]
- Angonese, M.; Motta, G.E.; de Farias, N.S.; Molognoni, L.; Daguer, H.; Brugnerotto, P.; de Oliveira Costa, A.C.; Olivera Muller, C.M. Organic dragon fruits (Hylocereus undatus and Hylocereus polyrhizus) grown at the same edaphoclimatic conditions: Comparison of phenolic and organic acids profiles and antioxidant activities. LWT-Food Sci. Technol. 2021, 149, 111924. [Google Scholar] [CrossRef]
- Yaodong, D.; Ruijun, H.; Hongyu, W. Characteristics and Impact of Weather and Climate in Guangdong Province from July to August 2023. Guangdong Meteorol. 2023, 5, 45. [Google Scholar]
- Chu, Y.; Chang, J. High temperature suppresses fruit/seed set and weight, and cladode regreening in red-fleshed ‘da hong’ pitaya (Hylocereus polyrhizus) under controlled conditions. Hortscience 2020, 55, 1259–1264. [Google Scholar] [CrossRef]
- Paśko, P.; Galanty, A.; Zagrodzki, P.; Ku, Y.G.; Luksirikul, P.; Weisz, M.; Gorinstein, S. Bioactivity and cytotoxicity of different species of pitaya fruits—A comparative study with advanced chemometric analysis. Food Biosci. 2021, 40, 100888. [Google Scholar] [CrossRef]
- Valcarcel, J.; Reilly, K.; Gaffney, M.; O Brien, N.M. Antioxidant activity, total phenolic and total flavonoid content in sixty varieties of potato (Solanum tuberosum L.) Grown in ireland. Potato Res. 2015, 58, 221–244. [Google Scholar] [CrossRef]
- Rytel, E.; Tajner-Czopek, A.; Kita, A.; Aniołowska, M.; Kucharska, A.Z.; Sokół-Bętowska, A.; Hamouz, K. Content of polyphenols in coloured and yellow fleshed potatoes during dices processing. Food Chem. 2014, 161, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Eghbaliferiz, S.; Iranshahi, M. Prooxidant activity of polyphenols, flavonoids, anthocyanins and carotenoids: Updated review of mechanisms and catalyzing metals. Phytother. Res. 2016, 30, 1379–1391. [Google Scholar] [CrossRef]
- Chen, Z.; Zhong, B.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. Identification of phenolic compounds in australian grown dragon fruits by lc-esi-qtof-ms/ms and determination of their antioxidant potential. Arab. J. Chem. 2021, 14, 103151. [Google Scholar] [CrossRef]
- Guan, H.; Zhang, W.; Sun-Waterhouse, D.; Jiang, Y.; Li, F.; Waterhouse, G.I.N.; Li, D. Phenolic-protein interactions in foods and post ingestion: Switches empowering health outcomes. Trends Food Sci. Technol. 2021, 118, 71–86. [Google Scholar] [CrossRef]
- Tao, X.; Wu, Q.; Li, J.; Huang, S.; Cai, L.; Mao, L.; Luo, Z.; Li, L.; Ying, T. Exogenous methyl jasmonate regulates phenolic compounds biosynthesis during postharvest tomato ripening. Postharvest Biol. Technol. 2022, 184, 111760. [Google Scholar] [CrossRef]
- Bovy, A.G.; Schijlen, E.G.W.M.; Hall, R.D. Metabolic engineering of flavonoids in tomato (Solanum lycopersicum): The potential for metabolomics. Metabolomics 2007, 3, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Ren, R.; Li, Z.; Zhang, L.; Zhou, H.; Jiang, X.; Liu, Y. Enzymatic and nonenzymatic antioxidant systems impact the viability of cryopreserved paeonia suffruticosa pollen. Plant Cell Tissue Organ Cult. 2021, 144, 233–246. [Google Scholar] [CrossRef]
- Munné-Bosch, S.; Queval, G.; Foyer, C.H. The impact of global change factors on redox signaling underpinning stress tolerance. Plant Physiol. 2013, 161, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Lou, T.; Zhao, N.; Gao, Y.; Dong, L.; Jiang, D.; Shen, W.; Huang, L.; Wang, R. Presoaking with hemin improves salinity tolerance during wheat seed germination. Acta Physiol. Plant. 2011, 33, 1173–1183. [Google Scholar] [CrossRef]
- Zhao, M.; Li, J.; Shi, X.; Sanaullah Malik, M.; Quan, Y.; Guo, D.; Wang, L.; Wang, S. Effects of exogenous plant regulators on growth and development of “kyoho” grape under salt alkali stress. Front. Plant Sci. 2023, 14, 1274684. [Google Scholar] [CrossRef]
- Meng, F.; Guo, J.; Feng, N.; Zheng, D.; Chen, X.; Chen, Z.; Jiang, H.; Jiang, X. Beneficial effects of hemin on antioxidative capacity and anatomical characters of nacl-stressed rice plants. J. Plant Growth Regul. 2024. [Google Scholar] [CrossRef]
- Keum, H.; Yoo, D.; Jon, S. Photomedicine based on heme-derived compounds. Adv. Drug Deliv. Rev. 2022, 182, 114134. [Google Scholar] [CrossRef]
- Li, Q.; Li, C.; Baryshnikov, G.; Ding, Y.; Zhao, C.; Gu, T.; Sha, F.; Liang, X.; Zhu, W.; Wu, X.; et al. Twisted-planar-twisted expanded porphyrinoid dimer as a rudimentary reaction-based methanol indicator. Nat. Commun. 2020, 11, 5289. [Google Scholar] [CrossRef]
- Lin, Y.; Li, M.; Huang, L.; Shen, W.; Ren, Y. Involvement of heme oxygenase-1 in β-cyclodextrin–hemin complex-induced cucumber adventitious rooting process. Plant Cell Rep. 2012, 31, 1563–1572. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, X.; Ren, Y.; Yang, B.; Liu, Z.; You, B.; Zhang, H.; Shen, W.; Chen, X. Β-cyclodextrin–hemin enhances tolerance against salinity in tobacco seedlings by reestablishment of ion and redox homeostasis. Plant Growth Regul. 2017, 81, 533–542. [Google Scholar] [CrossRef]
- Xie, Y.J.; Xu, S.; Han, B.; Wu, M.Z.; Yuan, X.X.; Han, Y.; Gu, Q.; Xu, D.K.; Yang, Q.; Shen, W.B. Evidence of arabidopsis salt acclimation induced by up-regulation of hy1 and the regulatory role of rbohd-derived reactive oxygen species synthesis. Plant J. Cell Mol. Biol. 2011, 66, 280–292. [Google Scholar]
- Sun, G.; Meng, Y.; Wang, Y.; Zhao, M.; Wei, S.; Gu, W. Exogenous hemin optimized maize leaf photosynthesis, root development, grain filling, and resource utilization on alleviating cadmium stress under field condition. J. Soil Sci. Plant Nut. 2022, 22, 631–646. [Google Scholar]
- Li, Y.; Li, H.; Wang, F.; Li, J.; Zhang, Y.; Wang, L.; Gao, J.; Sheng, Q. Comparative transcriptome analysis reveals effects of exogenous hematin on anthocyanin biosynthesis during strawberry fruit ripening. Int. J. Genom. 2016, 2016, 6762731. [Google Scholar] [CrossRef] [PubMed]
- Balestrasse, K.B.; Zilli, C.G.; Tomaro, M.L. Signal transduction pathways and haem oxygenase induction in soybean leaves subjected to salt stress. Redox Rep. 2008, 13, 255–262. [Google Scholar] [CrossRef]
- Chu, Y.; Chang, J. Heat stress leads to poor fruiting mainly due to inferior pollen viability and reduces shoot photosystem ii efficiency in “da hong” pitaya. Agronomy 2022, 12, 225. [Google Scholar] [CrossRef]
- Diop, A.; Meot, J.M.; Lechaudel, M.; Chiroleu, F.; Ndiaye, N.D.; Mertz, C.; Cisse, M.; Chillet, M. Impact of preharvest and postharvest on color changes during convective drying of mangoes. Foods 2021, 10, 490. [Google Scholar] [CrossRef] [PubMed]
- Campion, E.M.; Loughran, S.T.; Walls, D. Protein Quantitation and Analysis of Purity; Humana Press: Totowa, NJ, USA, 2011; Volume 681, pp. 229–258. [Google Scholar]
- Kuai, J.; Liu, Z.; Wang, Y.; Meng, Y.; Chen, B.; Zhao, W.; Zhou, Z.; Oosterhuis, D.M. Waterlogging during flowering and boll forming stages affects sucrose metabolism in the leaves subtending the cotton boll and its relationship with boll weight. Plant Sci. 2014, 223, 79–98. [Google Scholar] [CrossRef] [PubMed]
- Kanto, U.; Jutamanee, K.; Osotsapar, Y.; Jattupornpong, S. Effect of swine manure extract on leaf nitrogen concentration, chlorophyll content, total potassium in plant parts and starch content in fresh tuber yield of cassava. J. Plant Nutr. 2012, 35, 688–703. [Google Scholar] [CrossRef]
- Nayyar, H.; Bains, T.; Kumar, S. Low temperature induced floral abortion in chickpea: Relationship to abscisic acid and cryoprotectants in reproductive organs. Environ. Exp. Bot. 2005, 53, 39–47. [Google Scholar] [CrossRef]
- Du, Y.; Zhao, Q.; Chen, L.; Yao, X.; Zhang, W.; Zhang, B.; Xie, F. Effect of drought stress on sugar metabolism in leaves and roots of soybean seedlings. Plant Physiol. Biochem. 2020, 146, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zeraik, M.L.; Yariwake, J.H. Quantification of isoorientin and total flavonoids in passiflora edulis fruit pulp by hplc-uv/dad. Microchem. J. 2010, 96, 86–91. [Google Scholar] [CrossRef]
- Pirie, A.; Mullins, M.G. Changes in anthocyanin and phenolics content of grapevine leaf and fruit tissues treated with sucrose, nitrate, and abscisic acid. Plant Physiol. 1976, 58, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases, 1: Occurrence in higher plants [corn, oats, peas]. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Stewart, R.R.C.; Bewley, J.D. Lipid peroxidation associated with accelerated aging of soybean axes. Plant Physiol. 1980, 65, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Klapheck, S.; Zimmer, I.; Cosse, H. Scavenging of hydrogen peroxide in the endosperm of ricinus communis by ascorbate peroxidase. Plant Cell Physiol. 1990, 31, 1005–1013. [Google Scholar]
- Gupta, D.K.; Huang, H.G.; Yang, X.E.; Razafindrabe, B.H.N.; Inouhe, M. The detoxification of lead in sedum alfredii h. Is not related to phytochelatins but the glutathione. J. Hazard. Mater. 2010, 177, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, L.; Li, Y.; Ma, L.; Bu, N.; Ma, C. Changes in photosynthesis, antioxidant enzymes and lipid peroxidation in soybean seedlings exposed to uv-b radiation and/or cd. Plant Soil 2012, 352, 377–387. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K.K.U.U. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Xiao, Q.; Tang, W.; Zhang, C.; Zhou, L.; Feng, L.; Shen, J.; Yan, T.; Gao, P.; He, Y.; Wu, N. Spectral preprocessing combined with deep transfer learning to evaluate chlorophyll content in cotton leaves. Plant Phenomics 2022, 2022, 9813841. [Google Scholar] [CrossRef]
- Qian, G.; Li, X.; Zhang, H.; Zhang, H.; Zhou, J.; Ma, X.; Sun, W.; Yang, W.; He, R.; Wahab, A.; et al. Metabolomics analysis reveals the accumulation patterns of flavonoids and phenolic acids in quinoa (Chenopodium quinoa willd.) Grains of different colors. Food Chem. X 2023, 17, 100594. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Pan, Y.; Li, J.; Chen, J.; Yang, S.; Zhao, M.; Xue, Y. Transcriptome sequencing analysis of root in soybean responding to mn poisoning. Int. J. Mol. Sci. 2023, 24, 12727. [Google Scholar] [CrossRef]
- Magalhães, D.S.; Ramos, J.D.; Pio, L.A.S.; Vilas Boas, E.V.D.B.; Pasqual, M.; Rodrigues, F.A.; Rufini, J.C.M.; Santos, V.A.D. Physical and physicochemical modifications of white-fleshed pitaya throughout its development. Sci. Hortic. 2019, 243, 537–543. [Google Scholar] [CrossRef]
- Abdel-Hamid, H.A.; Marey, H.; Ibrahim, M. Hemin protects against cell stress induced by estrogen and progesterone in rat mammary glands via modulation of nrf2/ho-1 and nf-kappab pathways. Cell Stress Chaperones 2023, 28, 289–301. [Google Scholar] [CrossRef]
- Zhao, M.; Meng, Y.; Wang, Y.; Sun, G.; Liu, X.; Li, J.; Wei, S.; Gu, W. Exogenous hemin alleviates cadmium stress in maize by enhancing sucrose and nitrogen metabolism and regulating endogenous hormones. Int. J. Phytoremediat. 2023, 25, 368–380. [Google Scholar] [CrossRef]
- Mahawar, L.; Popek, R.; Shekhawat, G.S.; Alyemeni, M.N.; Ahmad, P. Exogenous hemin improves Cd2+ tolerance and remediation potential in Vigna radiata by intensifying the ho-1 mediated antioxidant defence system. Sci. Rep. 2021, 11, 2811. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, X.; Liu, Y.; Wei, J.; Shen, W.; Shen, Z.; Cui, J. Hemin-mediated alleviation of zinc, lead and chromium toxicity is associated with elevated photosynthesis, antioxidative capacity; Suppressed metal uptake and oxidative stress in rice seedlings. Plant Growth Regul. 2017, 81, 253–264. [Google Scholar] [CrossRef]
- Zhao, H.; Zheng, D.; Feng, N.; Zhou, G.; Khan, A.; Lu, X.; Deng, P.; Zhou, H.; Du, Y. Regulatory effects of hemin on prevention and rescue of salt stress in rapeseed (Brassica napus L.) Seedlings. BMC Plant Biol. 2023, 23, 558. [Google Scholar] [CrossRef] [PubMed]
- Shafiq, M.; Singh, Z.; Khan, A.S. Time of methyl jasmonate application influences the development of ‘cripps pink’ apple fruit colour. J. Sci. Food Agric. 2013, 93, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Shattir, A.; Abu-Goukh, A. Physico-chemical changes during growth and development of papaya fruit. Ι: Physical changes. Agric. Biol. J. N. Am. 2010, 1, 866–870. [Google Scholar] [CrossRef]
- de Lima, C.A.; Fábio, G.F.; Nilton, T.V.J.; Graciele, B. Avaliação de características físico-químicas de frutos de duas espécies de pitaya1. Ceres Mag. 2014, 3, 377–383. [Google Scholar] [CrossRef]
- Cordeiro, M.H.M.; Silva, J.M.D.; Mizobutsi, G.P.; Mizobutsi, E.H.; Mota, W.F.D. Caracterização física, química e nutricional da pitaia-rosa de polpa vermelha. Rev. Bras. Frutic. 2015, 37, 20–26. [Google Scholar] [CrossRef]
- Burger, Y.; Schaffer, A.A. Contribution of sucrose metabolism enzymes to sucrose accumulation in cucumis melo. J. Am. Soc. Hortic. Sci. 2007, 132, 704–712. [Google Scholar] [CrossRef]
- Wang, Y.; Ji, D.; Chen, T.; Li, B.; Zhang, Z.; Qin, G.; Tian, S. Production, signaling, and scavenging mechanisms of reactive oxygen species in fruit-pathogen interactions. Int. J. Mol. Sci. 2019, 20, 2994. [Google Scholar] [CrossRef]
- Kerch, G. Chitosan films and coatings prevent losses of fresh fruit nutritional quality: A review. Trends Food Sci. Technol. 2015, 46, 159–166. [Google Scholar] [CrossRef]
- Li, X.; Li, M.; Wang, J.; Wang, L.; Han, C.; Jin, P.; Zheng, Y. Methyl jasmonate enhances wound-induced phenolic accumulation in pitaya fruit by regulating sugar content and energy status. Postharvest Biol. Technol. 2018, 137, 106–112. [Google Scholar] [CrossRef]
- Ali, A.; Ong, M.K.; Forney, C.F. Effect of ozone pre-conditioning on quality and antioxidant capacity of papaya fruit during ambient storage. Food Chem. 2014, 142, 19–26. [Google Scholar] [CrossRef]
- Roleira, F.M.; Tavares-Da-Silva, E.J.; Varela, C.L.; Costa, S.C.; Silva, T.; Garrido, J.; Borges, F. Plant derived and dietary phenolic antioxidants: Anticancer properties. Food Chem. 2015, 183, 235–258. [Google Scholar] [CrossRef] [PubMed]
- Akyol, H.; Riciputi, Y.; Capanoglu, E.; Caboni, M.F.; Verardo, V. Phenolic compounds in the potato and its byproducts: An overview. Int. J. Mol. Sci. 2016, 17, 835. [Google Scholar] [CrossRef]
- Liu, X.; Meng, Y.; Wei, S.; Gu, W. Exogenous hemin confers cadmium tolerance by decreasing cadmium accumulation and modulating water status and matter accumulation in maize seedlings. Agronomy 2021, 11, 739. [Google Scholar] [CrossRef]
- Alañón, M.E.; Pimentel-Moral, S.; Arráez-Román, D.; Segura-Carretero, A. Hplc-dad-q-tof-ms profiling of phenolic compounds from mango (Mangifera indica L.) Seed kernel of different cultivars and maturation stages as a preliminary approach to determine functional and nutraceutical value. Food Chem. 2021, 337, 127764. [Google Scholar] [CrossRef]
- Trivellini, A.; Lucchesini, M.; Ferrante, A.; Massa, D.; Orlando, M.; Incrocci, L.; Mensuali-Sodi, A. Pitaya, an attractive alternative crop for mediterranean region. Agronomy 2020, 10, 1065. [Google Scholar] [CrossRef]
- Zhao, H.; Fan, Z.; Wu, J.; Zhu, S. Effects of pre-treatment with s-nitrosoglutathione-chitosan nanoparticles on quality and antioxidant systems of fresh-cut apple slices. Food Sci. Technol. 2021, 139, 110565. [Google Scholar] [CrossRef]
- Zhu, Z.; Huang, Y.; Wu, X.; Liu, Z.; Zou, J.; Chen, Y.; Su, N.; Cui, J. Increased antioxidative capacity and decreased cadmium uptake contribute to hemin-induced alleviation of cadmium toxicity in chinese cabbage seedlings. Ecotoxicol. Environ. Saf. 2019, 177, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Xuan, W.; Yu, T.; Fang, W.B.; Lou, T.L.; Gao, Y.; Chen, X.Y.; Xiao, X.; Shen, W.B. Exogenous hematin alleviates mercury-induced oxidative damage in the roots ofmedicago sativa. J. Integr. Plant Biol. 2007, 49, 1703–1713. [Google Scholar] [CrossRef]
- Xia, Y.; Chen, T.; Qin, G.; Li, B.; Tian, S. Synergistic action of antioxidative systems contributes to the alleviation of senescence in kiwifruit. Postharvest Biol. Technol. 2016, 111, 15–24. [Google Scholar] [CrossRef]
- Gayathri, V.; Asha, V.V.; John, J.A.; Subramoniam, A. Protection of immunocompromised mice from fungal infection with a thymus growth-stimulatory component from selaginella involvens, a fern. Immunopharm. Immunot. 2011, 33, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Li, S.; Gao, Q.; Qiao, L.; Cao, Q.; Yang, Z.; Chen, C.; Jiang, Y.; Wang, G.; Fu, S. Advances in the anti-tumor activity of biflavonoids in iselaginella/i. Int. J. Mol. Sci. 2023, 24, 7731. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C. The traditional and modern uses of Selaginella tamariscina (p.beauv.) Spring, in medicine and cosmetic: Applications and bioactive ingredients. J. Ethnopharmacol. 2021, 280, 114444. [Google Scholar] [CrossRef] [PubMed]
- Křížkovská, B.; Kumar, R.; Yehořová, K.; Sýkora, D.; Dobiasová, S.; Kučerová, D.; Tan, M.C.; Linis, V.; Oyong, G.; Ruml, T.; et al. Comparison of chemical composition and biological activities of eight selaginella species. Pharmaceuticals 2021, 14, 16. [Google Scholar] [CrossRef]
- Gao, F.; Khan, R.; Yang, L.; Chi, Y.X.; Wang, Y.; Zhou, X.B. Uncovering the potentials of long-term straw return and nitrogen supply on subtropical maize (Zea mays L.) Photosynthesis and grain yield. Field Crops Res. 2023, 302, 109062. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, S.; Ling, T.; Xu, L.; Shen, W. Heme oxygenase/carbon monoxide system participates in regulating wheat seed germination under osmotic stress involving the nitric oxide pathway. J. Plant Physiol. 2010, 167, 1371–1379. [Google Scholar] [CrossRef] [PubMed]


| Treatment | Single Fruit Weight (g) | Edible Rate (%) | Yield (g) | Transverse Diameter (mm) | Longitudinal Diameter (mm) | Fruit Shape Index |
|---|---|---|---|---|---|---|
| CK | 219.16 ± 8.61 b | 61.49%± 0.02 b | 6110.09 ± 206.15 b | 66.20 ± 2.72 a | 84.67 ± 3.28 b | 1.2767 ± 0.00 c |
| H1 | 247.62 ± 1.99 ab | 65.89%± 0.01 a | 6195.23 ± 69.88 b | 68.07 ± 1.15 a | 90.13 ± 1.91 ab | 1.3267 ± 0.01 b |
| H2 | 265.34 ± 14.04 a | 68.44% ± 0.01 a | 6954.10 ± 87.77 a | 67.63 ± 3.75 a | 90.67 ± 5.34 ab | 1.34 ± 0.01 ab |
| H3 | 247.42 ± 9.44 ab | 67.92%± 0.00 a | 6820.99 ± 230.08 a | 71.83 ± 0.66 a | 98.50 ± 1.82 a | 1.3667 ± 0.01 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, M.; Khan, A.; Wang, J.; Ding, L.; Yang, X.; Xiong, J.; Sun, Z.; Feng, N.; Zheng, D. Exogenous Hemin Increases the Yield, Phenolic Compound Content, and Antioxidant Enzyme Activity of Dragon Fruit during the High-Temperature Period. Agronomy 2024, 14, 1850. https://doi.org/10.3390/agronomy14081850
Sun M, Khan A, Wang J, Ding L, Yang X, Xiong J, Sun Z, Feng N, Zheng D. Exogenous Hemin Increases the Yield, Phenolic Compound Content, and Antioxidant Enzyme Activity of Dragon Fruit during the High-Temperature Period. Agronomy. 2024; 14(8):1850. https://doi.org/10.3390/agronomy14081850
Chicago/Turabian StyleSun, Minmin, Aaqil Khan, Jiahui Wang, Linchong Ding, Xiaohui Yang, Jian Xiong, Zhiyuan Sun, Naijie Feng, and Dianfeng Zheng. 2024. "Exogenous Hemin Increases the Yield, Phenolic Compound Content, and Antioxidant Enzyme Activity of Dragon Fruit during the High-Temperature Period" Agronomy 14, no. 8: 1850. https://doi.org/10.3390/agronomy14081850
APA StyleSun, M., Khan, A., Wang, J., Ding, L., Yang, X., Xiong, J., Sun, Z., Feng, N., & Zheng, D. (2024). Exogenous Hemin Increases the Yield, Phenolic Compound Content, and Antioxidant Enzyme Activity of Dragon Fruit during the High-Temperature Period. Agronomy, 14(8), 1850. https://doi.org/10.3390/agronomy14081850





