Characterization of Biochar Produced in a Mobile Handmade Kiln from Small-Sized Waste Biomass for Agronomic and Climate Change Benefits
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Kiln Construction and Biochar Production
3.2. Approximate Analyses
3.3. Elemental Analysis
3.4. pH and EC
3.5. Inorganic Matter
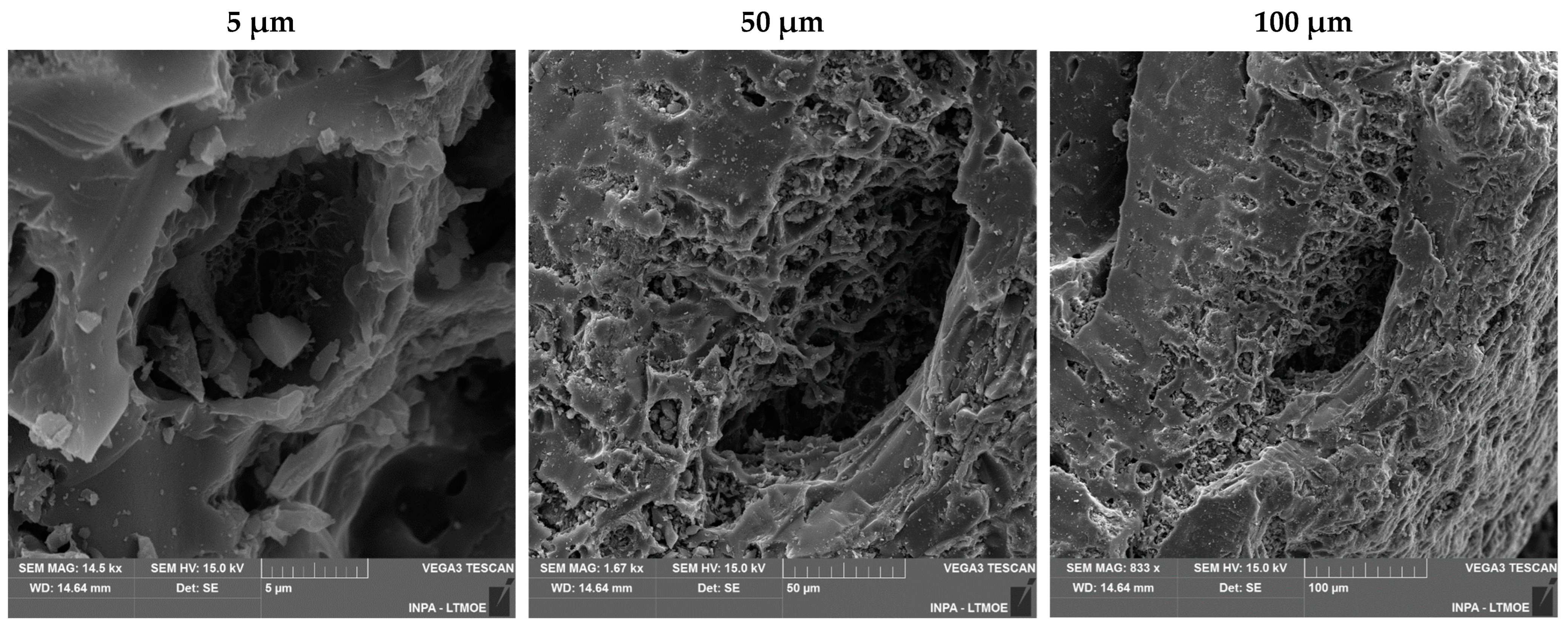
3.6. Morphological Analysis
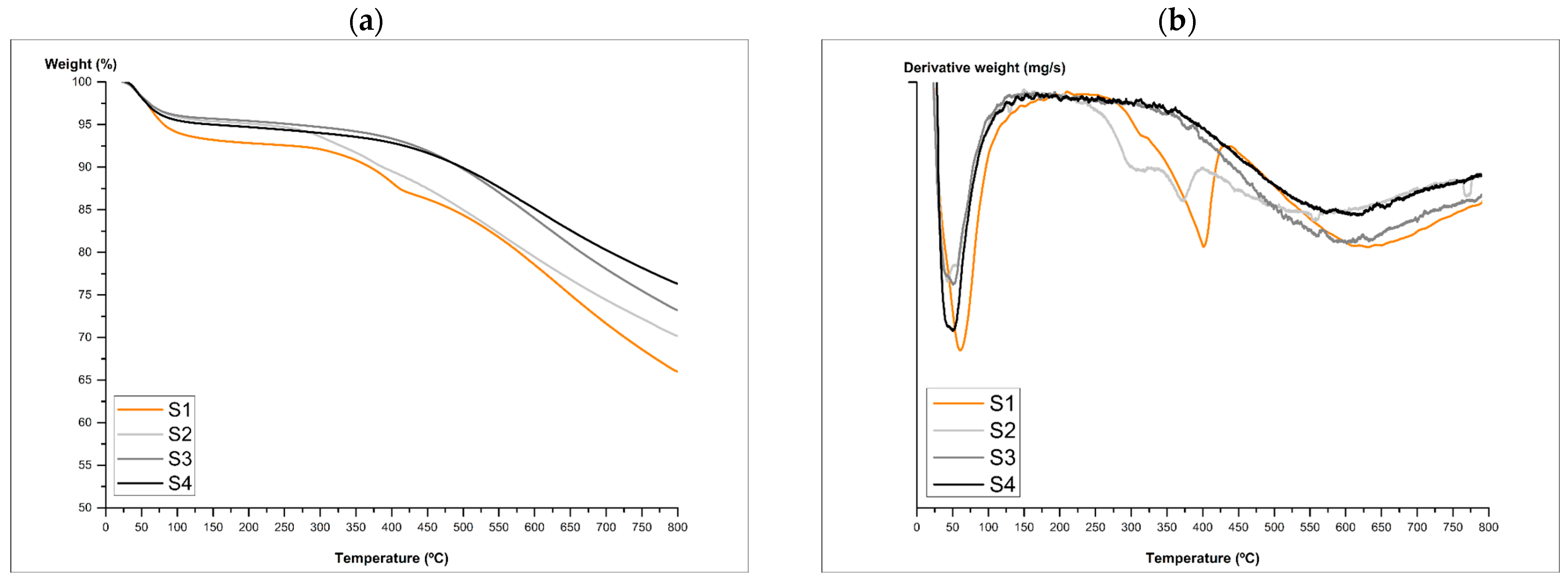
3.7. Thermal Decomposition
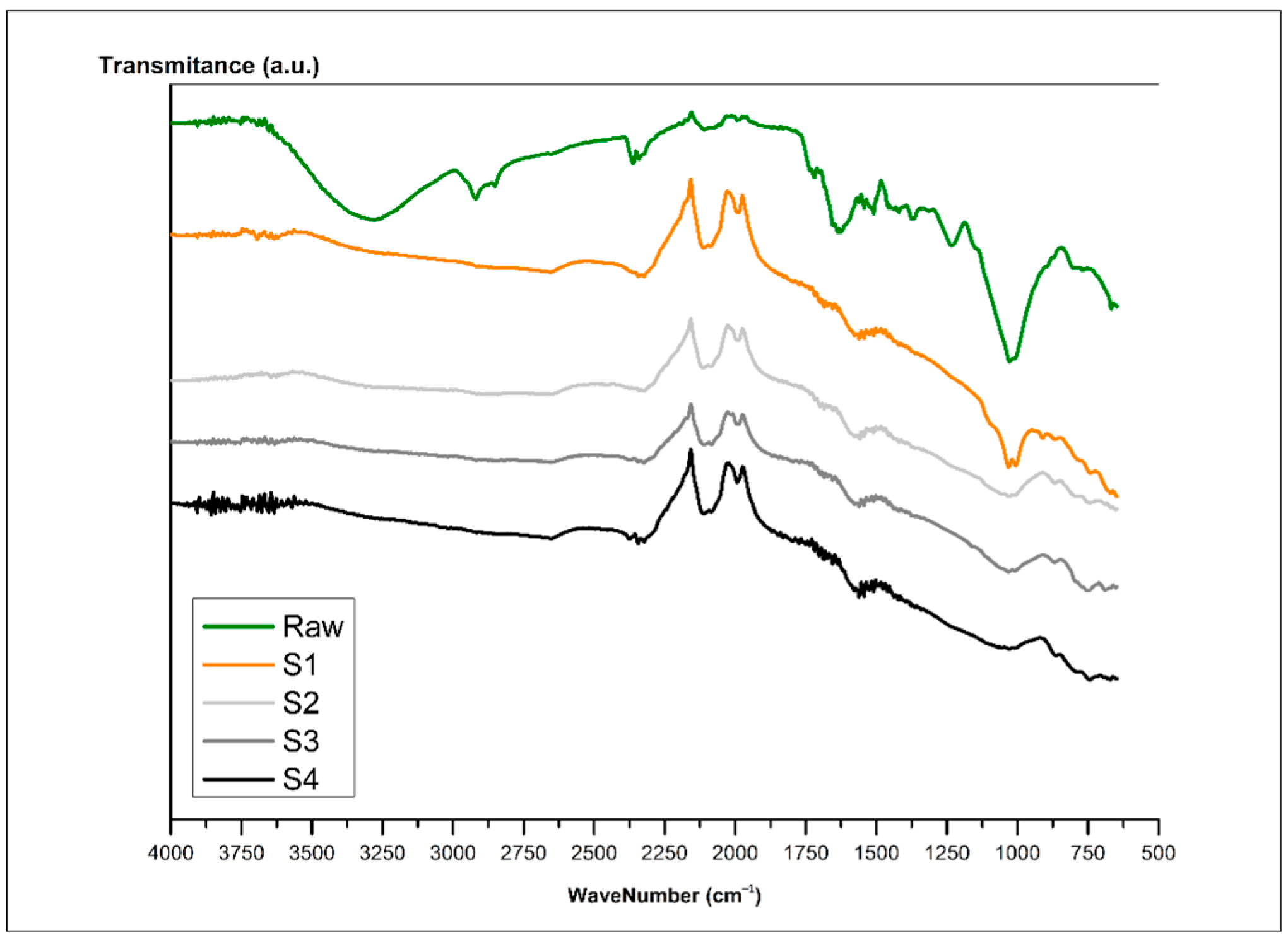
3.8. Functional Groups
3.9. Correlations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
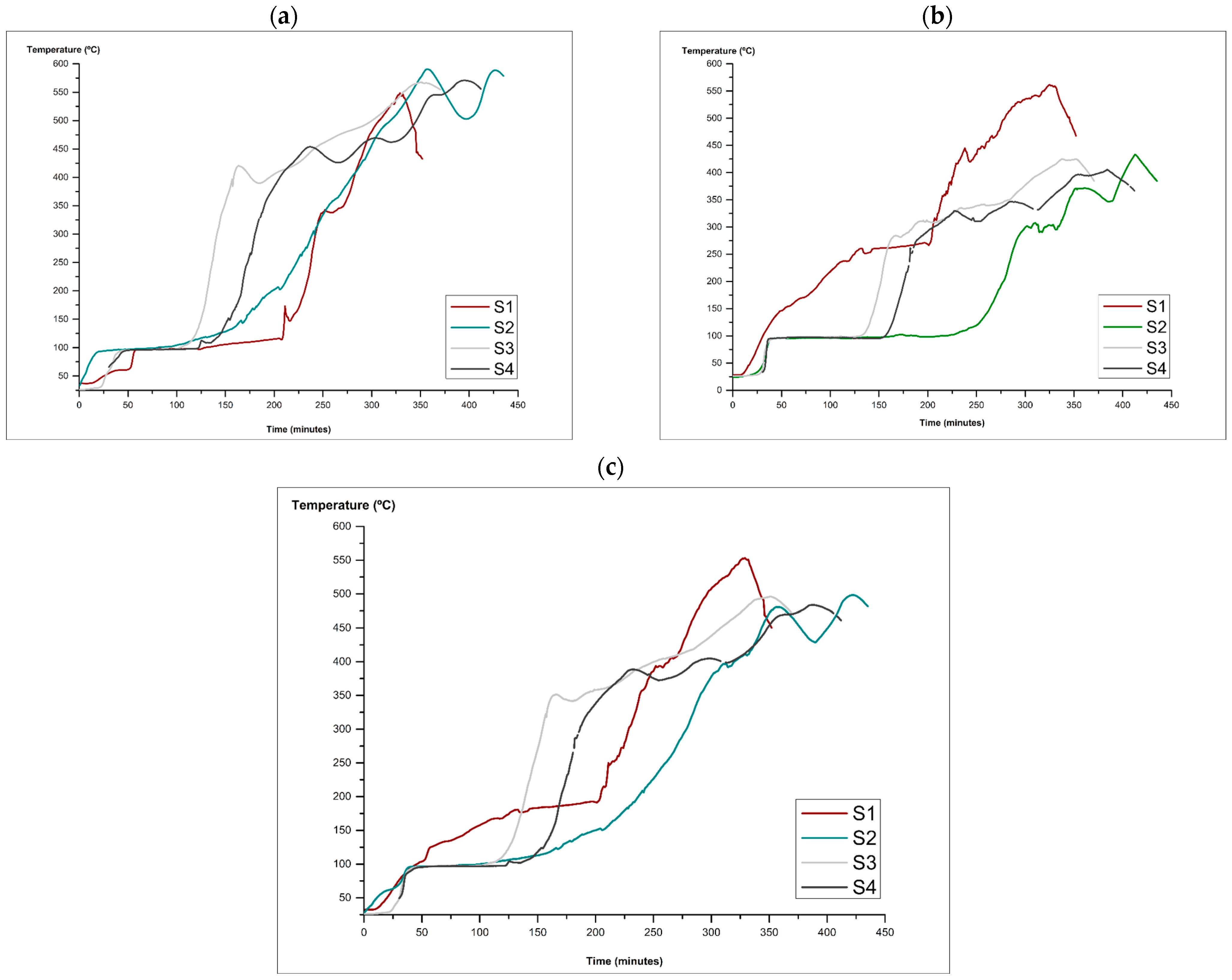
| Sample | Carbonisation Process Number | Biomass (kg) | Biochar (kg) | Gas (kg) | Maximum Temperature (°C) | Total Carbonisation Time (min) | |
|---|---|---|---|---|---|---|---|
| Thermocouple T1 | Thermocouple T2 | ||||||
| S1 | 1 | 37.8 | 10.3 | 4.4 | 548.6 | 561.6 | 351 |
| S2 | 10 | 57.4 | 16.0 | 5.6 | 590.3 | 433.3 | 375 |
| S3 | 16 | 45.0 | 13.0 | 2.8 | 567.7 | 425.2 | 351 |
| S4 | 17 | 47.3 | 12.9 | 3.3 | 570.8 | 405.6 | 400 |
| 95% Confidence Interval | ||||
|---|---|---|---|---|
| Mean | Lower Limit | Upper Limit | Standard Deviation | |
| Biomass (kg) | 46.89 | 34.03 | 59.75 | 8.081 |
| Biochar (kg) | 13.06 | 9.35 | 16.77 | 2.329 |
| Yield (biochar/biomass−%) | 27.83 | 26.62 | 29.04 | 0.759 |
| Gas consumption (kg) | 4.04 | 2.05 | 6.02 | 1.249 |
| Total carbonisation time (min) | 392.38 | 332.06 | 452.69 | 37.906 |
| Time above 250 °C (min) | 193 | 122.54 | 263.46 | 44.279 |
| Average temperature above 250 °C (°C) | 415.93 | 393.54 | 438.33 | 14.074 |
| Average maximum temperature (°C) | 507.94 | 458.79 | 557.09 | 30.889 |
| Total Carbonisation Time (min) | Time above 250 °C (min) | Average Temperature above 250 °C | Average Maximum Temperature (°C) | Moisture (%) | Ash (%) | pH (H2O) | Electrical Conductivity | C | N | O | H | Volatiles | Fixed C | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total carbonisation time (min) | — | ||||||||||||||||||
| Time above 250 °C (min) | 0.306 | — | |||||||||||||||||
| Average temperature above 250 °C | −0.499 | −0.963 | * | — | |||||||||||||||
| Average maximum temperature (°C) | −0.698 | −0.884 | 0.969 | * | — | ||||||||||||||
| Moisture (%) | −0.288 | −0.995 | ** | 0.971 | * | 0.885 | — | — | |||||||||||
| Ash (%) | 0.301 | 0.789 | −0.873 | −0.804 | −0.844 | ||||||||||||||
| pH (H2O) | −0.717 | 0.288 | −0.023 | 0.183 | −0.256 | −0.100 | — | ||||||||||||
| Electrical conductivity | 0.518 | 0.950 | * | −0.934 | −0.922 | −0.922 | 0.643 | 0.161 | — | ||||||||||
| C | −0.851 | 0.038 | 0.227 | 0.428 | −0.011 | −0.285 | 0.966 | * | −0.097 | — | |||||||||
| N | −0.205 | 0.556 | −0.570 | −0.408 | −0.632 | 0.862 | 0.164 | 0.289 | 0.068 | — | |||||||||
| O | 0.770 | −0.344 | 0.103 | −0.133 | 0.339 | −0.097 | −0.962 | * | −0.145 | −0.926 | −0.413 | — | |||||||
| H | 0.770 | 0.569 | −0.769 | −0.853 | −0.608 | 0.814 | −0.605 | 0.585 | −0.771 | 0.460 | 0.482 | — | |||||||
| Volatiles | −0.450 | −0.972 | * | 0.943 | 0.910 | 0.948 | −0.674 | −0.220 | −0.996 | ** | 0.038 | −0.354 | 0.219 | −0.564 | — | ||||
| Fixed C | 0.416 | 0.951 | * | −0.899 | −0.864 | −0.916 | 0.588 | 0.289 | 0.991 | ** | 0.035 | 0.273 | −0.260 | 0.474 | −0.993 | ** | — | ||
References
- CONAB. Boletim da Sociobiodiversidade; Companhia Nacional de Abastecimento: Brasília, DF, Brazil, 2022.
- IBGE. Produção Agrícola Municipal (PAM 2021). Available online: https://sidra.ibge.gov.br/tabela/1613 (accessed on 12 July 2023).
- IBGE. Produção da Extração Vegetal e da Silvicultura (PEVS 2021). Available online: https://sidra.ibge.gov.br/pesquisa/pevs/quadros/brasil/2021 (accessed on 12 July 2023).
- Tavares, G.d.S.; Homma, A.K.O.; de Menezes, A.A.; Palheta, M.P. Análise da Produção e Comercialização de Açaí no Estado do Pará, Brasil. In Sinergias de Mudança da Agricultura Amazônica: Conflitos e Oportunidades; Homma, A.K.O., Ed.; Embrapa: Brasília, DF, Brazil, 2022; pp. 444–463. [Google Scholar]
- Laurindo, L.F.; Barbalho, S.M.; Araújo, A.C.; Guiguer, E.L.; Mondal, A.; Bachtel, G.; Bishayee, A. Açaí (Euterpe oleracea Mart.) in Health and Disease: A Critical Review. Nutrients 2023, 15, 989. [Google Scholar] [CrossRef]
- IBGE. Produção de Açaí (Cultivo) No Brasil. Available online: https://www.ibge.gov.br/explica/producao-agropecuaria/acai-cultivo/br (accessed on 12 January 2024).
- Bufalino, L.; Guimarães, A.A.; Silva, B.M.d.S.e.; de Souza, R.L.F.; de Melo, I.C.N.A.; de Oliveira, D.N.P.S.; Trugilho, P.F. Local Variability of Yield and Physical Properties of Açaí Waste and Improvement of Its Energetic Attributes by Separation of Lignocellulosic Fibers and Seeds. J. Renew. Sustain. Energy 2018, 10, 053102. [Google Scholar] [CrossRef]
- Batista, J.G.; Correa, L.P. Destinação do Resíduo de Açaí em um Supermercado de Capanema-PA e a Redução de Emissões de Metano; Universidade Federal Rural da Amazônia: Capanema, PA, USA, 2021. [Google Scholar]
- Sato, M.K.; de Lima, H.V.; Noronha Costa, A.; Rodrigues, S.; Mooney, S.J.; Clarke, M.; Silva Pedroso, A.J.; de Freitas Maia, C.M.B. Biochar as a Sustainable Alternative to Açaí Waste Disposal in Amazon, Brazil. Process Saf. Environ. Prot. 2020, 139, 36–46. [Google Scholar] [CrossRef]
- de Castro, D.A.R.; Da Silva Ribeiro, H.; Hamoy Guerreiro, L.; Pinto Bernar, L.; Jonatan Bremer, S.; Costa Santo, M.; Da Silva Almeida, H.; Duvoisin, S.; Pizarro Borges, L.; Teixeira Machado, N. Production of Fuel-Like Fractions by Fractional Distillation of Bio-Oil from Açaí (Euterpe oleracea Mart.) Seeds Pyrolysis. Energies 2021, 14, 3713. [Google Scholar] [CrossRef]
- Mares, E.K.L.; Gonçalves, M.A.; Da Luz, P.T.S.; Da Rocha Filho, G.N.; Zamian, J.R.; Da Conceição, L.R.V. Acai Seed Ash as a Novel Basic Heterogeneous Catalyst for Biodiesel Synthesis: Optimization of the Biodiesel Production Process. Fuel 2021, 299, 120887. [Google Scholar] [CrossRef]
- Rendeiro, G. Combustão e Gasificação de Biomassa Sólida; Soluções Energéticas para a Amazônia, 1st ed.; Ministério de Minas e Energia: Brasília, DF, Brazil, 2008; ISBN 978-85-98341-05-7. [Google Scholar]
- Teixeira, M.A.; Escobar Palacio, J.C.; Sotomonte, C.R.; Silva Lora, E.E.; Venturini, O.J.; Aßmann, D. Assaí—An Energy View on an Amazon Residue. Biomass Bioenergy 2013, 58, 76–86. [Google Scholar] [CrossRef]
- Barbosa, A.d.M.; Rebelo, V.S.M.; Martorano, L.G.; Giacon, V.M. Caracterização de partículas de açaí visando seu potencial uso na construção civil. Matéria 2019, 24, e12435. [Google Scholar] [CrossRef]
- Santos, R.K.S.; Nascimento, B.F.; De Araújo, C.M.B.; Cavalcanti, J.V.F.L.; Bruckmann, F.S.; Rhoden, C.R.B.; Dotto, G.L.; Oliveira, M.L.S.; Silva, L.F.O.; Motta Sobrinho, M.A. Removal of Chloroquine from the Aqueous Solution by Adsorption onto Açaí-Based Biochars: Kinetics, Thermodynamics, and Phytotoxicity. J. Mol. Liq. 2023, 383, 122162. [Google Scholar] [CrossRef]
- Zaparoli, M.; Igansi, A.V.; Silveira, J.T.D.; Morais, M.G.D.; Costa, J.A.V. Biochar as a Sustainable Alternative for the Use of Residues from the Processing of Açaí and the Removal of Glyphosate. J. Environ. Chem. Eng. 2023, 11, 111162. [Google Scholar] [CrossRef]
- Dias, Y.N.; Souza, E.S.; da Costa, H.S.C.; Melo, L.C.A.; Penido, E.S.; do Amarante, C.B.; Teixeira, O.M.M.; Fernandes, A.R. Biochar Produced from Amazonian Agro-Industrial Wastes: Properties and Adsorbent Potential of Cd2+ and Cu2+. Biochar 2019, 1, 389–400. [Google Scholar] [CrossRef]
- Sato, M.K.; de Lima, H.V.; Costa, A.N.; Rodrigues, S.; Pedroso, A.J.S.; de Freitas Maia, C.M.B. Biochar from Acai Agroindustry Waste: Study of Pyrolysis Conditions. Waste Manag. 2019, 96, 158–167. [Google Scholar] [CrossRef]
- Santos, V.O.; Queiroz, L.S.; Araujo, R.O.; Ribeiro, F.C.P.; Guimarães, M.N.; da Costa, C.E.F.; Chaar, J.S.; de Souza, L.K.C. Pyrolysis of Acai Seed Biomass: Kinetics and Thermodynamic Parameters Using Thermogravimetric Analysis. Bioresour. Technol. Rep. 2020, 12, 100553. [Google Scholar] [CrossRef]
- Li, W.; Tao, E.; Hao, X.; Li, N.; Li, Y.; Yang, S. MMT and ZrO2 Jointly Regulate the Pore Size of Graphene Oxide-Based Composite Aerogel Materials to Improve the Selective Removal Ability of Cu(II). Sep. Purif. Technol. 2024, 331, 125506. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, S.; Zhou, K.; Zhao, J.; Wang, J.; Li, N.; Wang, Y.; Li, Y.; Tao, E. Preparation of Co-Doped Biochar to Improve Electron Transfer and Modulate 1O2 Generation: Unraveling the Radical-Unradical Mechanism. Chem. Eng. J. 2024, 491, 151985. [Google Scholar] [CrossRef]
- Adegbeye, M.J.; Ravi Kanth Reddy, P.; Obaisi, A.I.; Elghandour, M.M.M.Y.; Oyebamiji, K.J.; Salem, A.Z.M.; Morakinyo-Fasipe, O.T.; Cipriano-Salazar, M.; Camacho-Díaz, L.M. Sustainable Agriculture Options for Production, Greenhouse Gasses and Pollution Alleviation, and Nutrient Recycling in Emerging and Transitional Nations—An Overview. J. Clean. Prod. 2020, 242, 118319. [Google Scholar] [CrossRef]
- Joseph, S.; Cowie, A.L.; Van Zwieten, L.; Bolan, N.; Budai, A.; Buss, W.; Cayuela, M.L.; Graber, E.R.; Ippolito, J.A.; Kuzyakov, Y.; et al. How Biochar Works, and When It Doesn’t: A Review of Mechanisms Controlling Soil and Plant Responses to Biochar. GCB Bioenergy 2021, 13, 1731–1764. [Google Scholar] [CrossRef]
- Kumar, A.; Bhattacharya, T. Biochar: A Sustainable Solution. Environ. Dev. Sustain. 2021, 23, 6642–6680. [Google Scholar] [CrossRef]
- Arroyo-Kalin, M. Slash-Burn-and-Churn: Landscape History and Crop Cultivation in Pre-Columbian Amazonia. Quat. Int. 2012, 249, 4–18. [Google Scholar] [CrossRef]
- Bezerra, J.; Turnhout, E.; Vasquez, I.M.; Rittl, T.F.; Arts, B.; Kuyper, T.W. The Promises of the Amazonian Soil: Shifts in Discourses of Terra Preta and Biochar. J. Environ. Policy Plan. 2019, 21, 623–635. [Google Scholar] [CrossRef]
- Clement, C.R.; Denevan, W.M.; Heckenberger, M.J.; Junqueira, A.B.; Neves, E.G.; Teixeira, W.G.; Woods, W.I. The Domestication of Amazonia before European Conquest. Proc. R. Soc. B Biol. Sci. 2015, 282, 20150813. [Google Scholar] [CrossRef]
- Fraser, J.; Teixeira, W.; Falcão, N.; Woods, W.; Lehmann, J.; Junqueira, A.B. Anthropogenic Soils in the Central Amazon: From Categories to a Continuum: Anthropogenic Soils in the Central Amazon. Area 2011, 43, 264–273. [Google Scholar] [CrossRef]
- Glaser, B.; Birk, J.J. State of the Scientific Knowledge on Properties and Genesis of Anthropogenic Dark Earths in Central Amazonia (Terra Preta de Índio). Geochim. Cosmochim. Acta 2012, 82, 39–51. [Google Scholar] [CrossRef]
- Novotny, E.H.; Maia, C.M.B.d.F.; Carvalho, M.T.d.M.; Madari, B.E. Biochar: Pyrogenic Carbon for Agricultural Use—A Critical Review. Rev. Bras. Ciênc. Solo 2015, 39, 321–344. [Google Scholar] [CrossRef]
- Tripathi, M.; Sahu, J.N.; Ganesan, P. Effect of Process Parameters on Production of Biochar from Biomass Waste through Pyrolysis: A Review. Renew. Sustain. Energy Rev. 2016, 55, 467–481. [Google Scholar] [CrossRef]
- Kan, T.; Strezov, V.; Evans, T.J. Lignocellulosic Biomass Pyrolysis: A Review of Product Properties and Effects of Pyrolysis Parameters. Renew. Sustain. Energy Rev. 2016, 57, 1126–1140. [Google Scholar] [CrossRef]
- Wiedner, K.; Glaser, B. Traditional Use of Biochar. In Biochar for Environmental Management: Science, Technology and Implementation, 2nd ed.; Routledge: London, UK; Taylor & Francis Group: New York, NY, USA, 2015; pp. 15–37. [Google Scholar]
- Lefebvre, D.; Fawzy, S.; Aquije, C.A.; Osman, A.I.; Draper, K.T.; Trabold, T.A. Biomass Residue to Carbon Dioxide Removal: Quantifying the Global Impact of Biochar. Biochar 2023, 5, 65. [Google Scholar] [CrossRef]
- Sands, B.; Machado, M.R.; White, A.; Zent, E.; Gould, R. Moving towards an Anti-Colonial Definition for Regenerative Agriculture. Agric. Hum. Values 2023, 40, 1697–1716. [Google Scholar] [CrossRef]
- Tisserant, A.; Cherubini, F. Potentials, Limitations, Co-Benefits, and Trade-Offs of Biochar Applications to Soils for Climate Change Mitigation. Land 2019, 8, 179. [Google Scholar] [CrossRef]
- Ippolito, J.A.; Cui, L.; Kammann, C.; Wrage-Mönnig, N.; Estavillo, J.M.; Fuertes-Mendizabal, T.; Cayuela, M.L.; Sigua, G.; Novak, J.; Spokas, K.; et al. Feedstock Choice, Pyrolysis Temperature and Type Influence Biochar Characteristics: A Comprehensive Meta-Data Analysis Review. Biochar 2020, 2, 421–438. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar Physicochemical Properties: Pyrolysis Temperature and Feedstock Kind Effects. Rev. Environ. Sci. Biotechnol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Zhao, B.; O’Connor, D.; Zhang, J.; Peng, T.; Shen, Z.; Tsang, D.C.W.; Hou, D. Effect of Pyrolysis Temperature, Heating Rate, and Residence Time on Rapeseed Stem Derived Biochar. J. Clean. Prod. 2018, 174, 977–987. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. (Eds.) Biochar for Environmental Management: Science, Technology and Implementation, 2nd ed.; Routledge: London, UK; Taylor & Francis Group: New York, NY, USA, 2015; ISBN 978-0-415-70415-1. [Google Scholar]
- Cornelissen, G.; Pandit, N.R.; Taylor, P.; Pandit, B.H.; Sparrevik, M.; Schmidt, H.P. Emissions and Char Quality of Flame-Curtain “Kon Tiki” Kilns for Farmer-Scale Charcoal/Biochar Production. PLoS ONE 2016, 11, e0154617. [Google Scholar] [CrossRef]
- Luo, H.; Wang, X.; Liu, X.; Wu, X.; Shi, X.; Xiong, Q. A Review on CFD Simulation of Biomass Pyrolysis in Fluidized Bed Reactors with Emphasis on Particle-Scale Models. J. Anal. Appl. Pyrolysis 2022, 162, 105433. [Google Scholar] [CrossRef]
- Schwaber, K. Agile Project Management with Scrum; Microsoft Press: Redmond, WA, USA, 2004; ISBN 978-0-7356-1993-7. [Google Scholar]
- CarbonZero. Small Scale Biochar Production. Available online: http://biochar.ch/?p=en.small_scale_biochar_kiln (accessed on 17 February 2021).
- Sangsuk, S.; Buathong, C.; Suebsiri, S. High-Energy Conversion Efficiency of Drum Kiln with Heat Distribution Pipe for Charcoal and Biochar Production. Energy Sustain. Dev. 2020, 59, 1–7. [Google Scholar] [CrossRef]
- IBI-STD-2.1; IBI Standardized Product Definition and Product Testing Guidelines for Biochar That Is Used in Soil (Aka IBI Biochar Standards), Version 2.1. International Biochar Initiative: Bowdoinham, ME, USA, 2015.
- Leng, L.Y.; Husni, M.H.A.; Samsuri, A.W. Comparison of the Carbon-Sequestering Abilities of Pineapple Leaf Residue Chars Produced by Controlled Combustion and by Field Burning. Bioresour. Technol. 2011, 102, 10759–10762. [Google Scholar] [CrossRef]
- Lorenz, K.; Lal, R. Carbon Sequestration in Agricultural Ecosystems; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Benites, V.d.M.; Mendonça, E.d.S.; Schaefer, C.E.G.R.; Novotny, E.H.; Reis, E.L.; Ker, J.C. Properties of Black Soil Humic Acids from High Altitude Rocky Complexes in Brazil. Geoderma 2005, 127, 104–113. [Google Scholar] [CrossRef]
- Nan, H.; Yin, J.; Yang, F.; Luo, Y.; Zhao, L.; Cao, X. Pyrolysis Temperature-Dependent Carbon Retention and Stability of Biochar with Participation of Calcium: Implications to Carbon Sequestration. Environ. Pollut. 2021, 287, 117566. [Google Scholar] [CrossRef]
- MAPA. Manual de Métodos Analíticos Oficiais para Fertilizantes e Corretivos; Ministério da Agricultura, Pecuária e Abastecimento. Secretaria de Defesa Agropecuária: Brasília, DF, Brazil, 2017; ISBN 978-85-7991-109-5.
- Zhao, L.; Cao, X.; Mašek, O.; Zimmerman, A. Heterogeneity of Biochar Properties as a Function of Feedstock Sources and Production Temperatures. J. Hazard. Mater. 2013, 256–257, 1–9. [Google Scholar] [CrossRef]
- Adhikari, S.; Moon, E.; Paz-Ferreiro, J.; Timms, W. Comparative Analysis of Biochar Carbon Stability Methods and Implications for Carbon Credits. Sci. Total Environ. 2024, 914, 169607. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, L.; Li, H.; Westholm, L.J.; Carvalho, L.; Thorin, E.; Yu, Z.; Yu, X.; Skreiberg, Ø. A Critical Review on Production, Modification and Utilization of Biochar. J. Anal. Appl. Pyrolysis 2022, 161, 105405. [Google Scholar] [CrossRef]
- Namaswa, T.; Burslem, D.F.R.P.; Smith, J. Emerging Trends in Appropriate Kiln Designs for Small-Scale Biochar Production in Low to Middle Income Countries. Bioresour. Technol. Rep. 2023, 24, 101641. [Google Scholar] [CrossRef]
- Lehmann, J.; Cowie, A.; Masiello, C.A.; Kammann, C.; Woolf, D.; Amonette, J.E.; Cayuela, M.L.; Camps-Arbestain, M.; Whitman, T. Biochar in Climate Change Mitigation. Nat. Geosci. 2021, 14, 883–892. [Google Scholar] [CrossRef]
- Budai, A.; Zimmerman, A.R.; Cowie, A.L.; Webber, J.B.W.; Singh, B.P.; Glaser, B.; Masiello, C.A.; Shields, F.; Lehmann, J.; Arbestain, M.C.; et al. Biochar Carbon Stability Test Method: An Assessment of Methods to Determine Biochar Carbon Stability. Int. Biochar Initiat. 2013, 1, 1–20. [Google Scholar]
- Spokas, K.A. Review of the Stability of Biochar in Soils: Predictability of O:C Molar Ratios. Carbon Manag. 2010, 1, 289–303. [Google Scholar] [CrossRef]
- IBI. Biochar Classification Tool. Available online: https://biochar-international.org/resources/biochar-classification-tool/ (accessed on 4 May 2024).
- Mukherjee, A.; Zimmerman, A.R.; Harris, W. Surface Chemistry Variations among a Series of Laboratory-Produced Biochars. Geoderma 2011, 163, 247–255. [Google Scholar] [CrossRef]
- Sousa, E.D.T.d.S.; de Queiroz, D.M.; Coelho, A.L.d.F.; Valente, D.S.M. Development of Signal Analysis Algorithm for Apparent Soil Electrical Conductivity Sensor. Biosyst. Eng. 2021, 211, 183–191. [Google Scholar] [CrossRef]
- Bartoli, M.; Arrigo, R.; Malucelli, G.; Tagliaferro, A.; Duraccio, D. Recent Advances in Biochar Polymer Composites. Polymers 2022, 14, 2506. [Google Scholar] [CrossRef]
- Jemal, K. Role of Bio Char on the Amelioration of Soil Acidity. Agrotechnology 2021, 10, 212. [Google Scholar]
- Onorevoli, B.; Da Silva Maciel, G.P.; Machado, M.E.; Corbelini, V.; Caramão, E.B.; Jacques, R.A. Characterization of Feedstock and Biochar from Energetic Tobacco Seed Waste Pyrolysis and Potential Application of Biochar as an Adsorbent. J. Environ. Chem. Eng. 2018, 6, 1279–1287. [Google Scholar] [CrossRef]
- DeLuca, T.H.; Gundale, M.J.; MacKenzie, M.D.; Jones, D.L. Biochar Effects on Soil Nutrient Transformations. Biochar Environ. Manag. Sci. Technol. Implement. 2015, 2, 421–454. [Google Scholar]
- Buratto, R.T.; Cocero, M.J.; Martín, Á. Characterization of Industrial Açaí Pulp Residues and Valorization by Microwave-Assisted Extraction. Chem. Eng. Process.—Process Intensif. 2021, 160, 108269. [Google Scholar] [CrossRef]
- Jahirul, M.; Rasul, M.; Chowdhury, A.; Ashwath, N. Biofuels Production through Biomass Pyrolysis—A Technological Review. Energies 2012, 5, 4952–5001. [Google Scholar] [CrossRef]
- Narzari, R.; Bordoloi, N.; Sarma, B.; Gogoi, L.; Gogoi, N.; Borkotoki, B.; Kataki, R. Fabrication of Biochars Obtained from Valorization of Biowaste and Evaluation of Its Physicochemical Properties. Bioresour. Technol. 2017, 242, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Zornoza, R.; Moreno-Barriga, F.; Acosta, J.A.; Muñoz, M.A.; Faz, A. Stability, Nutrient Availability and Hydrophobicity of Biochars Derived from Manure, Crop Residues, and Municipal Solid Waste for Their Use as Soil Amendments. Chemosphere 2016, 144, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Dolk, M.M.; Shen, Q.; Camps-Arbestain, M. Biochar pH, Electrical Conductivity and Liming Potential. In Biochar: A Guide to Analytical Methods; CSIRO Publishing: Clayton, Australia, 2017; Volume 23. [Google Scholar]




| Temperature Range (°C) | Mean | ||
|---|---|---|---|
| Maximum Heating Rate (°C min−1) | Temperature (°C) | Residence Time (min) | |
| 25–119 | 4.1 | 84.6 | 122.6 |
| 120–249 | 6.5 | 171.8 | 76.8 |
| 250–349 | 5.5 | 305.8 | 31.0 |
| 350–600 | 3.2 | 436.9 | 162.0 |
| Element | Unit | Raw | Sample | Biochar Mean | 95% Confidence Interval | Standard Deviation | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | Lower Limit | Upper Limit | |||||
| Carbon (C) | % | 83.79 | 79.57 | 72.31 | 78.05 | 77.92 | 76.96 | 71.89 | 82.03 | 3.189 |
| Hydrogen (H) | 2.69 | 1.29 | 1.58 | 1.52 | 1.48 | 1.47 | 1.27 | 1.67 | 0.125 | |
| Nitrogen (N) | 0.24 | 0.32 | 0.37 | 0.59 | 0.36 | 0.41 | 0.21 | 0.60 | 0.122 | |
| Oxygen (O) | 11.44 | 15.00 | 20.48 | 13.58 | 15.08 | 16.04 | 11.20 | 20.87 | 3.041 | |
| H/C | Molar ratio | 0.38 | 0.19 | 0.26 | 0.23 | 0.23 | 0.23 | 0.19 | 0.27 | 0.028 |
| O/C | 0.10 | 0.14 | 0.21 | 0.13 | 0.15 | 0.16 | 0.10 | 0.22 | 0.037 | |
| C Retention | % | - | 25.84 | 24.05 | 26.91 | 25.44 | 25.56 | 23.68 | 27.44 | 1.183 |
| pH (H2O) | - | 5.58 | 9.21 | 7.77 | 9.18 | 9.28 | 8.86 | 7.70 | 10.02 | 0.728 |
| EC | μS m−1 | 687 | 183 | 259 | 292 | 358 | 273 | 157.22 | 388.78 | 72.760 |
| Element | Raw | Biochar Sample (g/kg Dry Matter) | CI 95% | ||||||
|---|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | Mean | SD | Lower Limit | Upper Limit | ||
| K | 3.73 | 5.79 | 8.89 | 9.77 | 10.27 | 8.68 | 2.0093 | 5.48 | 11.88 |
| P | 0.97 | 1.58 | 1.94 | 2.34 | 2.60 | 2.12 | 0.44822 | 1.40 | 2.83 |
| Ca | 0.88 | 1.33 | 1.37 | 1.76 | 1.58 | 1.51 | 0.1995 | 1.19 | 1.83 |
| Mg | 0.81 | 0.90 | 1.37 | 1.62 | 1.77 | 1.42 | 0.38092 | 0.81 | 2.02 |
| S | 0.19 | 0.41 | 0.70 | 0.64 | 0.57 | 0.58 | 0.1252 | 0.38 | 0.78 |
| Na | 0.13 | 0.22 | 0.07 | 0.07 | 0.04 | 0.10 | 0.0812 | −0.03 | 0.23 |
| Fe | 0.1875 | 0.6877 | 1.1875 | 0.9270 | 0.7654 | 0.8919 | 0.2208 | 0.5405 | 1.2433 |
| Zn | 0.0114 | 2.1044 | 0.0199 | 0.0251 | 0.0235 | 0.5432 | 1.0408 | −1.1129 | 2.1994 |
| Mn | 0.0690 | 0.1173 | 0.1482 | 0.1525 | 0.2016 | 0.1549 | 0.0349 | 0.0994 | 0.2104 |
| Cu | 0.0069 | 0.6279 | 0.0100 | 0.0140 | 0.0099 | 0.1655 | 0.3083 | −0.3251 | 0.6560 |
| Cr | 0.0034 | 0.0077 | 0.0188 | 0.0147 | 0.0206 | 0.0155 | 0.0057 | 0.0063 | 0.0246 |
| B | 0.0041 | 0.0180 | 0.0131 | 0.0125 | 0.0110 | 0.0137 | 0.0030 | 0.0088 | 0.0185 |
| Ni | 0.0030 | 0.0051 | 0.0074 | 0.0056 | 0.0091 | 0.0068 | 0.0018 | 0.0039 | 0.0097 |
| Pb | 0.0004 | 0.0054 | 0.0017 | 0.0018 | 0.0016 | 0.0026 | 0.0019 | −0.0003 | 0.0056 |
| Mo | 0.0003 | 0.0014 | 0.0007 | 0.0007 | 0.0007 | 0.0009 | 0.0004 | 0.0003 | 0.0014 |
| Cd | 0.0000 | 0.0002 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0000 | 0.0002 |
| Sample | Elements (Mass %) | |||||
|---|---|---|---|---|---|---|
| C | O | Ca | Fe | K | Nb | |
| S1 | 83.83 | 15.45 | 0.09 | 0.02 | 0.6 | 0.02 |
| S2 | 76.29 | 22.77 | 0.11 | 0.01 | 0.75 | 0.08 |
| S3 | 97.45 | - | 0.17 | 0.02 | 1.55 | 0.81 |
| S4 | 99.06 | - | 0.18 | 0.08 | 0.63 | 0.02 |
| Mean | 89.16 | - | 0.14 | 0.03 | 0.88 | 0.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
John, V.; Braga, A.R.d.O.; Danielli, C.K.A.d.O.; Sousa, H.M.; Danielli, F.E.; de Araujo, R.O.; Marques-dos-Santos, C.S.d.C.; Falcão, N.P.d.S.; Guerra, J.F.C. Characterization of Biochar Produced in a Mobile Handmade Kiln from Small-Sized Waste Biomass for Agronomic and Climate Change Benefits. Agronomy 2024, 14, 1861. https://doi.org/10.3390/agronomy14081861
John V, Braga ARdO, Danielli CKAdO, Sousa HM, Danielli FE, de Araujo RO, Marques-dos-Santos CSdC, Falcão NPdS, Guerra JFC. Characterization of Biochar Produced in a Mobile Handmade Kiln from Small-Sized Waste Biomass for Agronomic and Climate Change Benefits. Agronomy. 2024; 14(8):1861. https://doi.org/10.3390/agronomy14081861
Chicago/Turabian StyleJohn, Vinicius, Ana Rita de Oliveira Braga, Criscian Kellen Amaro de Oliveira Danielli, Heiriane Martins Sousa, Filipe Eduardo Danielli, Rayanne Oliveira de Araujo, Cláudia Saramago de Carvalho Marques-dos-Santos, Newton Paulo de Souza Falcão, and João Francisco Charrua Guerra. 2024. "Characterization of Biochar Produced in a Mobile Handmade Kiln from Small-Sized Waste Biomass for Agronomic and Climate Change Benefits" Agronomy 14, no. 8: 1861. https://doi.org/10.3390/agronomy14081861
APA StyleJohn, V., Braga, A. R. d. O., Danielli, C. K. A. d. O., Sousa, H. M., Danielli, F. E., de Araujo, R. O., Marques-dos-Santos, C. S. d. C., Falcão, N. P. d. S., & Guerra, J. F. C. (2024). Characterization of Biochar Produced in a Mobile Handmade Kiln from Small-Sized Waste Biomass for Agronomic and Climate Change Benefits. Agronomy, 14(8), 1861. https://doi.org/10.3390/agronomy14081861










