The Influence of Some Reactive Oxygen Species Treatments on the Yield and Changes in the Chemical Composition of Potato Tubers (Solanum tuberosum L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of the Experimental Model
2.2. Plant Material
2.3. Conditions for Conducting the Experiment
2.3.1. Soil Conditions
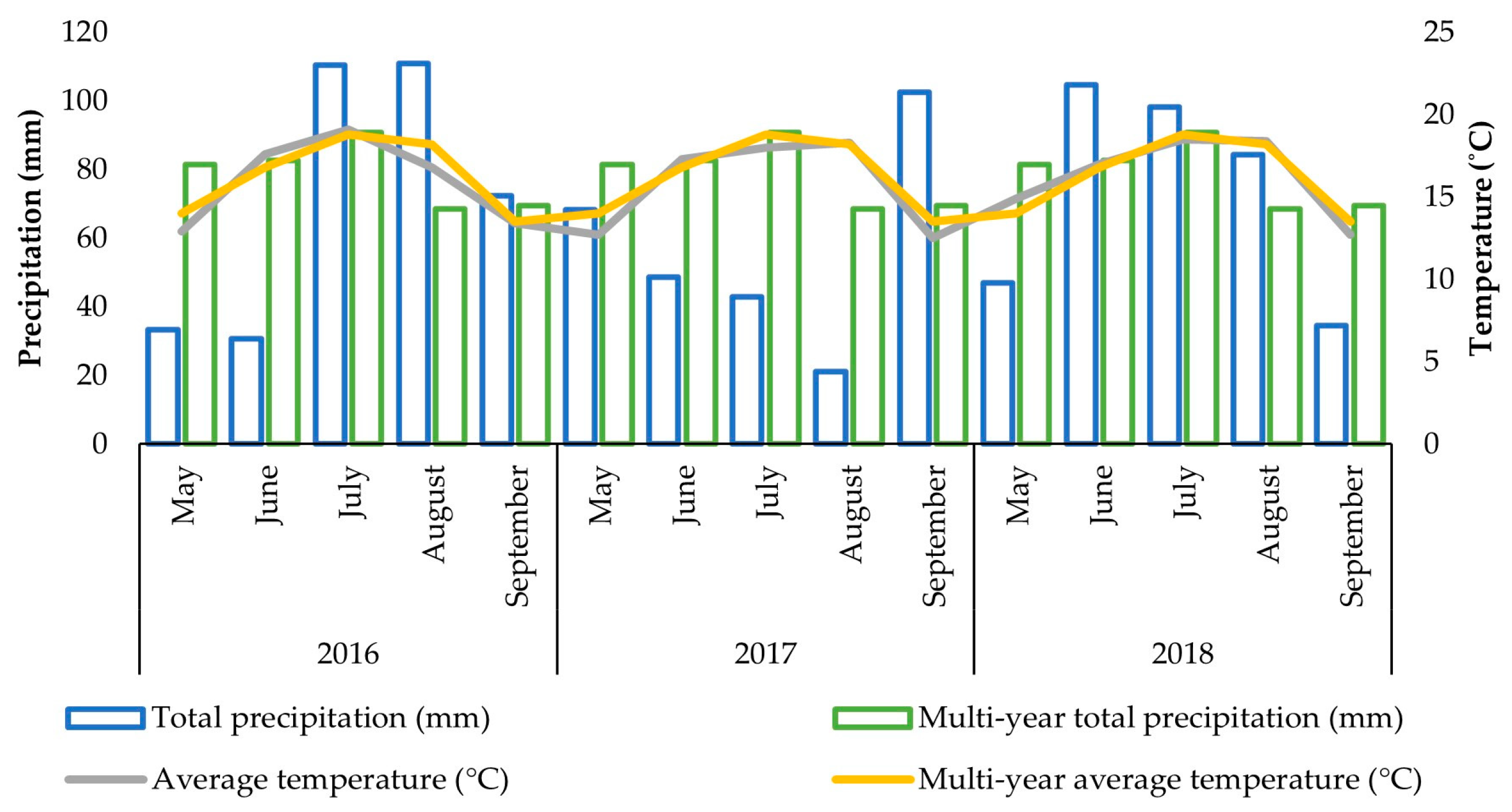
2.3.2. Weather Conditions
2.4. Device for Conducting the Plant Ozonation Process
2.5. Laboratory Evaluations
2.5.1. Starch Content
2.5.2. Vitamin C Content
2.5.3. Analysis of the Antioxidant Activity by the ABTS and DPPH Methods
2.5.4. Total Content of Polyphenolic Compounds
2.5.5. Total Antioxidant Capacity
2.6. Statistical Analysis
3. Results
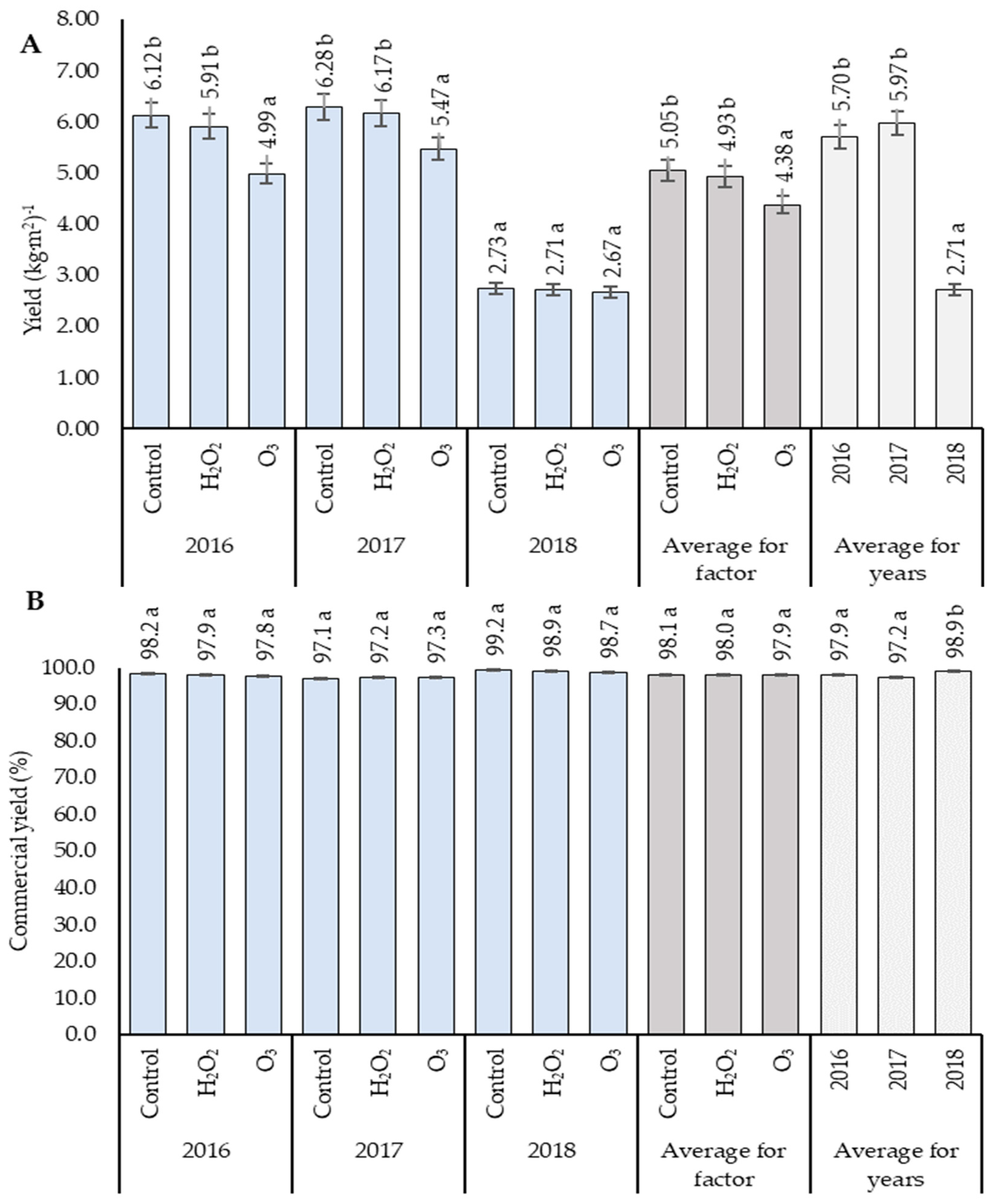
3.1. Yield Analysis
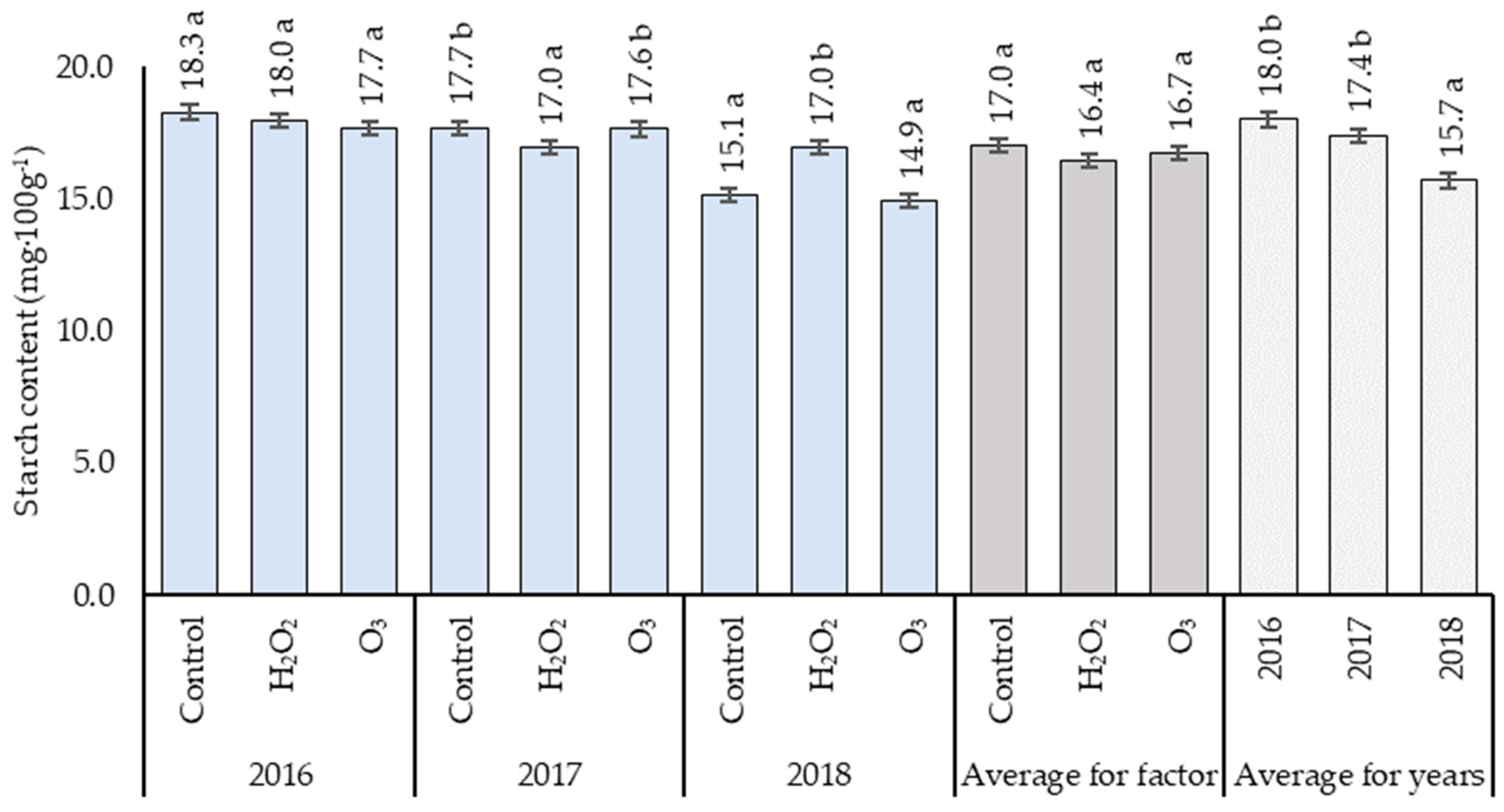
3.2. Starch Content
3.3. Vitamin C Content
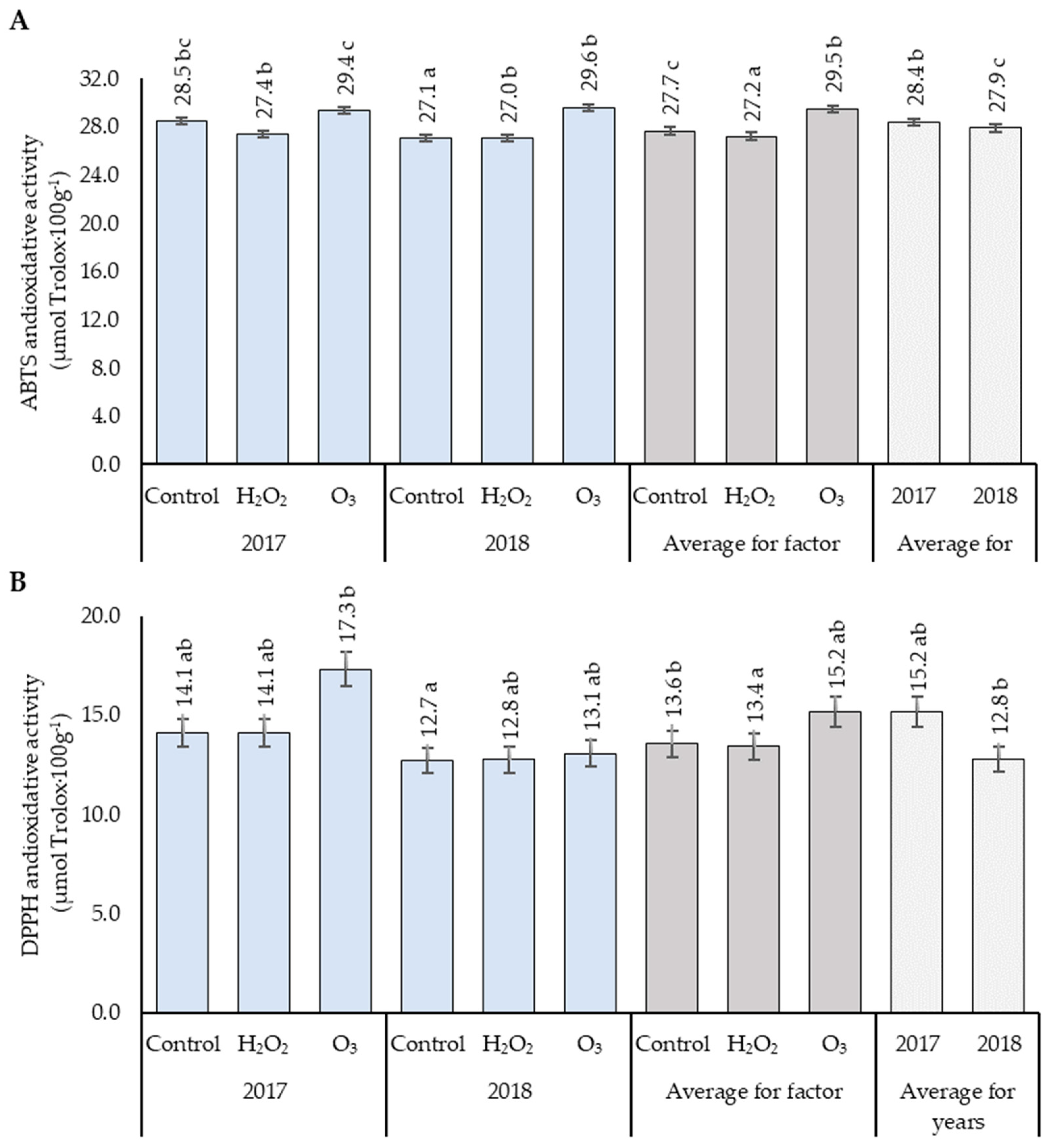
3.4. Antioxidant Activity
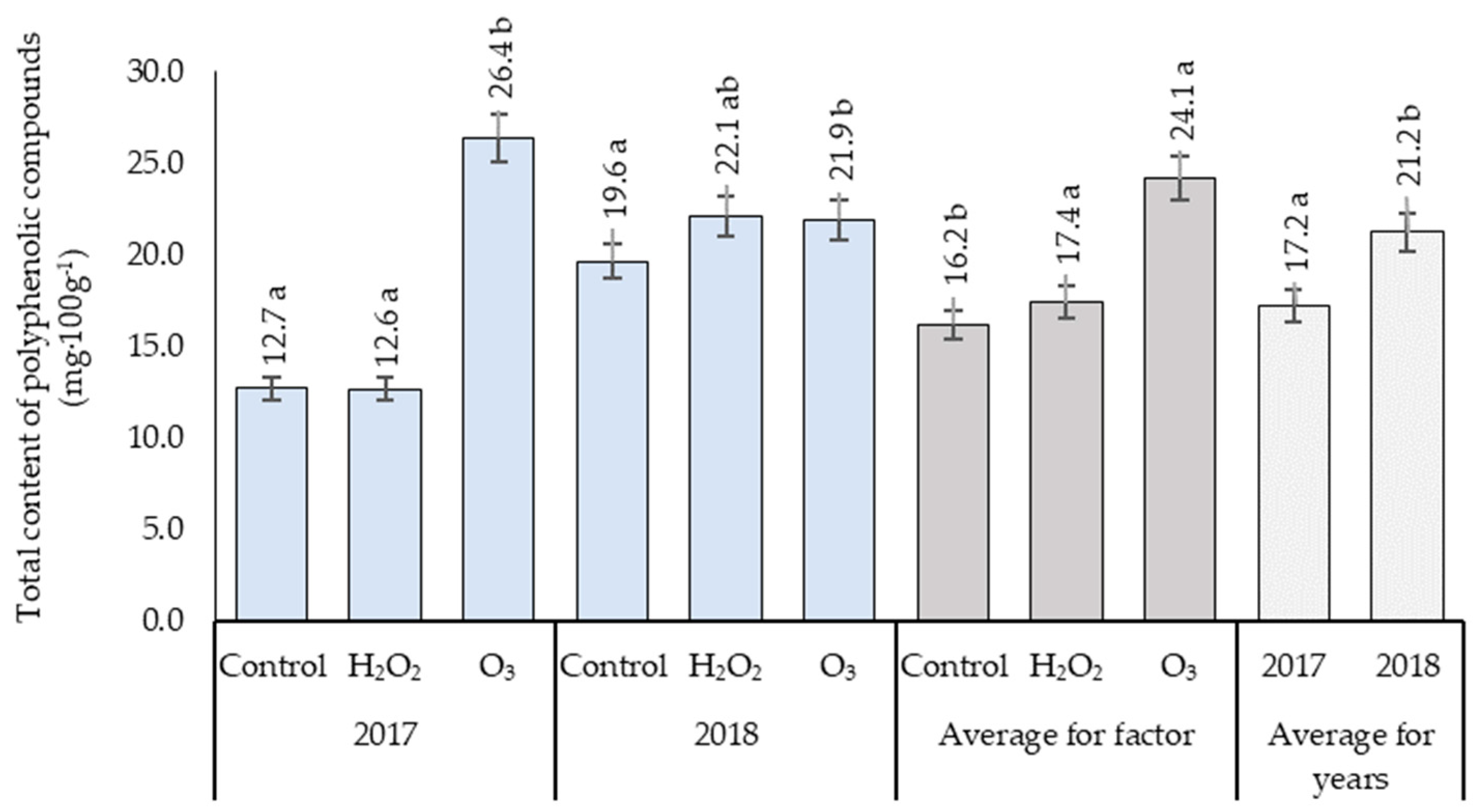
3.5. Total Content of Polyphenolic Compounds
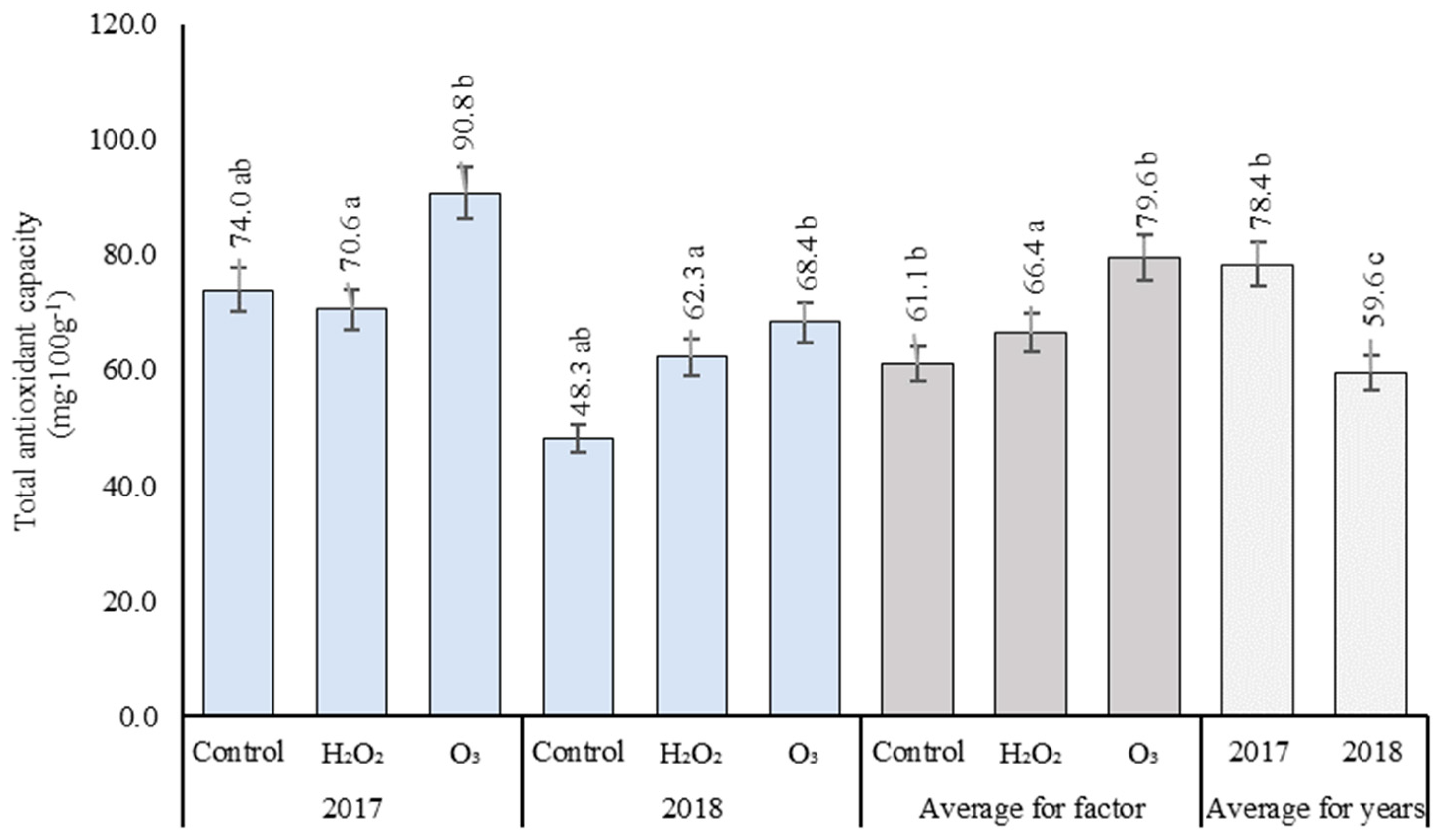
3.6. Total Antioxidant Capacity
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- You, J.; Chan, Z. ROS regulation during abiotic stress responses in crop plants. Front. Plant Sci. 2015, 6, 1092. [Google Scholar] [CrossRef]
- Sandalio, L.M.; Romero–Puertas, M.C. Peroxisomes sense and respond to environmental cues by regulating ROS and RNS signalling networks. Ann. Bot. 2015, 116, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Igamberdiev, A.U.; Lea, P.J. The role of peroxisomes in the integration of metabolism and evolutionary diversity of photosynthetic organisms. Phytochemistry 2002, 60, 651–674. [Google Scholar] [CrossRef] [PubMed]
- Raja, V.; Majeed, U.; Kang, H.; Andrabi, K.I.; John, R. Abiotic stress: Interplay between ROS, hormones and MAPKs. Environ. Exp. Bot. 2017, 137, 142–157. [Google Scholar] [CrossRef]
- Nath, M.; Bhatt, D.; Prasad, R.; Gill, S.S.; Anjum, N.A.; Tuteja, N. Reactive oxygen species generation–scavenging and signaling during plantarbuscular mycorrhizal and Piriformospora indica interaction under stress condition. Front. Plant Sci. 2016, 7, 1574. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Foyer, C.H. Ascorbate and Glutathione: Keeping Active Oxygen under Control. Annu. Rev. Plant Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef]
- Hancock, J.T. Cell Signalling; Oxford University Press: Oxford, UK, 2017. [Google Scholar]
- Jamil, M.; Rha, E.S. NaCl stress induced reduction in grwoth, photosynthesis and protein in mustard. J. Agric. Sci. 2013, 5, 114. [Google Scholar] [CrossRef]
- Abdel–Farid, I.B.; Marghany, M.R.; Rowezek, M.M.; Sheded, M.G. Effect of salinity stress on growth and metabolomic profiling of Cucumis sativus and Solanum lycopersicum. Plants 2020, 9, 1626. [Google Scholar] [CrossRef]
- Rai, M.K.; Kalia, R.K.; Singh, R.; Gangola, M.P.; Dhawan, A.K. Developing stress tolerant plants through in vitro selection—An overview of the recent progress. Environ. Exp. Bot. 2011, 71, 89–98. [Google Scholar] [CrossRef]
- Zhu, J.-K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 2002, 53, 247–273. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Savvides, A.; Ali, S.; Tester, M.; Fotopoulos, V. Chemical priming of plants against multiple abiotic stresses: Mission possible? Trends Plant Sci. 2016, 21, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef]
- Asthir, B. Mechanisms of heat tolerance in crop plants. Biol. Plant. 2015, 9, 620–628. [Google Scholar] [CrossRef]
- Suzuki, N.; Koussevitzky, S.; Mittler, R.; Miller, G. ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ. 2012, 35, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Gupta, K.; Dey, A.; Gupta, B. Plant polyamines in abiotic stress responses. Acta Physiol. Plant 2013, 35, 2015–2036. [Google Scholar] [CrossRef]
- Pál, M.; Szalai, G.; Janda, T. Speculation: Polyamines are important in abiotic stress signaling. Plant Sci. 2015, 237, 16–23. [Google Scholar] [CrossRef]
- Saha, J.; Brauer, E.K.; Sengupta, A.; Popescu, S.C.; Gupta, K.; Gupta, B. Polyamines as redox homeostasis regulators during salt stress in plants. Front. Environ. Sci. 2015, 3, 21. [Google Scholar] [CrossRef]
- Gadjev, I.; Stone, J.M.; Gechev, T.S. Programmed cell death in plants: New insights into redox regulation and the role of hydrogen peroxide. Int. Rev. Cell Mol. Biol. 2008, 270, 87–144. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Suzuki, N.; Miller, G.; Tognetti, V.B.; Vandepoele, K.; Gollery, M.; Shulaev, V.; Van Breusegem, F. ROS signaling: The new wave? Trends Plant Sci. 2011, 16, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Sengupta, A.; Chakraborty, M.; Gupta, B. Hydrogen peroxide and polyamines act as double edged swords in plant abiotic stress responses. Front. Plant Sci. 2016, 7, 1343. [Google Scholar] [CrossRef]
- Ainsworth, E.A. Understanding and improving global crop response to ozone pollution. Plant J. 2016, 90, 886–897. [Google Scholar] [CrossRef] [PubMed]
- Rai, R.; Agrawal, M. Impact of tropospheric ozone on crop plants. Proc. Natl. Acad. Sci. India Sect. B Boil. Sci. 2012, 82, 241–257. [Google Scholar] [CrossRef]
- Mina, U.; Kumar, P.; Varshney, C.K. Effect of ozone stress on different growth stages of potato (Solanum tuberosum). Phyton 2010, 49, 253–266. [Google Scholar]
- Asensi–Fabado, A.; García–Breijo, F.; Reig–Armiñana, J. Ozone–induced reductions in below–ground biomass: An anatomical approach in potato. Plant Cell Environ. 2010, 33, 1070–1083. [Google Scholar] [CrossRef]
- Guri, A. Variation in glutathione and ascorbic acid content among selected cultivars of Phaseolus vulgaris prior to and after exposure to ozone. Can. J. Plant Sci. 1983, 63, 733–737. [Google Scholar] [CrossRef]
- Chen, C.P.; Frank, T.D.; Long, S.P. Is a short, sharp shock equivalent to long–term punishment? Contrasting the spatial pattern of acute and chronic ozone damage to soybean leaves via chlorophyll fluorescence imaging. Plant Cell Environ. 2009, 32, 327–335. [Google Scholar] [CrossRef]
- Iriti, M.; Faoro, F. Oxidative stress, the paradigm of ozone toxicity in plants and animals. Water Air Soil Pollut. 2007, 187, 285–301. [Google Scholar] [CrossRef]
- Bandurska, H.; Borowiak, K.; Zielezińska, M. Oxidative stress enzymes in tobacco during a long–term exposure to ambient ozone at two different sites. Arch. Environ. Prot. 2018, 44, 3–11. [Google Scholar] [CrossRef]
- Rao, M.V.; Koch, J.R.; Davis, K. Ozone: A tool for probing programmed cell death in plants. Plant Mol. Biol. 2000, 44, 345–358. [Google Scholar] [CrossRef]
- Castagna, A.; Ranieri, A. Detoxification and repair process of ozone injury: From O3 uptake to gene expression adjustment. Environ. Pollut. 2009, 157, 1461–1469. [Google Scholar] [CrossRef] [PubMed]
- Krupa, S.V. Joint effects of elevated levels of ultraviolet–B radiation, carbon dioxide and ozone on plants. Photochem. Photobiol. 2003, 78, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Sandermann, H.; Ernst, D.; Heller, W.; Langebartels, C. Ozone: An abiotic elicitor of plant defence reactions. Trends Plant Sci. 1998, 3, 47–50. [Google Scholar] [CrossRef]
- Szpunar–Krok, E.; Jańczak–Pieniążek, M.; Migut, D.; Skrobacz, K.; Piechowiak, T.; Pawlak, R.; Balawejder, M. Physiological and biochemical properties of potato (Solanum tuberosum L.) in response to ozone–induced oxidative stress. Agronomy 2020, 10, 1745. [Google Scholar] [CrossRef]
- Szpunar–Krok, E.; Jańczak–Pieniążek, M.; Skrobacz, K.; Bobrecka–Jamro, D.; Balawejder, M. Response of potato (Solanum Tuberosum L.) plants to spraying by hydrogen peroxide. Sustainability 2020, 12, 2469. [Google Scholar] [CrossRef]
- Vainonen, J.P.; Kangasjärvi, J. Plant signalling in acute ozone exposure. Plant Cell Environ. 2014, 38, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Ueda, Y.; Uehara, N.; Sasaki, H.; Kobayashi, K.; Yamakawa, T. Impacts of acute ozone stress on superoxide dismutase (SOD) expression and reactive oxygen species (ROS) formation in rice leaves. Plant Physiol. Biochem. 2013, 70, 396–402. [Google Scholar] [CrossRef]
- Pandiselvam, R.; Subhashini, S.; Priya, E.B.; Kothakota, A.; Ramesh, S.; Shahir, S. Ozone based food preservation: A promising green technology for enhanced food safety. Ozone Sci. Eng. 2018, 41, 17–34. [Google Scholar] [CrossRef]
- Selma, M.V.; Ibáñez, A.M.; Cantwell, M.; Suslow, T. Reduction by gaseous ozone of Salmonella and microbial flora associated with fresh–cut cantaloupe. Food Microbiol. 2008, 25, 558–565. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Dummermuth, A.L.; Karsten, U.; Fisch, K.M.; Königc, G.M.; Wiencke, C. Responses of marine macroalgae to hydrogen–peroxide stress. J. Exp. Mar. Biol. Ecol. 2003, 289, 103–121. [Google Scholar] [CrossRef]
- Meloni, D.A.; Oliva, M.A.; Martinez, C.A.; Cambraia, J. Photosynthesis and activity of superoxide dismutase, peroxidase and glutathione reductase in cotton under salt stress. Environ. Exp. Bot. 2003, 49, 69–76. [Google Scholar] [CrossRef]
- Gechev, T.S.; Hille, J. Hydrogen peroxide as a signal controlling plant programmed cell death. J. Cell Biol. 2005, 168, 17–20. [Google Scholar] [CrossRef]
- Hossain, M.A.; Bhattacharjee, S.; Armin, S.M.; Qian, P.; Xin, W.; Li, H.Y.; Burritt, D.J.; Fujita, M.; Tran, L.S. Hydrogen peroxide priming modulates abiotic oxidative stress tolerance: Insights from ROS detoxification and scavenging. Front. Plant Sci. 2015, 6, 420. [Google Scholar] [CrossRef]
- Heyno, E.; Klose, C.; Krieger–Liszkay, A. Origin of cadmium–induced reactive oxygen species production: Mitochondrial electron transfer versus plasma membrane NADPH oxidase. New Phytol. 2008, 179, 687–699. [Google Scholar] [CrossRef]
- Petrov, V.D.; van Breusegem, F. Hydrogen peroxide—A central hub for information flow in plant cells. AoB Plants 2012, 2012, pls014. [Google Scholar] [CrossRef]
- Wang, X.; Shi, C.; Hu, Y.; Ma, Y.; Yi, Y.; Jia, H.; Li, F.; Sun, H.; Li, T.; Wang, X.; et al. Persulfidation maintains cytosolic G6PDs activity through changing tetrameric structure and competing cysteine sulfur oxidation under salt stress in Arabidopsis and tomato. New Phytol. 2023, 240, 626–643. [Google Scholar] [CrossRef]
- Bi, G.; Hu, M.; Fu, L.; Zhang, X.; Li, J.; Yang, J.; Zhou, J.-M. The cytosolic thiol peroxidase PRXIIB is an intracellular sensor for H2O2 that regulates plant immunity through a redox relay. Nat. Plants 2022, 8, 1160–1175. [Google Scholar] [CrossRef]
- Quan, L.J.; Zhang, B.; Shi, W.W.; Li, H.Y. Hydrogen peroxide in plants: A versatile molecule of the reactive oxygen species network. J. Integr. Plant Biol. 2008, 50, 2–18. [Google Scholar] [CrossRef]
- Dynowski, M.; Schaaf, G.; Loque, D.; Moran, O.; Ludewig, U. Plant plasma membrane water channel conduct the signalling molecule H2O2. Biochem. J. 2008, 414, 53–61. [Google Scholar] [CrossRef]
- Bienert, G.P.; Møller, A.L.B.; Kristiansen, K.A.; Schulz, A.; Møller, I.M.; Schjoerring, J.K.; Jahn, T.P. Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J. Biol. Chem. 2007, 282, 1183–1192. [Google Scholar] [CrossRef]
- Noctor, G.; Lelarge-Trouverie, C.; Mhamdi, A. The metabolomics of oxidative stress. Phytochemistry 2015, 112, 33–53. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization Corporate Statistical Database. Available online: http://www.fao.org/faostat/en/?#data/QC (accessed on 2 February 2024).
- Hardigan, M.A.; Laimbeer, F.P.E.; Newton, L.; Crisovan, E.; Hamilton, J.P.; Vaillancourt, B.; Wiegert–Rininger, K.; Wood, J.C.; Douches, D.S.; Farré, E.M. Genome diversity of tuber–bearing Solanum uncovers complex evolutionary history and targets of domestication in the cultivated potato. Proc. Natl. Acad. Sci. USA 2017, 114, E9999–E10008. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.; Abd El–Gawad, H.; Bondok, A. Physiological impacts of potassium citrate and folic acid on growth, yield and some viral diseases of potato plants. Middle East J. Agric. Res. 2015, 4, 577–589. [Google Scholar]
- Calliope, S.R.; Lobo, M.O.; Sammán, N.C. Biodiversity of Andean potatoes: Morphological, nutritional and functional characterization. Food Chem. 2018, 238, 42–50. [Google Scholar] [CrossRef]
- Alvani, K.; Qi, X.; Tester, R.F.; Snape, C.E. Physico-chemical properties of potato starches. Food Chem. 2011, 125, 958–965. [Google Scholar] [CrossRef]
- Waterschoot, J.; Gomand, S.V.; Fierens, E.; Delcour, J.A. Production, structure, physicochemical and functional properties of maize, cassava, wheat, potato and rice starches. Starch 2015, 67, 14–29. [Google Scholar] [CrossRef]
- Reyniers, S.; De Brier, N.; Matthijs, S.; Brijs, K.; Delcour, J.A. Impact of mineral ions on the release of starch and gel forming capacity of potato flakes in relation to water dynamics and oil uptake during the production of snacks made thereof. Food Res. Int. 2019, 122, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, J.M.; Cadoche, L.; López, C.; Gutierrez, G. Microstructural changes of potato cells and starch granules heated in oil. Food Res. Int. 2001, 34, 939–947. [Google Scholar] [CrossRef]
- PN-R-04031:1997; Agrochemical Soil Analyses—Sampling. Polish Committee for Standardization: Warsaw, Poland, 1997.
- PN-R-04024:1997; Agrochemical Soil Analyse—Determination of Available phosphorus, Potassium, Magnesium and Manganese Contents in Organic Soils. Polish Committee for Standardization: Warsaw, Poland, 1997.
- Roztropowicz, S. Metodyka Obserwacji, Pomiarów i Pobierania Prób w Agrotechnicznych Doświadczeniach z Ziemniakiem (Methodology of Observation, Measurement and Sampling in Agrotechnical Experiments with Potatoes); IHAR: Radzików, Poland, 1999; pp. 1–50. (In Polish) [Google Scholar]
- Molga, M. Meteorologia Rolnicza (Agricultural Meteorology); Powszechne Wydawnictwo Rolnicze i Leśne: Warszawa, Poland, 2008. (In Polish) [Google Scholar]
- Skowera, B.; Puła, J. Skrajne warunki pluwiotermiczne w okresie wiosennym na obszarze Polski w latach 1971–2000 (Extreme pluviothermal conditions in spring in Poland in 1971–2000). Acta Agrophys. 2004, 3, 171–177. (In Polish) [Google Scholar]
- PN-EN ISO 10520:2002; Native Starch—Determination of Starch Content—Ewers Polarimetric Method (ISO 10520:1997). Polish Committee for Standardization: Warsaw, Poland, 2002.
- PN-A-04019:1998; Food Products—Determination of Vitamin C. Polish Committee for Standardization: Warsaw, Poland, 1998.
- Yen, G.C.; Chen, H.Y. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J. Agric. Food Chem. 1995, 43, 27–32. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Bio. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Waterhouse, A.L. Determination of Total Phenolics. CPFAC 2002, 6, I1.1.1–I1.1.8. [Google Scholar] [CrossRef]
- Apak, R.; Guclu, K.; Ozyurek, M.; Karademir, S.E.; Altun, M. Total antioxidant capacity assay of human serum using copper(II)–neocuproine as chromogenic oxidant: The CUPRAC method. Free Radic. Res. 2005, 39, 949–961. [Google Scholar] [CrossRef]
- Larkin, R.P. Soil health paradigms and implications for disease management. Annu. Rev. Phytopathol. 2015, 53, 19–221. [Google Scholar] [CrossRef]
- Doran, J.W.; Zeiss, M.R. Soil health and sustainability: Managing the biotic component of soil quality. Appl. Soil Ecol. 2000, 15, 3–11. [Google Scholar] [CrossRef]
- Magdoff, F.; van Es, H. Building Soils for Better Crops, 3rd ed.; Sustainable Agriculture Research and Education: Waldorf, MD, USA, 2009. [Google Scholar]
- Grandy, A.S.; Porter, G.A.; Erich, M.S. Organic amendment and rotation crop effects on the recovery of soil organic matter and aggregation in potato cropping systems. Soil Sci. Soc. Am. J. 2002, 66, 1311–1319. [Google Scholar] [CrossRef]
- Ponisio, L.C.; M’Gonigle, L.K.; Mace, K.C.; Palomino, J.; de Valpine, P.; Kremen, C. Diversification practices reduce organic to conventional yield gap. Proc. R. Soc. Biol. Sci. 2015, 282, 20141396. [Google Scholar] [CrossRef]
- Kazimierczak, R.; Średnicka–Tober, D.; Hallmann, E.; Kopczyńska, K.; Zarzyńska, K. The Impact of Organic vs. Conventional Agricultural Practices on Selected Quality Features of Eight Potato Cultivars. Agronomy 2019, 9, 799. [Google Scholar] [CrossRef]
- Brazinskiene, V.; Asakaviciute, R.; Miezeliene, A.; Alencikiene, G.; Ivanauskas, L.; Jakstas, V.; Viskelis, P.; Razukas, A. Effect of farming systems on the yield, quality parameters and sensory properties of conventionally and organically grown potato (Solanum tuberosum L.) tubers. Food Chem. 2014, 145, 903–909. [Google Scholar] [CrossRef]
- Hagman, J.E.; Mårtensson, A.; Grandin, U. Cultivation practices and potato cultivars suitable for organic potato production. Potato Res. 2009, 52, 319–330. [Google Scholar] [CrossRef]
- Maggio, A.; Carillo, P.; Bulmetti, G.; Fuggi, A.; Barbieri, G.; De Pascale, S. Potato yield and metabolic profiling under conventional and organic farming. Eur. J. Agron. 2008, 28, 343–350. [Google Scholar] [CrossRef]
- Lloyd, J.R.; Kossmann, J. Transitory and storage starch metabolism: Two sides of the same coin? Curr Opin. Biotechnol. 2015, 32, 143–148. [Google Scholar] [CrossRef]
- Sonnewald, U.; Kossmann, J. Starches–from current models to genetic engineering. Plant Biotechnol. J. 2013, 11, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Van Harsselaar, J.K.; Lorenz, J.; Senning, M. Genome–wide analysis of starch metabolism genes in potato (Solanum tuberosum L.). BMC Genom. 2017, 18, 37. [Google Scholar] [CrossRef]
- Tester, R.F.; Karkalas, J. The Effects of Environmental Conditions on the Structural Features and Physico–chemical Properties of Starches. Starch–Stärke 2001, 53, 513–519. [Google Scholar] [CrossRef]
- Sheets, R.L. Amylolysis of Eight Different Starches with Four Different Species of Alpha–Amylase. Master’s Thesis, Iowa State University, Ames, IA, USA, 2016. Available online: https://lib.dr.iastate.edu/etd/15188 (accessed on 4 April 2024).
- Zgórska, K.; Grudzińska, M. Zmiany wybranych cech jakości bulw ziemniaka w czasie przechowywania (Changes in selected quality parameters of potato tubers during storage). Acta Agrophys. 2012, 19, 203–214. (In Polish) [Google Scholar]
- Fuentes–Zaragoza, E.; Sánchez–Zapata, E.; Sendra, E.; Sayas, E.; Navarro, C.; Fernández–López, J.; Pérez–Alvarez, J.A. Resistant starch as prebiotic: A review. Starch–Stärke 2011, 63, 406–415. [Google Scholar] [CrossRef]
- Zarzyńska, K.; Goliszewski, W. Zróżnicowanie jakości plonu ziemniaków uprawianych w systemie ekologicznym i integrowanym w zależności od odmiany i warunków glebowo-klimatycznych. Część, I. Udział wad zewnętrznych i wewnętrznych bulw (Variation in the quality of potato yields grown in organic and integrated systems depending on the variety and soil and climate conditions. Part I. Share of external and internal defects of tubers). Biul. Inst. Hod. Aklim. Rośl. 2012, 266, 73–79. (In Polish) [Google Scholar] [CrossRef]
- Turska, E.; Wielogórska, G.; Rymuza, K. Oddziaływanie wybranych czynników agrotechnicznych na jakość bulw ziemniaka (The impact of selected agrotechnical factors on the quality of potato tubers). Fragm. Agron. 2009, 26, 156–161. (In Polish) [Google Scholar]
- Wszelaczyńska, E.; Pobereżny, J.; Gruszczewski, M. Trwałość przechowalnicza i stabilność cech jakościowych wybranych odmian ziemniaka o różnych kierunkach użytkowania (Storage durability and stability of quality features of selected potato varieties for different uses). Inż. Ap. Chem. 2014, 53, 127–129. (In Polish) [Google Scholar]
- Żołnowski, A.C. Studia nad Zmiennością Plonowania i Jakością Ziemniaka Jadalnego (Solanum tuberosum L.) w Warunkach Zróżnicowanego Nawożenia Mineralnego (Studies on the Variability of Yield and Quality of Table Potatoes (Solanum tuberosum L.) under Conditions of Different Mineral Fertilization); Monographic Dissertation; UWM: Olsztyn, Poland, 2013; Volume 191, p. 259. [Google Scholar]
- Wierzbicka, A.; Trawczyński, C. Czynniki wpływające na zawartość i plon białka w bulwach ziemniaka (Factors influencing protein content and yield in potato tubers). Biul. IHAR 2012, 266, 181–190. (In Polish) [Google Scholar] [CrossRef]
- Krzysztofik, B. Wpływ uprawy roli na stopień wyrównania wielkości bulw ziemniaka i plon skrobi (The influence of tillage on the degree of uniformity of potato tuber size and starch yield). Acta Agrophys. 2009, 14, 355–365. [Google Scholar]
- Wierzbicka, A. Zawarto skadników mineralnych w bulwach ziemniaka uprawianego w systemie ekologicznym, ich warto żywieniowa i wzajemne relacje (Mineral content of potato tubers grown in the organic system their nutritional value and interaction). J. Res. Appl. Agric. Eng. 2012, 57, 188–192. (In Polish) [Google Scholar]
- Mystkowska, I. Wpływ stosowania biostymulatorów na zawartość suchej masy i skrobi w bulwach ziemniaka jadalnego (The effect of biostimulators on the dry matter and starch content in edible potato tubers). Fragm. Agron. 2019, 36, 45–53. (In Polish) [Google Scholar] [CrossRef]
- Rymuza, K.; Radzka, E.; Lenartowicz, T. Wpływ warunków środowiskowych na zawartość skrobi w bulwach odmian ziemniaka średnio wczesnego (The influence of environmental conditions on the starch content in tubers of medium-early potato varieties). Acta Agrophys. 2015, 22, 279–289. (In Polish) [Google Scholar]
- López–Delgado, H.; Zavaleta–Mancera, H.A.; Mora–Herrera, M.E.; Vázquez–Rivera, M.; Flores–Gutiérrez, F.X.; Scott, I.M. Hydrogen peroxide increases potato tuber and stem starch content, stem diameter, and stem lignin content. Am. J. Pot. Res. 2005, 82, 279. [Google Scholar] [CrossRef]
- Carr, A.C.; Vissers, M.C.M. Synthetic or food–derived vitamin C–are they equally bioavailable? Nutrients 2013, 5, 4284–4304. [Google Scholar] [CrossRef]
- Herencia, J.F.; García–Galavís, P.A.; Dorado, J.A.R.; Maqueda, C. Comparison of nutritional quality of the crops grown in an organic and conventional fertilized soil. Sci. Hortic. 2011, 129, 882–888. [Google Scholar] [CrossRef]
- Baranski, M.; Srednicka–Tober, D.; Volakakis, N.; Seal, C.; Sanderson, R.; Stewart, G.B.; Benbrook, C.; Biavati, B.; Markellou, E.; Giotis, C. Higher antioxidant and lower cadmium concentrations and lower incidence of pesticide residues in organically grown crops: A systematic literature review and meta–analyses. Br. J. Nutr. 2014, 112, 794–811. [Google Scholar] [CrossRef] [PubMed]
- Wegener, C.; Jansen, G.; Jurgens, H.U. Influence of drought and wounding stress on soluble phenols and proteins in potato tubers. Sustain. Agric. Res. 2014, 3, 1–15. [Google Scholar] [CrossRef]
- Zardzewiały, M.; Matłok, N.; Piechowiak, T.; Saletnik, B.; Balawejder, M.; Gorzelany, J. Preliminary Tests of Tomato Plant Protection Method with Ozone Gas Fumigation Supported with Hydrogen Peroxide Solution and Its Effect on Some Fruit Parameters. Sustainability 2024, 16, 3481. [Google Scholar] [CrossRef]
- Piechowiak, T.; Antos, P.; Kosowski, P.; Skrobacz, K.; Józefczyk, R.; Balawejder, M. Impact of ozonation process on the microbiological and antioxidant status of raspberry (Rubus ideaeus L.) fruit during storage at room temperature. Agric. Food Sci. 2019, 28, 35–44. [Google Scholar] [CrossRef]
- Piechowiak, T.; Balawejder, M. Impact of ozonation process on the level of selected oxidative stress markers in raspberries stored at room temperature. Food Chem. 2019, 298, 125093. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.; Yusuf, M.; Fariduddin, Q. Hydrogen peroxide in regulation of plant metabolism: Signalling and its effect under abiotic stress. Photosynthetica 2018, 56, 1237. [Google Scholar] [CrossRef]
- Borges, A.A.; Jiménez–Arias, D.; Expósito–Rodríguez, M.; Sandalio, L.M.; Pérez, J.A. Priming crops against biotic and abiotic stresses: MSB as a tool for studying mechanisms. Front. Plant Sci. 2014, 5, 642. [Google Scholar] [CrossRef]
- Hossain, M.A.; Fujita, M. Hydrogen peroxide priming stimulates drought tolerance in mustard (Brassica juncea L.) seedlings. Plant Gene Trait. 2013, 4, 20–109. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Rasheed, R.; Hussain, I.; Iqbal, M.; Haider, M.Z.; Parveen, S.; Sajid, M.A. Hydrogen peroxide modulates antioxidant system and nutrient relation in maize (Zea mays L.) under water deficit conditions. Arch. Agron. Soil Sci. 2014, 61, 507–523. [Google Scholar] [CrossRef]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2016, 90, 856–867. [Google Scholar] [CrossRef]
- Sarkar, A.; Singh, A.A.; Agrawal, S.B.; Ahmad, A.; Rai, S.P. Cultivar specific variations in antioxidative defense system, genome and proteome of two tropical rice cultivars against ambient and elevated ozone. Ecotoxicol. Environ. Saf. 2015, 115, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Rakwal, R.; Agrawal, S.B.; Shibato, J.; Ogawa, Y.; Yoshida, Y.; Agrawal, G.K.; Agrawal, S.B. Investigating the impact of elevated levels of ozone on tropical wheat using integrated phenotypical, physiological, biochemical, and proteomics approaches. J. Proteome Res. 2010, 9, 4565–4584. [Google Scholar] [CrossRef]
- Agrawal, G.K.; Rakwal, R.; Yonekura, M.; Saji, H. Rapid induction of defense/stress related proteins in leaves of rice (Oryza sativa) seedlings exposed to ozone is preceeded by newly phosphorylated proteins and changes in 66 K–Da ERK–type MAPK. J. Plant Physiol. 2002, 159, 361–369. [Google Scholar] [CrossRef]
- Wang, J.; Zeng, Q.; Zhu, J.; Liu, G.; Tang, H. Dissimilarity of ascorbate–glutathione (AsA–GSH) cycle mechanism in two rice (Oryza sativa L.) cultivars under experimental free–air ozone exposure. Agric. Ecosyst. Environ. 2013, 165, 39–49. [Google Scholar] [CrossRef]
- Singh, A.A.; Agrawal, S.B.; Shahi, J.P.; Agrawal, S.B. Assessment of growth and yield losses in two Zea mays L. cultivars (quality protein maize and nonquality protein maize) under projected levels of ozone. Environ. Sci. Pollut. Res. 2013, 21, 2628–2641. [Google Scholar] [CrossRef]
- Fuhrer, J.; Booker, F. Ecological issues related to ozone: Agricultural issues. Environ. Int. 2003, 29, 141–154. [Google Scholar] [CrossRef]
- Rai, R.; Agrawal, S.B.; Agrawal, S.B. Assessment of yield losses in tropical wheat using open top chambers. Atmos. Environ. 2007, 41, 9543–9554. [Google Scholar] [CrossRef]
- Grudzinska, M.; Czerko, Z.; Zarzynska, K.; Borowska–Komenda, M. Bioactive Compounds in Potato Tubers: Effects of Farming System, Cooking Method, and Flesh Color. PLoS ONE 2016, 11, e0153980. [Google Scholar] [CrossRef] [PubMed]
- Chirumbolo, S. Plant phytochemicals as new potential drugs for immune disorders and cancer therapy: Really a promising path? J. Sci. Food Agric. 2012, 92, 1573–1577. [Google Scholar] [CrossRef]
- Lombardo, S.; Lo Monaco, A.; Pandino, G.; Parisi, B.; Mauromicale, G. The phenology: Yield and tuber composition of ‘early’ crop potatoes: A comparison between organic and conventional cultivation systems. Renew. Agric. Food Syst. 2013, 28, 50–58. [Google Scholar] [CrossRef]
- Bélanger, G.; Walsh, J.R.; Richards, J.E.; Milburn, P.H.; Ziadi, N. Yield response of two potato cultivars to supplemental irrigation and N fertilization in New Brunswick. Am. J. Potato Res. 2000, 77, 11–21. [Google Scholar] [CrossRef]
- Licciardello, F.; Lombardo, S.; Rizzo, V.; Pitino, I.; Pandino, G.; Strano, M.G.; Muratore, G.; Restuccia, C.; Mauromicale, G. Integrated agronomical and technological approach for the quality maintenance of ready–to–fry potato sticks during refrigerated storage. Postharvest Biol. Technol. 2018, 136, 23–30. [Google Scholar] [CrossRef]
- Rizzo, V.; Amoroso, L.; Licciardello, F.; Mazzaglia, A.; Muratore, G.; Restuccia, C.; Lombardo, S.; Pandino, G.; Strano, M.G.; Mauromicale, G. The effect of sous vide packaging with rosemary essential oil on storage quality of fresh–cut potato. LWT Food Sci. Technol. 2018, 94, 111–118. [Google Scholar] [CrossRef]
- Brown, C.R. Antioxidants in potato. Am. J. Potato Res. 2005, 82, 163–172. [Google Scholar] [CrossRef]
- Hamouz, K.; Čepl, J.; Dvořák, P. Influence of environmental conditions on the quality of potato tubers. Hortic. Sci. 2005, 32, 89–95. [Google Scholar] [CrossRef]
- Lachman, J.; Hamouz, K.; Orsák, M.; Pivec, V.; Dvořák, P. The influence of flesh colour and growing locality on polyphenolic content and antioxidant activity in potatoes. Sci. Hortic. 2008, 117, 109–114. [Google Scholar] [CrossRef]
- Rosenthal, S.; Jansky, S. Effect of production site and storage on antioxidant levels in specialty potato (Solanum tuberosum L.) tubers. J. Sci. Food Agric. 2008, 88, 2087–2092. [Google Scholar] [CrossRef]
- Lachman, J.; Hamouz, K.; Musilová, J.; Hejtmánková, K.; Kotíková, Z.; Pazderů, K.; Domkárová, J.; Pivec, V.; Cimr, J. Effect of peeling and three cooking methods on the content of selected phytochemicals in potato tubers with various colour of flesh. Food Chem. 2013, 138, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Hajslova, J.; Schulzova, V.; Slanina, P.; Janne, K.; Hellenas, K.E.; Andersson, C. Quality of organically and conventionally grown potatoes: Four–year study of micronutrients, metals, secondary metabolites, enzymic browning and organoleptic properties. Food Addit. Contam. 2005, 22, 514–534. [Google Scholar] [CrossRef]
- Lombardo, S.; Pandino, G.; Mauromicale, G. The effect on tuber quality of an organic versus a conventional cultivation system in the early crop potato. J. Food Compos. Anal. 2017, 62, 189–196. [Google Scholar] [CrossRef]
- Chun, O.K.; Kim, D.O.; Smith, N.; Schroeder, D.; Han, J.T.; Lee, C.Y. Daily consumption of phenolics and total antioxidant capacity from fruit and vegetables in the American diet. J. Sci. Food Agric. 2005, 85, 1715–1724. [Google Scholar] [CrossRef]
- Vinson, J.A. Intracellular polyphenols: How little we know. J. Agric. Food Chem. 2019, 67, 3865–3870. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Zhang, H.; Qi, R.; Tsao, R.; Mine, Y. Recent advances in the understanding of the health benefits and molecular mechanisms associated with green tea polyphenols. J. Agric. Food Chem. 2019, 67, 1029–1043. [Google Scholar] [CrossRef]
- Andre, C.M.; Ghislain, M.; Bertin, P.; Oufir, M.; Herrera, M.D.R.; Hoffmann, L.; Hausman, J.F.; Larondelle, Y.; Evers, D. Andean potato cultivars (Solanum tuberosum L.) as a source of antioxidant and mineral micronutrients. J. Agric. Food Chem. 2007, 55, 366–378. [Google Scholar] [CrossRef] [PubMed]
- Teow, C.C.; Truong, V.D.; McFeeters, R.F.; Thompson, R.L.; Pecota, K.V.; Yencho, G.C. Antioxidant activities, phenolic and beta–carotene contents of sweet potato genotypes with varying flesh colours. Food Chem. 2007, 103, 829–838. [Google Scholar] [CrossRef]
- Arts, I.C.; Hollman, P.C. Polyphenols and disease risk in epidemiologic studies. Am. J. Clin. Nutr. 2005, 81, 317–325. [Google Scholar] [CrossRef]
- Vilcacundo, R.; Miralles, B.; Carrillo, W.; Hernández–Ledesma, B. In Vitro chemopreventive properties of peptides released from quinoa (Chenopodium quinoa Willd.) protein under simulated gastrointestinal digestion. Food Res. Int. 2018, 105, 403–411. [Google Scholar] [CrossRef]
- Vilcacundo, R.; Barrio, D.A.; Piñuel, L.; Boeri, P.; Tombari, A.; Pinto, A.; Welbaum, J.; Hernández–Ledesma, B.; Carrillo, W. Inhibition of lipid peroxidation of kiwicha (Amaranthus caudatus) hydrolyzed protein using zebrafish larvae and embryos. Plants 2018, 7, 69. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, W.; Gómez–Ruiz, J.A.; Miralles, B.; Ramos, M.; Barrio, D.; Recio, I. Identification of antioxidant peptides of hen egg–white lysozyme and evaluation of inhibition of lipid peroxidation and cytotoxicity in the Zebrafish model. Eur. Food Res. Technol. 2016, 242, 1777–1785. [Google Scholar] [CrossRef]
- Campos, D.; Noratto, G.; Chirinos, R.; Arbizu, C.; Roca, W.; Cisneros–Zevallos, L. Antioxidant capacity and secondary metabolites in four species of Andean tuber crops: Native potato (Solanum sp.), mashua (Tropaeolum tuberosum), Oca (Oxalis tuberosa Molina) and ulluco (Ullucus tuberosus Caldas). J. Sci. Food Agric. 2006, 86, 1481–1488. [Google Scholar] [CrossRef]
- Yang, C.S.; Landau, J.M.; Huang, M.T.; Newmark, H.L. Inhibition of carcinogenesis by dietary polyphenolic compounds. Ann. Rev. Nutr. 2001, 21, 381–406. [Google Scholar] [CrossRef]
- Zhao, D.; Simon, J.E.; Wu, Q. A critical review on grape polyphenols for neuroprotection: Strategies to enhance bioefficacy. Crit. Rev. Food Sci. Nut. 2020, 60, 597–625. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, S.; Suleria, H.A.R. Natural polyphenols: An overview. Int. J. Food Prop. 2017, 20, 1689–1699. [Google Scholar] [CrossRef]
- Moga, M.A.; Dimienescu, O.G.; Arvatescu, C.A.; Mironescu, A.; Dracea, L.; Ples, L. The role of natural polyphenols in the prevention and treatment of cervical cancer—An overview. Molecules 2016, 21, 1055. [Google Scholar] [CrossRef]
- Liu, R.H. Potential Synergy of Phytochemicals in cancer prevention: Mechanism of action. J. Nutr. 2004, 134, 3479S–3485S. [Google Scholar] [CrossRef] [PubMed]
- Samaniego, I.; Espin, S.; Cuesta, X.; Arias, V.; Rubio, A.; Llerena, W.; Angós, I.; Carrillo, W. Analysis of Environmental Conditions Effect in the Phytochemical Composition of Potato (Solanum tuberosum) Cultivars. Plants 2020, 9, 815. [Google Scholar] [CrossRef]
- Camire, M.E.; Kubow, S.; Donnelly, D.J. Potatoes and human health. Crit. Rev. Food Sci. Nutr. 2009, 49, 823–840. [Google Scholar] [CrossRef] [PubMed]
- Gumul, D.; Ziobro, R.; Noga, M.; Sabat, R. Characterisation of five potato cultivars according to their nutritional and pro–health components. Acta Sci. Pol. Technol. Aliment. 2011, 10, 77–81. [Google Scholar]
- Deusser, H.; Guignard, C.; Hoffmann, L.; Evers, D. Polyphenol and glycoalkaloid contents in potato cultivars grown in Luxembourg. Food Chem. 2012, 135, 2814–2824. [Google Scholar] [CrossRef]
- Al–Weshahy, A.; Rao, A.V. Isolation and characterization of functional components from peel samples of six potatoes varieties growing in Ontario. Food Res. Int. 2009, 42, 1062–1066. [Google Scholar] [CrossRef]

| Granulometric composition | clay loam |
| pH | pH: H2O 5.25 ± 0.11, KCl 4.72 ± 0.17 |
| Hydrolytic acidity | 3.2 ± 0.12 mmol(+)∙100 g−1 |
| Total nitrogen | ≤1 g∙kg−1 soil |
| Phosphorus | 14.2 ± 0.36 mg P2O5 100 g−1 soil |
| Potassium | 16.7 ± 0.19 mg K2O∙100 g−1 soil |
| Magnesium | 9.24 ± 0.32 mg∙100 g−1 soil |
| Contents of P, K, and Mg | average by abundance classes |
| Na+ | NH4+ | K+ | Mg2+ | Ca2+ | NO3− | SO42− | |
|---|---|---|---|---|---|---|---|
| 30 cm | 0.07 ± 0.02 | 0.04 ± 0.01 | 0.58 ± 0.03 | 0.05 ± 0.01 | 0.25 ± 0.03 | 2.57 ± 0.30 | 0.42 ± 0.02 |
| 60 cm | 0.16 ± 0.01 | 0.05 ± 0.03 | 0.20 ± 0.04 | 0.09 ± 0.03 | 0.63 ± 0.19 | 1.70 ± 0.15 | 1.01 ± 0.10 |
| 90 cm | 0.21 ± 0.05 | 0.03 ± 0.01 | 0.04 ± 0.01 | 0.06 ± 0.01 | 0.37 ± 0.03 | 2.00 ± 0.17 | 1.31 ± 0.16 |
| May | June | July | August | September | Average | |
|---|---|---|---|---|---|---|
| 2016 | 0.83 d | 0.58 vd | 1.86 rh | 2.18 h | 1.80 rh | 1.45 o |
| 2017 | 1.02 rd | 2.05 h | 1.71 rh | 1.48 o | 0.91 d | 1.43 o |
| 2018 | 1.02 rd | 2.05 h | 1.71 rh | 1.48 o | 0.91 d | 1.43 o |
| 1981–2010 | 1.93 rh | 1.71 rh | 1.61 rh | 1.26 rd | 1.92 rh | 1.69 rh |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skrobacz, K.; Szostek, M.; Balawejder, M. The Influence of Some Reactive Oxygen Species Treatments on the Yield and Changes in the Chemical Composition of Potato Tubers (Solanum tuberosum L.). Agronomy 2024, 14, 1865. https://doi.org/10.3390/agronomy14081865
Skrobacz K, Szostek M, Balawejder M. The Influence of Some Reactive Oxygen Species Treatments on the Yield and Changes in the Chemical Composition of Potato Tubers (Solanum tuberosum L.). Agronomy. 2024; 14(8):1865. https://doi.org/10.3390/agronomy14081865
Chicago/Turabian StyleSkrobacz, Karol, Małgorzata Szostek, and Maciej Balawejder. 2024. "The Influence of Some Reactive Oxygen Species Treatments on the Yield and Changes in the Chemical Composition of Potato Tubers (Solanum tuberosum L.)" Agronomy 14, no. 8: 1865. https://doi.org/10.3390/agronomy14081865





