Hidden Secrets of Mangrove Swamp Rice Stored Seeds in Guinea-Bissau: Assessment of Fungal Communities and Implications for Food Security
Abstract
:1. Introduction
2. Materials and Methods
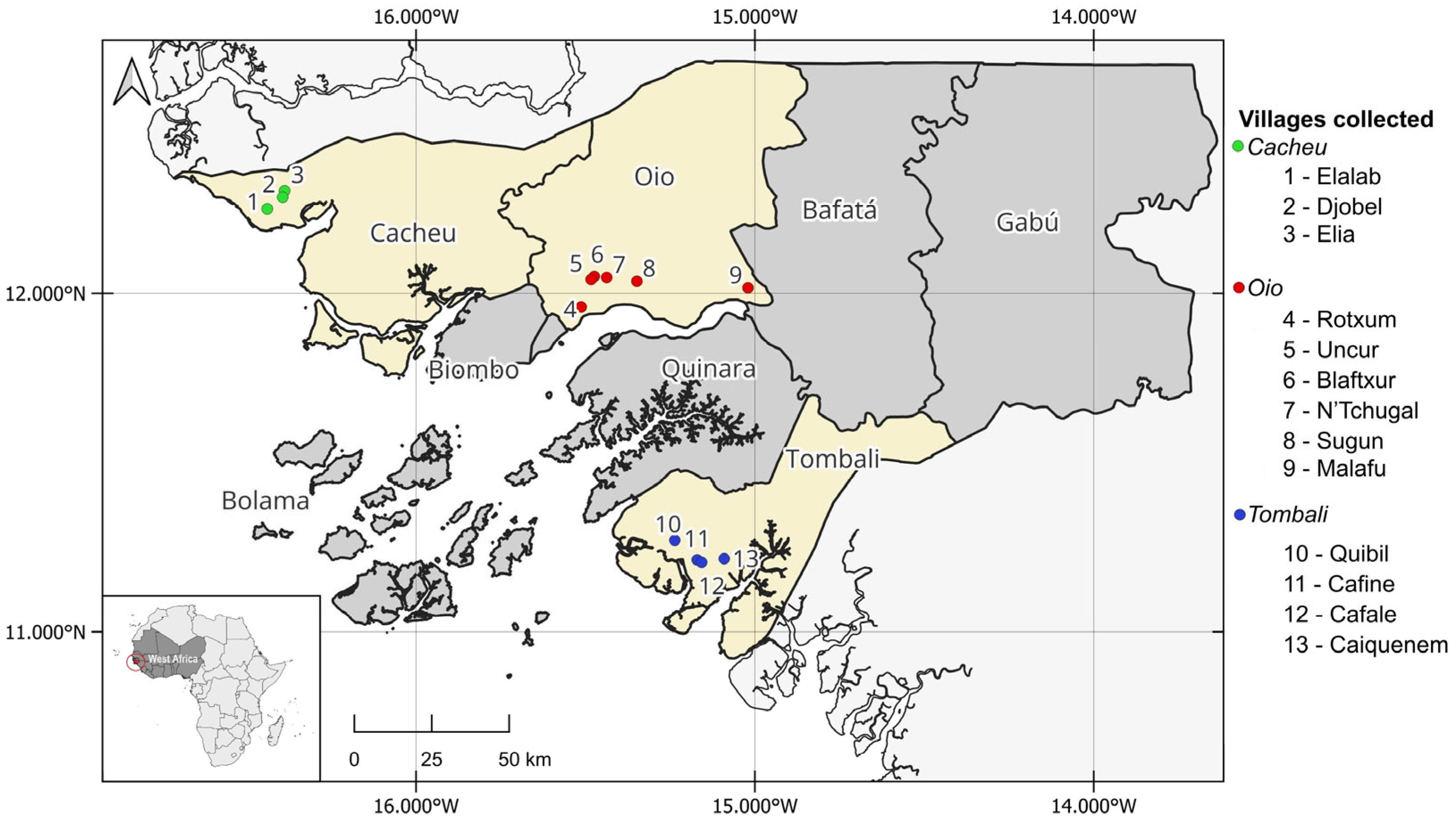
2.1. Field Collection
2.2. Seed Germination Rate
2.3. Fungal Identification, Distribution, and Trophic Modes
2.3.1. Fungal Isolation
2.3.2. DNA Extraction, Amplification, Sequencing, and Bioinformatics
2.3.3. Sequence Processing and Putative Taxa Assignment
2.3.4. Ecological Guild Classification
2.4. Aflatoxins Content Analyses
2.5. Statistical Analysis
3. Results
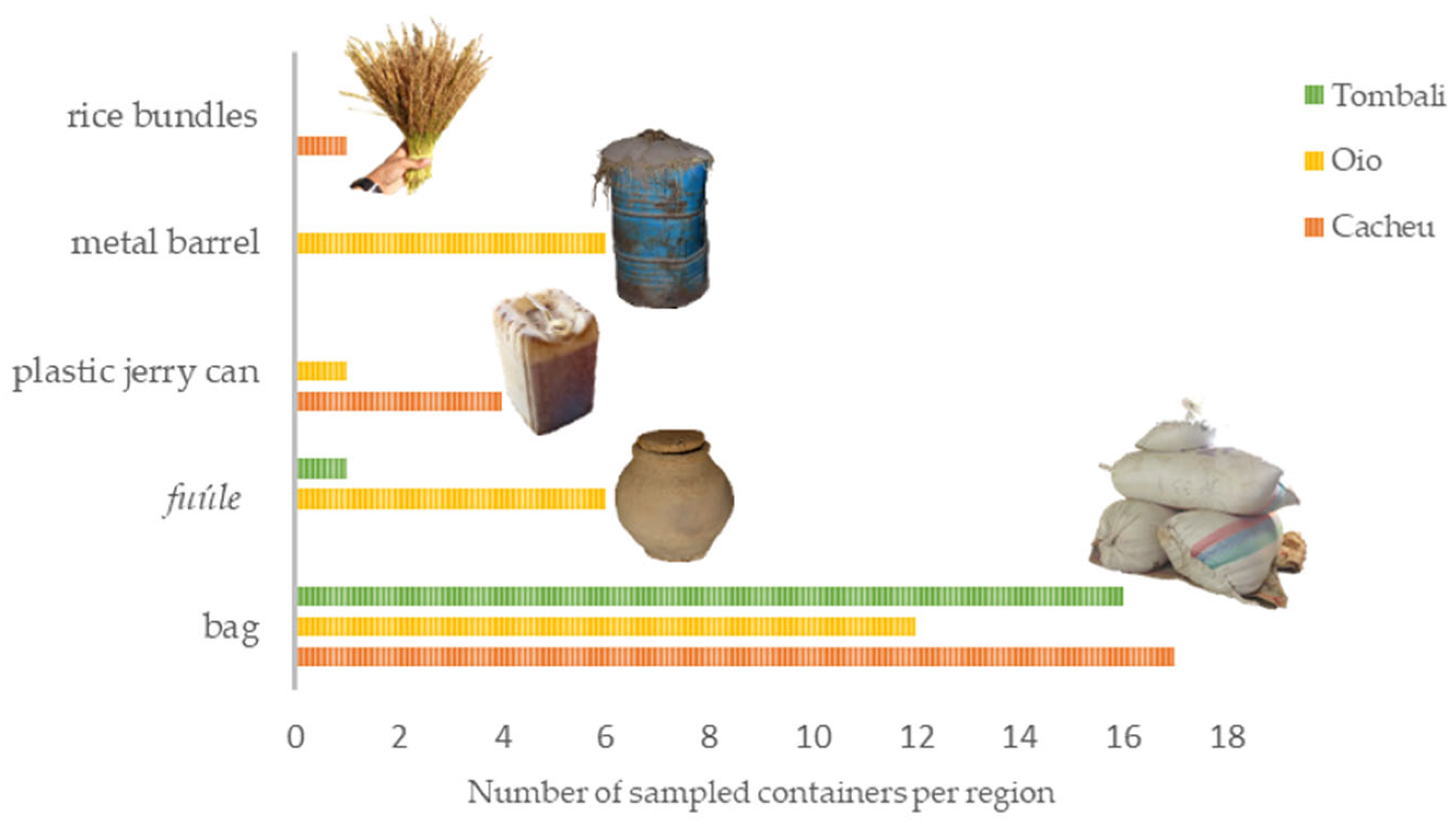
3.1. Characterisation and Distribution of Mangrove Rice Storage Facilities
3.2. Germination Rate Success of Stored Mangrove Rice
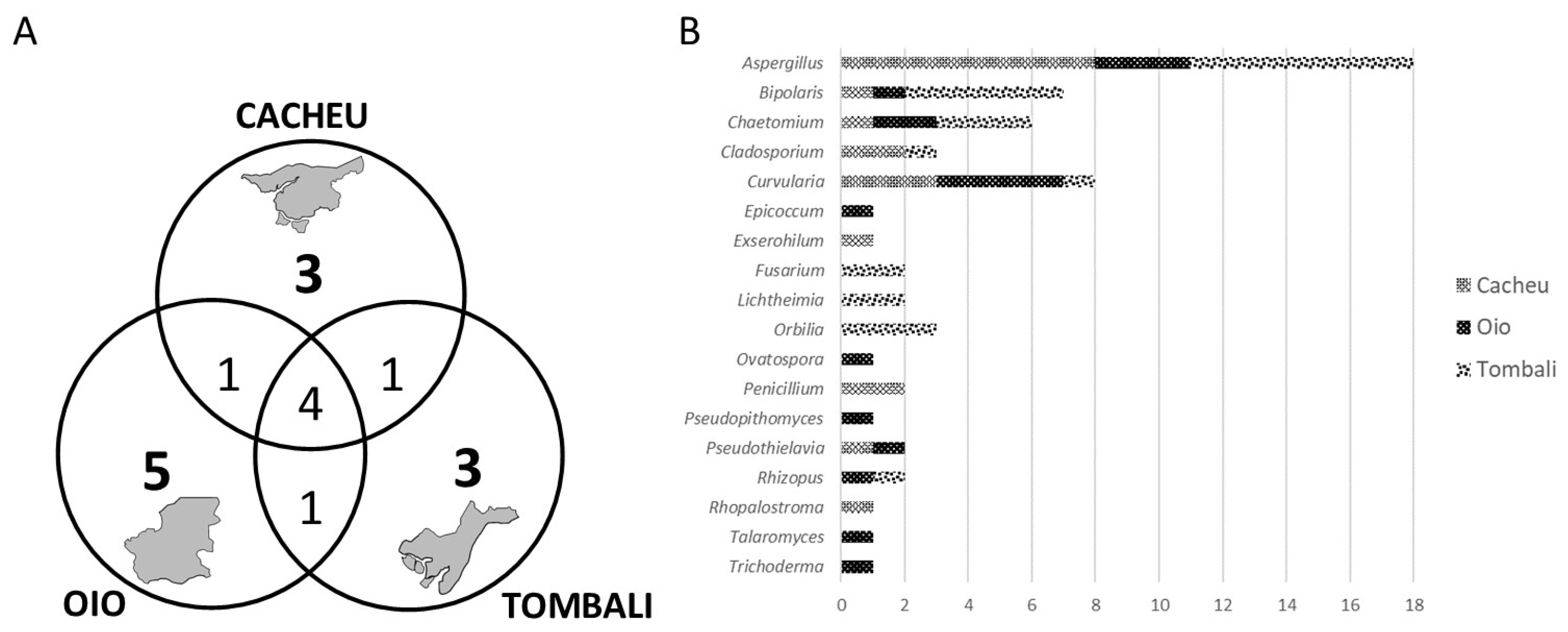
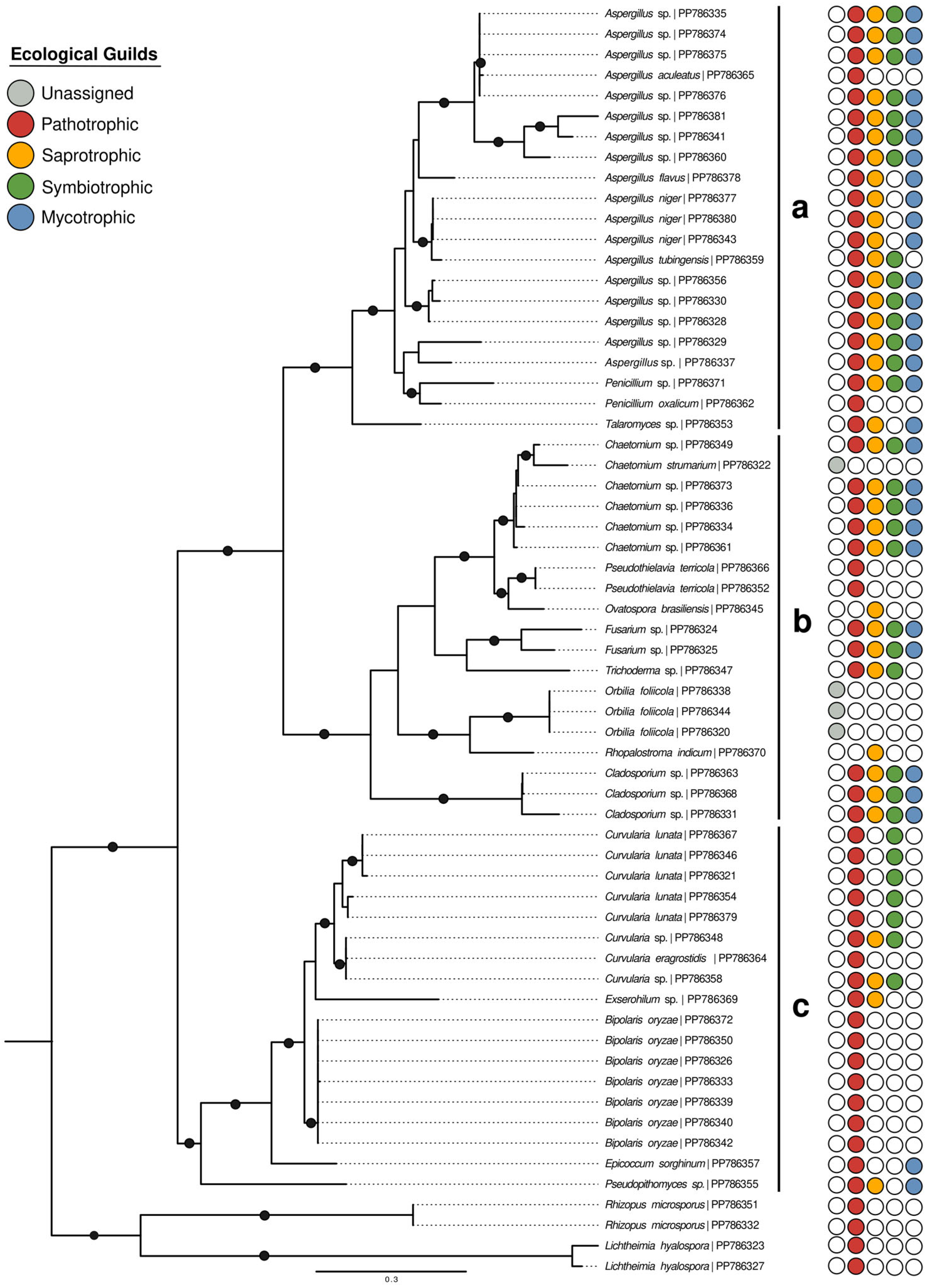
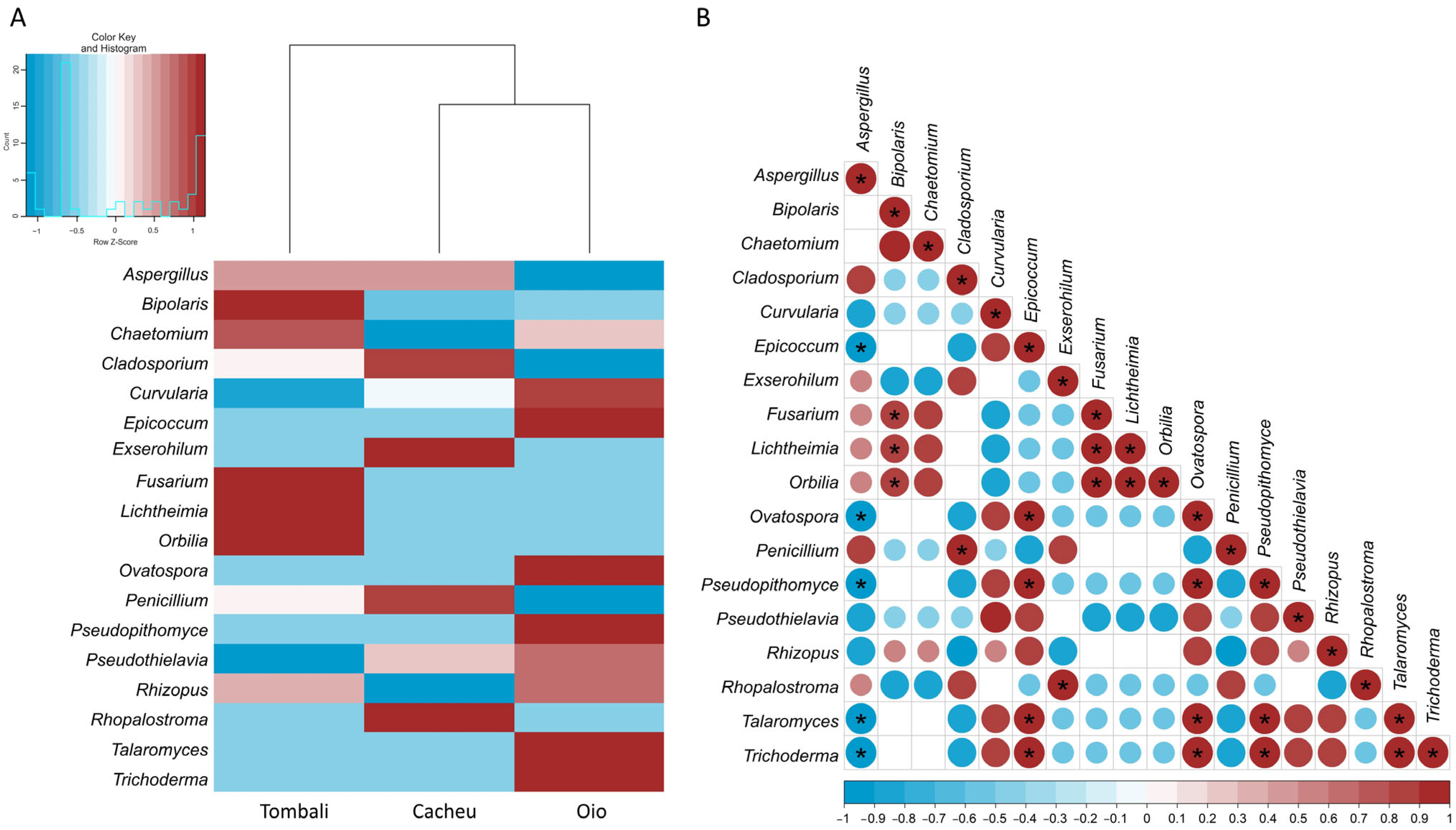
3.3. Identification and Distribution of Stored Fungi
3.4. Aflatoxin Content of Stored Mangrove Rice
4. Discussion
4.1. Traditional Storage Facilities for Mangrove Swamp Rice in Guinea-Bissau
4.2. Traditional Storage Practices and the Impact of Seed Storage Fungi on Food Safety and Plant Cycle
4.3. Fungal Communities in Seed Storage Mangrove Rice
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nukenine, E.N. Stored product protection in Africa: Past, present and future. In Proceedings of the 10th International Working Conference on Stored Product Protection, Estoril, Portugal, 27 June–2 July 2010. [Google Scholar] [CrossRef]
- Kumar, D.; Kalita, P. Reducing postharvest losses during storage of grain crops to strengthen food security in developing countries. Foods 2017, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- FAO. Seeds Toolkit—Module 6: Seed Storage; The Food and Agriculture Organization of the United Nations: Rome, Italy, 2018; 112p. [Google Scholar]
- Butt, A.R.; Yaseen, S.I.; Javaid, A. Seed-borne mycoflora of stored rice grains and its chemical control. J. Anim. Plant Sci. 2011, 21, 193–196. [Google Scholar]
- Moretti, A.; Susca, A. Mycotoxigenic Fungi: Methods and Protocols; Humana Press: Totowa, NJ, USA, 2017. [Google Scholar]
- Manandhar, A.; Milindi, P.; Shah, A. An overview of the post-harvest grain storage practices of smallholder farmers in developing countries. Agriculture 2018, 8, 57. [Google Scholar] [CrossRef]
- Nwaigwe, K.N. An overview of cereal grain storage techniques and prospects in Africa. IJBB 2019, 4, 19–25. [Google Scholar]
- Ackaah, F.M.; Nyaku, S.T.; Darkwa, E. Seed-Borne Fungi Associated with Diverse Rice Varieties Cultivated in the Western North Region of Ghana. Int. J. Microbiol. 2023, 2023, 8690464. [Google Scholar] [CrossRef]
- Loschiavo, S.R.; Sinha, R.N. Feeding, Oviposition, and Aggregation by the Rusty Grain Beetle, Cryptolestes ferrugineus (Coleoptera: Cucujidae) on Seed-Borne Fungi. Ann. Entomol. Soc. Am. 1966, 59, 578–585. [Google Scholar]
- Sharma, P.; Roy, M.; Roy, B.; Deka, S.D. Post harvest management strategies and storage approaches for quality seed production. In Emerging Issues in Agricultural Sciences Vol. 2; Al-Naggar, A.M.M., Ed.; BP International: London, UK, 2023; pp. 110–129. [Google Scholar] [CrossRef]
- WHO. WHO Estimates of the Global Burden of Foodborne Diseases; WHO Library Cataloguing-in-Publication Data; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- Ali, N. Aflatoxins in rice: Worldwide occurrence and public health perspectives. Toxicol. Rep. 2019, 6, 1188–1197. [Google Scholar] [CrossRef] [PubMed]
- Martín, I.; Gálvez, L.; Guasch, L.; Palmero, D. Fungal pathogens and seed storage in the dry state. Plants 2022, 11, 3167. [Google Scholar] [CrossRef]
- Dawlal, P.; Barros, E.; Marais, G.J. Evaluation of maize cultivars for their susceptibility towards mycotoxigenic fungi. J. Stored Prod. Res. 2011, 48, 114–119. [Google Scholar] [CrossRef]
- Balendres, M.A.O.; Karlovsky, P.; Cumagun, C.J.R. Mycotoxigenic fungi and mycotoxins in agricultural crop commodities in the Philippines: A review. Foods 2019, 8, 249. [Google Scholar] [CrossRef]
- El-Shanshoury, A.E.-R.R.; El-Sabbagh, S.M.; Emara, H.A.; Saba, H.E. Occurrence of moulds, toxicogenic capability of Aspergillus flavus and levels of aflatoxins in maize, wheat, rice and peanut from markets in central delta provinces, Egypt. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 852–865. [Google Scholar]
- Trucksess, M.W.; Abbas, H.K.; Weaver, C.M.; Shier, W.T. Distribution of aflatoxins in shelling and milling fractions of naturally contaminated rice. Food Addit. Contam. 2011, 28, 1076–1082. [Google Scholar] [CrossRef]
- Marin-Felix, Y.; Groenewald, J.Z.; Cai, L.; Chen, Q.; Marincowitz, S.; Bames, I.; Bensch, K.; Braun, U.; Camporesi, E.; Damm, U.; et al. Genera of phytopathogenic fungi: GOPHY 1. Stud. Mycol. 2017, 86, 99–216. [Google Scholar] [CrossRef] [PubMed]
- Idnurm, A.; Howlett, B.J. Pathogenicity genes of phytopathogenic fungi. Mol. Plant Pathol. 2001, 2, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Ascough, P.L.; Sturrock, J.; Bird, M.I. Investigation of growth response in saprophytic fungi to charred biomass. Isot. Environ. Health Stud. 2012, 46, 64–77. [Google Scholar] [CrossRef]
- Wutkowska, M.; Vader, A.; Mundra, S.; Cooper, E.J.; Eidesen, P.B. Dead or alive; or does it really matter? Level of congruency between trophic modes in total and active fungal communities in high arctic soil. Front. Microbiol. 2019, 9, 3243. [Google Scholar] [CrossRef]
- Hossain, M.A.; Awal, M.A.; Ali, M.R.; Alam, M.M. Hermetic bag: And effective storage technology for rice seed/hermetic bag: A key element in enhancement of food availability. In Proceedings of the 7th International Conference on Data Science & SDGs, Rajshahi, Bangladesh, 18–19 December 2019; Department of Statistics, University of Rajshahi: Rajshahi, Bangladesh, 2019; pp. 717–723. [Google Scholar]
- Espírito-Santo, J. Notas sobre a cultura do arroz entre os balantas. Bol. Cult. Guiné Port. 1949, 14, 197–232. [Google Scholar]
- Teeken, B.; Nuijten, E.; Temudo, M.P.; Okry, F.; Mokuwa, A.; Struik, P.C.; Richards, P. Maintaining or abandoning African rice: Lessons for understanding processes of seed innovation. Hum. Ecol. 2012, 40, 879–892. [Google Scholar] [CrossRef]
- De Oliveira, O.B.; Havik, P.J.; Schiefer, U. Armazenamento Tradicional na Guiné-Bissau: Produtos, Sementes e Celeiros Utilizados Pelas Etnias na Guiné-Bissau; Centro de Pesquisa Copin; Münster, FB 06 Erziehungswissenschaft und Sozialwissenschaften; Institut für Soziologie: Hanover, Germany, 1996; p. 449. [Google Scholar]
- Temudo, M.P. Inovação e Mudança em Sociedades Rurais Africanas. Gestão de Recursos Naturais, Saber Local e Instituições de Desenvolvimento Induzido: Estudo de caso na Guiné-Bissau (Vol. I). Ph.D. Thesis, University of Lisbon, Lisbon, Portugal, 1998. [Google Scholar]
- Honger, J.; Oppong, C.; Amoatey, C. Mycofloral diversity and molecular characterization of species isolated from farmer-saved rice seeds in the irrigated rice production districts of the coastal savannah zones of Ghana. Ghana J. Sci. 2022, 63, 1–13. [Google Scholar] [CrossRef]
- Piepenbring, M.; Maciá-Vicente, J.G.; Codjia, J.E.I.; Glatthorn, C.; Kirk, P.; Meswaet, Y.; Minter, D.; Olou, B.A.; Reschke, K.; Schmidt, M.; et al. Mapping mycological ignorance—Checklists and diversity patterns of fungi known for West Africa. IMA Fungus 2020, 11, 13. [Google Scholar] [CrossRef]
- Basu, S.; Groot, S.P.C. Seed vigour and invigoration. In Seed Science and Technology; Dadlani, M., Yadava, D.K., Eds.; Springer: Singapore, 2023. [Google Scholar] [CrossRef]
- Abdul-Baki, A.A.; Anderson, J.D. Vigor Determination in Soybean Seed by Multiple Criteria. Crop Sci. 1973, 13, 630–633. [Google Scholar] [CrossRef]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes—Application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols, a Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: Cambridge, MA, USA, 1990; pp. 315–322. [Google Scholar] [CrossRef]
- Monteiro, F.; Diniz, I.; Pena, A.R.; Baldé, A.; Catarino, L.; Batista, D. First report of three Lasiodiplodia species (L. theobromae, L. pseudotheobromae, and L. caatinguensis) causing cashew gummosis in Guinea-Bissau (West Africa). Plant Dis. 2020, 104, 2522. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit v.7.0.9: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Android, iOS, Windows, Mac, and Linux. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Nguyen, N.H.; Song, Z.; Bates, S.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Serra, R. Microflora das uvas Portuguesas e seu Potencial para a Contaminação das uvas com Micotoxinas, com Destaque para a Ocratoxina A. Doctoral Dissertation, University of Minho, Braga, Portugal, 2005. [Google Scholar]
- Reddy, K.R.N.; Reddy, C.S.; Abbas, H.K.; Abel, C.A.; Muralidharan, K. Mycotoxigenic fungi, mycotoxins, and management of rice grains. Toxin Rev. 2008, 27, 287–317. [Google Scholar] [CrossRef]
- Moretti, A.; Sarrocco, S. Fungi. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Oxford, UK, 2016; pp. 162–168. [Google Scholar] [CrossRef]
- Perelló, A.; Aulicino, M.; Stenglein, S.A.; Labuda, R.; Moreno, M.V. Pseudopithomyces chartarum associated with wheat seeds in Argentina, pathogenicity and evaluation of toxigenic ability. Eur. J. Plant Pathol. 2017, 148, 491–496. [Google Scholar] [CrossRef]
- Tirumale, S.; Wani, N. Biological control of phytopathogenic fungi using different extracts of Chaetomium cupreum. Asian J. Pharm. Clin. Res. 2018, 11, 328–332. [Google Scholar] [CrossRef]
- Salvatore, M.M.; Andolfi, A. Phytopathogenic fungi and toxicity. Toxins 2021, 13, 689. [Google Scholar] [CrossRef] [PubMed]
- Taguiam, J.D.; Evallo, E.; Balendres, M.A. Epicoccum species: Ubiquitous plant pathogens and effective biological control agents. Eur. J. Plant Pathol. 2021, 159, 713–725. [Google Scholar] [CrossRef]
- Xu, X.; Li, J.; Yang, X.; Zhang, L.; Wang, S.; Shen, G.; Hui, B.; Xiao, J.; Zhou, C.; Wang, X.; et al. Epicoccum spp. Causing maize leaf spot in Heilongjiang province, China. Plant Dis. 2022, 106, 3050–3060. [Google Scholar] [CrossRef]
- Stępień, Ł. Plant-Pathogenic Fusarium species. Fungi 2022, 9, 13. [Google Scholar] [CrossRef]
- Shao, M.-W.; Lu, Y.-H.; Miao, S.; Zhang, Y.; Chen, T.-T.; Zhang, Y.-L. Diversity, bacterial symbionts and antibacterial potential of gut-associated fungi isolated from the Pantala flavescens larvae in China. PLoS ONE 2015, 10, e0134542. [Google Scholar] [CrossRef]
- Rodriguez, R.J.; Redman, R.S.; Henson, J.M. The role of fungal symbioses in the adaptation of plants to high stress environments. Mitig. Adapt. Strateg. Glob. Chang. 2004, 9, 261–272. [Google Scholar] [CrossRef]
- Perrone, G.; Gallo, A. Aspergillus species and their associated mycotoxins. In Mycotoxigenic fungi: Methods and Protocols, Methods in Molecular Biology; Moretti, A., Susca, A., Eds.; Springer Science+Business Media LLC: Berlin/Heidelberg, Germany, 2016; Volume 1542. [Google Scholar] [CrossRef]
- Noguchi, M.T.; Nagase, H.; Yamauchi, N.; Mimuro, G.; Sakai, H.; Furusawa, A.; Miki, S.; Yoshida, S. Taxonomical identification of Chinese cabbage yellows inhibitory fungus isolated from disease suppressive soil. J. Gen. Plant Pathol. 2022, 88, 239–245. [Google Scholar] [CrossRef]
- Madbouly, A.K.; Abdel-Wareth, M.T.A. The use of Chaetomium taxa as biocontrol agents. In Recent Developments on Genus Chaetomium. Fungal Biology; Abdel-Azeem, A.M., Ed.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Gnanesh, B.N.; Tejaswi, A.; Arunakumar, G.S.; Supriya, M.; Manojkumar, H.B.; Tewary, P. Molecular phylogeny, identification and pathogenicity of Rhizopus oryzae associated with root rot of mulberry in India. J. Appl. Microbiol. 2021, 131, 360–374. [Google Scholar] [CrossRef] [PubMed]
- Fogle, M.R.; Douglas, D.R.; Jumper, C.A.; Straus, D. Growth and mycotoxin production by Chaetomium globosum is favored in a neutral pH. Int. J. Mol. Sci. 2008, 9, 2357–2365. [Google Scholar] [CrossRef]
- Daranagama, D.; Liu, X.; Chamyuang, S.; Stadler, M.; Bahkali, A.H.; Hyde, K. Rhopalostroma brevistipitatum sp. Nov. from Thailand with an extended generic description for Rhopalostroma. Phytotaxa 2015, 227, 229–242. [Google Scholar] [CrossRef]
- Ajmal, M.; Hussain, A.; Ali, A.; Chen, H.; Lin, H. Strategies for Controlling the Sporulation in Fusarium spp. J. Fungi 2022, 9, 10. [Google Scholar] [CrossRef]
- Yu, X.; Li, Z.; Han, R.; Zhang, W.; Zhang, J.; Cao, W.; Ma, Y.; Wang, X.; Zhao, J.; Xiang, W. Epicoccum sorghinum as leaf spot disease-causing pathogen in ginger (Zingiber officinale Rosc.) in China. Crop Prot. 2023, 170, 106263. [Google Scholar] [CrossRef]
- Katoh, K.; Toh, H. Recent developments in the MAFFT multiple sequence alignment program. Brief. Bioinform. 2008, 9, 286–298. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A. FigTree v1.4.4, a Graphical Viewer of Phylogenetic Trees. 2007. Available online: https://tree.bio.ed.ac.uk/software/figtree/ (accessed on 20 May 2024).
- Inkscape Project. Inkscape. 2020. Available online: https://inkscape.org (accessed on 20 May 2024).
- Martos, P.A.; Thompson, W.; Diaz, G.J. Multiresidue mycotoxin analysis in wheat, barley, oats, rye and maize grain by high-performance liquid chromatography-tandem mass spectrometry. World Mycotoxin J. 2010, 3, 205–223. [Google Scholar]
- Stahl-Zeng, J.; Lock, S.; Kreppenhofer, S.; von Czapiewski, K. The Quantitation of Mycotoxins in Cereals Using a Simple Sample Extraction and LC-MS/MS with Fast Polarity Switching and the Schenduled MRM™ Algorithm; AB SCIEX: Framingham, MA, USA, 2011; Publ. nº 4700211-01. [Google Scholar]
- Bardsley, J.; Oliver, M. Determination of Multiple Mycotoxins in Grain Using a QuEChERS Sample Preparation Approach and LC-MS/MS Detection; Thermo Fisher Scientific: Waltham, MA, USA, 2015; Application Note 21121. [Google Scholar]
- EN 15662:2018; Foods of Plant Origin—Multimethod for the Determination of Pesticides Residues Using GC- and LC-Based Analysis Following Acetonitrile Extraction/Portioning and Clean-Up by Dispersive SPE—Modular Quechers-Method. European Standards: Plzen, Czech Republic, 2018.
- SANTE/11312/2021 v2; Analytical Quality Control and Method Validation Procedures for Pesticides Residues Analysis in Food and Feed. European Union: Brussels, Belgium, 2024.
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria. Available online: https://www.R-project.org/ (accessed on 1 April 2024).
- WHO. WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action. 2022. Available online: https://www.who.int/publications/i/item/9789240060241 (accessed on 5 March 2024).
- Montero-Vargas, M.; Escudero-Leyva, E.; Díaz-Valerio, S.; Chaverri, P. Step-by-step pipeline for the ecological analysis of endophytic fungi using ITS nrDNA data. Curr. Protoc. Microbiol. 2020, 56, e96. [Google Scholar] [CrossRef]
- Ministério das Finanças: Direção Geral da Previsão e Estudos Económicos. Nota do Enquadramento Macroeconómico e Orçamental. Governo da Guiné-Bissau. 2020. Available online: https://www.mef.gw/publicacoes/boletim-trimestral-de-conjuntura/13-nota-de-enqudramento-macroeconomico-setembro-de-2020/file (accessed on 14 June 2024).
- Maia, A. Métodos de Armazenamento de Cereais em Países do Continente Africano; Complementary Work for Access Tests to Assistant Researcher; Tropical Research Institute: Lisbon, Portugal, 1994; 46p. [Google Scholar]
- Mobolade, A.J.; Bunindro, N.; Sahoo, D.; Rajashekar, Y. Traditional methods of food grains preservation and storage in Nigeria and India. Ann. Agric. Sci. 2019, 64, 196–205. [Google Scholar] [CrossRef]
- Da Silva, C.S. Os Ecossistemas orizicolas na Guiné Bissau. Comunicações (Portugal). Ser. Ciências Agrárias 1993, 13, 367–388. [Google Scholar]
- Kyle, S. Rice Sector Policy Options in Guinea Bissau; Working Paper; Cornell University: New York, NY, USA, 2015. [Google Scholar]
- FAOSTAT. Food and Agriculture Organizations of the United Nations Statistics on Food Balance 2021, Rice and Products and Food Supply Quantity (kg/capita/year). Available online: https://www.fao.org/faostat/en/#data/FBS (accessed on 20 June 2024).
- Fidalgo, H. Guiné-Bissau compra fora o arroz que podia produzir duas vezes por ano—especialistas. Lusa, 12 May 2024. Available online: https://www.agroportal.pt/guine-bissau-compra-fora-o-arroz-que-podia-produzir-duas-vezes-por-ano-especialistas/ (accessed on 27 June 2024).
- Monajjem, S.; Zainali, E.; Ghaderi-Far, F.; Solain, E.; Chaleshtari, M.H.; Khoshkdaman, M. Evaluation seed-born fungi of rice (Oryza sativa L.) and that effect on seed quality. J. Plant Pathol. Microbiol. 2014, 5, 1000239. [Google Scholar] [CrossRef]
- Dorley, O.S.; Were, J.O.; Ochuodho, J.O.; Auma, E.O. Fungal pathogens affecting the quality of rice (Oryza sativa L.) seed in selected agro-ecological zones of Liberia. World J. Agric. Res. 2023, 11, 8–15. [Google Scholar] [CrossRef]
- Aremu, M.B.; Kurah, I.A.; Okparavero, N.F.; Okunade, S.O.; Adebola, M.O.; Rahman, M.O.; Aina, O.B. Assessment of seed-borne plant pathogenic fungi, insect emergence and percentage germinability associated with harvested and stored rice in Kwara state, Nigeria. Afr. J. Agric. Food Sci. 2021, 4, 44–54. [Google Scholar] [CrossRef]
- Thuy, T.T.T.; Lübeck, M.; Smedegaard-Petersen, V.; de Neergaard, E.; Jørgensen, H.J.L. Infection Biology of Bipolaris oryzae in Rice and Defence Responses in Compatible and Less Compatible Interactions. Agronomy 2023, 13, 231. [Google Scholar] [CrossRef]
- Daou, R.; Joubrane, K.; Maround, R.G.; Khabbaz, L.R.; Ismail, A.; El Khoury, A. Mycotoxins: Factors influencing production and control strategies. AIMS Agric. Food 2021, 6, 416–447. [Google Scholar] [CrossRef]
- Commission Regulation (EU) No 165/2010. Official Journal of the European Union, 50/8. (Point 2.1.12 of the Annex). 2010. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:050:0008:0012:EN:PDF (accessed on 25 June 2024).
- Islam, M.R.; Chowdhury, R.; Roy, A.S.; Islam, M.N.; Mita, M.M.; Bashar, S.; Saha, P.; Rahat, R.A.; Hasan, M.; Akter, M.A.; et al. Native Trichoderma induced the defense-related enzymes and genes in rice against Xanthomonas oryzae pv. oryzae (Xoo). Plants 2023, 12, 1864. [Google Scholar] [CrossRef]
- Doni, F.; Isahak, A.; Fathurrahman, F.; Yusoff, W.M.W. Rice plant’s resistance to sheath blight infection is increased by the synergistic effects of Trichoderma inoculation with SRI management. Agronomy 2023, 13, 711. [Google Scholar] [CrossRef]
- Mau, Y.S.; Prayetno, R.S.; Kaka, H.; Naat, K.D.; Henuk, J.B.D.; Hahuly, M.V.; Widinugraheni, S.; Gandut, Y.R.Y. Efficacy of indigenous Trichoderma isolates of West Timor, Indonesia, as biocontrol agents of brown spot (Drechslera oryzae) on two upland rice varieties. Egypt. J. Biol. Pest Control 2022, 32, 62. [Google Scholar] [CrossRef]
- Datta, D.; Senapati, A.K.; Behera, L.; Zaidi, N.W.; Kumar, S.; Dey, P.; Kumar, S. Alleviating drought stress in rice plant through intervention of Trichoderma spp. J. Environ. Biol. 2022, 44, 373–379. [Google Scholar] [CrossRef]
- Abdul-Halim, A.M.A.; Shivanand, P.; Taha, H. Performance of a selected Trichoderma strain as plant pathogen inhibitor and biofertilizer. Malays. J. Microbiol. 2022, 18, 446–454. [Google Scholar] [CrossRef]

| Region | Village | Rice Variety [Vernacular Name] | Storage Container | Storage Structure |
|---|---|---|---|---|
| Cacheu | Djobel | Iacai branco | bag | bentém |
| Edjur | bag | storage room | ||
| Iacai preto | bundles | raised platform * | ||
| Edjur | bag | storage room | ||
| Edjur | plastic jerry can | storage room | ||
| Iacai preto | plastic jerry can | storage room | ||
| Etele | bag | bentém | ||
| Elalab | Batumpaiabo | bag | bentém | |
| Caublak/Caublac | bag | storage room | ||
| Tomor/Etomoray | bag | storage room | ||
| Bakongabu | bag | storage room | ||
| Batumpaiabo | bag | storage room | ||
| Iacai iadi | bag | storage room | ||
| Balenabu | bag | storage room | ||
| Tomor/Etomoray | bag | bentém | ||
| Elia | Iacai vermelho | bag | bentém | |
| Edjur | bag | bentém | ||
| Etele | bag | bentém | ||
| Edjur | plastic jerry can | house hallway | ||
| Edjur | bag | bentém | ||
| Etele | plastic jerry can | house hallway | ||
| Etele | bag | bentém | ||
| Oio | Blaftxur | Caublak/Caublac | fuúle/vuúle | front porche |
| Caublak/Caublac | fuúle/vuúle | front porche | ||
| Caublak/Caublac | fuúle/vuúle | front porche | ||
| Seli/Sili | fuúle/vuúle | front porche | ||
| Tanham/Atanham | fuúle/vuúle | front porche | ||
| Malafu | Sampena/Quisampena | metal barrel | storage room | |
| DEPA | bag | storage room | ||
| Yaca sau/xau | bag | storage room | ||
| DEPA | bag | bentém | ||
| Caublak/Caublac | bag | bentém | ||
| Caublak/Caublac | bag | storage room | ||
| N’Tchugal | Malu rassa | bag | storage room | |
| Caublak/Caublac | metal barrel | house hallway | ||
| Caublak/Caublac | fuúle/vuúle | front porche | ||
| Yaca sau/xau | bag | storage room | ||
| Caublak/Caublac | metal barrel | storage room | ||
| Rotxum | Yaca ieie | bag | storage room | |
| Barakonde | bag | front porche | ||
| Sugun | Caublak/Caublac | metal barrel | storage room | |
| Caublak/Caublac | metal barrel | front porche | ||
| Yaca sau/xau | metal barrel | front porche | ||
| Yaca ieie | bag | storage room | ||
| Yaca sau/xau | bag | front porche | ||
| Uncur | Caublak/Caublac | bag | storage room | |
| Caublak/Caublac | plastic jerry can | storage room | ||
| Tombali | Cafale | Caublak/Caublac | bag | bentém |
| Caublak/Caublac | bag | bentém | ||
| Yaca sau/xau | bag | bentém | ||
| Cafine | Caublak/Caublac | bag | bentém | |
| Yaca sau/xau | bag | bentém | ||
| Var 29 (Ianda Arruz project) | bag | biré/psangá | ||
| Mamusso | fuúle/vuúle | front porche | ||
| Yaca branco | bag | biré/psangá | ||
| Yaca sau/xau | bag | storage room | ||
| Yaca branco | bag | bentém | ||
| Caiquenem | Yaca sau/xau | bag | storage room | |
| Yaca sau/xau | bag | bentém | ||
| Caublak/Caublac | bag | storage room | ||
| Quibil | Yaca sau/xau | bag | biré/psangá | |
| Caublak/Caublac | bag | storage room | ||
| Caublak/Caublac | bag | biré/psangá | ||
| Yaca sau/xau | bag | storage room |
| Rice species | Rice Varieties [Vernacular Name] | Language | Region |
|---|---|---|---|
| Oryza glaberrima | Edjur | Baiote | C |
| Etele | C | ||
| Malu rassa | Balanta | O | |
| Bakongabu | Felupe | C | |
| Balenabu | C | ||
| Batumpaiabo | C | ||
| Oryza sativa | Tanham/Atanham | Balanta | O |
| Caublak/Caublac | Creole | C/O/T | |
| DEPA | O | ||
| Seli/Sili | O | ||
| Var 29 (Ianda Arruz project) | T | ||
| Yaca branco | T | ||
| Yaca ieie | O | ||
| Yaca sau/xau | O/T | ||
| Tomor/Etomoray | Felupe | C | |
| Iacai branco | Felupe/Baiote/Creole | C | |
| Iacai adi | C | ||
| Iacai preto | C | ||
| Iacai vermelho | C | ||
| Barakonde | Mandinga | O | |
| Mamusso | Susu | T | |
| Sampena/Quisampena | O |
| Rice Varieties [Vernacular Name] | % Germ. Day 3 | % Germ. Day 5 | Root Length [mm] | % Germ. Day 10 | % Germ. Day 14 | Seedling Length [mm] | SVI | |
|---|---|---|---|---|---|---|---|---|
| Oryza glaberrima | Bakongabu | 93% | 95% | 43.3 ± 13.1 | 99% | 100% | 64.3 ± 14.6 | 6425 |
| Balenabu | 85% | 88% | 49.8 ± 12.4 | 99% | 100% | 73.4 ± 9.1 | 7341 | |
| Batumpaiabo | 88% | 93% | 36.4 ± 15.5 | 99% | 100% | 79.3 ± 15.3 | 7891 | |
| Edjur | 51% | 92% | 55.4 ± 15.9 | 95% | 97% | 70 ± 11.8 | 6806 | |
| Etele | 8% | 96% | 48.2 ± 18.8 | 99% | 100% | 69 ± 12.8 | 6869 | |
| Malu rassa | 92% | 95% | 70.4 ± 16.6 | 98% | 99% | 67 ± 12 | 6636 | |
| Oryza sativa | Barakonde | 97% | 99% | 60.9 ± 17.2 | 100% | 100% | 79.9 ± 11.5 | 7965 |
| Caublak/Caublac | 94% | 95% | 54.4 ± 13.9 | 98% | 99% | 80.8 ± 13.9 | 8013 | |
| DEPA | 73% | 95% | 39.3 ± 18.9 | 98% | 100% | 69.1 ± 15.9 | 6873 | |
| Iacai branco | 93% | 98% | 50.8 ± 19.4 | 98% | 99% | 69.7 ± 10.5 | 6905 | |
| Iacai iadi | 73% | 83% | 51.3 ± 13.6 | 96% | 99% | 70.3 ± 13.3 | 6959 | |
| Iacai preto | 58% | 97% | 53.3 ± 18.8 | 98% | 100% | 72.9 ± 13.9 | 7254 | |
| Iacai vermelho | 98% | 98% | 56.8 ± 12.4 | 98% | 100% | 68.1 ± 14.8 | 6785 | |
| Mamusso | 95% | 96% | 42.5 ± 7.2 | 98% | 99% | 62.9 ± 8.9 | 6208 | |
| Sampena/Quisampena | 68% | 93% | 16.8 ± 11.8 | 98% | 99% | 57.9 ± 16.8 | 5737 | |
| Seli/Sili | 97% | 97% | 51.8 ± 10.1 | 99% | 99% | 91.9 ± 14.8 | 9128 | |
| Tanham/Atanham | 97% | 97% | 44.3 ± 9.3 | 99% | 100% | 112.2 ± 12.8 | 11,219 | |
| Tomor/Etomoray | 95% | 97% | 54.4 ± 13.9 | 99% | 100% | 77.2 ± 13 | 7694 | |
| Var 29 (Ianda Arruz project) | 88% | 97% | 20.6 ± 10.1 | 95% | 99% | 75.6 ± 11.6 | 7454 | |
| Yaca branco | 68% | 82% | 32.3 ± 13.5 | 98% | 99% | 71.2 ± 11 | 7052 | |
| Yaca ieie | 97% | 98% | 55.8 ± 12.9 | 99% | 100% | 78.7 ± 12.7 | 7829 | |
| Yaca sau/xau | 67% | 86% | 45.6 ± 12.9 | 95% | 99% | 72.9 ± 11.4 | 7188 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conde, S.; Barai, A.; Catarino, S.; Costa, G.J.; Ferreira, S.; Tavares, I.; Ferreira, M.R.; Temudo, M.P.; Monteiro, F. Hidden Secrets of Mangrove Swamp Rice Stored Seeds in Guinea-Bissau: Assessment of Fungal Communities and Implications for Food Security. Agronomy 2024, 14, 1870. https://doi.org/10.3390/agronomy14081870
Conde S, Barai A, Catarino S, Costa GJ, Ferreira S, Tavares I, Ferreira MR, Temudo MP, Monteiro F. Hidden Secrets of Mangrove Swamp Rice Stored Seeds in Guinea-Bissau: Assessment of Fungal Communities and Implications for Food Security. Agronomy. 2024; 14(8):1870. https://doi.org/10.3390/agronomy14081870
Chicago/Turabian StyleConde, Sofia, Amidu Barai, Sílvia Catarino, Gonçalo J. Costa, Sónia Ferreira, Idília Tavares, Maria Rosa Ferreira, Marina Padrão Temudo, and Filipa Monteiro. 2024. "Hidden Secrets of Mangrove Swamp Rice Stored Seeds in Guinea-Bissau: Assessment of Fungal Communities and Implications for Food Security" Agronomy 14, no. 8: 1870. https://doi.org/10.3390/agronomy14081870







