Response of Wheat to Pre-Emergence and Early Post-Emergence Herbicides
Abstract
1. Introduction
2. Materials and Methods
3. Results
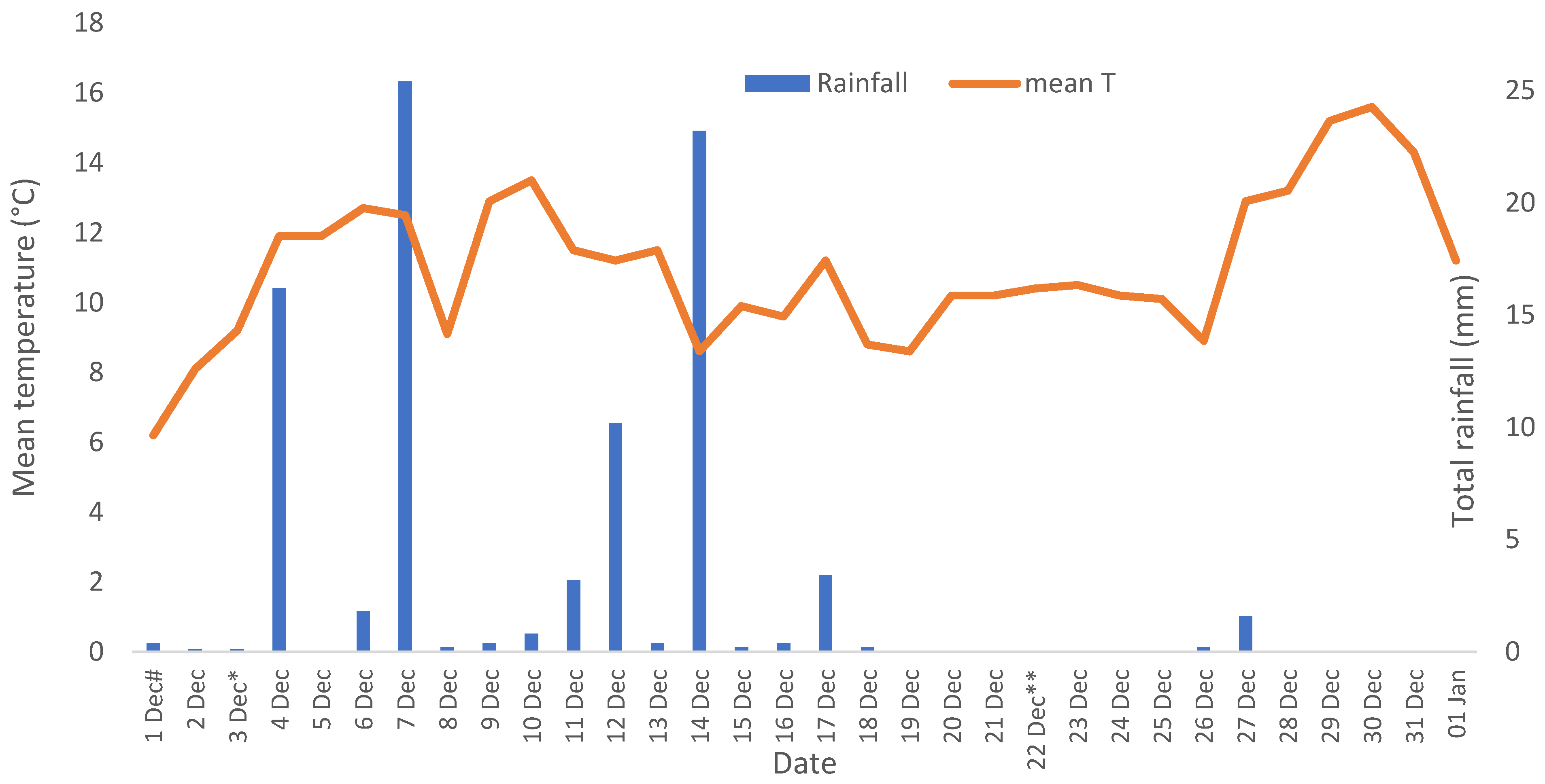
3.1. Trial A (2020–2021)
3.1.1. Crop Density, Plant Height and Injury Symptoms for Trial A
3.1.2. Number of Spikes and Crop Yield for Trial A
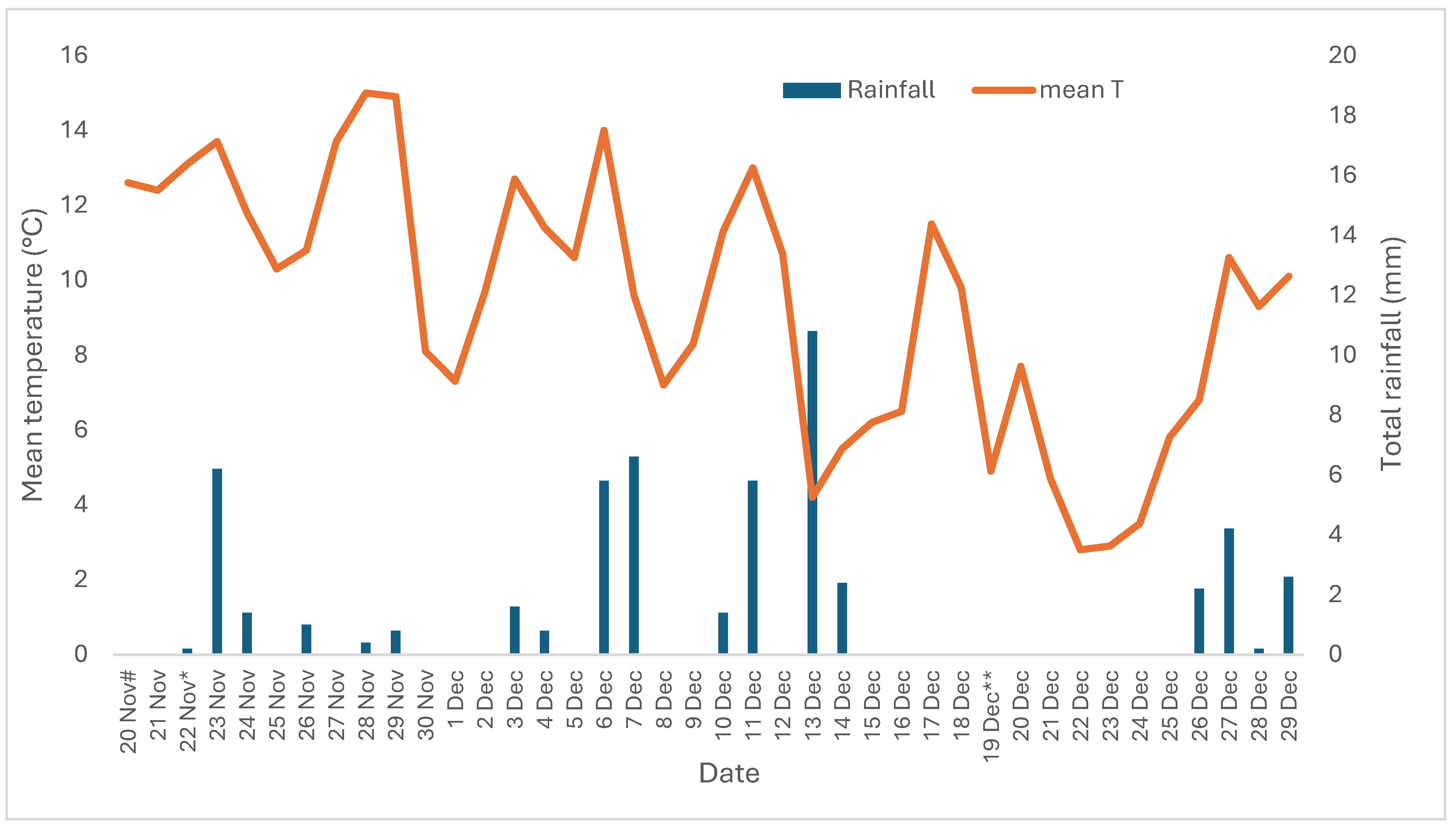
3.2. Trial B (2020–2021)
3.2.1. Crop Density, Plant Height and Injury Symptoms for Trial B
3.2.2. Number of Spikes and Crop Yield for Trial B
3.3. Weed Control
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Broster, J.C.; Koetz, E.A.; Wu, H. Herbicide resistance levels in annual ryegrass (Lolium rigidum Gaud.) in southern New South Wales. Plant Prot. Q. 2011, 26, 22–28. [Google Scholar]
- Heap, I. The International Herbicide-Resistant Weed Database. Available online: https://www.weedscience.org/Home.aspx (accessed on 10 January 2024).
- Kotoula-Syka, E.; Tal, A.; Rubin, B. Diclofop-resistant Lolium rigidum from northern Greece with cross-resistance to ACCase inhibitors and multiple resistance to chlorsulfuron. Pest Manag. Sci. 2000, 56, 1054–1058. [Google Scholar] [CrossRef]
- Travlos, I.S.; Giannopolitis, C.N.; Economou, G. Diclofop resistance in sterile wild oat (Avena sterilis L.) in wheat fields in Greece and its management by means of other post-emergence herbicides. Crop Prot. 2011, 30, 1449–1454. [Google Scholar] [CrossRef]
- Papapanagiotou, A.P.; Kaloumenos, N.S.; Eleftherohorinos, I.G. Sterile oat (Avena sterilis L.) cross-resistance profile to ACCase-inhibiting herbicides in Greece. Crop Prot. 2012, 35, 118–126. [Google Scholar] [CrossRef]
- Papapanagiotou, A.P.; Paresidou, M.I.; Kaloumenos, N.S.; Eleftherohorinos, I.G. ACCase mutations in Avena sterilis populations and their impact on plant fitness. Pestic. Biochem. Physiol. 2015, 123, 40–48. [Google Scholar] [CrossRef]
- Kaloumenos, S.N.; Tsioni, V.C.; Daliani, E.G.; Papavassileiou, S.E.; Vassileiou, A.G.; Laoutidou, P.N.; Eleftherohorinos, I.G. Multiple Pro-197 substitutions in the acetolactate synthase of rigid ryegrass (Lolium rigidum) and their impact on chlorsulfuron activity and plant growth. Crop Prot. 2011, 38, 35–43. [Google Scholar] [CrossRef]
- Scarabel, L.; Panozzo, S.; Loddo, D.; Mathiassen, S.K.; Kristensen, M.; Kudsk, P.; Gitsopoulos, T.; Travlos, I.; Tani, E.; Chachalis, D.; et al. Diversified resistance mechanisms in multi-resistant Lolium spp. in three European countries. Front. Plant Sci. 2020, 11, 608845. [Google Scholar] [CrossRef]
- Papapanagiotou, A.P.; Damalas, C.A.; Bosmali, I.; Madesis, P.; Menexes, G.; Eleftherohorinos, I.G. Multiple resistance of silky windgrass to acetolactate synthase- and acetyl-CoA synthase-inhibiting herbicides. Weed Technol. 2022, 36, 334–343. [Google Scholar] [CrossRef]
- Papapanagiotou, A.P.; Loukovitis, D.; Anthimidou, E.; Eleftherohorinos, I.G. Impact of ALS Herbicide-Resistant Perennial Ryegrass (Lolium perenne) Population on Growth Rate and Competitive Ability against Wheat. Agronomy 2023, 13, 1641. [Google Scholar] [CrossRef]
- Tomlin, C.D. The Pesticides Manual, 11th ed.; British Crop Protection Council: Farnham, UK, 2000. [Google Scholar]
- Andreasen, C.; Høgh, K.L.; Jensen, S.M. The Effect of Foliar and Soil Application of Flufenacet and Prosulfocarb on Italian Ryegrass (Lolium multiflorum L.) Control. Agriculture 2020, 10, 552. [Google Scholar] [CrossRef]
- PPDB: Pesticide Properties DataBase. University of Hertforshire. Available online: https://sitem.herts.ac.uk/aeru/ppdb/en/atoz_herb.htm (accessed on 10 January 2024).
- Fuerst, E. Understanding the Mode of Action of the Chloroacetamide and Thiocarbamate Herbicides. Weed Technol. 1987, 1, 270–277. [Google Scholar] [CrossRef]
- Chamovitz, D.; Sandmann, G.; Hirschberg, J. Molecular and biochemical characterization of herbicide-resistant mutants of cyanobacteria reveals that phytoene desaturation is a rate-limiting step in carotenoid biosynthesis. J. Biol. Chem. 1993, 268, 17348–17353. [Google Scholar] [CrossRef] [PubMed]
- Sandmann, G.; Schneider, C.; Böger, P. A new non-radioactive assay of phytoene desaturase to evaluate bleaching herbicides. Z. Naturf. C J. Biosci. 1996, 51, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Congreve, M.; Cameron, J. Soil Behaviour of Pre-Emergent Herbicides in Australian Farming Systems—A National Reference Manual for Advisers, 3rd ed.; GRDC Publication: Barton, Australia, 2023; Available online: https://grdc.com.au/__data/assets/pdf_file/0037/593884/SoilBehavPre-EmerHerbicides_2307_Web.pdf (accessed on 15 April 2024).
- Shaner, D.L. Herbicide Handbook, 10th ed.; Weed Science Society of America: Lawrence, KS, USA, 2014. [Google Scholar]
- Lechelt-Kunze, C.; Meissner, R.C.; Drewes, M.; Tietjen, K. Flufenacet herbicide treatment phenocopies the fiddlehead mutant in Arabidopsis thaliana. Pest Manag. Sci. 2003, 59, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Senseman, S.A. Herbicide Handbook, 9th ed.; Weed Science society of America: Champaign, IL, USA, 2007; p. 458. [Google Scholar]
- Deege, R.; Forster, H.; Schmidt, R.R.; Thielert, W.; Tice, M.A.; Aagesen, G.J.; Bloomberg, J.R.; Santel, H.J. BAY 5043: A new low rate herbicide for pre-emergence grass control in corn, cereals, soybeans and other selected crops. In Proceedings of the Brighton Crop Protection Conference, Brighton, UK, 20–23 November 1995; Volume 1, pp. 43–48. [Google Scholar]
- Grey, T.L.; Bridges, D.C. Alternatives to Diclofop for the Control of Italian Ryegrass (Lolium multiflorum) in Winter Wheat (Triticum aestivum). Weed Technol. 2003, 17, 219–223. [Google Scholar] [CrossRef]
- Koepke-Hill, R.; Armel, G.; Bradley, K.; Bailey, W.; Wilson, H.; Hines, T. Evaluation of Flufenacet plus Metribuzin Mixtures for Control of Italian Ryegrass in Winter Wheat. Weed Technol. 2011, 25, 563–567. [Google Scholar] [CrossRef]
- Cole, D.J. Detoxification and activation of agrochemicals in plants. Pestic. Sci. 1994, 42, 209–222. [Google Scholar] [CrossRef]
- Cole, D.J.; Van Oorshot, J.L.P. The effects of environmental factors on the metabolism of herbicides in plants. Asp. Appl. Biol. 1983, 4, 245–252. [Google Scholar]
- Hatzios, K.K.; Penner, D. Metabolism of Herbicides in Higher Plants; CEPCO Division; Burgess Pub. Co.: Minneapolis, MN, USA, 1982. [Google Scholar]
- Ferreira, K.L.; Baker, T.K.; Peeper, T.F. Factors Influencing Winter Wheat (Triticum aestivum) Injury from Sulfonylurea Herbicides. Weed Technol. 1990, 4, 724–730. [Google Scholar] [CrossRef]
- Robinson, M.A.; Letarte, J.; Cowbrough, M.J.; Sikkema, P.H.; Tardif, F.J. Winter wheat (Triticum aestivum L.) response to herbicides as affected by application timing and temperature. Can. J. Plant Sci. 2015, 95, 325–333. [Google Scholar] [CrossRef]
- Bough, R.A.; Gaines, T.A.; Dayan, F.E. Low Temperature Delays Metabolism of Quizalofop in Resistant Winter Wheat and Three Annual Grass Weed Species. Front. Agron. 2022, 3, 800731. [Google Scholar] [CrossRef]
- Kleemann, S.G.L.; Preston, C.; Gill, G.S. Influence of seeding system disturbance on preplant incorporated herbicide control of rigid ryegrass (Lolium rigidum) in wheat in Southern Australia. Weed Technol. 2014, 28, 323–331. [Google Scholar] [CrossRef]
- Tanji, A.; Boutfirass, M. Effective Preemergence Herbicides for Rigid Ryegrass (Lolium rigidum Gaud.) Control in Irrigated Bread Wheat (Triticum aestivum L.). J. Agric. Sci. 2018, 10, 79–85. [Google Scholar] [CrossRef][Green Version]
- Cirujeda, A.; Taberner, A. Chemical control of herbicide-resistant Lolium rigidum Gaud. in north-eastern Spain. Pest Manag. Sci. 2010, 66, 1380–1388. [Google Scholar] [CrossRef] [PubMed]
- Mamnoie, E.; Karaminejad, M.R. Evaluation Time and Rate Application of Prosulfocarb Herbicide in the Weed Control of Wheat. J. Crop Prod. 2020, 13, 51–66. [Google Scholar] [CrossRef]
- Boutsalis, P.; Gill, G.S.; Preston, C. Control of rigid ryegrass in Australian wheat production with pyroxasulfone. Weed Technol. 2014, 28, 332–339. [Google Scholar] [CrossRef]
- Delchev, G. Sowing characteristics of the durum wheat seeds (Triticum durum Desf.) by use of some antigraminaceous and combined herbicides. Agric. Sci. Technol. 2022, 14, 54–59. [Google Scholar] [CrossRef]
- Siminszky, B.; Corbin, F.T.; Ward, E.R.; Fleischmann, T.J.; Dewey, R.E. Expression of a soybean cytochrome P450 monooxygenase cDNA in yeast and tobacco enhances the metabolism of phenylurea herbicides. Proc. Natl. Acad. Sci. USA 1999, 96, 1750–1755. [Google Scholar] [CrossRef]
- Werck-Reichhart, D.; Hehn, A.; Didierjean, L. Cytochromes P450 for engineering herbicide tolerance. Trends Plant Sci. 2000, 5, 116–123. [Google Scholar] [CrossRef]
- Cabanne, F.; Gaillardon, P.; Scalla, R. Phytotoxicity and metabolism of chlortoluron in two wheat varieties. Pestic. Biochem. Physiol. 1985, 23, 212–220. [Google Scholar] [CrossRef]
- Cabanne, F.; Huby, D.; Gaillardon, P.; Scalla, R.; Durst, F. Effect of the cytochrome P-450 inhibitor 1-aminobenzotriazole on metabolism of chlorotoluron and isoproturon in wheat. Pestic. Biochem. Physiol. 1987, 28, 371–380. [Google Scholar] [CrossRef]
- Mougin, C.; Scalla, R.; Cabanne, F. Monooxygenases responsible for the metabolism of chlorotoluron in wheat microsomes. Rencontres Interdiscip. Biochim. 1990, ffhal01600676f. [Google Scholar]
- Song, N.H.; Zhang, S.; Hong, M.; Yang, H. Impact of dissolved organic matter on bioavailability of chlorotoluron to wheat. Environ. Pollut. 2010, 158, 906–912. [Google Scholar] [CrossRef]
- Haynes, C.; Kirkwood, R.C. Studies on the mode of action of diflufenican in selected crop and weed species: Basis of selectivity of pre-and early post-emergence applications. Pest Manag. Sci. 1992, 35, 161–165. [Google Scholar] [CrossRef]
- Bieseler, B.; Fedtke, C.; Neuefeind, W.; Etzel, W.; Prade, L.; Reinemer, P. Maize selectivity of FOE 5043: Breakdown of active ingredient by glutathione-S-transferases. Pflanzenschutz-Nachrichten Bayer 1997, 50, 117–142. [Google Scholar]
- Gould, T.J.; Grace, T.J.; Krolski, M.E.; Lemke, V.J.; Murphy, J.J. An analytical method for the determination of FOE 5043 residues in plant matrices and analysis of field residues using the method. Pflanzenschutz-Nachr. Bayer 1997, 50, 199–232. [Google Scholar]
- Devlin, D.L.; Gealy, D.R.; Morrow, L.A. Differential metabolism of metribuzin by downy brome (Bromus tectorum) and winter wheat (Triticum aestivum). Weed Sci. 1987, 35, 741–745. [Google Scholar] [CrossRef]
- Gawronski, S.W.; Haderlie, L.C.; Stark, J.C. Metribuzin Absorption and Translocation in Two Barley (Hordeum vulgare) Cultivars. Weed Sci. 1986, 34, 491–495. [Google Scholar] [CrossRef]
- Shaw, D.R.; Wesley, M.T. Wheat Cultivar Tolerance and Italian Ryegrass (Lolium multiflorum) Control with Diclofop, BAY SMY 1500, and Metribuzin. Weed Technol. 1991, 5, 776–781. [Google Scholar] [CrossRef]
- VanGessel, M.J.; Johnson, Q.R.; Scott, B.A. Effect of Application Timing on Winter Wheat Response to Metribuzin. Weed Technol. 2017, 31, 94–99. [Google Scholar] [CrossRef]
- Schroeder, J.; Banks, P.A.; Nichols, R.L. Soft Red Winter Wheat (Triticum Aestivum) Cultivar Response to Metribuzin. Weed Sci. 1986, 34, 66–69. [Google Scholar] [CrossRef]
- Gitsopoulos, T.; Georgoulas, I.; Vazanelli, E.; Botsoglou, D. Selectivity of the Premixtures Flufenacet, Diflufenican and Flufenacet, Diflufenican, Metribuzin on Bread Wheat (Triticum aestivum L.) and Barley (Hordeum vulgare L.) and Efficacy on ALS/ACCase-Resistant Populations of Lolium rigidum Gaudin. Agronomy 2024, 14, 949. [Google Scholar] [CrossRef]
- Hajjaj, B.; Bouhache, M.; Mrabet, R.; Taleb, A.; Douaik, A. Efficacité de quelques herbicides des céréales dans une culture de blé tendre conduit en semis direct. Rev. Mar. Sci. Agron. Vét. 2016, 4, 48–56. [Google Scholar]
- Sobiech, Ł.; Joniec, A.; Loryś, B.; Rogulski, J.; Grzanka, M.; Idziak, R. Autumn Application of Synthetic Auxin Herbicide for Weed Control in Cereals in Poland and Germany. Agriculture 2023, 13, 32. [Google Scholar] [CrossRef]
- Bonin, L.; Gautellier-Vizioz, L. Study of New Herbicides in Winter Cereals (Etude des nouveaux herbicides sur les cereales d’hiver). In Proceedings of the 24e Conférence du COLUMA: Journées Internationales sur la Lutte Contre les Mauvaises Herbes, Orleans, France, 3–5 December 2019. [Google Scholar]
| Herbicide | Soil Adsorption and Mobility (mL g−1) * | Solubility in Water (mg L−1) ** | Soil Degradation (Days) (Aerobic) *** |
|---|---|---|---|
| Prosulfocarb | 1693 | 13.2 | 9.8 |
| Chlorotoluron | 147.22 | 76 | 12.5 |
| Diflufenican | 2215 | 0.05 | 64.6 |
| Flufenacet | 273.3 | 51 | 39 |
| Metribuzin | 48.3 | 10,700 | 19 |
| Herbicide Ai | HRAC Group | Mode of Action (MoA) | Product Information | Herbicide Rate (g ai ha−1) # | |
|---|---|---|---|---|---|
| 1. | prosulfocarb | 15 | Inhibition of very long-chain fatty acid synthesis | Boxer 80 EC (80% ai) Syngenta Crop Protection AG(Basel, Switzerland) | 3200 |
| 2. | chlorotoluron + diflufenican | 5 + 12 | Inhibition of photosynthesis at PS-II Serine 264 Binders + Inhibition of Phytoene Desaturase | Carmina Max SC (60% + 4% ai) Nufarm GmbH & Co KG (Linz, Austria) | 1380 + 92 |
| 3. | flufenacet + diflufenican | 15 + 12 | Inhibition of very long-chain fatty acid synthesis + Inhibition of Phytoene Desaturase | Fosburi 600 SC (40% + 20% ai) Bayer AG (Leverkusen, Germany) | 240 + 120 |
| 4. | flufenacet + diflufenican + metribuzin | 15 + 12 + 5 | Inhibition of very long-chain fatty acid synthesis + Inhibition of Phytoene Desaturase + Inhibition of photosynthesis at PS-II Serine 264 Binders | Herold Trio SC (17.1% + 17.1% + 6.4% ai) Bayer AG (Leverkusen, Germany) | 119.7 + 119.7 + 44.8 |
| Treatment | Wheat Density | 13.1.21 (44 DAS ^) | 11.2.21 (73 DAS) | 26.3.21 (116 DAS) |
|---|---|---|---|---|
| Plants m−1 | cm | |||
| Prosulfocarb | 71.0 b * (5.55) # | 15.4 (0.34) | 21.9 ab (0.79) | 49.0 (2.10) |
| Chlorotoluron + Diflufenican | 84.7 a (5.49) | 15.9 (0.40) | 20.6 b (1.56) | 46.8 (1.21) |
| Flufenacet + Diflufenican | 92.5 a (6.59) | 15.6 (0.62) | 17.2 c (0.44) | 47.5 (1.22) |
| Flufenacet + Diflufenican + Metribuzin | 86.4 a (4.70) | 16.5 (0.30) | 22.7 ab (1.22) | 50.8 (1.66) |
| Untreated control | 88.7 a (4.67) | 15.9 (0.50) | 23.4 ab (0.52) | 50.2 (0.88) |
| Weed-free | 95.2 a (4.09) | 16.1 (0.61) | 24.3 a (1.15) | 52.1 (1.20) |
| LSD ## (5%) | 12.92 | ns ** | 3.09 | ns |
| Treatment | 2020–2021 | |||
|---|---|---|---|---|
| Spikes m−2 | t ha−1 ** | |||
| Prosulfocarb | 443 b * | (22.3) # | 4.30 bc | (0.28) |
| Chlorotoluron + Diflufenican | 526 ab | (49.3) | 4.06 c | 0.39) |
| Flufenacet + Diflufenican | 567 a | (31.3) | 4.99 abc | (0.37) |
| Flufenacet + Diflufenican + Metribuzin | 558 a | (23.9) | 5.12 ab | (0.33) |
| Untreated control | 602 a | (17.9) | 5.43 a | (0.27) |
| Weed-free | 622 a | (33.5) | 5.79 a | (0.21) |
| LSD ## (5%) | 96.6 | 0.945 | ||
| Treatment | 5.1.22 (44 DAS *) | 12.2.22 (82 DAS) | 24.3.22 (122 DAS) |
|---|---|---|---|
| cm | |||
| Prosulfocarb | 9.65 (0.39) # | 12.33 (1.21) | 39.7 (1.94) |
| Chlorotoluron + Diflufenican | 9.48 (0.63) | 12.26 (1.45) | 40.5 (3.07) |
| Flufenacet + Diflufenican | 9.50 (0.33) | 11.86 (0.68) | 38.6 (2.21) |
| Flufenacet + Diflufenican + Metribuzin | 10.23 (0.13) | 12.53 (0.67) | 42.2 (1.34) |
| Untreated control | 9.96 (0.49) | 12.25 (0.87) | 37.6 (1.63) |
| Weed-free | 10.09 (0.36) | 13.19 (0.41) | 41.2 (1.29) |
| LSD ## (5%) | ns ** | ns | ns |
| Treatment | 2021–2022 | |||
|---|---|---|---|---|
| Spikes m−2 | t ha−1 ** | |||
| Prosulfocarb | 498 a * | (23.0) # | 5.04 a | (0.13) |
| Chlorotoluron + Diflufenican | 486 ab | (20.2) | 4.49 ab | (0.20) |
| Flufenacet + Diflufenican | 424 bc | (26.4) | 4.10 b | (0.02) |
| Flufenacet + Diflufenican + Metribuzin | 464 ab | (18.3) | 4.48 ab | (0.17) |
| Untreated control | 394 c | (20.5) | 4.14 b | 0.27) |
| Weed-free | 493 a | (31.0) | 5.01 a | (0.18) |
| LSD ## (5%) | 66.4 | 0.566 | ||
| Herbicides/Weed Species | Veronica spp. | Lamium spp. | F. officinalis | C. bursa-pastoris | M. recutita | P. rhoeas | L. rigidum |
|---|---|---|---|---|---|---|---|
| % control * | |||||||
| Prosulfocarb | 96 (1.25) # | 94 (1.13) | 92 (0.92) b ** | 91 (1.30) b | 92 (2.14) | 41 (4.79) b | 94 (1.55) |
| Chlorotoluron + Diflufenican | 98 (0.92) | 97 (0.92) | 96 (1.13) a | 93 (1.49) b | 94 (1.70) | 94 (1.87) a | 93 (1.34) |
| Flufenacet + Diflufenican | 96 (1.57) | 98 (0.95) | 97 (0.92) a | 98 (1.64) a | 94 (1.25) | 99 (0.92) a | 97 (0.92) |
| Flufenacet + Diflufenican + Metribuzin | 98 (0.92) | 98 (0.92) | 96 (1.13) a | 95 (0.95) ab | 97 (0.92) | 97 (1.01) a | 96 (1.48) |
| LSD ^ (5%) | ns *** | ns | 3.01 | 4.11 | ns | 5.26 | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gitsopoulos, T.; Georgoulas, I.; Botsoglou, D.; Vazanelli, E. Response of Wheat to Pre-Emergence and Early Post-Emergence Herbicides. Agronomy 2024, 14, 1875. https://doi.org/10.3390/agronomy14081875
Gitsopoulos T, Georgoulas I, Botsoglou D, Vazanelli E. Response of Wheat to Pre-Emergence and Early Post-Emergence Herbicides. Agronomy. 2024; 14(8):1875. https://doi.org/10.3390/agronomy14081875
Chicago/Turabian StyleGitsopoulos, Thomas, Ioannis Georgoulas, Despoina Botsoglou, and Eirini Vazanelli. 2024. "Response of Wheat to Pre-Emergence and Early Post-Emergence Herbicides" Agronomy 14, no. 8: 1875. https://doi.org/10.3390/agronomy14081875
APA StyleGitsopoulos, T., Georgoulas, I., Botsoglou, D., & Vazanelli, E. (2024). Response of Wheat to Pre-Emergence and Early Post-Emergence Herbicides. Agronomy, 14(8), 1875. https://doi.org/10.3390/agronomy14081875






