Assessing Salinity Tolerance in Pinto Bean Varieties: Implications for Sustainable Agriculture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Germination Experiment
2.2. Greenhouse Experiment
2.3. Statistical Analysis
3. Results
3.1. Germination Experiment
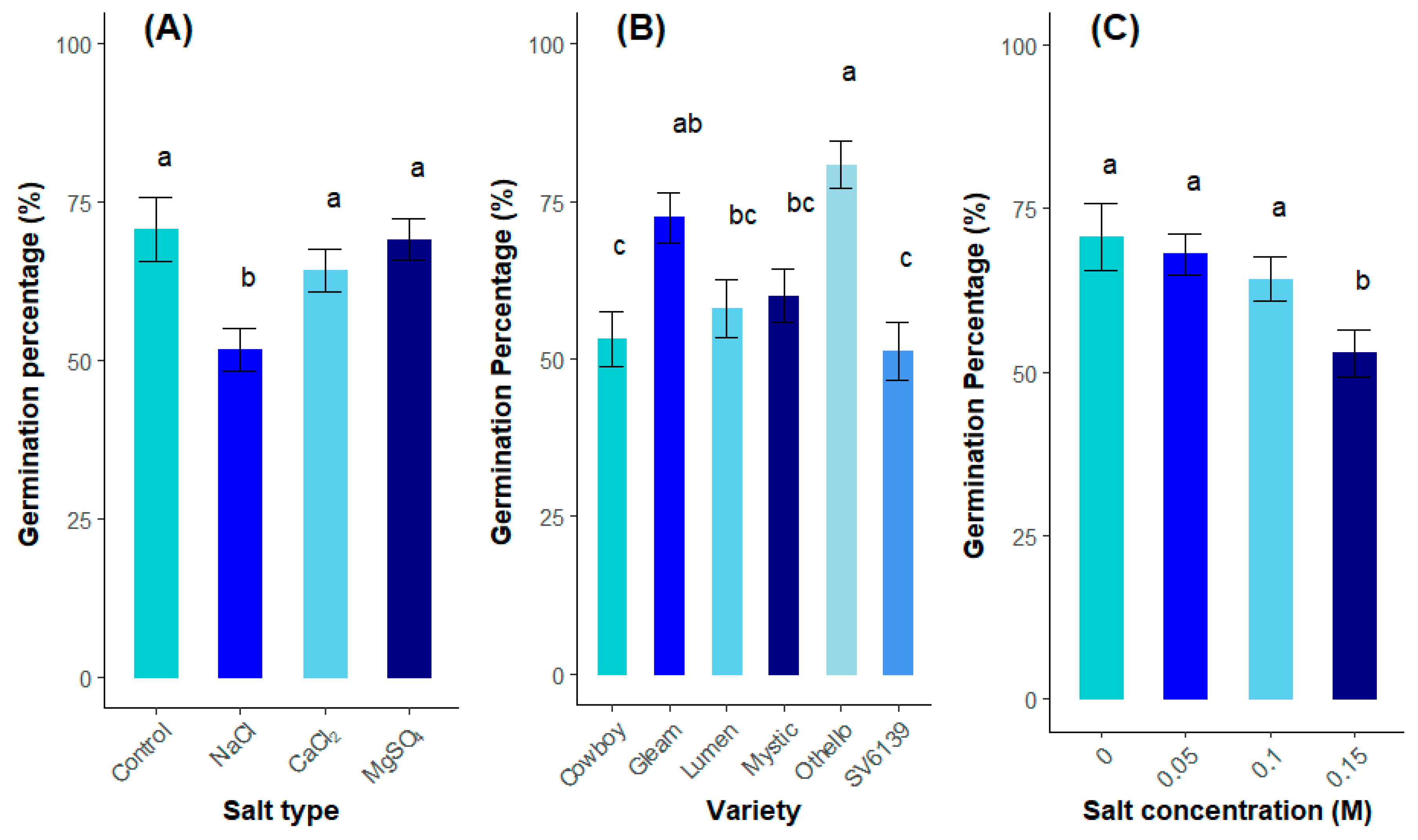
3.1.1. Germination Percentage
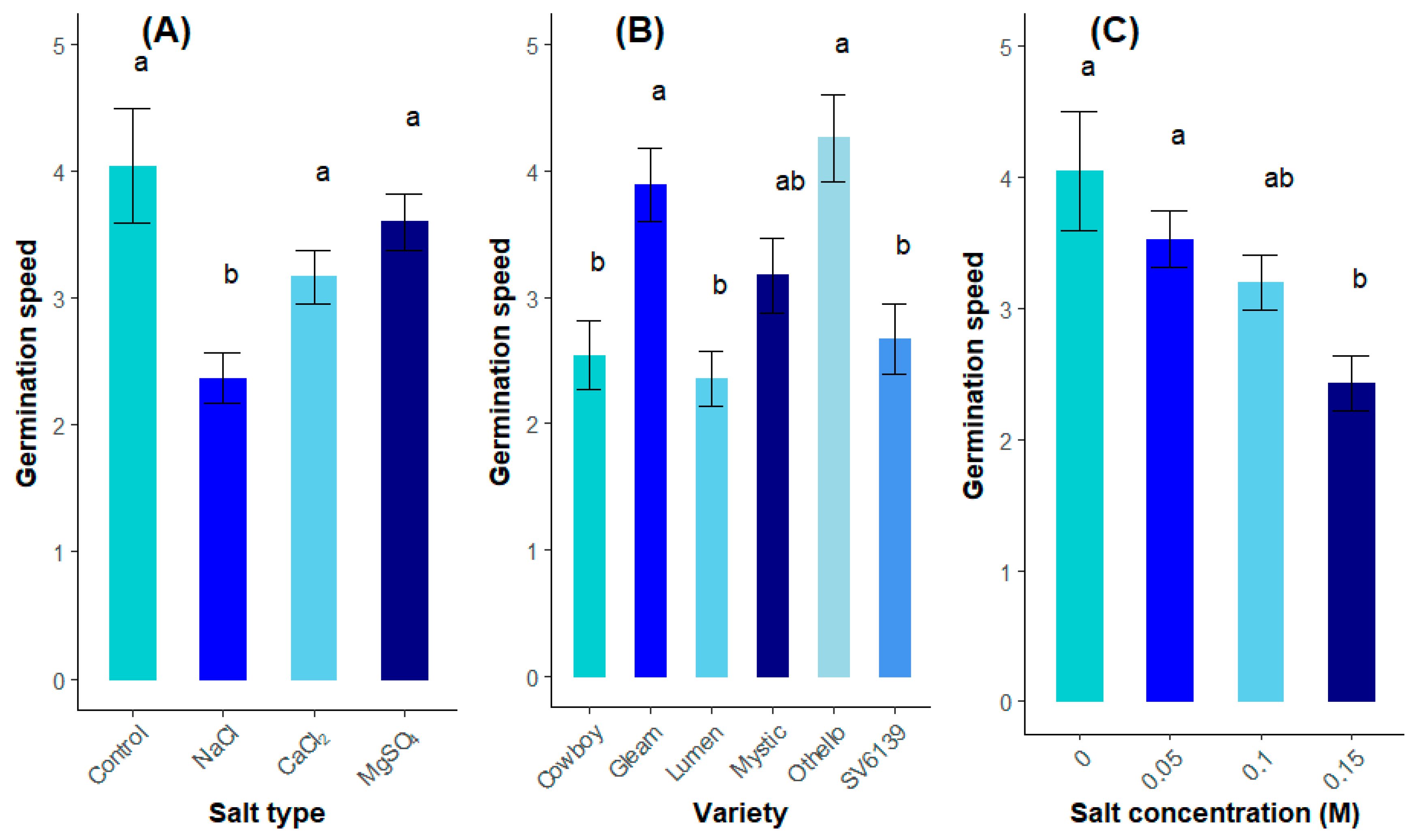
3.1.2. Speed of Germination
3.1.3. Hypocotyl and Radicle Length
3.2. Greenhouse Experiment
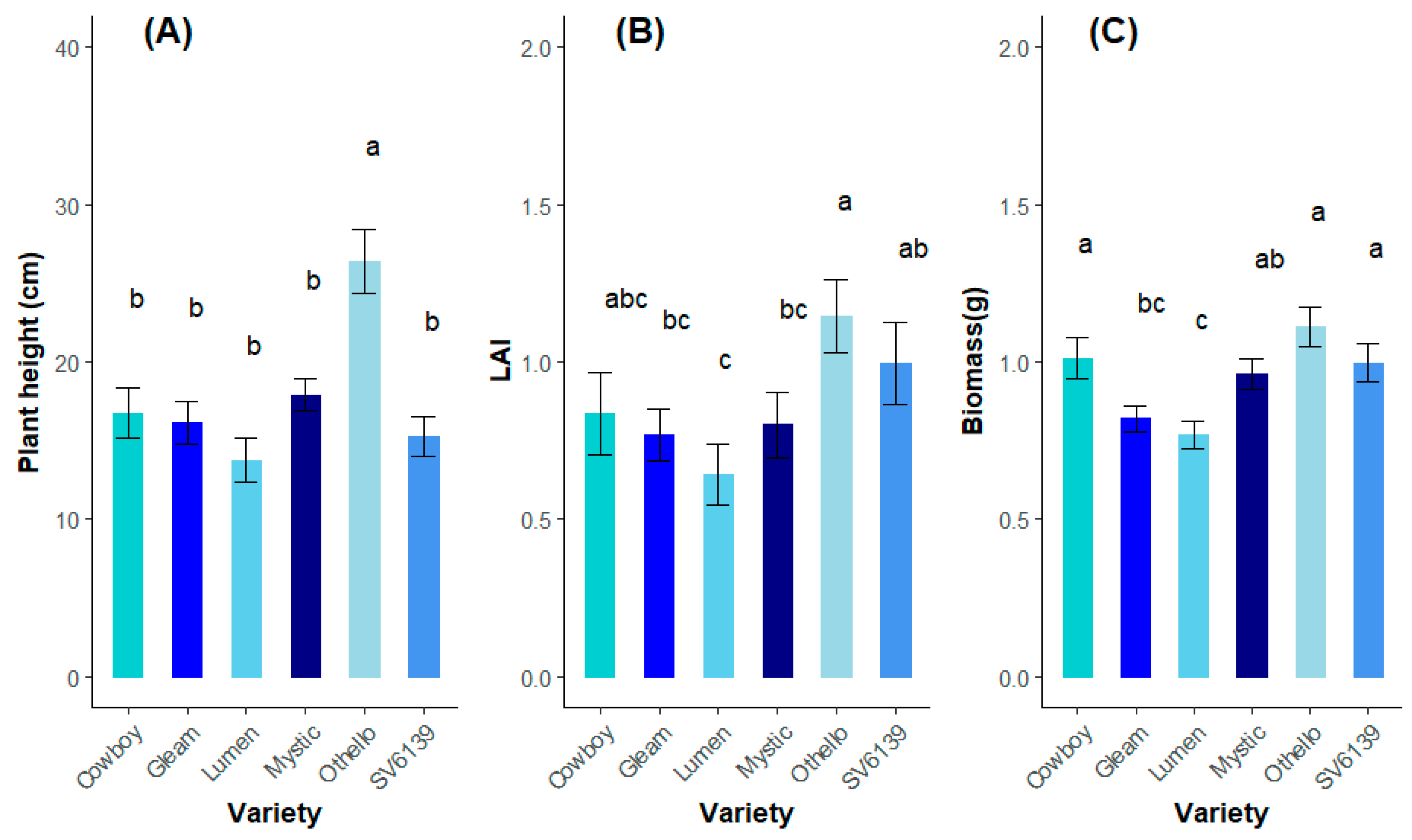
3.2.1. Growth Comparisons among Varieties
3.2.2. Flower and Pod Number
3.2.3. ECe Analysis of Saturated Paste Extracts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Keshavarz, R.; Didinger, C.; Duncan, A.; Thompson, H.J. Pulse Crops and Their Key Role as Staple Foods in Healthful Eating Patterns. In Colorado State University Extension Factsheet 0.312; Colorado State University: Fort Collins, CO, USA, 2020; Available online: https://extension.colostate.edu/topic-areas/agriculture/pulse-crops-and-their-key-role-as-staple-foods-in-healthful-eating-patterns-0-313/ (accessed on 15 July 2024).
- Didinger, C.; Thompson, H.J. The role of pulses in improving human health: A review. Legume Sci. 2022, 4, e147. [Google Scholar] [CrossRef]
- Keeping the Spotlight on Pulses: “Roots” for Sustainable Agriculture and Food Security. United Nations. Available online: https://www.un.org/en/un-chronicle/keeping-spotlight-pulses-%E2%80%9Croots%E2%80%9D-sustainable-agriculture-and-food-security (accessed on 15 July 2024).
- Ghassemi-Golezani, K.; Nikpour-Rashidabad, N.; Zehtab-Salmasi, S. Effect of salinity on yield and yield components of pinto bean cultivars. Int. J. Plant Anim. Environ. Sci. 2012, 2, 47–51. [Google Scholar]
- Schwartz, H.F.; Brick, M.A. Eco-Tillage, Biopesticide and Resistance Management of White Mold in Dry Bean. Available online: https://www.ars.usda.gov/ARSUserFiles/30000000/WhiteMoldResearch/AnnualMeetingFiles/2004/Schwartz.pdf (accessed on 15 July 2024).
- Colorado Beans Are a Magical Ingredient—Colorado Proud’s January Recipe of the Month. Colorado Department of Agriculture. Available online: https://ag.colorado.gov/press-release/colorado-beans-are-a-magical-ingredient-colorado-prouds-january-recipe-of-the-month (accessed on 15 July 2024).
- Dealers—Colorado Dry Beans. Available online: https://coloradodrybeans.org/colorado-dry-bean-committee/dealers/ (accessed on 15 July 2024).
- Duwadi, K.; Austin, R.S.; Mainali, H.R.; Bett, K.; Marsolais, F.; Dhaubhadel, S. Slow darkening of pinto bean seed coat is associated with significant metabolite and transcript differences related to proanthocyanidin biosynthesis. BMC Genom. 2018, 19, 260. [Google Scholar] [CrossRef]
- Park, D.; Maga, J.A. Dry bean (Phaseolis vulgaris) color stability as influenced by time and moisture content. J. Food Process. Preserv. 1999, 23, 515–522. [Google Scholar] [CrossRef]
- Hildebrant, D. Slow-Darkening Pinto Beans Developed by NDSU. Farm & Ranch Guide 2020. Available online: https://agupdate.com/farmandranchguide/news/crop/slow-darkening-pinto-beans-developed-by-ndsu/article_f8bf7bf4-d745-11ea-8d06-5fdf555291a3.html (accessed on 15 July 2024).
- Rhoades, J.D.; Kandiah, A.; Mashali, A.M. The Use of Saline Waters for Crop Production; FAO Irrigation and Drainage Paper; Food and Agriculture Organization, United Nations: Rome, Italy, 1992; ISBN 978-92-5-103237-4. [Google Scholar]
- Cardon, G.E.; Davis, J.G.; Bauder, T.A.; Waskom, R.M. Managing Saline Soils. In Colorado State University Extension Factsheet 0.503; Colorado State University: Fort Collins, CO, USA, 2014; Available online: https://extension.colostate.edu/topic-areas/agriculture/managing-saline-soils-0-503/ (accessed on 15 July 2024).
- Bernstein, N. Chapter 5—Plants and salt: Plant response and adaptations to salinity. In Model Ecosystems in Extreme Environments; Seckbach, J., Rampelotto, P., Eds.; Astrobiology Exploring Life on Earth and Beyond; Academic Press: Cambridge, MA, USA, 2019; pp. 101–112. ISBN 978-0-12-812742-1. [Google Scholar]
- Joshi, R.; Durgesh, K. Salt stress in pulses: A learning from global research on salinity in crop plants. Indian J. Genet. Plant Breed. 2021, 81, 159–185. [Google Scholar] [CrossRef]
- Keshtiban, R.K.; Carvani, V.; Imandar, M. Effects of salinity stress and drought due to different concentrations of sodium chloride and polyethylene glycol 6000 on germination and seedling growth characteristics of pinto bean (Phaseolus vulgaris L.). Adv. Environ. Biol. 2015, 9, 229–235. [Google Scholar]
- Dasgan, H.; Koc, S. Evaluation of salt tolerance in common bean genotypes by ion regulation and searching for screening parameters. J. Food Agric. Environ. 2009, 77, 363–372. [Google Scholar]
- Niknam, S.R.; McComb, J. Salt tolerance screening of selected Australian woody species—A review. For. Ecol. Manag. 2000, 139, 1–19. [Google Scholar] [CrossRef]
- Kökten, K.; Karaköy, T.; Bakoglu, A.; Akçura, M. Determination of salinity tolerance of some lentil (Lens culinaris M.) varieties. J. Food Agric. Env. 2009, 8, 140–143. [Google Scholar]
- Goertz, S.H.; Coons, J.M. Tolerance of tepary and navy beans to NaCl during germination and emergence. HortScience 1991, 26, 246–249. [Google Scholar] [CrossRef]
- Salim, M.; Pitman, M.G. Salinity tolerance of mung bean (Vigna radiata L.): Seed production. Biol. Plant. 1988, 30, 53–57. [Google Scholar] [CrossRef]
- Afkairin, A.; Dixon, M.M.; Buchanan, C.; Ippolito, J.A.; Manter, D.K.; Davis, J.G.; Vivanco, J.M. Harnessing phosphorous (P) fertilizer-insensitive bacteria to enhance rhizosphere P bioavailability in legumes. Microorganisms 2024, 12, 353. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Casal, A.E.; Castillo, J.M.; Luque, C.J.; Figueroa, M.E. Influence of salinity on germination and seeds viability of two primary colonizers of Mediterranean salt pans. J. Arid. Environ. 2003, 53, 145–154. [Google Scholar] [CrossRef]
- Ehtaiwesh, A.; Emsahel, M. Impact of salinity stress on germination and growth of pea (Pisum sativum L.) plants. Al-Mukhtar J. Sci. 2020, 35, 146–159. [Google Scholar] [CrossRef]
- Official Series Description—GARRETT Series. United States Department of Agriculture. Available online: https://soilseries.sc.egov.usda.gov/OSD_Docs/G/GARRETT.html (accessed on 15 July 2024).
- Rhoades, J.D. Salinity: Electrical conductivity and total dissolved solids. In Methods of Soil Analysis; John Wiley & Sons, Ltd.: New York, NY, USA, 1996; pp. 417–435. ISBN 978-0-89118-866-7. [Google Scholar]
- Olsen, S.R. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; United States Department of Agriculture: Washington, DC, USA, 1954.
- Davis, J.; Brick, M. Fertilizing Dry Beans. In Colorado State University Extension Factsheet 0.539; Colorado State University: Fort Collins, CO, USA, 2009; Available online: https://extension.colostate.edu/topic-areas/agriculture/fertilizing-dry-beans-0-539/ (accessed on 15 July 2024).
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Gutierrez, M.; Escalante-Estrada, J.; Rodriguez-Gonzalez, M. Differences in salt tolerance between Phaseolus vulgaris and Phaseolus coccineus cultivars. Int. J. Agric. Res. 2009, 4, 270–278. [Google Scholar] [CrossRef]
- Wolde, G.; Adamu, C. Impact of salinity on seed germination and biomass yields of field pea (Pasum satrium L.). Asian J. Sci. Tech. 2018, 9, 7565–7569. [Google Scholar]
- Kaymakanova, M. Effect of salinity on germination and seed physiology in bean (Phaseolus vulgaris L.). Biotechnol. Biotechnol. Equip. 2009, 23, 326–329. [Google Scholar] [CrossRef]
- Atta, K.; Mondal, S.; Gorai, S.; Singh, A.P.; Kumari, A.; Ghosh, T.; Roy, A.; Hembram, S.; Gaikwad, D.J.; Mondal, S.; et al. Impacts of salinity stress on crop plants: Improving salt tolerance through genetic and molecular dissection. Front. Plant Sci. 2023, 14, 1241736. [Google Scholar] [CrossRef]
- Munns, R.; Passioura, J.B.; Colmer, T.D.; Byrt, C.S. Osmotic adjustment and energy limitations to plant growth in saline soil. New Phytol. 2020, 225, 1091–1096. [Google Scholar] [CrossRef]
- Daszkowska-Golec, A. Arabidopsis seed germination under abiotic stress as a concert of action of phytohormones. OMICS J. Integr. Biol. 2011, 15, 763–774. [Google Scholar] [CrossRef]
- EL Sabagh, A.; Islam, M.S.; Skalicky, M.; Ali Raza, M.; Singh, K.; Anwar Hossain, M.; Hossain, A.; Mahboob, W.; Iqbal, M.A.; Ratnasekera, D.; et al. Salinity stress in wheat (Triticum aestivum L.) in the changing climate: Adaptation and management strategies. Front. Agron. 2021, 3, 661932. [Google Scholar] [CrossRef]
- Weiss, D.; Ori, N. Mechanisms of cross talk between gibberellin and other hormones. Plant Physiol. 2007, 144, 1240–1246. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Raihan, M.R.H.; Masud, A.A.C.; Rahman, K.; Nowroz, F.; Rahman, M.; Nahar, K.; Fujita, M. Regulation of reactive oxygen species and antioxidant defense in plants under salinity. Int. J. Mol. Sci. 2021, 22, 9326. [Google Scholar] [CrossRef] [PubMed]
- Hepler, P.K. Calcium: A central regulator of plant growth and development. Plant Cell 2005, 17, 2142–2155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.P.; Ma, C.X.; Sun, L.R.; Hao, F.S. Roles and mechanisms of Ca2+ in regulating primary root growth of plants. Plant Signal. Behav. 2020, 15, 1748283. [Google Scholar] [CrossRef] [PubMed]
- Bergonci, T.; Ribeiro, B.; Ceciliato, P.H.O.; Guerrero-Abad, J.C.; Silva-Filho, M.C.; Moura, D.S. Arabidopsis Thaliana RALF1 opposes brassinosteroid effects on root cell elongation and lateral root formation. J. Exp. Bot. 2014, 65, 2219–2230. [Google Scholar] [CrossRef]
- Wei, Z.; Li, J. Brassinosteroids regulate root growth, development, and symbiosis. Mol. Plant 2016, 9, 86–100. [Google Scholar] [CrossRef]
- Campos, W.F.; Dressano, K.; Ceciliato, P.H.; Guerrero-Abad, J.C.; Silva, A.L.; Fiori, C.S.; Canto, A.M.D.; Bergonci, T.; Claus, L.A.; Silva-Filho, M.C.; et al. Arabidopsis thaliana rapid alkalinization factor 1–mediated root growth inhibition is dependent on calmodulin-like protein 38. J. Biol. Chem. 2018, 293, 2159–2171. [Google Scholar] [CrossRef]
- Feng, W.; Kita, D.; Peaucelle, A.; Cartwright, H.N.; Doan, V.; Duan, Q.; Liu, M.-C.; Maman, J.; Steinhorst, L.; Schmitz-Thom, I.; et al. The feronia receptor kinase maintains cell-wall integrity during salt stress through Ca2+ signaling. Curr. Biol. 2018, 28, 666–675. [Google Scholar] [CrossRef]
- Cakmak, I.; Kirkby, E.A. Role of magnesium in carbon partitioning and alleviating photooxidative damage. Physiol. Plant. 2008, 133, 692–704. [Google Scholar] [CrossRef] [PubMed]
- Senbayram, M.; Gransee, A.; Wahle, V.; Thiel, H. Role of magnesium fertilisers in agriculture: Plant–soil continuum. Crop Pasture Sci. 2015, 66, 1219–1229. [Google Scholar] [CrossRef]
- Cakmak, I.; Yazici, A. Magnesium: A forgotten element in crop production. Better Crops 2010, 94, 23–25. [Google Scholar]
- Gerendás, J.; Führs, H. The significance of magnesium for crop quality. Plant Soil 2013, 368, 101–128. [Google Scholar] [CrossRef]
- Marschner, H. Preface to second edition. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Academic Press: San Diego, CA, USA, 2012; p. ix. ISBN 978-0-12-384905-2. [Google Scholar]
- White, P.J.; Broadley, M.R. Biofortification of crops with seven mineral elements often lacking in human diets—Iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol. 2009, 182, 49–84. [Google Scholar] [CrossRef] [PubMed]
- Gransee, A.; Führs, H. Magnesium mobility in soils as a challenge for soil and plant analysis, magnesium fertilization and root uptake under adverse growth conditions. Plant Soil 2013, 368, 5–21. [Google Scholar] [CrossRef]
- Wang, Z.; Hassan, M.U.; Nadeem, F.; Wu, L.; Zhang, F.; Li, X. Magnesium fertilization improves crop yield in most production systems: A meta-analysis. Front. Plant Sci. 2020, 10, 1727. [Google Scholar] [CrossRef]
- Biswas, B.; Dey, D.; Pal, S.; Kole, N. Integrative effect of magnesium sulphate on the growth of flowers and grain yield of paddy: A chemist’s perspective. Rasayan J. Chem. 2013, 6, 300–302. [Google Scholar]
- Narayan, O.P.; Kumar, P.; Yadav, B.; Dua, M.; Johri, A.K. Sulfur nutrition and its role in plant growth and development. Plant Signal. Behav. 2023, 18, 2030082. [Google Scholar] [CrossRef]
- Kopriva, S.; Malagoli, M.; Takahashi, H. Sulfur nutrition: Impacts on plant development, metabolism, and stress responses. J. Exp. Bot. 2019, 70, 4069–4073. [Google Scholar] [CrossRef]
- Dietz, K.-J.; Heilos, L. Carbon metabolism in spinach leaves as affected by leaf age and phosphorus and sulfur nutrition. Plant Physiol. 1990, 93, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Lopez-Baltazar, J.; Rachii, F.; Tremblay, N.; Gauthier, L.; Desjardins, Y.; Gosselin, A. Effect of sulphate on photosynthesis in greenhouse–grown tomato plants. Physiol. Plant. 2006, 96, 722–726. [Google Scholar] [CrossRef]
- SO42− Deprivation Has an Early Effect on the Content of Ribulose-1,5-Bisphosphate Carboxylase/Oxygenase and Photosynthesis in Young Leaves of Wheat. Rothamsted Research. Available online: https://repository.rothamsted.ac.uk/item/8w157/so42-deprivation-has-an-early-effect-on-the-content-of-ribulose-1-5-bisphosphate-carboxylase-oxygenase-and-photosynthesis-in-young-leaves-of-wheat (accessed on 15 July 2024).
- Lencioni, L.; Ranieri, A.; Fergola, S.; Soldatini, G.F. Photosynthesis and metabolic changes in leaves of rapeseed grown under long-term sulfate deprivation. J. Plant Nutr. 1997, 20, 405–415. [Google Scholar] [CrossRef]
- Blake-Kalff, M.M.A.; Harrison, K.R.; Hawkesford, M.J.; Zhao, F.J.; McGrath, S.P. Distribution of sulfur within oilseed rape leaves in response to sulfur deficiency during vegetative growth. Plant Physiol. 1998, 118, 1337–1344. [Google Scholar] [CrossRef]
- Resurreccion, A.P.; Makino, A.; Bennett, J.; Mae, T. Effects of sulfur nutrition on the growth and photosynthesis of rice. Soil Sci. Plant Nutr. 2001, 47, 611–620. [Google Scholar] [CrossRef]
- Place, S.; Kilcer, T.; Ketterings, Q.; Cherney, D.; Cherney, J. Sulfur for Field Crops. Cornell University Cooperative Extension, Agronomy Fact Sheet 34. Available online: http://nmsp.cals.cornell.edu/guidelines/factsheets.html (accessed on 19 July 2024).
- Jones, M.B. Sulfur availability indexes. In Sulfur in Agriculture; John Wiley & Sons, Ltd.: New York, NY, USA, 1986; pp. 549–566. ISBN 978-0-89118-220-7. [Google Scholar]
- Zhao, F.; Tausz, M.; De Kok, L.J. Role of sulfur for plant production in agricultural and natural ecosystems. In Sulfur Metabolism in Phototrophic Organisms; Hell, R., Dahl, C., Knaff, D., Leustek, T., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 417–435. ISBN 978-1-4020-6863-8. [Google Scholar]
- Burke, D.W.; Silbernagel, M.J.; Kraft, J.M.; Koehler, H.H. Registration of ‘Othello’ pinto bean. Crop Sci. 1995, 35, 943. [Google Scholar] [CrossRef]
- Singh, S.P.; Miklas, P.N.; Brick, M.A.; Schwartz, H.F.; Urrea, C.A.; Terán, H.; Centeno, C.; Ogg, B.; Otto, K.; Soler, A. Pinto bean cultivars Blackfoot, Nez Perce, and Twin Falls. J. Plant Regist. 2017, 11, 212–217. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, J.-G. Osmotic adjustment and plant adaptation to environmental changes related to drought and salinity. Environ. Rev. 2010, 18, 309–319. [Google Scholar] [CrossRef]
- Munns, R. Why measure osmotic adjustment? Funct. Plant Biol. 1988, 15, 717–726. [Google Scholar] [CrossRef]
- Hsiao, T.C.; Acevedo, E.; Fereres, E.; Henderson, D.W.; Monteith, J.L.; Weatherley, P.E. Water stress, growth and osmotic adjustment. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1997, 273, 479–500. [Google Scholar] [CrossRef]
- Stepien, P.; Johnson, G.N. Contrasting responses of photosynthesis to salt stress in the glycophyte Arabidopsis and the halophyte Thellungiella: Role of the plastid terminal oxidase as an alternative electron sink. Plant Physiol. 2009, 149, 1154–1165. [Google Scholar] [CrossRef] [PubMed]
- Keisham, M.; Mukherjee, S.; Bhatla, S.C. Mechanisms of sodium transport in plants—Progresses and challenges. Int. J. Mol. Sci. 2018, 19, 647. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.-K. Regulation of ion homeostasis under salt stress. Curr. Opin. Plant Biol. 2003, 6, 441–445. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Value |
|---|---|
| pH 1 | 8.1 |
| Electrical conductivity 2 (dS m−1) | 0.78 |
| OM 3 (%) | 1.6 |
| Nitrate-N 4 (mg kg−1) | 3.2 |
| Olsen-P 5 (mg kg−1) | 6 |
| Salt | Concentration (M) | EC 1 (dS m−1) |
|---|---|---|
| NaCl | 0.05 | 5.04 |
| CaCl2 | 0.10 | 5.01 |
| MgSO4·7H2O | 0.15 | 5.00 |
| Factors | Hypocotyl Length | Radicle Length |
|---|---|---|
| -------------------------cm------------------------- | ||
| Salt types | ||
| Control | 1.09 (0.04) 1 a | 2.47 (0.18) ab |
| NaCl | 0.85 (0.02) c | 1.61 (0.08) c |
| CaCl2 | 0.87 (0.02) c | 3.02 (0.17) a |
| MgSO4 | 0.98 (0.03) b | 2.38 (0.11) b |
| Variety | ||
| Cowboy | 0.92 (0.04) a | 2.67 (0.19) a |
| Gleam | 0.94 (0.02) a | 2.60 (0.18) a |
| Lumen | 0.76 (0.03) b | 2.17 (0.16) a |
| Mystic | 0.95 (0.03) a | 2.16 (0.16) a |
| Othello | 1.01 (0.03) a | 2.59 (0.16) a |
| SV6139 | 0.93 (0.05) a | 2.05 (0.17) a |
| Salt concentrations (M) | ||
| 0 | 1.09 (0.04) a | 2.47 (0.18) a |
| 0.05 | 0.94 (0.03) b | 2.77 (0.14) a |
| 0.1 | 0.92 (0.02) bc | 2.53 (0.14) a |
| 0.15 | 0.83 (0.03) c | 1.72 (0.09) b |
| Factors | Young Leaves | Old Leaves |
|---|---|---|
| Salt types (Control) | ||
| Cowboy | 31.9 (1.43) 1 ab | 34.9 (1.92) ab |
| Gleam | 22.3 (1.37) c | 34.3 (1.28) ab |
| Lumen | 24.6 (3.12) bc | 31.7 (4.16) b |
| Mystic | 29.1 (1.96) abc | 36.3 (1.61) ab |
| Othello | 35.3 (1.29) a | 41.5 (0.91) a |
| SV6139 | 28.1 (3.45) abc | 36.2 (2.32) ab |
| Salt type (NaCl) | ||
| Cowboy | 13.1 (3.36) b | 5.9 (3.96) b |
| Gleam | 16.0 (2.21) ab | 13.7 (4.78) ab |
| Lumen | 15.9 (1.720 ab | 7.9 (4.08) b |
| Mystic | 25.7 (4.07) a | 28.3 (3.94) a |
| Othello | 26.8 (2.36) a | 26.0 (2.47) a |
| SV6139 | 9.3 (2.81) b | 12.4 (4.61) ab |
| Salt type (CaCl2) | ||
| Cowboy | 11.4 (3.16) a | 3.0 (3.02) c |
| Gleam | 22.4 (3.15) a | 5.6 (3.76) bc |
| Lumen | 16.1 (3.34) a | 11.3 (4.12) abc |
| Mystic | 22.5 (2.57) a | 20.8 (4.82) ab |
| Othello | 24.0 (3.54) a | 24.8 (3.17) a |
| SV6139 | 17.5 (4.60) a | 13.3 (4.93) abc |
| Salt type (MgSO4) | ||
| Cowboy | 33.1 (1.13) ab | 42.4 (1.15) a |
| Gleam | 29.6 (2.52) b | 35.1 (4.14) a |
| Lumen | 31.0 (1.88) b | 39.9 (1.77) a |
| Mystic | 31.8 (2.68) b | 40.2 (1.56) a |
| Othello | 40.4 (0.95) a | 44.3 (1.47) a |
| SV6139 | 37.5 (2.05) ab | 39.4 (4.64) a |
| Factors | Flower Number 30 Days after Planting | Flower Number 38 Days after Planting | Pod Number 35 Days after Planting | Pod Number 47 Days after Planting |
|---|---|---|---|---|
| Salt types | ||||
| Control | 0.2 (0.09) 1 a | 1.3 (0.16) a | 0.2 (0.10) b | 2.0 (0.21) b |
| NaCl | 0.2 (0.07) a | 0.7 (0.20) b | 0.2 (0.10) b | 0.3 (0.10) c |
| CaCl2 | 0.1 (0.05) a | 1.0 (0.18) ab | 0.3 (0.12) ab | 1.0 (0.21) c |
| MgSO4 | 0.3 (0.10) a | 1.4 (0.18) a | 1.0 (0.20) a | 3.2 (0.28) a |
| Variety | ||||
| Cowboy | 0.0 (0.00) b | 0.4 (0.12) b | 0.0 (0.00) b | 1.1 (0.20) b |
| Gleam | 0.0 (0.00) b | 1.5 (0.21) a | 0.0 (0.00) b | 1.6 (0.32) b |
| Lumen | 0.0 (0.00) b | 0.4 (0.10) b | 0.0 (0.00) b | 1.0 (0.24) b |
| Mystic | 0.0 (0.00) b | 1.1 (0.24) ab | 0.0 (0.00) b | 1.4 (0.34) b |
| Othello | 1.1 (0.20) a | 2.0 (0.30) a | 2.0 (0.30) a | 3.0 (0.33) a |
| SV6139 | 0.0 (0.00) b | 1.4 (0.24) a | 0.0 (0.00) b | 1.6 (0.31) b |
| Factors | ECe (dS m−1) |
|---|---|
| Salt type (Control) | |
| Cowboy | 1.06 (0.05) 1 ab |
| Gleam | 1.04 (0.10) ab |
| Lumen | 0.90 (0.05) b |
| Mystic | 1.00 (0.06) ab |
| Othello | 0.85 (0.06) b |
| SV6139 | 1.32 (0.12) a |
| Salt type (NaCl) | |
| Cowboy | 4.06 (0.33) a |
| Gleam | 5.36 (0.39) a |
| Lumen | 5.36 (0.41) a |
| Mystic | 5.10 (0.25) a |
| Othello | 4.69 (0.45) a |
| SV6139 | 5.35 (0.56) a |
| Salt type (CaCl2) | |
| Cowboy | 3.71 (0.25) b |
| Gleam | 5.96 (0.41) a |
| Lumen | 4.44 (0.47) ab |
| Mystic | 4.95 (0.41) ab |
| Othello | 4.60 (0.49) ab |
| SV6139 | 5.14 (0.69) ab |
| Salt type (MgSO4) | |
| Cowboy | 4.00 (0.11) a |
| Gleam | 3.81 (0.26) ab |
| Lumen | 3.21 (0.22) b |
| Mystic | 4.08 (0.16) a |
| Othello | 3.86 (0.15) ab |
| SV6139 | 4.05 (0.10) a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paul, W.S.; Afkairin, A.; Andales, A.A.; Qian, Y.; Davis, J.G. Assessing Salinity Tolerance in Pinto Bean Varieties: Implications for Sustainable Agriculture. Agronomy 2024, 14, 1877. https://doi.org/10.3390/agronomy14091877
Paul WS, Afkairin A, Andales AA, Qian Y, Davis JG. Assessing Salinity Tolerance in Pinto Bean Varieties: Implications for Sustainable Agriculture. Agronomy. 2024; 14(9):1877. https://doi.org/10.3390/agronomy14091877
Chicago/Turabian StylePaul, Winie S., Antisar Afkairin, Allan A. Andales, Yaling Qian, and Jessica G. Davis. 2024. "Assessing Salinity Tolerance in Pinto Bean Varieties: Implications for Sustainable Agriculture" Agronomy 14, no. 9: 1877. https://doi.org/10.3390/agronomy14091877





