Nocturnal LED Supplemental Lighting Improves Quality of Tomato Seedlings by Increasing Biomass Accumulation in a Controlled Environment
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Experimental Design
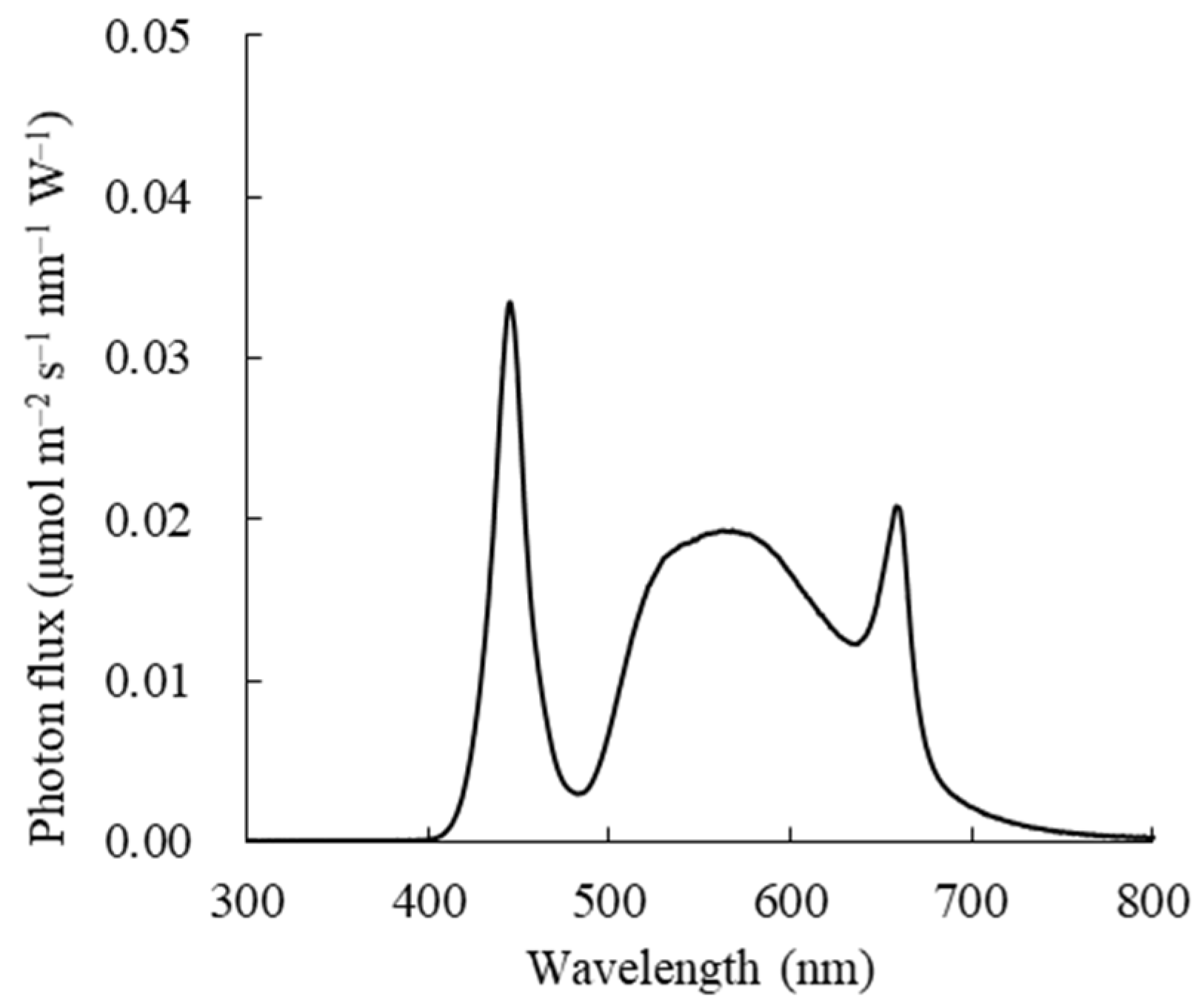
2.3. Measurement Indicators and Methods
2.3.1. Measurement of Growth Morphology
2.3.2. Measurement of Photosynthetic Capacity
2.3.3. Measurement of Root Activity
2.3.4. Measurement of Chlorophyll Fluorescence Induction Characteristics
2.3.5. Measurement of Proline
2.3.6. Measurement of MDA
2.3.7. Measurement of Biomass Accumulation
2.3.8. Measurement of Health Index
2.4. Statistical Analysis
3. Results
3.1. Growth
3.2. Photosynthetic Capacity
3.2.1. Photosynthetic Pigments
3.2.2. Gas Exchange
3.3. Resistance Physiology
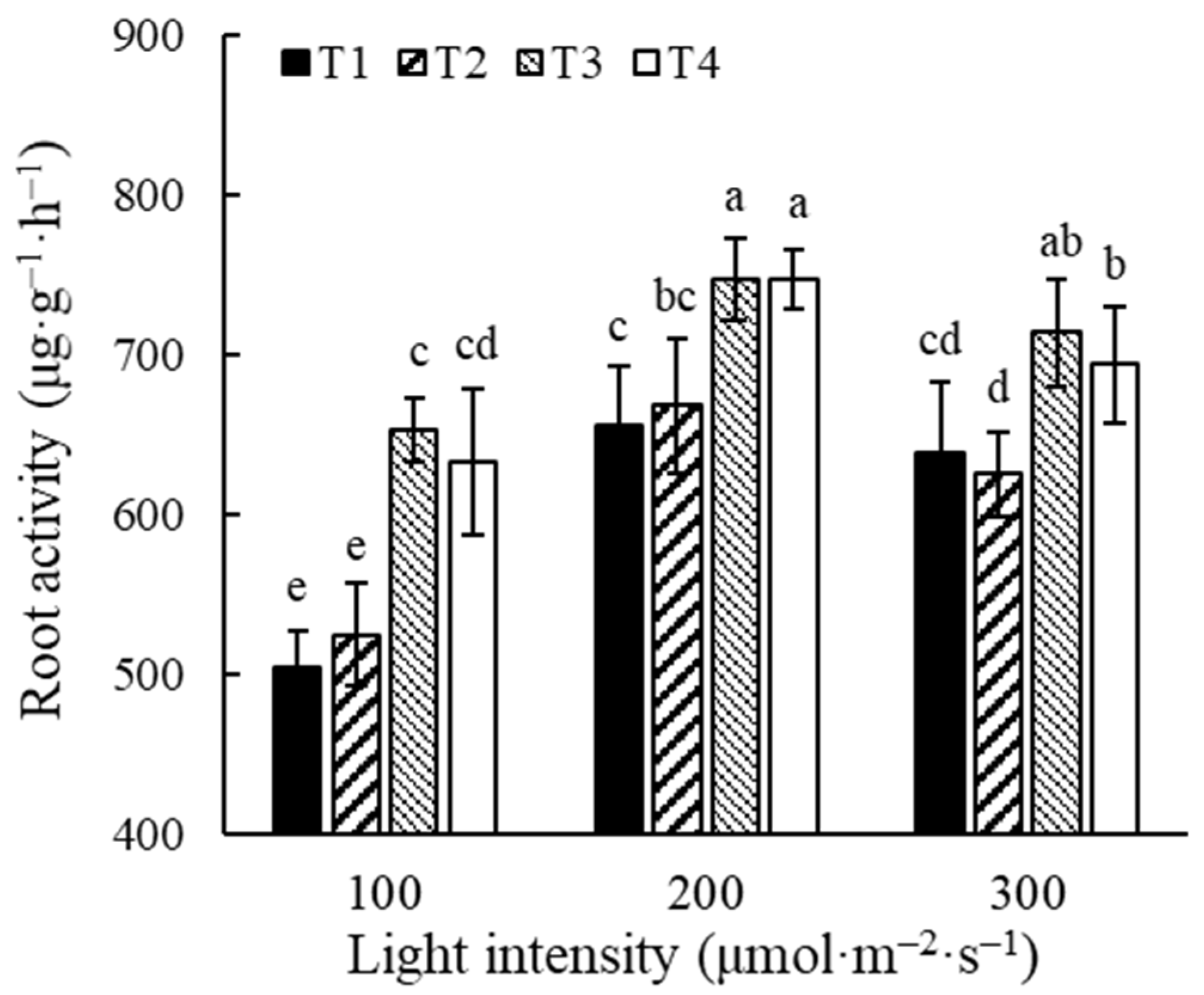
3.3.1. Root Activity
3.3.2. Chlorophyll Fluorescence
3.3.3. Stress Resistance
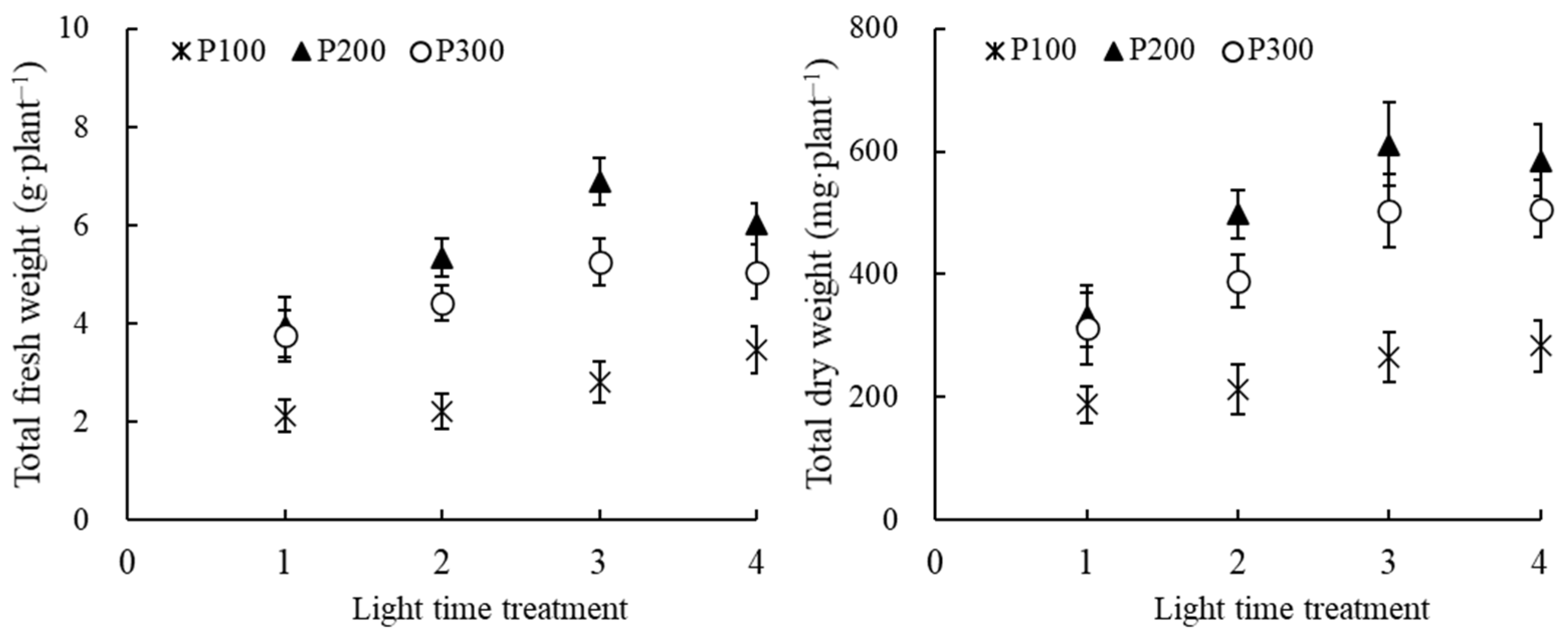
3.4. Biomass Accumulation
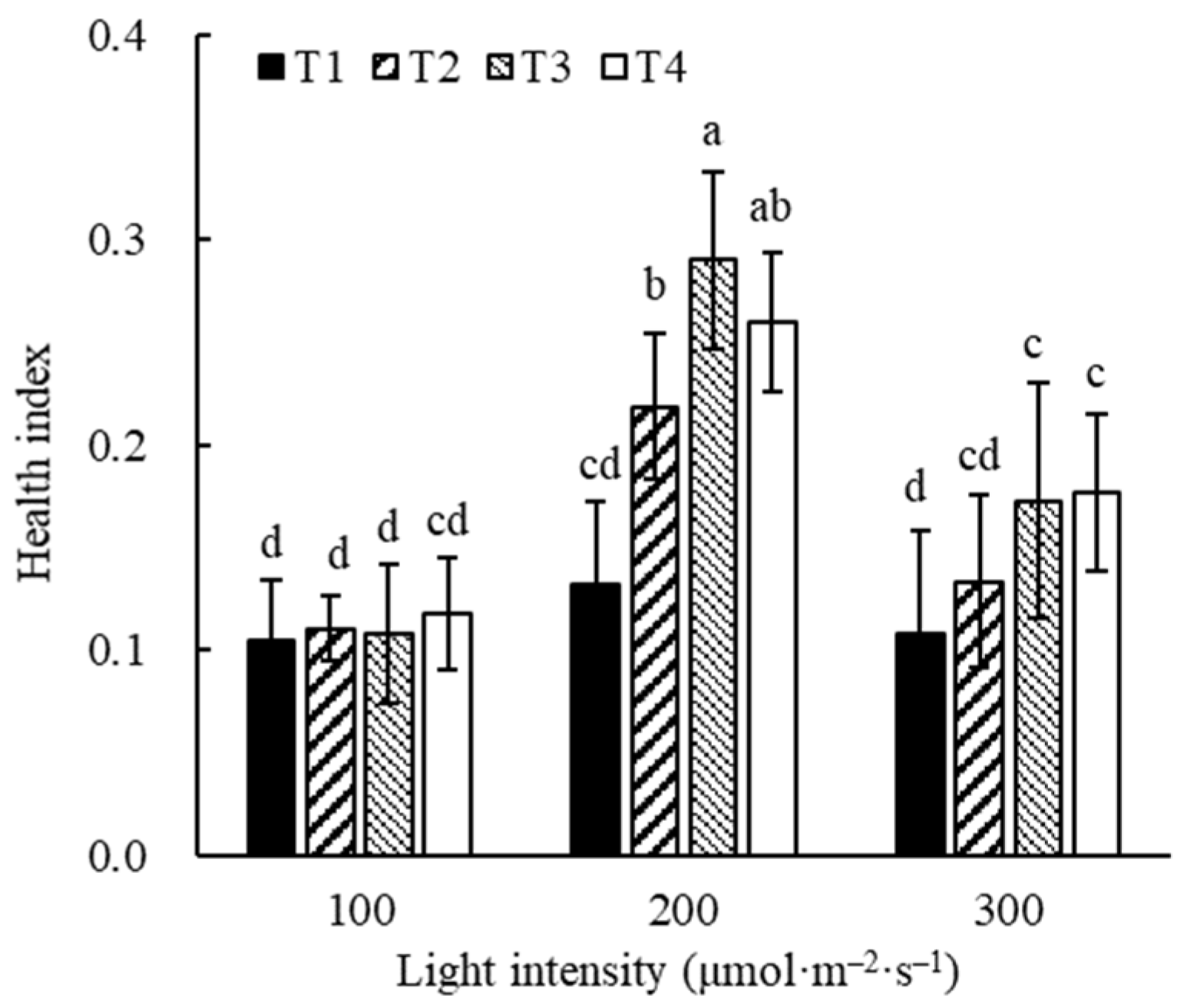
3.5. Seedling Quality
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sun, Y.; He, J.; Wei, F.; Yang, W. Evaluation on the development and international competitiveness of China’s tomato industry during the 13th Five-Year Plan period. China Cucurbits Veg. 2023, 36, 112–116. (In Chinese) [Google Scholar]
- Zhou, M.; Sun, H.; Xu, X.; Yang, J.; Wang, G.; Wei, Z.; Xu, T.; Yin, J. Study on the method and mechanism of seedling picking for pepper (Capsicum annuum L.) plug seedlings. Agriculture 2024, 14, 11. [Google Scholar] [CrossRef]
- Xu, X.; Yang, F.; Song, J.; Zhang, R.; Cai, W. Does the daily light integral influence the sowing density of tomato plug seedlings in a controlled environment? Horticulturae 2024, 10, 730. [Google Scholar] [CrossRef]
- Li, Q.; Kubota, C. Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce. Environ. Exp. Bot. 2009, 67, 59–64. [Google Scholar] [CrossRef]
- Neo, D.; Ong, M.; Lee, Y.; Teo, E.; Ong, Q.; Tanoto, H.; Xu, J.; Ong, K.; Suresh, V. Shaping and tuning lighting conditions in controlled environment agriculture: A Review. ACS Agric. Sci. Technol. 2022, 2, 3–16. [Google Scholar] [CrossRef]
- Han, L.; Mo, M.; Gao, Y.; Ma, H.; Xiang, D.; Ma, G.; Mao, H. Effects of new compounds into substrates on seedling qualities for efficient transplanting. Agronomy 2022, 12, 983. [Google Scholar] [CrossRef]
- Kozai, T.; Niu, G. Plant Factory: Chapter 4: Plant Factory as a Resource–Efficient Closed Plant Production System; Academic Press: Cambridge, MA, USA, 2016; pp. 69–90. [Google Scholar]
- Fylladitakis, E. Controlled LED lighting for horticulture: A Review. Open J. Appl. Sci. 2023, 13, 175–188. [Google Scholar] [CrossRef]
- Song, J.; Fan, Y.; Li, X.; Li, Y.; Mao, H.; Zuo, Z.; Zou, Z. Effects of daily light integral on tomato (Solanum lycopersicon L.) grafting and quality in a controlled environment. Int. J. Agric. Biol. Eng. 2022, 15, 44–50. [Google Scholar] [CrossRef]
- Kucharewicz, W.; Distelfeld, A.; Bilger, W.; Müller, M.; Munné-Bosch, S.; Hensel, G.; Krupinska, K. Acceleration of leaf senescence is slowed down in transgenic barley plants deficient in the DNA/RNA-binding protein WHIRLY1. J. Exp. Bot. 2017, 68, 983–996. [Google Scholar] [CrossRef]
- Li, Q.; Chai, L.; Tong, N.; Yu, H.; Jiang, W. Potential carbohydrate regulation mechanism underlying starvation-induced abscission of tomato flower. Int. J. Mol. Sci. 2022, 23, 1952. [Google Scholar] [CrossRef]
- Paradiso, R.; Proietti, S. Light-quality manipulation to control plant growth and photomorphogenesis in greenhouse horticulture: The state of the art and the opportunities of modern LED systems. J. Plant Growth Regul. 2022, 41, 742–780. [Google Scholar] [CrossRef]
- Gupta, S.; Agarwal, A. Light Emitting Diodes for Agriculture: Artificial Lighting System for Plant Growth and Development: Chronological Advancement, Working Principles, and Comparative Assessment; Springer: Singapore, 2017; pp. 1–25. [Google Scholar]
- Sena, S.; Kumari, S.; Kumar, V.; Husen, A. Light emitting diode (LED) lights for the improvement of plant performance and production: A comprehensive review. Curr. Res. Biotechnol. 2024, 7, 100184. [Google Scholar] [CrossRef]
- Shibaeva, T.; Sherudilo, E.; Rubaeva, A.; Titov, A. Continuous LED lighting enhances yield and nutritional value of four genotypes of Brassicaceae microgreens. Plants 2022, 11, 176. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yang, M.; Cheng, F.; Liu, S.; Liang, Y. Effects of LED photoperiods and light qualities on in vitro growth and chlorophyll fluorescence of Cunninghamia lanceolata. BMC Plant Biol. 2020, 20, 269. [Google Scholar] [CrossRef]
- Hamedalla, A.; Ali, M.; Ali, W.; Ahmed, M.; Kaseb, M.; Kalaji, H.; Gajc-Wolska, J.; Yousef, A. Increasing the performance of cucumber (Cucumis sativus L.) seedlings by LED illumination. Scientific. Rep. 2022, 12, 00852. [Google Scholar] [CrossRef]
- Li, Y.; Xin, G.; Wei, M.; Shi, Q.; Yang, F.; Wang, X. Carbohydrate accumulation and sucrose metabolism responses in tomato seedling leaves when subjected to different light qualities. Sci. Hortic. 2017, 225, 490–497. [Google Scholar] [CrossRef]
- Modarelli, G.; Paradiso, R.; Arena, C.; De Pascale, S.; Van Labeke, M. High light intensity from blue-red LEDs enhance photosynthetic performance, plant growth, and optical properties of red lettuce in controlled environment. Horticulturae 2022, 8, 114. [Google Scholar] [CrossRef]
- Li, J.; Guo, X.; Zhang, S.; Zhang, Y.; Chen, L.; Zheng, W.; Xue, X. Effects of light quality on growth, nutritional characteristics, and antioxidant properties of winter wheat seedlings (Triticum aestivum L.). Front. Plant Sci. 2022, 13, 978468. [Google Scholar] [CrossRef]
- Cao, K.; Cuil, R.; Ye, L.; Zhou, X.; Bao, E.; Zhao, H.; Zou, Z. Effects of red light night break treatment on growth and flowering of tomato plants. Front. Plant Sci. 2016, 7, 00527. [Google Scholar] [CrossRef]
- Wang, X.; Xu, X.; Cui, J. The importance of blue light for leaf area expansion, development of photosynthetic apparatus, and chloroplast ultrastructure of Cucumis sativus grown under weak light. Photosynth. Res. 2015, 53, 213–222. [Google Scholar] [CrossRef]
- Li, X.; Wang, C.; Liu, J.; Guo, Y.; Cheng, F.; Yang, Y.; Yan, Z. Long supplementary light duration under same daily light integral provided by white plus blue light-emitting diodes improves quality of greenhouse-grown tomato seedlings. Hortic. Environ. Biotechnol. 2023, 64, 963–975. [Google Scholar] [CrossRef]
- Ying, Q.; Kong, Y.; Zheng, Y. Applying blue light alone, or in combination with far-red light, during nighttime increases elongation without compromising yield and quality of indoor-grown microgreens. HortScience 2020, 55, 876–881. [Google Scholar] [CrossRef]
- Hou, M.; Ni, J.; Mao, H. Effects of airflow disturbance on the content of biochemical components and mechanical properties of cucumber seedling stems. Agronomy 2023, 13, 1125. [Google Scholar] [CrossRef]
- Paucek, I.; Appolloni, E.; Pennisi, G.; Quaini, S.; Gianquinto, G.; Orsini, F. LED lighting systems for horticulture: Business growth and global distribution. Sustainability 2020, 12, 7516. [Google Scholar] [CrossRef]
- Stamford, J.; Stevens, J.; Mullineaux, P.; Lawson, T. LED Lighting: A grower’s guide to light spectra. HortScience 2023, 58, 180–196. [Google Scholar] [CrossRef]
- Wang, S.; Fang, H.; Xie, J.; Wu, Y.; Tang, Z.; Liu, Z.; Lv, J.; Yu, J. Physiological responses of cucumber seedlings to different supplemental light duration of red and blue LED. Front. Plant Sci. 2021, 12, 709313. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Wang, C.; Wang, L.; Wang, G.; Yang, Y. The combinations of white, blue, and UV-A light provided by supplementary Light-Emitting Diodes promoted the quality of greenhouse-grown cucumber seedlings. Agriculture 2022, 12, 1593. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Wu, Z.; Yuan, S.; Li, W.; Zhang, P. Effects of different prolonged light durations on survival, growth and physiology of the Eelgrass Zostera marina. Front. Environ. Sci. 2022, 10, 893377. [Google Scholar] [CrossRef]
- Snowden, M.; Cope, K.; Bugbee, B. Sensitivity of seven diverse species to blue and green light: Interactions with photon flux. PLoS ONE 2016, 11, e0163121. [Google Scholar] [CrossRef]
- Brian, E.; Paul, C.; James, T. Night interruption lighting equally effective as daylength extension in retaining the vegetative state of Cannabis mother plants. Crop Forage Turfgrass Manag. 2020, 6, e20001. [Google Scholar]
- Tewolde, F.; Lu, N.; Shiinas, K.; Maruo, T.; Takagaki, M.; Kozai, T.; Yamori, W. Nighttime supplemental LED inter-lighting improves growth and yield of single-truss tomatoes by enhancing photosynthesis in both winter and summer. Front. Plant Sci. 2016, 7, 00448. [Google Scholar] [CrossRef]
- Yu, H.; Liu, P.; Xu, J.; Wang, T.; Lu, T.; Gao, J.; Li, Q.; Jiang, W. The effects of different durations of night-time supplementary lighting on the growth, yield, quality and economic returns of tomato. Plants 2024, 13, 1516. [Google Scholar] [CrossRef]
- Gao, W.; He, D.; Ji, F.; Zhang, S.; Zheng, J. Effects of daily light integral and LED spectrum on growth and nutritional quality of hydroponic spinach. Agronomy 2020, 10, 1082. [Google Scholar] [CrossRef]
- Middelberg, A.; Zhang, J.; Xia, X. An optimal control model for load shifting –With application in the energy management of a colliery. Appl. Energy 2009, 86, 1266–1273. [Google Scholar] [CrossRef]
- Liu, Y.; Ren, X.; Jeong, B. Carbon dioxide enrichment combined with supplemental light improve growth and quality of plug seedlings of astragalus membranaceus bunge and Codonopsis lanceolata benth. et hook. f. Agronomy 2019, 9, 715. [Google Scholar] [CrossRef]
- Ma, G.; Mao, H.; Bu, Q.; Han, L.; Shabbir, A.; Gao, F. Effect of Compound Biochar Substrate on the Root Growth of Cucumber Plug Seedlings. Agronomy 2020, 10, 1080. [Google Scholar] [CrossRef]
- Wang, J.; He, D.; Song, J.; Dou, H.; Du, W. Non-destructive measurement of chlorophyll in tomato leaves using spectral transmittance. Int. J. Agric. Biol. Eng. 2015, 8, 73–78. [Google Scholar]
- Garland, K.; Burnett, S.; Day, M.; Iersel, M. Influence of substrate water content and daily light integral on photosynthesis, water use efficiency, and morphology of heuchera americana. J. Am. Soc. Hortic. Sci. 2012, 137, 57–67. [Google Scholar] [CrossRef]
- Zeeshan, M.; Prasad, S. Differential response of growth, photosynthetic pigments and antioxidant enzymes to UV-b radiation in tomato (Solanum lycopersicum L.) seedlings. Fresenius Environ. Bull. 2016, 25, 4192–4196. [Google Scholar]
- Yang, F.; Wang, Y.; Miao, L. Comparative physiological and proteomic responses to drought stress in two poplar species originating from different altitudes. Physiol. Plant. 2010, 139, 388–400. [Google Scholar] [CrossRef]
- Ma, X.; Liu, Y.; Huang, S.; Zhang, C.; Sun, K.; Wang, Y.; Shen, S.; Sun, Z.; Pu, F.; Zhang, T. Effects of different LED illumination intensity at night on the growth and development of tomato seedlings. Plant Physiol. J. 2022, 58, 2411–2420. (In Chinese) [Google Scholar]
- Gautam, B.; Dubey, R.; Kaur, N.; Choudhary, O. Growth response of indoor ornamental plant species to various artificial light intensities (LED) in an indoor vertical garden. Plant Arch. 2021, 21, 695–700. [Google Scholar] [CrossRef]
- Matsuda, R.; Yamano, T.; Murakami, K.; Fujiwara, K. Effects of spectral distribution and photosynthetic photon flux density for overnight LED light irradiation on tomato seedling growth and leaf injury. Sci. Hortic. 2016, 198, 363–369. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, Z.; Cui, J.; Gu, A.; Guo, Y. Effects of light quality on the growth and chloroplast ultrastructure of tomato and lettuce seedlings. Chin. J. Appl. Ecol. 2010, 21, 959–965. [Google Scholar]
- Knop, E.; Zoller, L.; Ryser, R.; Gerpe, C.; Hörler, M.; Fontaine, C. Artificial light at night as a new threat to pollination. Nature 2017, 10, 206–209. [Google Scholar] [CrossRef]
- Dannehl, D.; Schwend, T.; Veit, D.; Schmidt, U. Increase of yield, lycopene, and lutein content in tomatoes grown under continuous PAR spectrum LED lighting. Front. Plant Sci. 2021, 12, 611236. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, Q.; Guo, Y.; Guo, D.; Shah, G.; Liu, H.; Mao, A. Stem-swelling and photosynthate partitioning in stem mustard are regulated by photoperiod and plant hormones. Environ. Exp. Bot. 2008, 62, 160–167. [Google Scholar] [CrossRef]
- Ali, B.; Khandaker, L.; Oba, S. Comparative study on functional components, antioxidant activity and color parameters of selected colored leafy vegetables as affected by photoperiods. J. Food Agric. Environ. 2009, 37, 392–398. [Google Scholar]
- Fanwoua, J.; Vercambre, G.; Buck-Sorlin, G.; Dieleman, J.; Visser, P.; Génard, M. Supplemental LED lighting affects the dynamics of tomato fruit growth and composition. Sci. Hortic. 2019, 256, 108571. [Google Scholar] [CrossRef]
- Kwak, M.; Je, S.; Cheng, H.; Seo, S.; Park, J.; Baek, S.; Khaine, I.; Lee, T.; Jang, J.; Li, Y.; et al. Night light-adaptation strategies for photosynthetic apparatus in yellow-poplar (Liriodendron tulipifera L.) exposed to artificial night lighting. Forests 2018, 9, 74. [Google Scholar] [CrossRef]
- Li, H.; Tang, C.; Xu, Z.; Liu, X.; Han, X. Effects of different light sources on the growth of non-heading Chinese cabbage. J. Agric. Sci. 2012, 4, 262–273. [Google Scholar]
- Hogewoning, S.; Wientjes, E.; Douwstra, P.; Trouwborst, G.; Ieperen, W.; Croce, R.; Harbinson, J. Photosynthetic quantum yield dynamics: From photosystems to leaves. Plant Cell 2012, 24, 1921–1935. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; He, D.; Niu, G.; Yan, Z.; Song, J. Effects of environment lighting on the growth, photosynthesis, and quality of hydroponic lettuce in a plant factory. Int. J. Agric. Biol. Eng. 2018, 11, 33–40. [Google Scholar] [CrossRef]
- Yan, Z.; He, D.; Niu, G.; Zhou, Q.; Qu, Y. Growth, nutritional quality, and energy use efficiency of hydroponic lettuce as influenced by daily light integrals exposed to white versus white plus red light-emitting diodes. Hortscience 2019, 54, 1737–1744. [Google Scholar] [CrossRef]
- Wahidin, S.; Idris, A.; Shaleh, S. The influence of light intensity and photoperiod on the growth and lipid content of microalgae Nannochloropsis sp. Bioresour. Technol. 2013, 129, 7–11. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, X.; Liu, Y.; Wang, Q.; Li, C.; Zhang, C.; Jiang, J. LED lighting periods at night affects the growth and development of tomato seedlings. Chin. Cucurbits Veg. 2022, 35, 79–85. [Google Scholar]
- Ighodaro, O.; Akinloye, O. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Zhang, C.; Akhlaq, M.; Yan, H.; Ni, Y.; Liang, S.; Zhou, J.; Xue, R.; Li, M.; Adnan, R.; Li, J. Chlorophyll fluorescence parameter as a predictor of tomato growth and yield under CO2 enrichment in protective cultivation. Agric. Water Manag. 2023, 284, 108333. [Google Scholar] [CrossRef]
- Fan, X.; Xu, Z.; Liu, X.; Tang, C.; Wang, L.; Han, X. Effects of light intensity on the growth and leaf development of young tomato plants grown under a combination of red and blue light. Sci. Hortic. 2013, 153, 50–55. [Google Scholar] [CrossRef]
- Giavi, S.; Fontaine, C.; Knop, E. Impact of artificial light at night on diurnal plant-pollinator interactions. Nat. Commun. 2021, 12, 1690. [Google Scholar] [CrossRef]
- Ning, Y.; Deng, H.; Li, Q.; Mi, Q.; Han, B.; Ai, X. Effects of red and blue light quality on the metabolites and key enzyme activities of carbon-nitrogen metabolism in Celer. Plant Physiol. J. 2015, 51, 112–118. [Google Scholar]
- Hernández, R.; Kubota, C. Growth and morphological response of cucumber seedlings to supplemental red and blue photon flux ratio under varied solar daily light integrals. Sci. Hortic. 2014, 173, 92–99. [Google Scholar] [CrossRef]
- Li, H.; Tang, C.; Xu, Z. The effects of different light qualities on rapeseed (Brassica napus L.) plantlet growth and morphogenesis in vitro. Sci. Hortic. 2014, 150, 117–124. [Google Scholar] [CrossRef]
- Kalaitzoglou, P.; Taylor, C.; Calders, K.; Hogervorst, M.; Ieperen, W.; Harbinson, J.; Visser, P.; Nicole, C.; Marcelis, L. Unraveling the effects of blue light in an artificial solar background light on growth of tomato plants. Environ. Exp. Bot. 2021, 184, 104377. [Google Scholar] [CrossRef]


| Treatment | PPFD | Light Time | Light Period-of-Time |
|---|---|---|---|
| (μmol·m−2·s−1) | (h) | ||
| CK | 0 | 0 | -- |
| P1T1 | 100 | 1 | 23:30–00:30 |
| P1T2 | 1 | 21:40–22:10 and 01:50–02:20 | |
| P1T3 | 2 | 23:00–01:00 | |
| P1T4 | 2 | 21:20–22:20 and 01:40–02:40 | |
| P2T1 | 200 | 1 | 23:30–00:30 |
| P2T2 | 1 | 21:40–22:10 and 01:50–02:20 | |
| P2T3 | 2 | 23:00–01:00 | |
| P2T4 | 2 | 21:20–22:20 and 01:40–02:40 | |
| P3T1 | 300 | 1 | 23:30–00:30 |
| P3T2 | 1 | 21:40–22:10 and 01:50–02:20 | |
| P3T3 | 2 | 23:00–01:00 | |
| P3T4 | 2 | 21:20–22:20 and 01:40–02:40 |
| Treatment | Plant Height | Stem Diameter | Leaf Number | Leaf Area |
|---|---|---|---|---|
| (cm) | (mm) | (piece) | (cm2) | |
| CK | 6.9 ± 0.8 e | 3.09 ± 0.23 c | 3.5 ± 0.3 d | 51.4 ± 11.2 d |
| P1T1 | 6.1 ± 0.4 e | 3.40 ± 0.53 c | 3.6 ± 0.4 cd | 62.3 ± 10.7 c |
| P1T2 | 6.3 ± 0.3 e | 3.27 ± 0.23 c | 3.9 ± 0.3 c | 66.8 ± 14.2 c |
| P1T3 | 8.2 ± 0.5 d | 3.33 ± 0.17 c | 3.7 ± 0.4 cd | 72.0 ± 8.9 bc |
| P1T4 | 8.6 ± 0.4 c | 3.58 ± 0.28 bc | 3.6 ± 0.3 cd | 86.9 ± 12.8 b |
| P2T1 | 9.3 ± 0.8 bc | 3.69 ± 0.30 b | 4.1 ± 0.2 bc | 119.1 ± 24.6 ab |
| P2T2 | 9.3 ± 0.6 bc | 4.08 ± 0.47 ab | 4.3 ± 0.4 bc | 157.6 ± 36.9 ab |
| P2T3 | 9.6 ± 0.8 b | 4.55 ± 0.62 a | 4.6 ± 0.4 ab | 186.9 ± 73.4 a |
| P2T4 | 9.5 ± 0.7 b | 4.22 ± 0.52 ab | 4.5 ± 0.4 b | 187.6 ± 52.3 a |
| P3T1 | 10.6 ± 1.2 ab | 3.65 ± 0.47 bc | 4.2 ± 0.6 bc | 104.0 ± 40.8 bc |
| P3T2 | 10.4 ± 0.6 a | 3.57 ± 0.38 bc | 4.5 ± 0.4 b | 110.7 ± 39.5 b |
| P3T3 | 10.9 ± 0.8 a | 3.74 ± 0.31 b | 4.9 ± 0.3 a | 107.4 ± 22.6 b |
| P3T4 | 10.6 ± 0.7 a | 3.69 ± 0.39 bc | 4.8 ± 0.3 ab | 101.9 ± 35.7 b |
| Treatment | Total Chlorophyll Content | Chlorophyll a/b | SPAD Values |
|---|---|---|---|
| (mg·g−1) | |||
| CK | 2.44 ± 0.16 c | 2.43 ± 0.13 d | 35.8 ± 1.6 f |
| P1T1 | 2.64 ± 0.22 bc | 2.52 ± 0.16 cd | 38.5 ± 1.4 e |
| P1T2 | 2.75 ± 0.18 b | 2.49 ± 0.17 cd | 41.6 ± 1.4 d |
| P1T3 | 2.90 ± 0.31 b | 2.76 ± 0.23 bc | 44.3 ± 1.6 c |
| P1T4 | 2.80 ± 0.26 b | 2.88 ± 0.21 ab | 45.3 ± 1.2 bc |
| P2T1 | 2.71 ± 0.19 b | 2.76 ± 0.17 bc | 41.8 ± 1.1 d |
| P2T2 | 2.84 ± 0.24 b | 2.83 ± 0.16 b | 44.9 ± 1.3 bc |
| P2T3 | 3.03 ± 0.24 ab | 2.96 ± 0.21 ab | 48.7 ± 1.7 a |
| P2T4 | 3.15 ± 0.17 a | 3.03 ± 0.17 a | 48.6 ± 1.4 a |
| P3T1 | 2.85 ± 0.22 b | 2.75 ± 0.19 bc | 41.6 ± 1.6 d |
| P3T2 | 2.88 ± 0.18 b | 2.59 ± 0.16 c | 45.8 ± 1.7 bc |
| P3T3 | 2.89 ± 0.16 b | 2.56 ± 0.11 c | 46.2 ± 1.2 b |
| P3T4 | 2.81 ± 0.16 b | 2.52 ± 0.09 cd | 46.5 ± 1.4 b |
| Treatment | Net Photosynthetic Rate | Stomatal Conductivity | Intercellular CO2 Concentration | Transpiration Rate |
|---|---|---|---|---|
| (μmol·m−2·s−1) | (mol·m−2·s−1) | (μmol·mol−1) | (mmol·m−2·s−1) | |
| CK | 15.6 ± 0.4 f | 0.295 ± 0.036 d | 722 ± 20 a | 2.12 ± 0.19 c |
| P1T1 | 18.2 ± 1.2 e | 0.321 ± 0.042 cd | 702 ± 16 ab | 2.39 ± 0.07 b |
| P1T2 | 18.6 ± 1.3 e | 0.347 ± 0.038 c | 693 ± 33 bc | 2.41 ± 0.14 b |
| P1T3 | 20.6 ± 0.8 cd | 0.404 ± 0.043 bc | 676 ± 19 bc | 2.62 ± 0.26 ab |
| P1T4 | 21.8 ± 0.9 bc | 0.475 ± 0.047 a | 669 ± 34 bc | 2.69 ± 0.24 ab |
| P2T1 | 19.5 ± 0.7 d | 0.382 ± 0.054 bc | 688 ± 22 bc | 2.38 ± 0.16 b |
| P2T2 | 21.7 ± 1.2 bc | 0.429 ± 0.059 b | 672 ± 28 bc | 2.47 ± 0.19 b |
| P2T3 | 24.9 ± 1.3 a | 0.449 ± 0.028 ab | 657 ± 14 c | 2.69 ± 0.14 ab |
| P2T4 | 25.2 ± 1.3 a | 0.463 ± 0.047 ab | 641 ± 18 c | 2.82 ± 0.24 a |
| P3T1 | 18.8 ± 0.4 de | 0.336 ± 0.039 c | 701 ± 36 b | 2.36 ± 0.22 b |
| P3T2 | 19.9 ± 0.9 cd | 0.374 ± 0.052 bc | 688 ± 34 bc | 2.48 ± 0.24 b |
| P3T3 | 22.7 ± 0.9 b | 0.424 ± 0.022 b | 672 ± 18 bc | 2.67 ± 0.18 ab |
| P3T4 | 21.4 ± 1.3 c | 0.416 ± 0.037 b | 669 ± 25 bc | 2.66 ± 0.23 ab |
| Treatment | Fv/Fm | ψo | φEo | PI_ABS |
|---|---|---|---|---|
| CK | 0.863 ± 0.023 a | 0.663 ± 0.006 a | 0.567 ± 0.012 b | 4.89 ± 1.12 ab |
| P1T1 | 0.857 ± 0.016 a | 0.653 ± 0.035 ab | 0.580 ± 0.030 ab | 5.83 ± 1.11 a |
| P1T2 | 0.861 ± 0.021 a | 0.656 ± 0.034 ab | 0.583 ± 0.012 a | 5.75 ± 0.35 a |
| P1T3 | 0.860 ± 0.017 a | 0.653 ± 0.021 ab | 0.563 ± 0.006 b | 4.85 ± 0.21 ab |
| P1T4 | 0.864 ± 0.029 a | 0.656 ± 0.019 a | 0.567 ± 0.023 b | 4.69 ± 0.75 b |
| P2T1 | 0.853 ± 0.021 a | 0.660 ± 0.017 a | 0.560 ± 0.026 b | 2.79 ± 1.66 cd |
| P2T2 | 0.853 ± 0.031 a | 0.642 ± 0.019 ab | 0.556 ± 0.019 b | 4.63 ± 0.86 b |
| P2T3 | 0.857 ± 0.032 a | 0.593 ± 0.035 bc | 0.507 ± 0.028 cd | 3.65 ± 1.40 c |
| P2T4 | 0.865 ± 0.024 a | 0.574 ± 0.022 c | 0.469 ± 0.024 d | 2.94 ± 1.12 cd |
| P3T1 | 0.860 ± 0.017 a | 0.660 ± 0.035 ab | 0.563 ± 0.021 b | 4.97 ± 0.16 a |
| P3T2 | 0.862 ± 0.019 a | 0.632 ± 0.034 b | 0.556 ± 0.019 b | 4.33 ± 0.46 bc |
| P3T3 | 0.850 ± 0.026 a | 0.572 ± 0.026 c | 0.520 ± 0.026 c | 3.67 ± 0.79 c |
| P3T4 | 0.857 ± 0.033 a | 0.557 ± 0.024 c | 0.476 ± 0.024 d | 2.68 ± 1.07 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, J.; Zhang, R.; Yang, F.; Wang, J.; Cai, W.; Zhang, Y. Nocturnal LED Supplemental Lighting Improves Quality of Tomato Seedlings by Increasing Biomass Accumulation in a Controlled Environment. Agronomy 2024, 14, 1888. https://doi.org/10.3390/agronomy14091888
Song J, Zhang R, Yang F, Wang J, Cai W, Zhang Y. Nocturnal LED Supplemental Lighting Improves Quality of Tomato Seedlings by Increasing Biomass Accumulation in a Controlled Environment. Agronomy. 2024; 14(9):1888. https://doi.org/10.3390/agronomy14091888
Chicago/Turabian StyleSong, Jinxiu, Rong Zhang, Fulin Yang, Jianfeng Wang, Wei Cai, and Yue Zhang. 2024. "Nocturnal LED Supplemental Lighting Improves Quality of Tomato Seedlings by Increasing Biomass Accumulation in a Controlled Environment" Agronomy 14, no. 9: 1888. https://doi.org/10.3390/agronomy14091888
APA StyleSong, J., Zhang, R., Yang, F., Wang, J., Cai, W., & Zhang, Y. (2024). Nocturnal LED Supplemental Lighting Improves Quality of Tomato Seedlings by Increasing Biomass Accumulation in a Controlled Environment. Agronomy, 14(9), 1888. https://doi.org/10.3390/agronomy14091888






