Alterations in Physiological Parameters and Secondary Metabolites of Astragalus adsurgens Infected by the Pathogen Alternaria gansuensis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Greenhouse Trials
2.2. Stomatal Conductance (Sc), Photosynthesis (P), and Water Use Efficiency (WUE)
2.3. Toxicity Determination of A. gansuensis Culture Filtrate (CF)
2.4. Phytochemical Investigation
3. Results
3.1. Infection Rate and Lesion Length
3.2. Sc, P, and WUE in Standing Milkvetch Inoculated with A. gansuensis
3.3. Toxic Effects of CF of A. gansuensis
3.4. Relationship between the Relative Decline in Net P and Extracellular Increase in Conductivity, Extracellular Decrease in pH, and Percentage of Necrosis
3.5. Phytochemical Investigation
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, X.; Wang, Y.; Guo, P.; Zhang, Z.; Cui, X.; Hao, B.; Guo, W. Arbuscular mycorrhizal fungi facilitate Astragalus adsurgens growth and stress tolerance in cadmium and lead contaminated saline soil by regulating rhizosphere bacterial community. Appl. Soil Ecol. 2023, 187, 104842. [Google Scholar] [CrossRef]
- Su, S.F. Astragalus Adsurgens; Agriculture Press: Beijing, China, 1985. [Google Scholar]
- Yin, Y.L.; Nan, Z.B.; Li, C.J.; Hou, F.J. Root-invading fungi of milk vetch on the Loess Plateau, China. Agric. Ecosyst. Environ. 2008, 124, 51–59. [Google Scholar] [CrossRef]
- Liu, Y.; Feng, X.; Gao, P.; Li, Y.; Christensen, M.J.; Duan, T. Arbuscular mycorrhiza fungi increased the susceptibility of Astragalus adsurgens to powdery mildew caused by Erysiphe pisi. Mycology 2018, 28, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Nan, Z.B. Seedborne fungi of Astragelus adsurgen-environment, pathogenicity and disease control. Acta Prataculturae Sin. 1998, 1, 12–18. [Google Scholar]
- Wang, Z.; Wang, Q. Cultivating Erect Milkvetch (Astragalus adsurgens Pall.) (Leguminosae) Improved Soil Properties in Loess Hilly and Gullies in China. J. Integr. Agric. 2013, 12, 1652–1658. [Google Scholar] [CrossRef]
- Li, Y.Z.; Nan, Z.B. First report of yellow stunt and root rot of standing milkvetch caused by Embellisia sp. from China. Plant. Pathol. 2008, 57, 780. [Google Scholar] [CrossRef]
- Li, Y.Z.; Nan, Z.B. Nutritional study on Embellisia astragali, a fungal pathogen of milk vetch (Astragalus adsurgens). Anton. Leeuw. Int. J. G. 2009, 95, 275–284. [Google Scholar] [CrossRef]
- Liu, J.L.; Li, Y.Z.; Nan, Z.B. Design of species-specific PCR method for the detection of pathogen Embellisia astragali in standing milk vetch seeds. Lett. Appl. Microbiol. 2015, 60, 372–378. [Google Scholar] [CrossRef]
- Yu, B.H.; Nan, Z.B.; Li, Y.Z.; Lin, H.L. Resistance of standing milkvetch (Astragalus adsurgens) varieties to Embellisia astragali. Crop Pasture Sci. 2012, 63, 351–359. [Google Scholar] [CrossRef]
- Zeng, C.Y.; Zhu, X.; Cui, Z.; Li, Y.Z. Antifungal activity of plant extracts against Embellisia astragali, the fungal causal agent of yellow dwarf and root-rot disease of standing milkvetch. Crop Pasture Sci. 2017, 66, 735–739. [Google Scholar] [CrossRef]
- Collins, B.; Parke, J.; Lachenbruch, B.; Hansen, E. The effects of Phytophthora ramorum infection on hydraulic conductivity and tylosis formation in tanoak sapwood. Can. J. For. Res. 2009, 39, 1766–1776. [Google Scholar] [CrossRef]
- Huang, C.; Cun, Y.; Yu, H.; Tong, Z.; Xiao, B.; Song, Z.; Wang, B.; Li, Y.; Liu, Y. Transcriptomic profile of tobacco in response to Tomato zonate spot orthotospovirus infection. Virol. J. 2017, 14, 153. [Google Scholar] [CrossRef]
- Prokopová, J.; Mieslerová, B.; Hlaváčková, V.; Hlavinka, J.; Lebeda, A.; Nauš, J.; Špundová, M. Changes in photosynthesis of Lycopersicon spp. plants induced by tomato powdery mildew infection in combination with heat shock pre-treatment. Physiol. Mol. Plant Pathol. 2010, 74, 205–213. [Google Scholar] [CrossRef]
- Vélez, M.L.; Silva, P.V.; Troncoso, O.A.; Greslebin, A.G. Alteration of physiological parameters of Austrocedrus chilensis by the pathogen Phytophthora austrocedrae. Plant Pathol. 2012, 61, 877–888. [Google Scholar] [CrossRef]
- Chang, Q.; Liu, J.; Wang, Q.; Han, L.N.; Liu, J.; Li, M.; Huang, L.L.; Yang, J.R.; Kang, Z.S. The effect of Puccinia striiformis f. sp. tritici on the levels of water-soluble carbohydrates and the photosynthetic rate in wheat leaves. Physiol. Mol. Plant Pathol. 2013, 84, 131–137. [Google Scholar]
- Dawson, P.; Weste, G. Changes in water relations associated with infection by Phytophthora cinnamomi. Aust. J. Bot. 1982, 30, 393–400. [Google Scholar] [CrossRef]
- Manter, D.K.; Kelsey, R.G.; Karchesy, J.J. Photosynthetic declines in Phytophthora ramorum-infected plants develop prior to water stress and in response to exogenous application of elicitins. Phytopathology 2007, 97, 850–856. [Google Scholar] [CrossRef]
- Hardham, A.R.; Cahill, D.M. The role of oomycete effectors in plant–pathogen interactions. Funct. Plant Biol. 2010, 37, 919–925. [Google Scholar] [CrossRef]
- Gyenge, J.E.; Fernández, M.E.; Schlichter, T. Influence of radiation and drought on gas exchange of Austrocedrus chilensis seedlings. Bosque 2007, 28, 220–225. [Google Scholar] [CrossRef]
- Gyenge, J.E.; Fernández, M.E.; Schlichter, T.M. Are differences in productivity between native and exotic trees in N.W. Patagonia related to differences in hydraulic conductance. Trees 2008, 22, 483–490. [Google Scholar] [CrossRef]
- Ezeibekwe, I.O.; Ofong, A.U.; Mbagwu, F.N.; Unamba, C.I.N. Toxin production by fungi isolated from rotten pawpaw fruits in parts of Imo and Abia states of Nigeria. Rep. Opin. 2009, 1, 41–44. [Google Scholar]
- Liu, Q. Studies on the Structures and Bioactivities of Secondary Metabolites from Three Southern Plants. Ph.D. Thesis, Lanzhou University, Lanzhou, China, 2010. [Google Scholar]
- Rao, S.R.; Ravishankarb, G.A. Plant cell cultures: Chemical factories of secondary metabolites. Biotechnol. Adv. 2002, 20, 101–153. [Google Scholar] [PubMed]
- Parke, J.L.; Oh, E.; Voelker, S.; Hansen, E.M.; Buckles, G.; Lachenbruch, B. Phytophthora ramorum colonizes tanoak xylem and is associated with reduced stem water transport. Phytopathology 2007, 97, 1559–1567. [Google Scholar] [CrossRef]
- Costet, L.; Cordelier, S.; Dorey, S.; Baillieul, F.; Fritig, B.; Kauffmann, S. Relationship between localized acquired resistance (LAR) and the hypersensitive response (HR): HR is necessary for LAR to occur and salicylic acid is not sufficient to trigger LAR. Mol. Plant Microbe Interact. 1999, 12, 655–662. [Google Scholar] [CrossRef]
- Kamoun, S. A catalogue of the effector secretome of plant pathogenic oomycetes. Annu. Rev. Phytopathol. 2006, 44, 41–60. [Google Scholar] [CrossRef]
- Brummer, M.; Arend, M.; Fromm, J.; Schlenzig, A.; Oßwald, W.F. Ultrastructural changes in immunocytochemical localization of the elicitin quercinin in Quercus robur L. roots infected with Phytophthora quercina. Physiol. Mol. Plant Pathol. 2002, 61, 109–120. [Google Scholar] [CrossRef]
- Desender, S.; Andrivon, D.; Val, F. Activation of defence reactions in Solanaceae: Where is the specificity? Cell Microbiol. 2007, 9, 21–30. [Google Scholar] [CrossRef]
- Fleischmann, F.; Koehl, J.; Portz, R.; Beltrame, A.B.; Oßwald, W. Physiological changes of Fagus sylvatica seedlings infected with Phytophthora citricola and the contribution of its elicitin ‘‘citricolin’’ to pathogenesis. Plant Biol. 2005, 7, 650–658. [Google Scholar] [CrossRef]
- Vleeshouwers, V.G.A.A.; Van, D.W.; Govers, F.; Kamoun, S.; Colon, L. The hypersensitive response is associated with host and nonhost resistance to Phytophthora infestans. Planta 2000, 210, 853–864. [Google Scholar] [CrossRef]
- Nie, H.X.; Gao, F.; Duan, T.Y.; Li, Y.Z. Progress in research on the diseases of Onobrychis viciaefolia. Acta Prataculturae Sin. 2014, 23, 302–312. [Google Scholar]
- Clemenz, C.; Fleischmann, F.; Ha¨berle, K.H.; Matyssek, R.; Oßwald, W. Photosynthetic and leaf water potential responses of Alnus glutinosa saplings to stem-base inoculation with Phytophthora alni subsp. alni. Tree Physiol. 2008, 11, 1703–1711. [Google Scholar] [CrossRef] [PubMed]
- Robin, C.; Capron, G.; Desprez-loustau, M.L. Root infection by Phytophthora cinnamomi in seedlings of three oak species. Plant Pathol. 2001, 50, 708–716. [Google Scholar] [CrossRef]
- Kostic, I.; Nikolic, N.; Milanovic, S.; Milenkovic, I.; Pavlovic, J.; Paravinja, A.; Nikolic, M. Silicon modifies leaf nutriome and improves growth of oak seedlings exposed to phosphorus deficiency and Phytophthora plurivora infection. Front. Plant Sci. 2023, 29, 1265782. [Google Scholar] [CrossRef] [PubMed]
- Wink, M.; Mohamed, G.I.A. Evolution of chemical defense traits in the Leguminosae: Mapping of distribution patterns of secondary metabolites on a molecular phylogeny inferred from nucleotide sequences of the rbcL gene. Biochem. Syst. Ecol. 2003, 31, 897–917. [Google Scholar] [CrossRef]
- Song, Q.Y.; Nan, Z.B.; Gao, K.; Song, H.; Tian, P.; Zhang, X.X.; Li, C.J.; Xu, W.B.; Li, X.Z. Antifungal, phytotoxic, and cytotoxic activities of metabolites from Epichloë bromicola, a fungus obtained from Elymus tangutorum grass. J. Agric. Food Chem. 2015, 63, 8787–8792. [Google Scholar] [CrossRef]
- Xia, C.; Li, N.N.; Zhang, Y.W.; Li, C.J.; Zhang, X.X.; Nan, Z.B. Role of Epichloë Endophytes in defense responses of cool-season grasses to pathogens: A review. Plant Dis. 2018, 102, 2061–2073. [Google Scholar] [CrossRef]




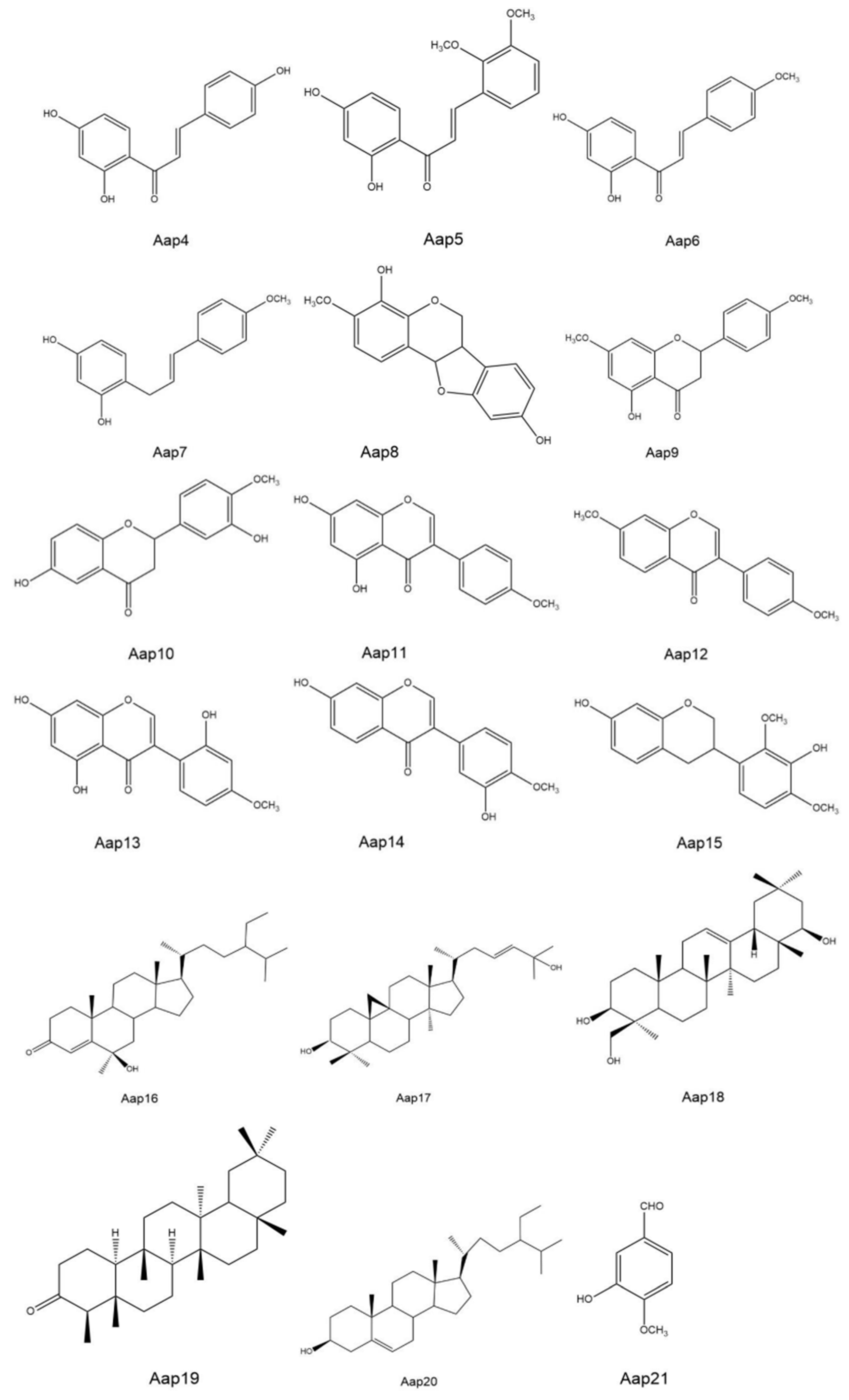
| Secondary Metabolite | Relative Content (%) in: | |||
|---|---|---|---|---|
| Control Treatment | Inoculated with A. gansuensis | |||
| Shanxi | Ningxia | Shanxi | Ningxia | |
| Aap 1: Methyl 3- (2, 4-dihydroxyphenyl) -5- (4-methoxyphenyl) -5-oxopentanoate | 0.00 | 0.00 | 5.36 | 2.85 |
| Aap 2: 2,4,4’trihydroxy-3-isopentenyl chalcone | 4.92 | 4.51 | 11.21 | 8.41 |
| Aap 3: 2, 4-dihydroxy-3’,4’ -dimethoxy-chalcone | 0.97 | 0.26 | 2.51 | 1.36 |
| Aap 4: 2,4,4 ’-trihydroxy-chalcone | 0.95 | 1.53 | 0.95 | 3.25 |
| Aap 5: 2, 4-dihydroxy-2’,3’ -dimethoxy-chalcone | 0.21 | 0.33 | 0.00 | 0.00 |
| Aap 6: 2, 4-dihydroxy-4’ methoxychalcone | 0.48 | 0.25 | 0.00 | 0.00 |
| Aap 7: 2, 4-dihydroxy-4’-methoxy- chalalkyl | 0.81 | 1.64 | 2.37 | 1.27 |
| Aap 8: 8-4’ dihydroxy-7-methoxy- hydroxypterocarpan | 0.00 | 0.00 | 1.31 | 0.95 |
| Aap 9: 5-hydroxy-7,4’-dimethoxy- dihydroflavone | 0.00 | 0.00 | 8.33 | 5.68 |
| Aap 10: 6,3’-dihydroxy-4’-methoxy- dihydroflavone | 0.00 | 0.00 | 12.01 | 9.33 |
| Aap 11: 5, 7-dihydroxy-4’-methoxy-isoflavone | 0.00 | 0.00 | 2.17 | 3.58 |
| Aap 12: 7,4’-dimethoxy-isoflavone | 6.68 | 8.12 | 0.00 | 0.00 |
| Aap 13: 5,7,2’-trihydroxy-4’ methoxy-isoflavone | 13.56 | 11.93 | 0.00 | 0.00 |
| Aap 14: 7,3’-dihydroxy-4’ -methoxy-isoflavone | 10.23 | 5.56 | 0.00 | 0.00 |
| Aap 15: 7, 3-dihydroxy-2’,4’-dimethoxy-isoflavane | 0.00 | 0.00 | 0.62 | 0.45 |
| Aap 16: steroid-4-ene-6 -alcohol-3-ketone | 0.00 | 0.00 | 11.97 | 14.68 |
| Aap 17: cycloaltin-26-ene 3β, 28-diol | 0.00 | 0.00 | 28.21 | 26.56 |
| Aap 18: Oleanolic-12-ene 3β,22β, 23-triol | 24.65 | 30.98 | 1.25 | 2.48 |
| Aap 19: friedelin | 8.22 | 6.56 | 0.00 | 0.00 |
| Aap 20: β-sitosterol | 8.05 | 6.83 | 0.00 | 0.00 |
| Aap 21: vanillin | 1.72 | 3.43 | 0.00 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, X.; Li, X.; White, J.F.; Creamer, R.; Li, C.; Yu, B. Alterations in Physiological Parameters and Secondary Metabolites of Astragalus adsurgens Infected by the Pathogen Alternaria gansuensis. Agronomy 2024, 14, 1892. https://doi.org/10.3390/agronomy14091892
Han X, Li X, White JF, Creamer R, Li C, Yu B. Alterations in Physiological Parameters and Secondary Metabolites of Astragalus adsurgens Infected by the Pathogen Alternaria gansuensis. Agronomy. 2024; 14(9):1892. https://doi.org/10.3390/agronomy14091892
Chicago/Turabian StyleHan, Xinyao, Xiaopeng Li, James F. White, Rebecca Creamer, Chunjie Li, and Binhua Yu. 2024. "Alterations in Physiological Parameters and Secondary Metabolites of Astragalus adsurgens Infected by the Pathogen Alternaria gansuensis" Agronomy 14, no. 9: 1892. https://doi.org/10.3390/agronomy14091892





