Establishment of a Serology- and Molecular-Combined Detection System for Youcai Mosaic Virus and Its Application in Various Host Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Construction of Recombinant CPYoMV-YZ Protein Expression Vector
2.2. Prokaryotic Expression and Purification of Recombinant CPYoMV-YZ Protein
2.3. Production of Polyclonal Antibody against His-CPYoMV-YZ Protein
2.4. Western Blotting, Immuno-Dot Blotting, and ELISA Detection of YoMV
2.5. RT-PCR for YoMV
2.6. S-RT-LAMP Assay for YoMV Using Prepared PAb-CPYoMV-YZ
2.7. Multiple Sequence Alignment and Phylogenetic Tree Construction of the CPYoMV Gene.
3. Results
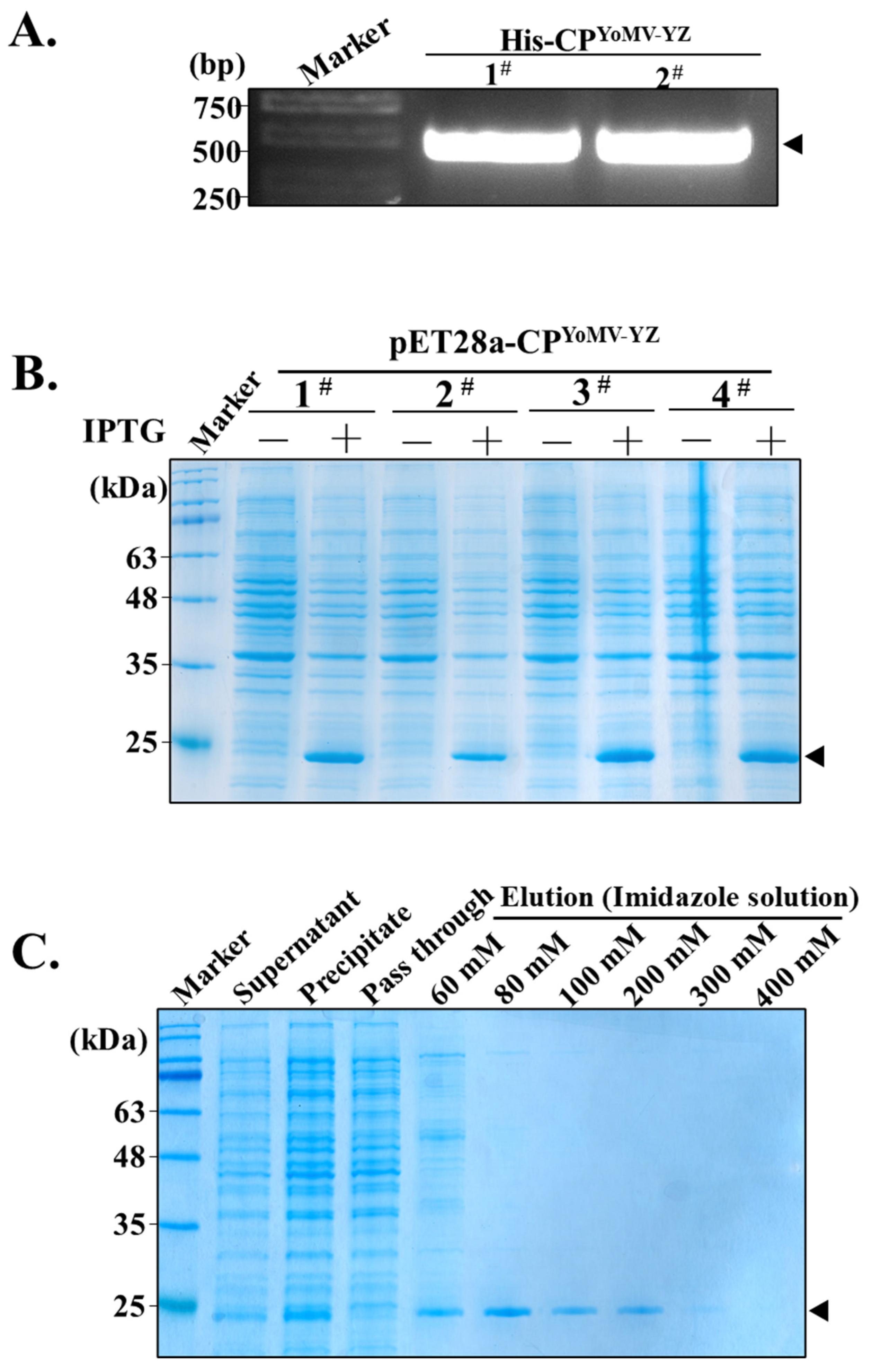
3.1. Production of Recombinant CPYoMV-YZ Protein
3.2. Antiserum Production Using Recombinant His-CPYoMV-YZ Protein
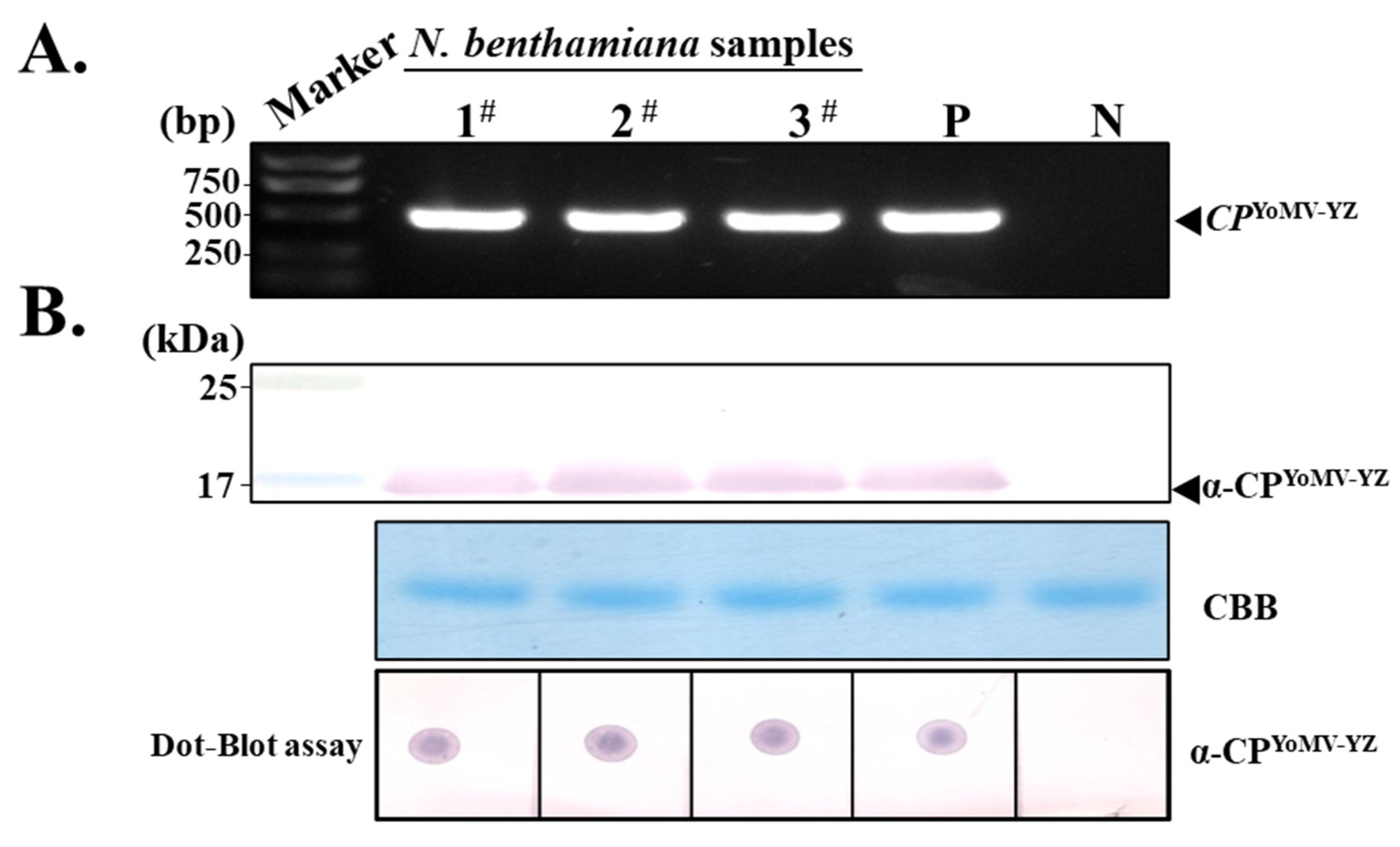
3.3. Evaluation of Purified PAb-CPYoMV-YZ by Western Blotting and Immuno-Dot Blotting
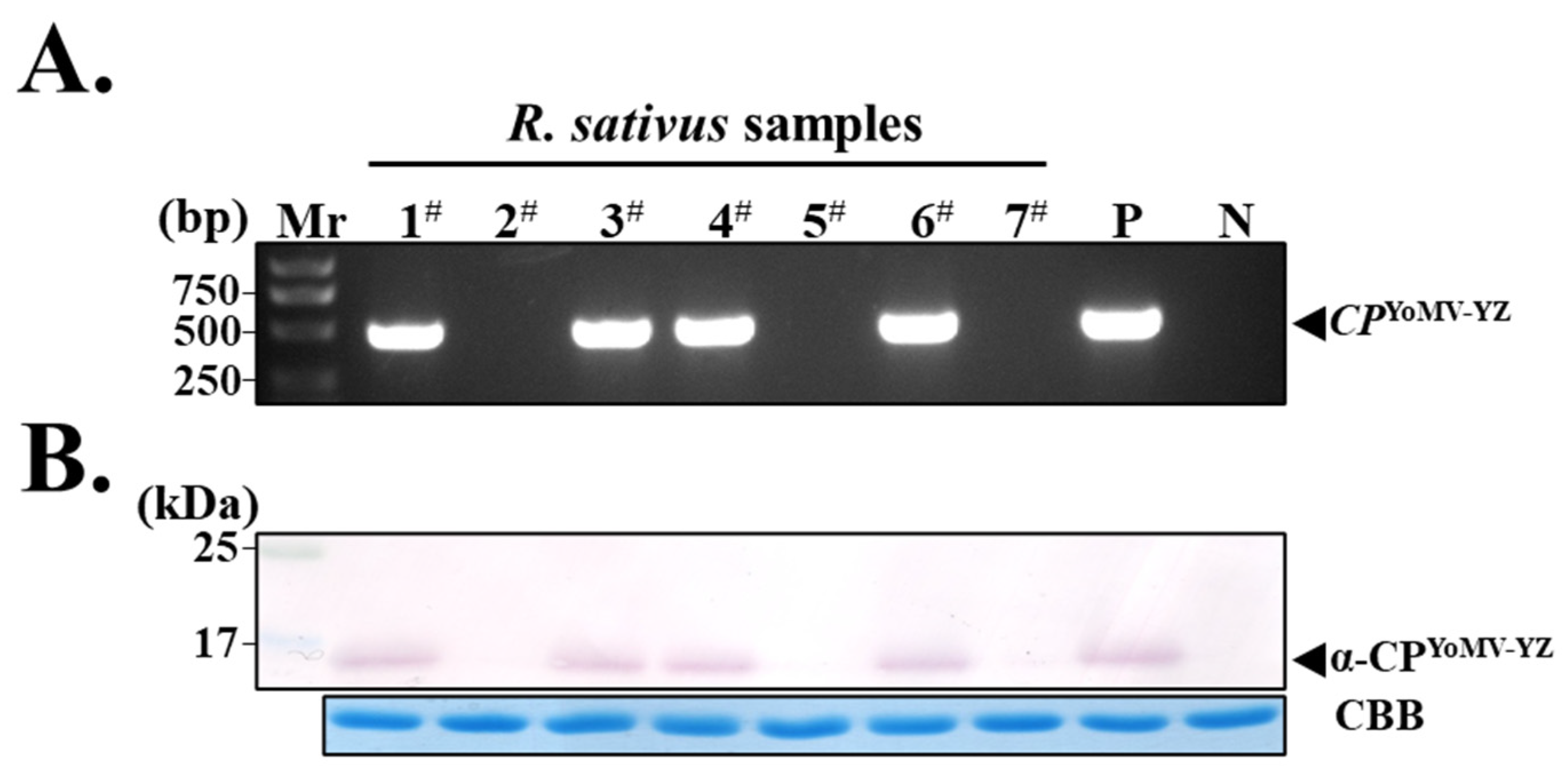
3.4. Screening of YoMV-Positive and Negative Radish Plants by Molecular and Serological Methods
3.5. Establishment of ELISA and Immuno-Dot Blotting Assays for YoMV in Radish
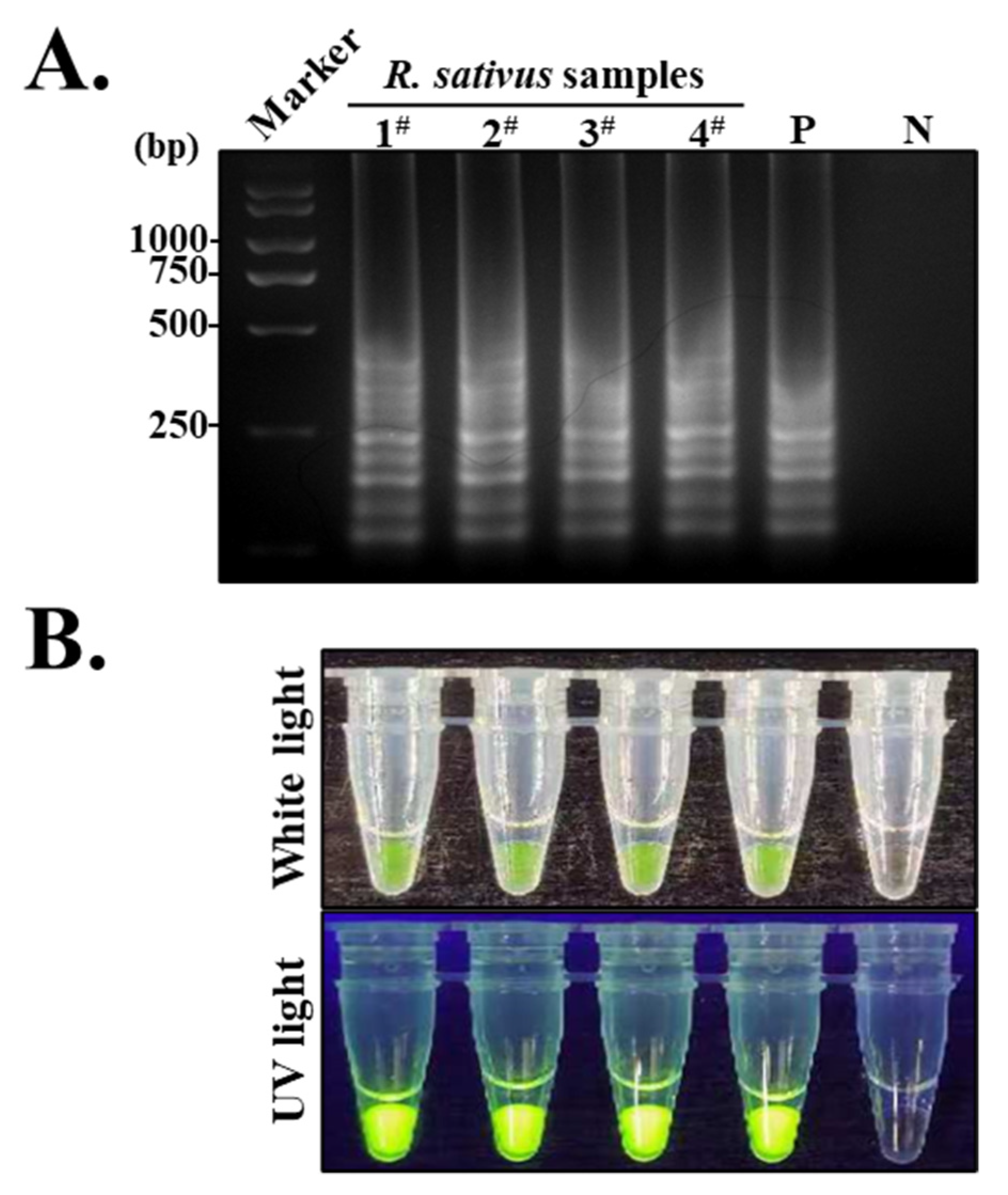
3.6. Establishment of an S-RT-LAMP Assay Based on the CPYoMV-YZ Gene
3.7. Purified PAb-CPYoMV-YZ for YoMV Detection in a Wide Range of Plants
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bao, C.; Wang, J.; Hu, T.; Hu, H.; Wang, W.; Wei, Q. Discussion on the Current Situation of Development and Breeding Direction of Radish Industry in China. J. Zhejiang Agric. Sci. 2019, 60, 707–710. [Google Scholar]
- Yuan, W.; Yuan, S.; Gan, C.; Cui, L.; Deng, X. Molecular Identification and Control of Radish Virus Disease in Hubei Province. J. CJ. Veg. 2015, 8, 61–62. [Google Scholar]
- Cai, L.; Xu, Z.; Chen, K.; Yan, L.; Hou, M. Identification and Characterization of Oilseed Rape Mosaic Virus (ORMV) Wh Strain. J. Plant Prot. 2005, 32, 4. [Google Scholar]
- Wang, L.; Chen, X.; Chen, W.; Tan, Q.; Yang, X. Detection of Main Viral Species of Vegetable Virus Diseases in Guizhou. GD. Agric. Sci. 2015, 20, 63–67. [Google Scholar]
- Chung, J.; Han, J.-Y.; Kim, J.; Ju, H.; Gong, J.; Seo, E.-Y.; Hammond, J.; Lim, H.-S. Survey of Viruses Present in Radish Fields in 2014. Res. Plant Dis. 2015, 21, 235–242. [Google Scholar] [CrossRef]
- Choi, S.-K.; Park, S.-H.; Jeon, B.-W.; Jang, S.-W.; Yoon, J.-Y. First Report of Youcai Mosaic Virus in Raphanus Raphanistrum Subsp. Sativus in Korea. Plant Dis. 2017, 101, 1959. [Google Scholar] [CrossRef]
- Chen, X.-R.; Wang, Y.; Zhao, H.-H.; Zhang, X.-Y.; Wang, X.-B.; Li, D.-W.; Yu, J.-L.; Han, C.-G. Brassica Yellows Virus’ Movement Protein Upregulates Anthocyanin Accumulation, Leading to the Development of Purple Leaf Symptoms on Arabidopsis Thaliana. Sci. Rep. 2018, 8, 16273. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Tan, Q.; Xiao, Q.; Mei, S.; Zhang, J. Pathogen Identification of Virus Disease and Evaluation for Germplasm Disease Resistance in Radish, Acta Hortic. Sin. 2020, 47, 1947–1955. [Google Scholar]
- Liu, Y.; Li, F.; Li, Y.; Zhang, S.; Gao, X.; Xie, Y.; Yan, F.; Zhang, A.; Dai, L.; Cheng, Z.; et al. Identification, Distribution and Occurrence of Viruses in the Main Vegetables of China. Sci. Agric. Sin. 2019, 52, 239–261. [Google Scholar]
- Lartey, R.T.; Voss, T.C.; Melcher, U. Tobamovirus Evolution: Gene Overlaps, Recombination, and Taxonomic Implications. Mol. Biol. Evol. 1996, 13, 1327–1338. [Google Scholar] [CrossRef]
- Gibbs, A.; Tien, P.; Kang, L.; Tian, Y.; Randles, J. Classification of several tobamoviruses isolated in China on the basis of the amino acid composition of their virion proteins. Intervirology 1982, 18, 160–163. [Google Scholar] [CrossRef] [PubMed]
- Palukaitis, P.; Randles, J.W.; Tian, Y.T.; Kang, L.Y.; Tien, P. Taxonomy of several tobamoviruses from China as determined by molecular hybridization analysis with complementary DNA. Intervirology. 1981, 16, 136–141. [Google Scholar] [CrossRef]
- Ju, H.-K.; Kim, I.-H.; Hu, W.-X.; Kim, B.; Choi, G.-W.; Kim, J.; Lim, Y.P.; Domier, L.L.; Hammond, J.; Lim, H.-S. A Single Nucleotide Change in the Overlapping MP and CP Reading Frames Results in Differences in Symptoms Caused by Two Isolates of Youcai Mosaic Virus. Arch. Virol. 2019, 164, 1553–1565. [Google Scholar] [CrossRef]
- Wei, J.; Shen, S.; Wang, J.; Zhang, C.; Zhu, Y. A Preliminary Study of Brassica Campestris and Turnip Mosaic Virus in East China. Acta Phytophy. Acta Pharm. Sin. 1958, 4, 2. [Google Scholar]
- Aguilar, I.; Sanchez, F.; Martin, A.M.; Martinez-Herrera, D.; Ponz, F. Nucleotide Sequence of Chinese Rape Mosaic Virus (Oilseed Rape Mosaic Virus), a Crucifer Tobamovirus Infectious on Arabidopsis thaliana. Plant Mol. Biol. 1996, 30, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Fuji, S.; Mochizuki, N.; Fujinaga, M.; Ikeda, M.; Shinoda, K.; Uematsu, S.; Furuya, H.; Naito, H.; Fukumoto, F. Incidence of Viruses in Alstroemeria Plants Cultivated in Japan and Characterization of Broad Bean Wilt Virus-2, Cucumber Mosaic Virus and Youcai Mosaic Virus. J. Gen. Plant Pathol. 2007, 73, 216–221. [Google Scholar] [CrossRef]
- Zhu, H.; Hong, J.; Ye, R.; Chen, J.; Yu, S.; Adams, M.J. Sequence Analysis Shows That Ribgrass Mosaic Virus Shanghai Isolate (RMV-Sh) Is Closely Related to Youcai Mosaic Virus. Arch. Virol. 2001, 146, 1231–1238. [Google Scholar] [CrossRef]
- Kwon, S.-J.; Kim, Y.-B.; Back, C.-K.; Chung, B.-N. First Report of a Mixed Infection of Youcai Mosaic Virus and Rehmannia Mosaic Virus in Rehmannia Glutinosa in Korea. Plant Dis. 2017, 07, 1128. [Google Scholar] [CrossRef]
- Li, Z.; Tang, Y.; She, X.; Yu, L.; Lan, G.; He, Z. Molecular Characteristics and Pathogenicity Analysis of Youcai Mosaic Virus Guangdong Isolate Infecting Radish. Sci. Agric. Sin. 2022, 55, 2752–2761. [Google Scholar]
- Gu, T.; Feng, C.; Hua, Y.; Liu, D.; Chen, H.; He, Z.; Xu, K.; Zhang, K. Molecular Characterization and Pathogenicity of an Infectious cDNA Clone of Youcai Mosaic Virus on Solanum nigrum. Int. J. Mol. Sci. 2024, 25, 1620. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, X.; Yang, F.; Niu, Y. Identification and Analysis of Complete Genomic Sequence of Youcai Mosaic Virus Rehmannia Glutinosa Isolate in Shanxi. Acta Phytophy. Sin. 2016, 46, 598–604. [Google Scholar]
- Mansilla, C.; Aguilar, I.; Martínez-Herrera, D.; Sánchez, F.; Ponz, F. Physiological Effects of Constitutive Expression of Oilseed Rape Mosaic Tobamovirus (ORMV) Movement Protein in Arabidopsis Thaliana. Transgenic Res. 2006, 15, 761–770. [Google Scholar] [CrossRef]
- Pang, X. Identification of Virus Infecting Atractylodes macrocephala Koidz, Pinellia ternata Breit and Rehmannia glutinosa Libosch by Small RNA Deep Sequencing. SXAU. 2018, 6, 45–50. [Google Scholar]
- Qin, Y.; Wang, F.; Wen, Y.; Zhao, Z.; Gao, S.; Zhang, D.; Li, S.; Liu, Y.; Liu, Y.; Lu, S.; et al. Establishment of RT-LAMP Method for Rapid Detection of Youcai Mosaic Virus. Acta Phytophy. Sin. 2023, 1024, 001. [Google Scholar]
- Hua, Y.; Feng, C.; Gu, T.; Chen, H.; Liu, D.; Xu, K.; Zhang, K. Development of Polyclonal Antibodies and a Serological-Based Reverse-Transcription Loop-Mediated Isothermal Amplification (S-RT-LAMP) Assay for Rice Black-Streaked Dwarf Virus Detection in Both Rice and Small Brown Planthopper. Viruses 2023, 15, 2127. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhuang, X.; Xu, H.; Gan, H.; He, Z.; Chen, J. Development of Polyclonal Antibodies-Based Serological Methods and a Dig-Labelled DNA Probe-Based Molecular Method for Detection of the Vicia Cryptic Virus-M in Field Plants. J. Virol. Methods. 2022, 299, 114331. [Google Scholar] [CrossRef]
- Zhang, K.; Zhuang, X.; Xu, H.; Guo, X.; He, Z.; Xu, K.; Liu, F. Sensitive and High-Throughput Polyclonal Antibody-Based Serological Methods for Rice Stripe Virus Detection in Both Rice and Small Brown Planthopper. Crop Prot. 2021, 144, 105599. [Google Scholar] [CrossRef]
- Barker, K. At the Bench: A Laboratory Navigator; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2004. [Google Scholar]
- Qin, Y.; Wang, F.; Cai, L.; Gao, S.; Wen, Y.; Liu, Y.; Lu, C.; Yang, J.; Li, X.; Qi, W.; et al. First Report of Youcai Mosaic Virus Infecting Yam in China. Plant Dis. 2023, 107, 1247. [Google Scholar] [CrossRef]
- Chen, J.; Feng, C.; Guo, X.; Zhou, Y.; Gu, T.; Zhuang, X.; Cheng, L.; Zhang, K. Development of Polyclonal Antibodies-Based Serological Methods for Detection of the Rehmannia Mosaic Virus in Field Plants. Front. Sustain. Food Syst. 2022, 6, 1013470. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, C.; Hua, Y.; Liu, D.; Chen, H.; Wu, M.; Hua, J.; Zhang, K. Establishment of a Serology- and Molecular-Combined Detection System for Youcai Mosaic Virus and Its Application in Various Host Plants. Agronomy 2024, 14, 1900. https://doi.org/10.3390/agronomy14091900
Feng C, Hua Y, Liu D, Chen H, Wu M, Hua J, Zhang K. Establishment of a Serology- and Molecular-Combined Detection System for Youcai Mosaic Virus and Its Application in Various Host Plants. Agronomy. 2024; 14(9):1900. https://doi.org/10.3390/agronomy14091900
Chicago/Turabian StyleFeng, Chenwei, Yanhong Hua, Duxuan Liu, Haoyu Chen, Mingjie Wu, Jing Hua, and Kun Zhang. 2024. "Establishment of a Serology- and Molecular-Combined Detection System for Youcai Mosaic Virus and Its Application in Various Host Plants" Agronomy 14, no. 9: 1900. https://doi.org/10.3390/agronomy14091900






