Genetic Evaluation of Kazakhstani Potato Germplasm for Pathogen and Pest Resistance Using DNA Markers
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. DNA Isolation
2.3. PCR and Restriction Analysis
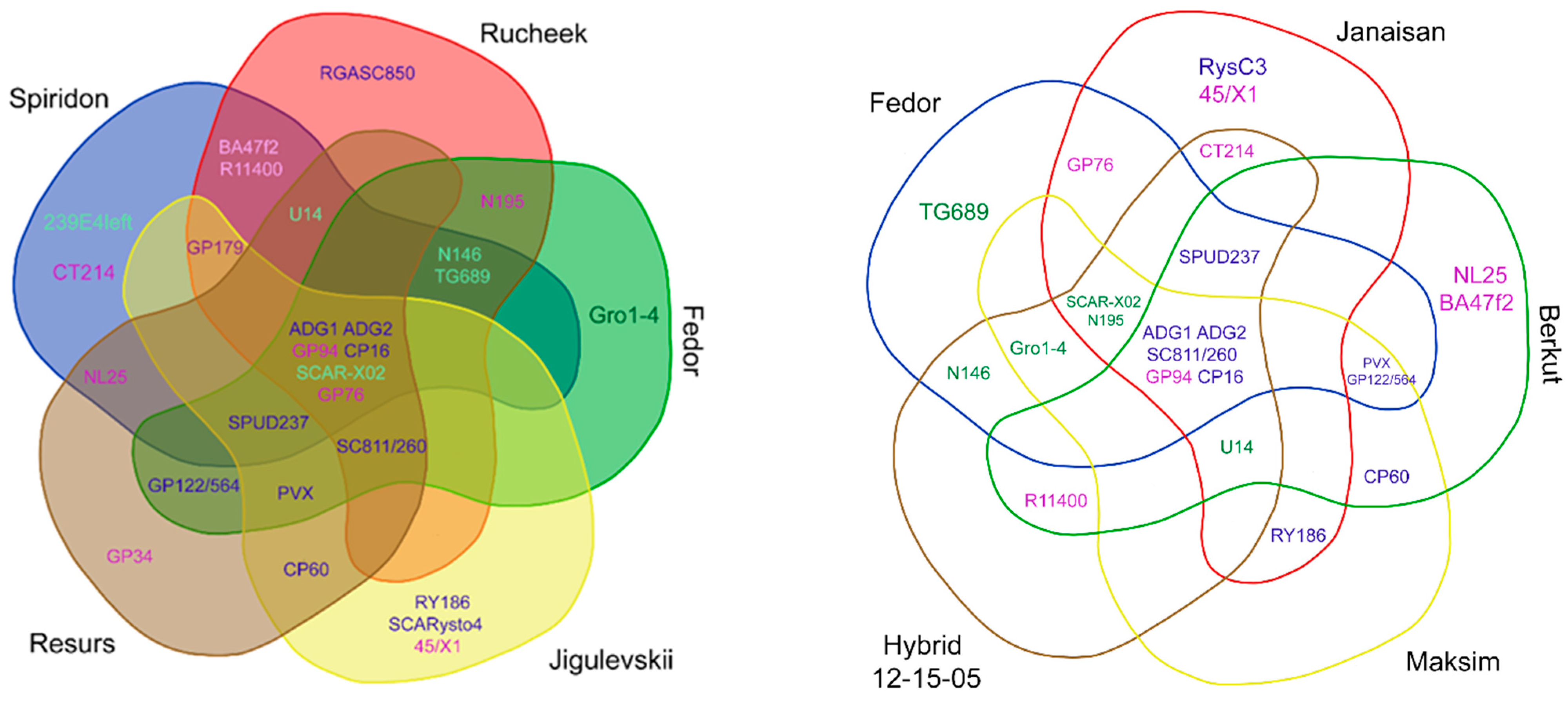
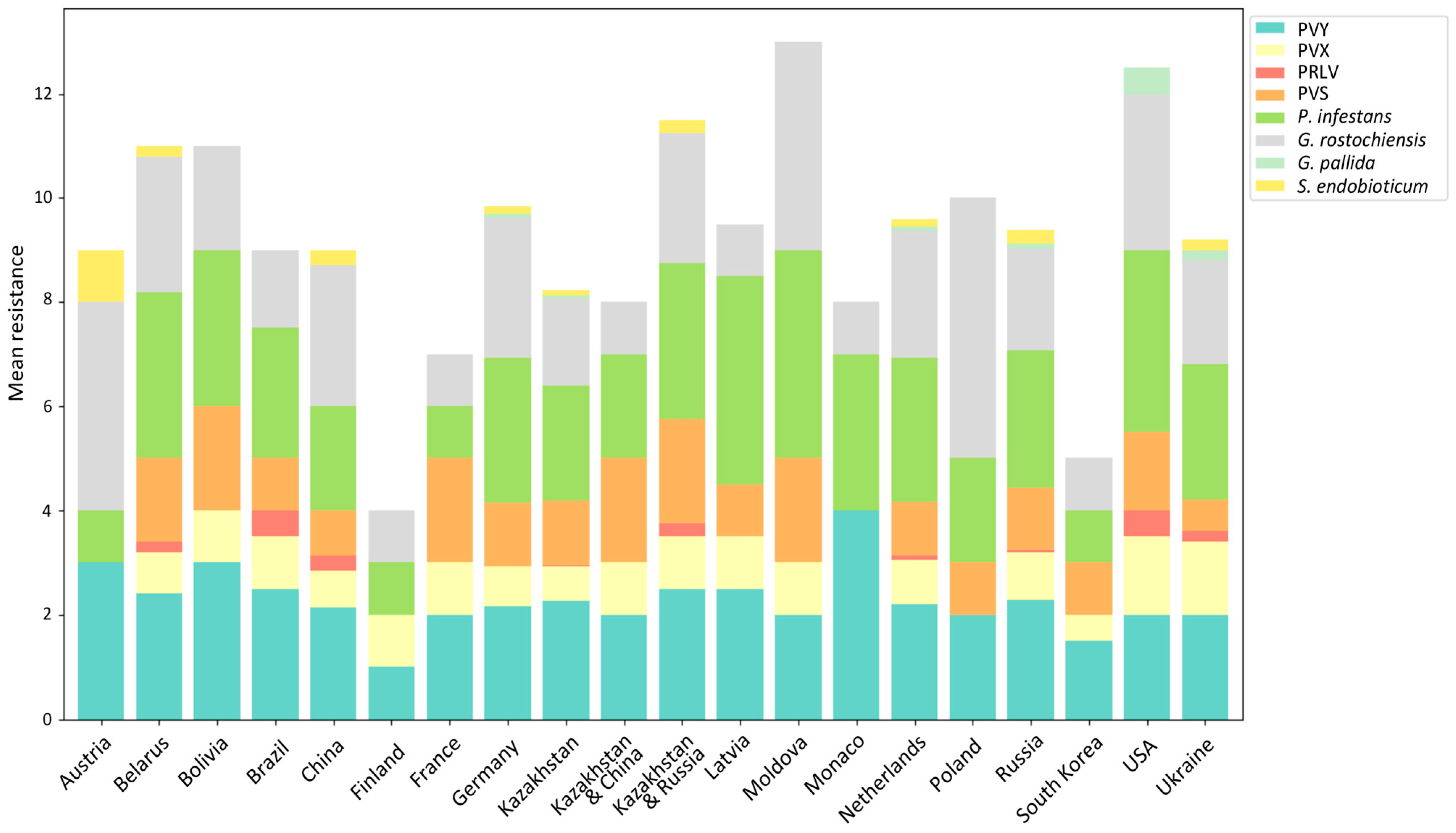
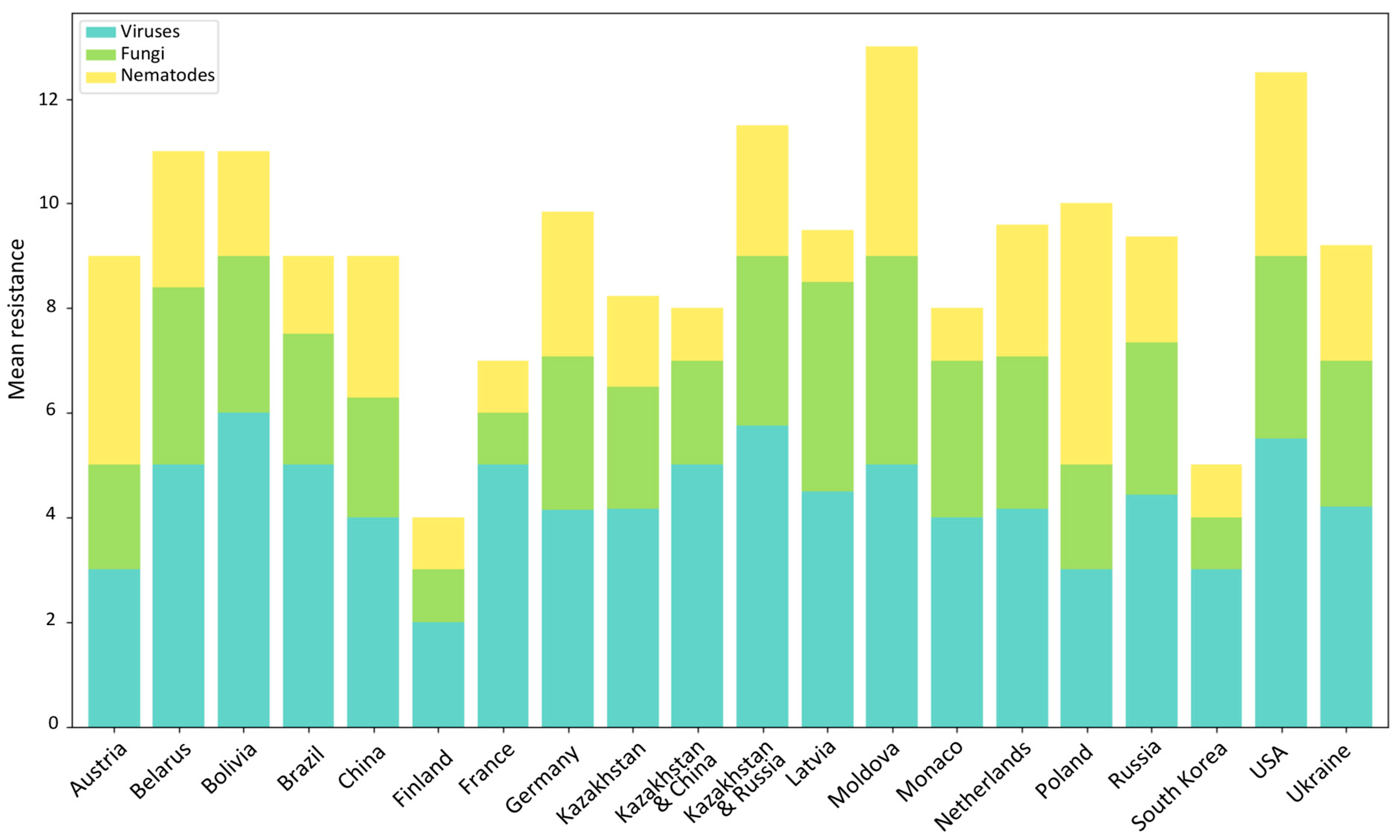
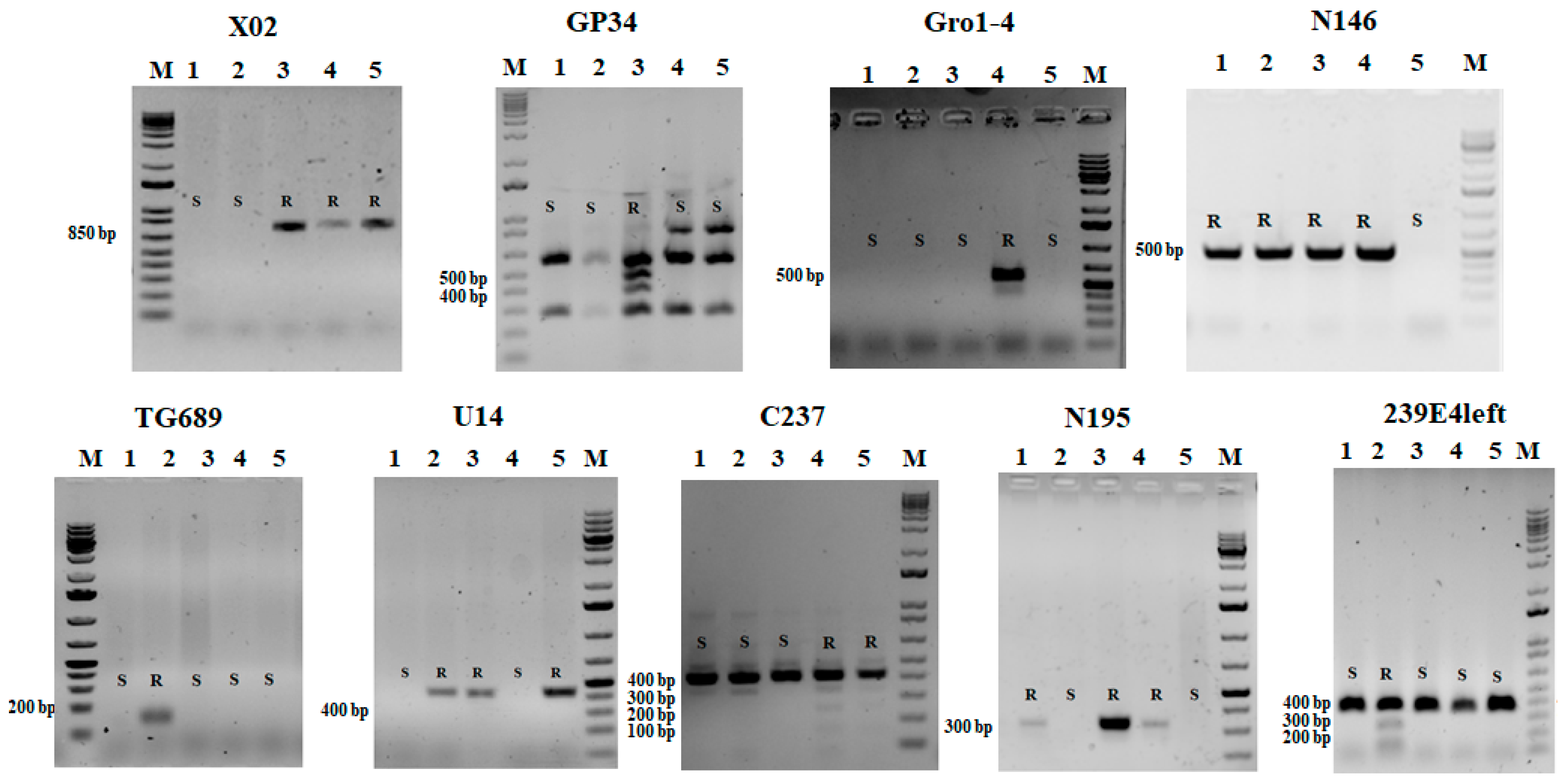
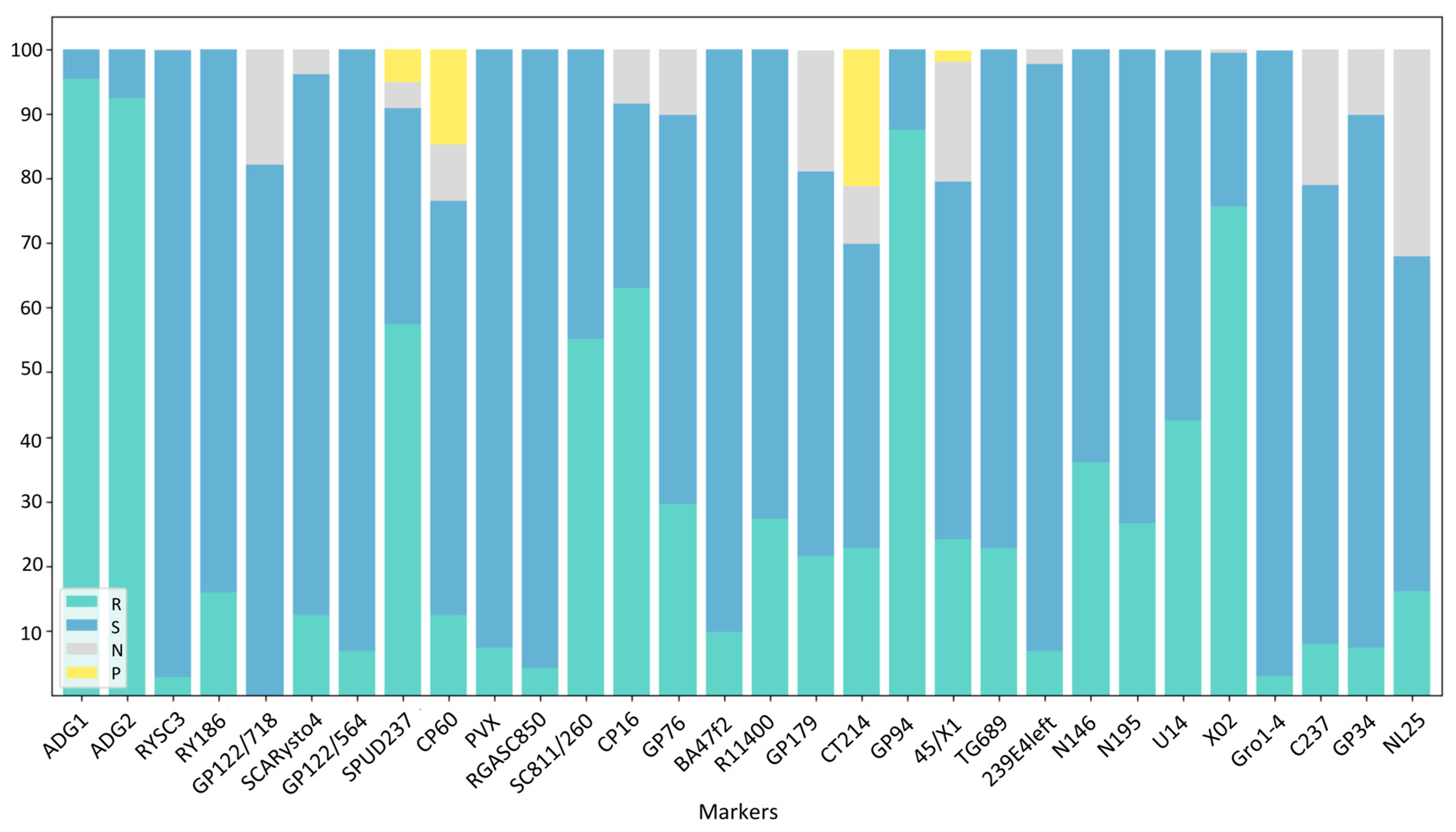
3. Results
3.1. Virus Resistance Screening
3.2. Late Blight and Potato Wart Screening
3.3. PCN Resistance Screening
4. Discussion
4.1. Top Global Cultivars
4.2. Top Local Cultivars
4.3. DNA Marker Applicability
4.4. Perspectives on Potato Breeding
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lopez-Pardo, R.; Barandalla, L.; Ritter, E.; Ruiz De Galarreta, J.I. Validation of Molecular Markers for Pathogen Resistance in Potato. Plant Breed. 2013, 132, 246–251. [Google Scholar] [CrossRef]
- Chikh-Ali, M.; Karasev, A.V. Virus Diseases of Potato and Their Control. In Potato Production Worldwide; Elsevier: Amsterdam, The Netherlands, 2023; pp. 199–212. [Google Scholar]
- Makarova, S.; Makarov, V.; Taliansky, M.; Kalinina, N. Resistance to Viruses of Potato: Current Status and Prospects. 2017. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/20173272111 (accessed on 10 April 2024).
- Flis, B.; Hennig, J.; Strzelczyk-Żyta, D.; Gebhardt, C.; Marczewski, W. The Ry-Fsto Gene from Solanum stoloniferum for Extreme Resistant to Potato Virus Y Maps to Potato Chromosome XII and Is Diagnosed by PCR Marker GP122718 in PVY Resistant Potato Cultivars. Mol. Breed. 2005, 15, 95–101. [Google Scholar] [CrossRef]
- Herrera, M.D.R.; Vidalon, L.J.; Montenegro, J.D.; Riccio, C.; Guzman, F.; Bartolini, I.; Ghislain, M. Molecular and Genetic Characterization of the Ryadg Locus on Chromosome XI from Andigena Potatoes Conferring Extreme Resistance to Potato Virus Y. Theor. Appl. Genet. 2018, 131, 1925–1938. [Google Scholar] [CrossRef]
- Li, G.; Shao, J.; Wang, Y.; Liu, T.; Tong, Y.; Jansky, S.; Xie, C.; Song, B.; Cai, X. Rychc Confers Extreme Resistance to Potato Virus Y in Potato. Cells 2022, 11, 2577. [Google Scholar] [CrossRef]
- Ballvora, A.; Ercolano, M.R.; Weiß, J.; Meksem, K.; Bormann, C.A.; Oberhagemann, P.; Salamini, F.; Gebhardt, C. The R1 Gene for Potato Resistance to Late Blight (Phytophthora infestans) Belongs to the Leucine Zipper/NBS/LRR Class of Plant Resistance Genes. Plant J. 2002, 30, 361–371. [Google Scholar] [CrossRef]
- Rauscher, G.M.; Smart, C.D.; Simko, I.; Bonierbale, M.; Mayton, H.; Greenland, A.; Fry, W.E. Characterization and Mapping of R Pi-Ber, a Novel Potato Late Blight Resistance Gene from Solanum Berthaultii. Theor. Appl. Genet. 2006, 112, 674–687. [Google Scholar] [CrossRef]
- Śliwka, J.; Jakuczun, H.; Lebecka, R.; Marczewski, W.; Gebhardt, C.; Zimnoch-Guzowska, E. The Novel, Major Locus Rpi-Phu1 for Late Blight Resistance Maps to Potato Chromosome IX and Is Not Correlated with Long Vegetation Period. Theor. Appl. Genet. 2006, 113, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Rietman, H.; Bijsterbosch, G.; Cano, L.M.; Lee, H.-R.; Vossen, J.H.; Jacobsen, E.; Visser, R.G.F.; Kamoun, S.; Vleeshouwers, V.G.A.A. Qualitative and Quantitative Late Blight Resistance in the Potato Cultivar Sarpo Mira Is Determined by the Perception of Five Distinct RXLR Effectors. Mol. Plant-Microbe Interact. 2012, 25, 910–919. [Google Scholar] [CrossRef] [PubMed]
- Dalamu, D.; Tiwari, J.K.; Bairwa, A.; Bhatia, N.; Zinta, R.; Kaushal, N.; Kumar, V.; Sharma, A.K.; Sharma, S.; Choudhary, B.; et al. Resistance Evaluation for Native Potato Accessions against Late Blight Disease and Potato Cyst Nematodes by Molecular Markers and Phenotypic Screening in India. Life 2022, 13, 33. [Google Scholar] [CrossRef] [PubMed]
- On Approval of the List of Quarantine Objects and Alien Species in Relation to Which Plants Quarantine Measures Shall Be Established and Implemented, and the List of Especially Dangerous Harmful Organisms—LIS “Adilet”). Available online: https://adilet.zan.kz/rus/docs/V1500011739 (accessed on 13 July 2024).
- EPPO Standards. Available online: https://www.eppo.int/RESOURCES/eppo_standards (accessed on 14 July 2024).
- Turner, S.J.; Subbotin, S.A. Cyst Nematodes. In Plant Nematology; CAB International: Wallingford, UK, 2006; pp. 91–122. [Google Scholar]
- Paal, J.; Henselewski, H.; Muth, J.; Meksem, K.; Menéndez, C.M.; Salamini, F.; Ballvora, A.; Gebhardt, C. Molecular Cloning of the Potato Gro1-4 Gene Conferring Resistance to Pathotype Ro1 of the Root Cyst Nematode Globodera Rostochiensis, Based on a Candidate Gene Approach. Plant J. 2004, 38, 285–297. [Google Scholar] [CrossRef]
- Van Der Voort, J.R.; Kanyuka, K.; Van Der Vossen, E.; Bendahmane, A.; Mooijman, P.; Klein-Lankhorst, R.; Stiekema, W.; Baulcombe, D.; Bakker, J. Tight Physical Linkage of the Nematode Resistance Gene Gpa2 and the Virus Resistance Gene Rx on a Single Segment Introgressed from the Wild Species Solanum tuberosum subsp. Andigena CPC 1673 into Cultivated Potato. Mol. Plant-Microbe Interact. 1999, 12, 197–206. [Google Scholar] [CrossRef]
- Williamson, V.; Kumar, A. Nematode Resistance in Plants: The Battle Underground. Trends Genet. 2006, 22, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Karpova, O.; Alexandrova, A.M.; Nargilova, R.; Kryldakov, R.; Yekaterinskaya, E.; Romadanova, N.; Kushnarenko, S.; Iskakov, B. Diagnosis of potato viruses in Kazakhstan: Molecular characterisation of isolates. Eurasian J. Appl. Biotechnol. 2019. [Google Scholar] [CrossRef]
- Beisembina, B.; Kuzminova, O.A.; Udovitsky, A.S.; Musynov, K.M.; Khasanov, V.T.; Vologin, S.G. First results of assessment potato varieties of Kazakhstan breeding on the availability of resistance genes to potato virus Y with the help of molecular markers. Agric. Sci. 2019, 326, 23–27. [Google Scholar] [CrossRef]
- Daurov, D.; Daurova, A.; Karimov, A.; Tolegenova, D.; Volkov, D.; Raimbek, D.; Zhambakin, K.; Shamekova, M. Determining Effective Methods of Obtaining Virus-Free Potato for Cultivation in Kazakhstan. Am. J. Potato Res. 2020, 97, 367–375. [Google Scholar] [CrossRef]
- Gritsenko, D.; Aubakirova, K.; Pozharskiy, A. SSR Profiling of Potato Cultivars Resistant to Pathogens. 2021, p. 88. Available online: https://www.elibrary.ru/item.asp?id=46169889 (accessed on 5 April 2024).
- Azhimakhan, M.; Khassanov, V.; Vologin, S.; Baigeng, H. Evaluation of potato breeding material for the presence of DNA markers of resistance to potato X-virus. Sci. J. Bull. Sci. Kazakh Agrotech. Res. Univ. Named S. seifullin 2023, 1, 102–109. [Google Scholar]
- Aboul-Maaty, N.A.-F.; Oraby, H.A.-S. Extraction of High-Quality Genomic DNA from Different Plant Orders Applying a Modified CTAB-Based Method. Bull. Natl. Res. Cent. 2019, 43, 25. [Google Scholar] [CrossRef]
- Gallagher, S.R. Quantitation of DNA and RNA with Absorption and Fluorescence Spectroscopy. In Current Protocols in Human Genetics; Wiley Online Library: Hoboken, NJ, USA, 1994; p. A-3D. [Google Scholar]
- Valkonen, J.P.T.; Wiegmann, K.; Hämäläinen, J.H.; Marczewski, W.; Watanabe, K.N. Evidence for Utility of the Same PCR-based Markers for Selection of Extreme Resistance to Potato Virus Y Controlled by Rysto of Solanum stoloniferum Derived from Different Sources. Ann. Appl. Biol. 2008, 152, 121–130. [Google Scholar] [CrossRef]
- Ottoman, R.J.; Hane, D.C.; Brown, C.R.; Yilma, S.; James, S.R.; Mosley, A.R.; Crosslin, J.M.; Vales, M.I. Validation and Implementation of Marker-Assisted Selection (MAS) for PVY Resistance (Ry Adg Gene) in a Tetraploid Potato Breeding Program. Am. J. Pot Res. 2009, 86, 304–314. [Google Scholar] [CrossRef]
- Schultz, L.; Cogan, N.O.; McLean, K.; Dale, M.F.B.; Bryan, G.J.; Forster, J.W.; Slater, A.T. Evaluation and Implementation of a Potential Diagnostic Molecular Marker for H1-conferred Potato Cyst Nematode Resistance in Potato (Solanum tuberosum L.). Plant Breed. 2012, 131, 315–321. [Google Scholar] [CrossRef]
- Oberhagemann, P.; Chatot-Balandras, C.; Schäfer-Pregl, R.; Wegener, D.; Palomino, C.; Salamini, F.; Bonnel, E.; Gebhardt, C. A Genetic Analysis of Quantitative Resistance to Late Blight in Potato: Towards Marker-Assisted Selection. Mol. Breed. 1999, 5, 399–415. [Google Scholar] [CrossRef]
- Hämäläinen, J.H.; Sorri, V.A.; Watanabe, K.N.; Gebhardt, C.; Valkonen, J.P.T. Molecular Examination of a Chromosome Region That Controls Resistance to Potato Y and A Potyviruses in Potato. Theor. Appl. Genet. 1998, 96, 1036–1043. [Google Scholar] [CrossRef]
- Kasai, K.; Morikawa, Y.; Sorri, V.A.; Valkonen, J.P.T.; Gebhardt, C.; Watanabe, K.N. Development of SCAR Markers to the PVY Resistance Gene Ryadg Based on a Common Feature of Plant Disease Resistance Genes. Genome 2000, 43, 1–8. [Google Scholar] [CrossRef]
- Mori, K.; Mukojima, N.; Nakao, T.; Tamiya, S.; Sakamoto, Y.; Sohbaru, N.; Hayashi, K.; Watanuki, H.; Nara, K.; Yamazaki, K.; et al. Germplasm Release: Saikai 35, a Male and Female Fertile Breeding Line Carrying Solanum Phureja-Derived Cytoplasm and Potato Cyst Nematode Resistance (H1) and Potato Virus Y Resistance (RyChc) Genes. Am. J. Pot Res. 2012, 89, 63–72. [Google Scholar] [CrossRef]
- Cernák, I.; Taller, J.; Wolf, I.; Fehér, E.; Babinszky, G.; Alföldi, Z.; Csanádi, G.; Polgár, Z. Analysis of the Applicability of Molecular Markers Linked to the PVY Extreme Resistance Gene Rysto, and the Identification of New Markers. Acta Biol. Hung. 2008, 59, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Cernák, I.; Decsi, K.; Nagy, S.; Wolf, I.; Polgár, Z.; Gulyás, G.; Hirata, Y.; Taller, J. Development of a Locus-Specific Marker and Localization of the Rysto Gene Based on Linkage to a Catalase Gene on Chromosome XII in the Tetraploid Potato Genome. Breed. Sci. 2008, 58, 309–314. [Google Scholar] [CrossRef][Green Version]
- De Jong, W.; Forsyth, A.; Leister, D.; Gebhardt, C.; Baulcombe, D.C. A Potato Hypersensitive Resistance Gene against Potato Virus X Maps to a Resistance Gene Cluster on Chromosome 5. Theory Appl. Genet. 1997, 95, 246–252. [Google Scholar] [CrossRef]
- Bendahmane, A.; Kanyuka, K.; Baulcombe, D.C. High-Resolution Genetical and Physical Mapping of the Rx Gene for Extreme Resistance to Potato Virus X in Tetraploid Potato. Theor. Appl. Genet. 1997, 95, 153–162. [Google Scholar] [CrossRef]
- Mori, K.; Sakamoto, Y.; Mukojima, N.; Tamiya, S.; Nakao, T.; Ishii, T.; Hosaka, K. Development of a Multiplex PCR Method for Simultaneous Detection of Diagnostic DNA Markers of Five Disease and Pest Resistance Genes in Potato. Euphytica 2011, 180, 347–355. [Google Scholar] [CrossRef]
- Mihovilovich, E.; Aponte, M.; Lindqvist-Kreuze, H.; Bonierbale, M. An RGA-Derived SCAR Marker Linked to PLRV Resistance from Solanum Tuberosum ssp. Andigena. Plant Mol. Biol. Rep. 2014, 32, 117–128. [Google Scholar] [CrossRef]
- Witek, K.; Strzelczyk-Żyta, D.; Hennig, J.; Marczewski, W. A Multiplex PCR Approach to Simultaneously Genotype Potato towards the Resistance Alleles Ry-f Sto and Ns. Mol. Breed. 2006, 18, 273–275. [Google Scholar] [CrossRef]
- Marczewski, W.; Hennig, J.; Gebhardt, C. The Potato Virus S Resistance Gene Ns Maps to Potato Chromosome VIII. Theory Appl. Genet. 2002, 105, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Gebhardt, C.; Ballvora, A.; Walkemeier, B.; Oberhagemann, P.; Schüler, K. Assessing Genetic Potential in Germplasm Collections of Crop Plants by Marker-Trait Association: A Case Study for Potatoes with Quantitative Variation of Resistance to Late Blight and Maturity Type. Mol. Breed. 2004, 13, 93–102. [Google Scholar] [CrossRef]
- Park, T.-H.; Foster, S.; Brigneti, G.; Jones, J.D.G. Two Distinct Potato Late Blight Resistance Genes from Solanum Berthaultii Are Located on Chromosome 10. Euphytica 2009, 165, 269–278. [Google Scholar] [CrossRef]
- Śliwka, J.; Jakuczun, H.; Kamiński, P.; Zimnoch-Guzowska, E. Marker-Assisted Selection of Diploid and Tetraploid Potatoes carryingRpi-Phu1, a Major Gene for Resistance toPhytophthora Infestans. J. Appl. Genet. 2010, 51, 133–140. [Google Scholar] [CrossRef]
- Tomczyńska, I.; Stefańczyk, E.; Chmielarz, M.; Karasiewicz, B.; Kamiński, P.; Jones, J.D.G.; Lees, A.K.; Śliwka, J. A Locus Conferring Effective Late Blight Resistance in Potato Cultivar Sárpo Mira Maps to Chromosome XI. Theory Appl. Genet. 2014, 127, 647–657. [Google Scholar] [CrossRef]
- Galek, R.; Rurek, M.; De Jong, W.S.; Pietkiewicz, G.; Augustyniak, H.; Sawicka-Sienkiewicz, E. Application of DNA Markers Linked to the Potato H1 Gene Conferring Resistance to Pathotype Ro1 of Globodera Rostochiensis. J. Appl. Genet. 2011, 52, 407–411. [Google Scholar] [CrossRef]
- Bakker, E.; Achenbach, U.; Bakker, J.; Van Vliet, J.; Peleman, J.; Segers, B.; Van Der Heijden, S.; Van Der Linde, P.; Graveland, R.; Hutten, R.; et al. A High-Resolution Map of the H1 Locus Harbouring Resistance to the Potato Cyst Nematode Globodera Rostochiensis. Theory Appl. Genet. 2004, 109, 146–152. [Google Scholar] [CrossRef]
- Moloney, C.; Griffin, D.; Jones, P.W.; Bryan, G.J.; McLean, K.; Bradshaw, J.E.; Milbourne, D. Development of Diagnostic Markers for Use in Breeding Potatoes Resistant to Globodera Pallida Pathotype Pa2/3 Using Germplasm Derived from Solanum Tuberosum ssp. Andigena CPC 2802. Theory Appl. Genet. 2010, 120, 679–689. [Google Scholar] [CrossRef]
- Van De Vossenberg, B.T.L.H.; Prodhomme, C.; Vossen, J.H.; Van Der Lee, T.A.J. Synchytrium Endobioticum, the Potato Wart Disease Pathogen. Mol. Plant Pathol. 2022, 23, 461–474. [Google Scholar] [CrossRef]
- Jacobs, J.M.E.; van Eck, J.; Horsman, K.; Arens, P.F.P.; Verkerk-Bakker, B.; Jacobsen, E.; Pereira, A.; Stiekema, J. Mapping of Resistance to the Potato Cyst Nematode Globodera Rostochiensis from the Wild Potato Species Solarium Vernei. Mol. Breed. 1996, 2, 51–60. [Google Scholar] [CrossRef]
- Gebhardt, C.; Bellin, D.; Henselewski, H.; Lehmann, W.; Schwarzfischer, J.; Valkonen, J.P.T. Marker-Assisted Combination of Major Genes for Pathogen Resistance in Potato. Theory Appl. Genet. 2006, 112, 1458–1464. [Google Scholar] [CrossRef] [PubMed]
- Biryukova, V.; Shmyglya, I.; Abrosimova, S.; Zapekina, T.; Meleshin, A.; Mityushkin, A.; Manankov, V. The search for sources of resistance genes to pathogens among the samples of plant breeding and genetics collections of All-Russian A.G. Lorh Research Institute of Potato Farming using molecular markers. Potato Prot. 2015, 1, 3–7. [Google Scholar]
- Krasavin, V.; Moshnyakov, A.; Sharipova, D.; Koibagarov, E.; Krasavina, V. Study collection of the potatoes for main economic and securities signs in the South-East of Kazakhstan. Potato Grow. 2022, 21, 104–111. [Google Scholar]
- Sudha, R.; Venkatasalam, E.P.; Bairwa, A.; Bhardwaj, V.; Dalamu; Sharma, R. Identification of Potato Cyst Nematode Resistant Genotypes Using Molecular Markers. Sci. Hortic. 2016, 198, 21–26. [Google Scholar] [CrossRef]
- Totsky, I.V.; Rozanova, I.V.; Safonova, A.D.; Batov, A.S.; Gureeva, Y.A.; Khlestkina, E.K.; Kochetov, A.V. Genotyping of Potato Samples from the GenAgro ICG SB RAS Collection Using DNA Markers of Genes Conferring Resistance to Phytopathogens. Vavilov J. Genet. Breed. 2021, 25, 677–686. [Google Scholar] [CrossRef]
- Khiutti, A.; Afanasenko, O.; Antonova, O.; Shuvalov, O.; Novikova, L.; Krylova, E.; Chalaya, N.; Mironenko, N.; Spooner, D.M.; Gavrilenko, T. Characterization of Resistance to Synchytrium Endobioticum in Cultivated Potato Accessions from the Collection of Vavilov Institute of Plant Industry. Plant Breed. 2012, 131, 744–750. [Google Scholar] [CrossRef]
- Milczarek, D.; Flis, B.; Przetakiewicz, A. Suitability of Molecular Markers for Selection of Potatoes Resistant to Globodera spp. Am. J. Pot Res. 2011, 88, 245–255. [Google Scholar] [CrossRef]
- Antonova, O.Y.; Shvachko, N.A.; Novikova, L.Y.; Shuvalov, O.Y.; Kostina, L.I.; Klimenko, N.S.; Shuvalova, A.R.; Gavrilenko, T.A. Genetic diversity of potato varieties bred in Russia and near-abroad countries based on polymorphism of SSR-loci and markers associated with resistance R-genes. Vavilov J. Genet. Breed. 2016, 20, 596–606. [Google Scholar] [CrossRef]
- Khiutti, A.; Antonova, O.Y.; Mironenko, N.; Gavrilenko, T.; Afanasenko, O. Potato Resistance to Quarantine Diseases. Russ. J. Genet. Appl. Res. 2017, 7, 833–844. [Google Scholar] [CrossRef]
- Saynakova, A.; Romanova, M.; Krasnikov, S.; Litvinchuk, O.; Alekseev, Y.I.; Nikulin, A.; Terentjeva, E. Testing Potato Collection Samples for the Presence of Genes for Resistance to Phytopathogens by Means of DNA Markers. 2018. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/20193461113 (accessed on 20 June 2024).
- Rogozina, E.V.; Ulianich, P.S.; Volkov, V.A.; Chalaya, N.A.; Potokina, E.K. Genetic Diversity of Solanum Pinnatisectum Dun. and Solanum Chacoense Bitt. by Resistance to Potato Virus Y and Results of DNA Analysis. Russ. J. Genet. 2019, 55, 1330–1337. [Google Scholar] [CrossRef]
- Sereda, G.; Zhukova, M.; Gurlenya, N. Potato Testing for Wart Resistance in Belarus. Agronomijas Vēstis 2008, 11, 141–146. [Google Scholar]
- Dimitrova, L.; Laginova, M.; Becheva, A.; Van Leeuwen, G.C.M. Occurrence of Potato Wart Disease (Synchytrium endobioticum) in Bulgaria: Identification of Pathotype(s) Present. EPPO Bull. 2011, 41, 195–202. [Google Scholar] [CrossRef]
- Prodhomme, C.; Van Arkel, G.; Plich, J.; Tammes, J.E.; Rijk, J.; Van Eck, H.J.; Visser, R.G.F.; Vossen, J.H. A Hitchhiker’s Guide to the Potato Wart Disease Resistance Galaxy. Theor. Appl. Genet. 2020, 133, 3419–3439. [Google Scholar] [CrossRef]
- Elison, G.L.; Park, J.; Novy, R.G.; Whitworth, J.L. A Potential New Source of Extreme Resistance to Potato Virus Y in the Potato Variety Bistra. Am. J. Potato Res. 2024, 101, 248–256. [Google Scholar] [CrossRef]
- Funke, C.N.; Tran, L.T.; Karasev, A.V. Screening Three Potato Cultivars for Resistance to Potato Virus Y Strains: Broad and Strain-Specific Sources of Resistance. Am. J. Potato Res. 2024, 101, 132–141. [Google Scholar] [CrossRef]
- Sajeevan, R.S.; Abdelmeguid, I.; Saripella, G.V.; Lenman, M.; Alexandersson, E. Comprehensive Transcriptome Analysis of Different Potato Cultivars Provides Insight into Early Blight Disease Caused by Alternaria Solani. BMC Plant Biol. 2023, 23, 130. [Google Scholar] [CrossRef]
- Li, C.; Yuan, B.; Zhang, C.; Yao, Q.; He, H.; Wang, Q.; Liang, J.; Li, N.; Zhu, X.; Wang, Z. Revealing Key Genes and Pathways in Potato Scab Disease Resistance through Transcriptome Analysis. Agronomy 2024, 14, 291. [Google Scholar] [CrossRef]
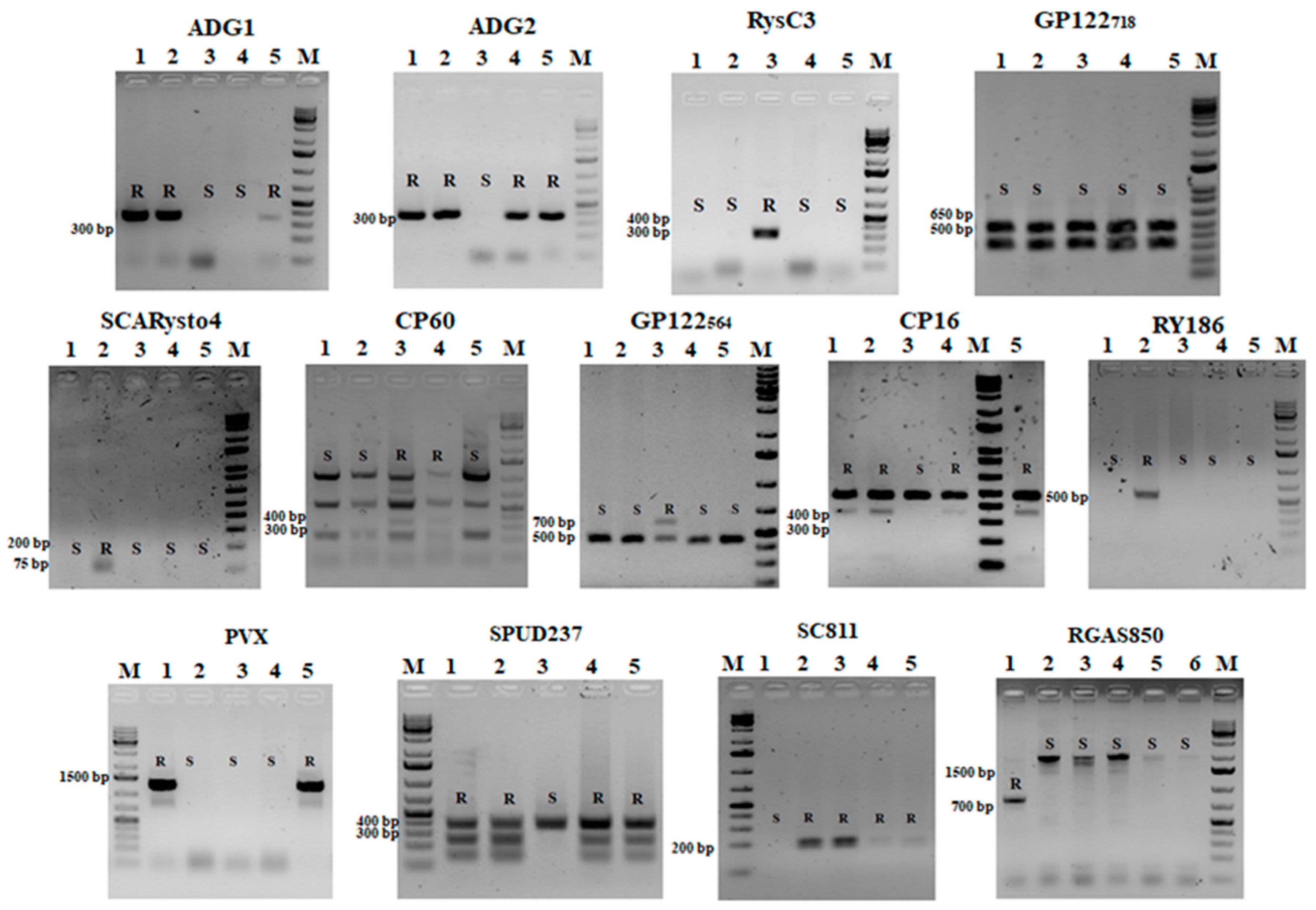
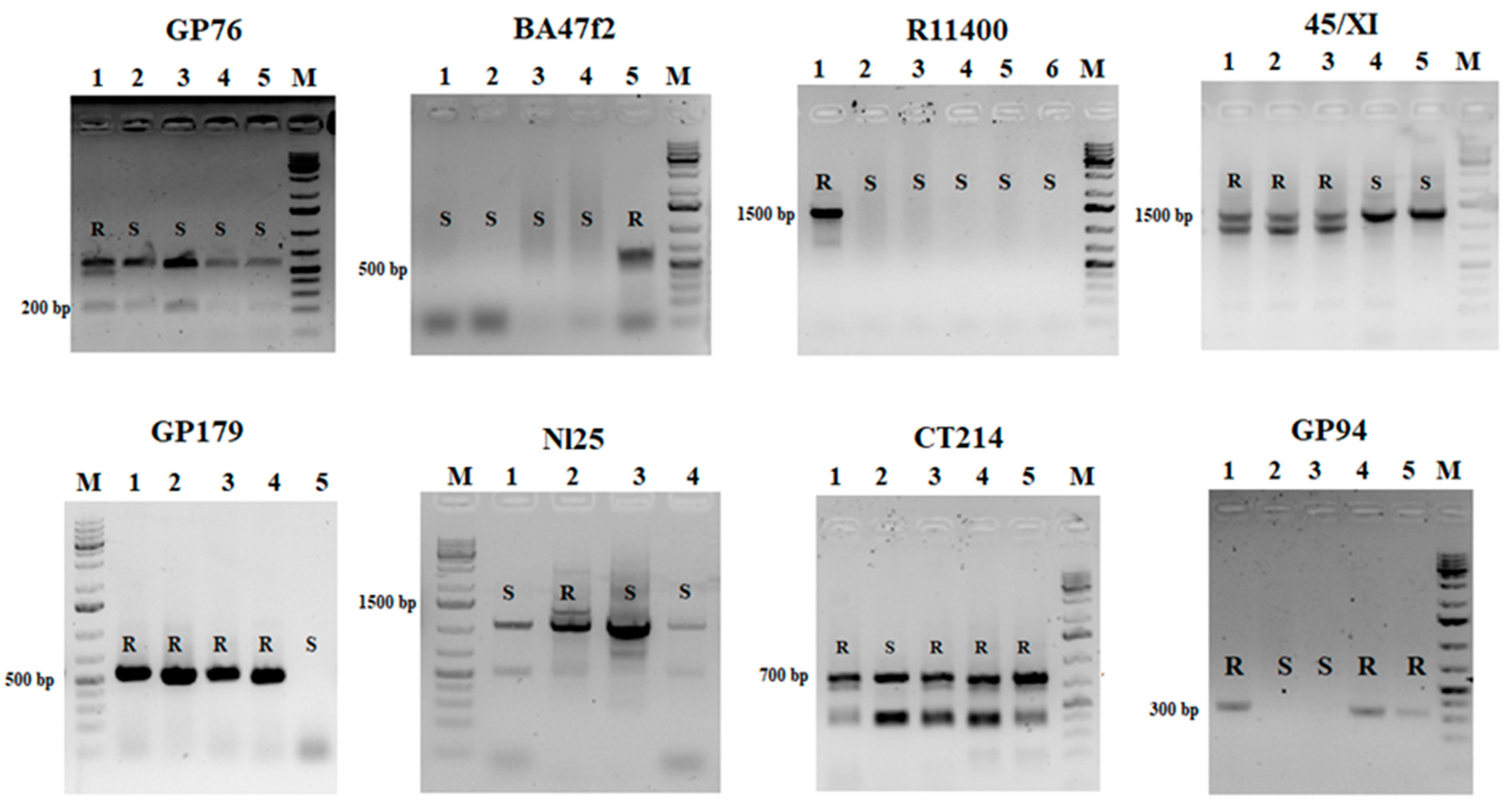
| Marker | Gene/QTL | Type | Forward and Reverse Primer Sequences (5′-3′) | PCR Conditions | Size (bp) | Resistance | References |
|---|---|---|---|---|---|---|---|
| Potato wart | |||||||
| Potato virus | |||||||
| GP122564 | Ry-fsto | CAPS/EcoRV | F-TATTTTAGGGGTACTTCTTTCTTATGTT; R-CTGTCAAAAAAATTCACTTGCATAACTAC | 94 °C—1 min, 40 cycles (93 °C—20 s, 53 °C—25 s, 72 °C—1 min), 72 °C—5 min | 718 bp 564 bp | PVYN, | [1] |
| GP122718 | Ry-fsto | CAPS/EcoRV | F-TATTTTAGGGTACTTCTTTCTTA; R-GCACTCAATAGCCCTTCTT | 94 °C—1 min, 40 cycles (93 °C—20 s, 53 °C—25 s, 72 °C—1 min), 72 °C—5 min | 718 bp 564 bp 400 bp | PVYN, | [4] |
| ADG1 | Ryadg | PCR-baced, AS | F-CACACTCTCGTATCAGTTTGA; R-ATTTAATAGCGTGACAGT CAAC | 93 °C—2 min; 35 × (93 °C—45 s, 55 °C—45 s, 72 °C—80 s), 72 °C –10 min. | 356 bp | PVYO, PVYN, PVA | [29] |
| ADG2 | Ryadg | PCR-baced, AS | F-CACACTCTCGTATCAGTTTGA; R-ATTTAATAGCGTGACAGT CAAC | 93 °C—2 min; 35 × (93 °C—45 s, 55 °C—45 s, 72 °C—80 s), 72 °C –10 min. | 354 bp | PVYO, PVYN, PVA | [29] |
| RysC3 | Ryadg | SCAR | F-ATACACTCATCTAAATTTGATGG; R-AGGATATACGGCATCATTTTTCCGA | 93 °C—9 min, 35 × (94 °C—45 s, 60 °C—45 s, 72 °C—60 s), 72 °C—5 min | 321 bp | PVY | [30] |
| RY186 | Rychc | PCR-baced, AS | F-TGGTAGGGATATTTTCCTTAGA; R-GCAAATCCTAGGTTATCAACTCA | 95 °C—10 min, 35 × (94 °C—30 s, 55 °C—30 s, 72 °C—1.5 min), 72 °C—5 min | 587 bp | PVY | [31] |
| SCARysto4 | Rysto | SCAR | F-ATTCGTTCGCCTCTCTCCT R-TCATCACCCCTAACAAATACAA | 94 °C—1 min, 35 × (94 °C—30 s; 54 °C –60 s; 72 °C—60 s), 72 °C—10 min | 100 bp | PVY | [32,33] |
| SPUD237 | Nb | CAPS/AluI | F-TTCCTGCTGATACTGACTAGAAAACC R-AGCCAAGGAAAAGCTAGCATCCAAG | 94 °C—1 min, 35 × (94 °C—15 s; 55 °C—15 s; 72 °C—40 s), 72 °C—10 min | 3 resistance products | PVX | [34] |
| CP60 | Rx1 | CAPS/DdeI | F-CAGCCTACCGCGAAAGTGCCTTCG R-GCCAACCCCACGAGTTTCTCACTGAC | 94 °C—3 min, 35 × (92 °C—45 s, 58 °C—45 s, 72 °C—1 min), 72 °C—10 min | 350 bp | PVX | [35] |
| PVX | Rx1 | PCR-baced, AS | ATCTTGGTTTGAATACATGG CACAATATTGGAAGGATTCA | 94 °C—10 min, 35 × (94 °C—30 s, 58 °C—30, 72 °C—1 min), 72 °C—5 min | 1230 bp | PVX | [36] |
| RGASC850 | Rladg | SCAR | F-GAATGCCAGGAATGGGAAAGACTACTTT R-TCACATCCAAGCAAAACCAA | 95 °C—4 min 32 × (95 °C—45 s, 53 °C—1 min, 72 °C—1 min), 72 °C—10 min | 850 bp | PLRV | [37] |
| SC811 | Ns | CAPS/MboI | F-CGAACAAAATACGTAATGCATTGAATAA R-GACCT ATATCAGTCCCTTCTAATCCACTAT | 94 °C—60 s, 30 × (93 °C—15 s, 56 °C—20 s, 72 °C—60 s), 72 °C—5 min | 260 bp | PVS | [38] |
| CP16 | Ns | CAPS/HindIII | F-CTTAAACGCGTCAAGTGAAACT R-TTAGGGACATACAAACAAACCTCA | 94 °C—60 s, 35 × (94 °C—15 s, 55 °C—15 s, 72 °C—60 s), 72 °C—3 min | 460 bp | PVS | [39] |
| Potato late blight | |||||||
| GP76 | unknown | CAPS/RsaI | F-ATGAAGCAACACTGATGCAA R-TTCTCCAATGAACGCAAACT | 93 °C—2 min, 40 × (93 °C—30 s, 55 °C—30 s, 72 °C—1 min), 72 °C—10 min | 500 bp | Phytophthora infestans | [28] |
| BA47f2 | R1 | PCR-baced, AS | F-TAACCAACATTATCTTCTTTGCC R-GAATTTGGAGAGGGGTTTGCTG | 93 °C—2 min, 40 × (93 °C—45 s, 55 °C—45 s and 72 °C—90 s), 72 °C—10 min | 650 bp | Phytophthora infestans | [40] |
| R11400 | R1 | PCR-baced, AS | F-CACTCGTGACATATCCTCACTA R-CAACCCTGGCATGCCACG | 93 °C—2 min, 40 × (93 °C—45 s, 55 °C—45 s and 72 °C—90 s), 72 °C—10 min | 1400 bp | Phytophthora infestans | [7] |
| GP179 | R1 | PCR-baced, AS | F-GGTTTTAGTGATTGTGCTGC R-AATTTCAGACGAGTAGGCACT | 93 °C—2 min, 40 × (93 °C—45 s; 55 °C—45 s; 72 °C—1min 20 s), 72 °C—10 min | 570 bp | Phytophthora infestans | [40] |
| CT214 | Rpi-ber1 | CAPS/HinfI | F-CGCGAAAGAGTGCTGATAG R-CCGCTGCCTATGGAGAGT | 94 °C—5 min, 35 × (94 °C—45s, 55 °C—45 s, 72 °C—1 min), 72 °C—8 min | 3 resistance products | Phytophthora infestans | [41] |
| GP94 | Rpi-phu1 | PCR-baced, AS | F-ATGTATCACAATCACATTCTTGCTC R-TGTAAAACCAACAAGTAGTGTTGC | 2 min— 95 °C, 40 × (93 °C—1 min, 56 °C—1 min, 72 °C—1 min), 72 °C—10 min | 350 bp | Phytophthora infestans | [42] |
| 45/XI | Rpi-Smira1 | PCR-baced, AS | F-AGAGAGGTTGTTTCCGATAGACC R-TCGTTGTAGTTGTCATTCCACAC | 94 °C—3 min; 39 × (94 °C—15 s, 55 °C—15 s, 72 °C—60 s), 72 °C– 7 min | 900–1500 bp | Phytophthora infestans | [43] |
| Potato cyst nematodes | |||||||
| TG689 | H1 | PCR-baced, AS | F-TAAAACTCTTGGTTATAGCCTAT R-CAATAGAATGTGTTGTTTCACCAA | 94 °C—5 min, 35 × (94 °C—45 s, 55 °C—45 s, and 72 °C—1 min), 72 °C—8 min | 141 bp | Globodera rostochiensis, Ro 1 | [44] |
| 239E4left | H1 | CAPS/AluI | F-GGCCCCACAAACAAGAAAAC R-AGGTACCTCCATCTCATTTTGTAAG | 94 °C—3 min 35 × (94 °C—30 s, 51 °C—30s, 72 °C—90 s), 72 °C 5 min | 230 bp | Globodera rostochiensis | [45] |
| Gro1-4 | Gro1-4 | SCAR | F-TCTTTGGAGATACTGATTCTCA R-CGACCTAAAATGAAAAGCATCT | 94 °C—3 min, 35 × (92 °C—45 s, 52 °C—45 s, 72 °C—1 min), 72 °C—10 min | 602 bp | Globodera rostochiensis, Ro 1 | [15] |
| N146 | H1 | SCAR | F-AAGCTCTTGCCTAGTGCTC R-AGGCGGAACATGCCATG | 94 °C—10 min, 35 × (94 °C—30 s, 55 °C—30 s, 72 °C—1 min), 72 °C—5 min | 506 bp | Globodera rostochiensis | [36] |
| N195 | H1 | SCAR | F-TGGAAATGGCACCCACTA R-CATCATGGTTTCACTTGTCAC | 94 °C—10 min, 35 × (94 °C—30 s, 55 °C—30 s, 72 °C—1 min), 72 °C—5 min | 337 bp | Globodera rostochiensis | [36] |
| U14 | GroV1 | SCAR | F-GGGCTTGTATAAGACCTCCGAGAGG R-CCCTTCCTTGGGTAGTTTGAGCG | 92 °C, 7 min; 25 × (92 °C, 1 min; 57 °C, 1 min; 72 °C, 2 min); 72 °C, 5 min | 366 bp | Globodera rostochiensis | [46] |
| X02 | GroV1 | PCR-baced, AS | F-CCACCAAACCCATAAAGCTGC R-TGTGAATTGGTATGAATCTGCAACC | 92 °C, 7 min; 25 × (92 °C, 1 min; 50 °C, 1 min; 72 °C, 2 min); 72 °C, 5 min | 854 bp | Globodera rostochiensis | [46] |
| C237 | GpaIVSadg | CAPS/TaqI | F-GCAGTCCTAATTGCACGTAACA R-CTTACTTGGGCAACCCAGAAT | 94 °C—10 min, 35 × (at 94 °C—30 s, 60 °C—30 s, 72 °C—1 min), 72 °C—5 min | 423 bp | Globodera pallida, Pa2/3 | [47] |
| GP34 | Gpa2 | CAPS/TaqI | F-CGTTGCTAGGTAAGCATGAAGAAG R-GTTATCGTTGATTTCTCGTTCCG | 94 °C—3 min, 35 × (94 °C—15 s, 62 °C—15 s, 72 °C—1 min), 72 °C, 5 min | 495 bp | Globodera pallida, Pa2 | [35] |
| Nl25 | Sen1 | Pcr-baced, AS | F-TATTGTTAATCGTTACTCCCTC; R-AGAGTCGTTTTACCGACTCC | 93 °C—3 min, 35 × (93 °C—2 min, 93 °C—45s, 72 °C—1.5 min), 72 °C—10 min. | 1400 bp 1200 bp | Synchytrium endobioticum | [48] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adilbayeva, K.; Moisseyev, R.; Kolchenko, M.; Kenzhebekova, R.; Khassanov, V.; Beisembina, B.; Azhimakhan, M.; Tokbergenova, Z.; Sharipova, D.; Krasavin, V.; et al. Genetic Evaluation of Kazakhstani Potato Germplasm for Pathogen and Pest Resistance Using DNA Markers. Agronomy 2024, 14, 1923. https://doi.org/10.3390/agronomy14091923
Adilbayeva K, Moisseyev R, Kolchenko M, Kenzhebekova R, Khassanov V, Beisembina B, Azhimakhan M, Tokbergenova Z, Sharipova D, Krasavin V, et al. Genetic Evaluation of Kazakhstani Potato Germplasm for Pathogen and Pest Resistance Using DNA Markers. Agronomy. 2024; 14(9):1923. https://doi.org/10.3390/agronomy14091923
Chicago/Turabian StyleAdilbayeva, Kamila, Ruslan Moisseyev, Mariya Kolchenko, Roza Kenzhebekova, Vadim Khassanov, Bibigul Beisembina, Moldir Azhimakhan, Zhursinkul Tokbergenova, Dinara Sharipova, Valeriy Krasavin, and et al. 2024. "Genetic Evaluation of Kazakhstani Potato Germplasm for Pathogen and Pest Resistance Using DNA Markers" Agronomy 14, no. 9: 1923. https://doi.org/10.3390/agronomy14091923
APA StyleAdilbayeva, K., Moisseyev, R., Kolchenko, M., Kenzhebekova, R., Khassanov, V., Beisembina, B., Azhimakhan, M., Tokbergenova, Z., Sharipova, D., Krasavin, V., Pozharskiy, A., & Gritsenko, D. (2024). Genetic Evaluation of Kazakhstani Potato Germplasm for Pathogen and Pest Resistance Using DNA Markers. Agronomy, 14(9), 1923. https://doi.org/10.3390/agronomy14091923






