Characterization of Strubbelig-Receptor Family (SRF) Related to Drought and Heat Stress Tolerance in Upland Cotton (Gossypium hirsutum L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sequence Retrieval of SRF Protein Family Members
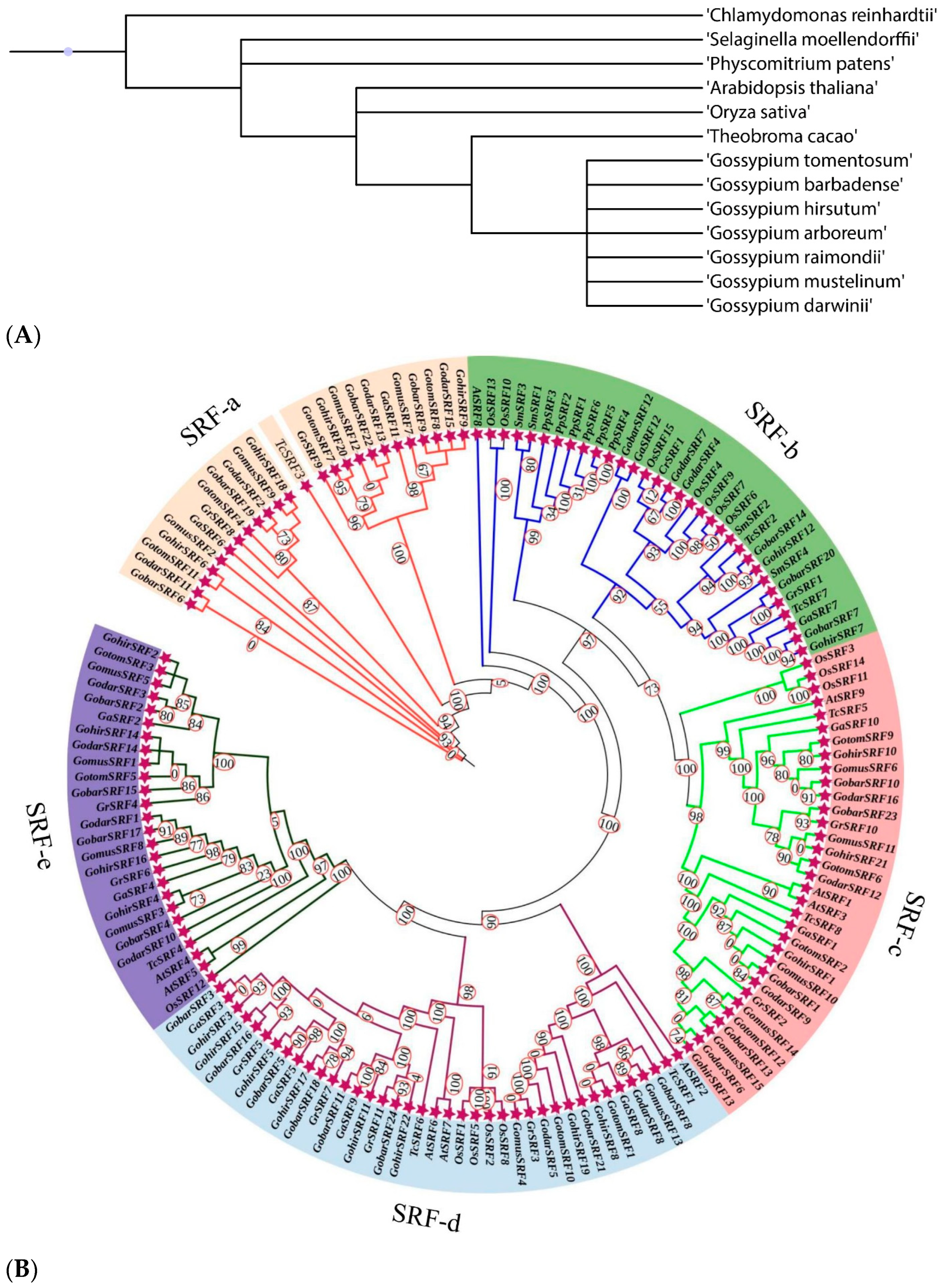
2.2. Construction of Phylostratum, and Phylogeny Tree
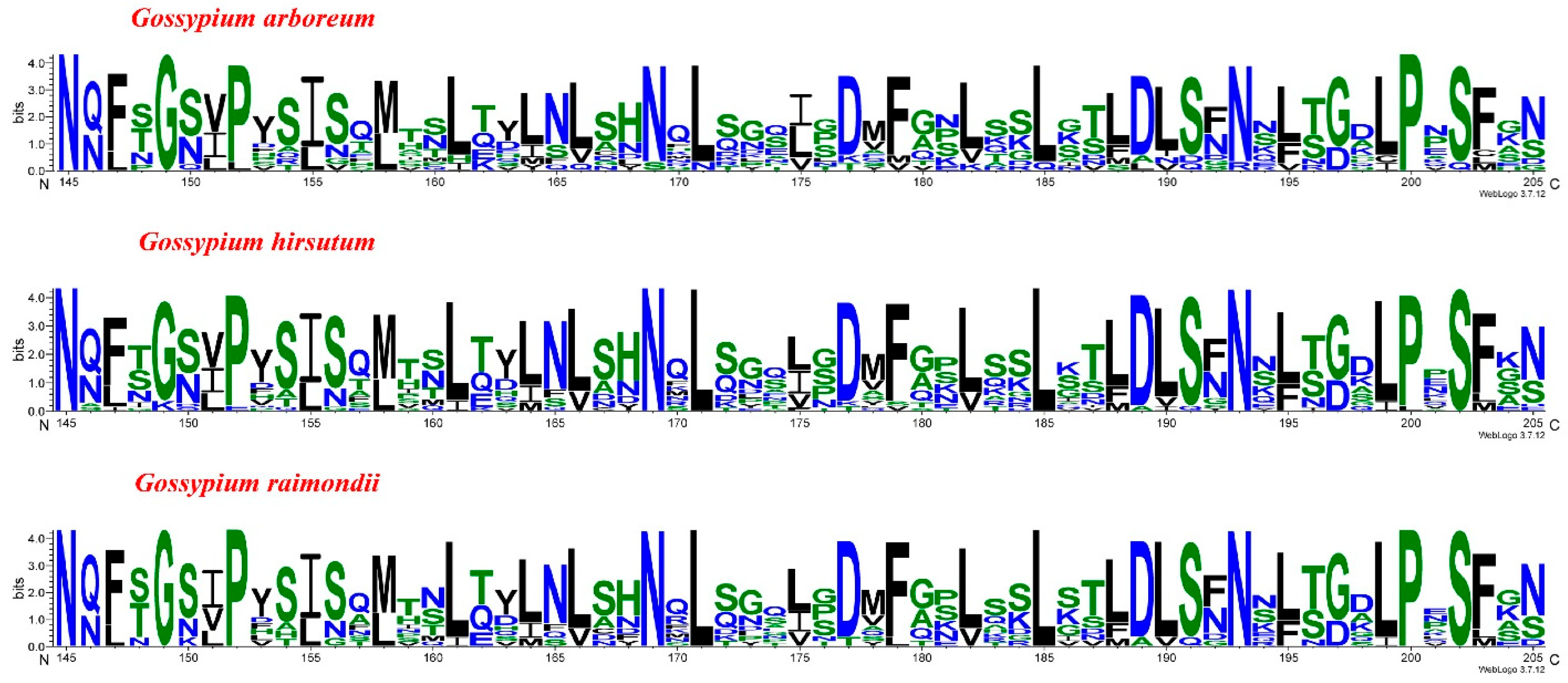
2.3. Sequence Logos and Conserved Amino Acid Analysis
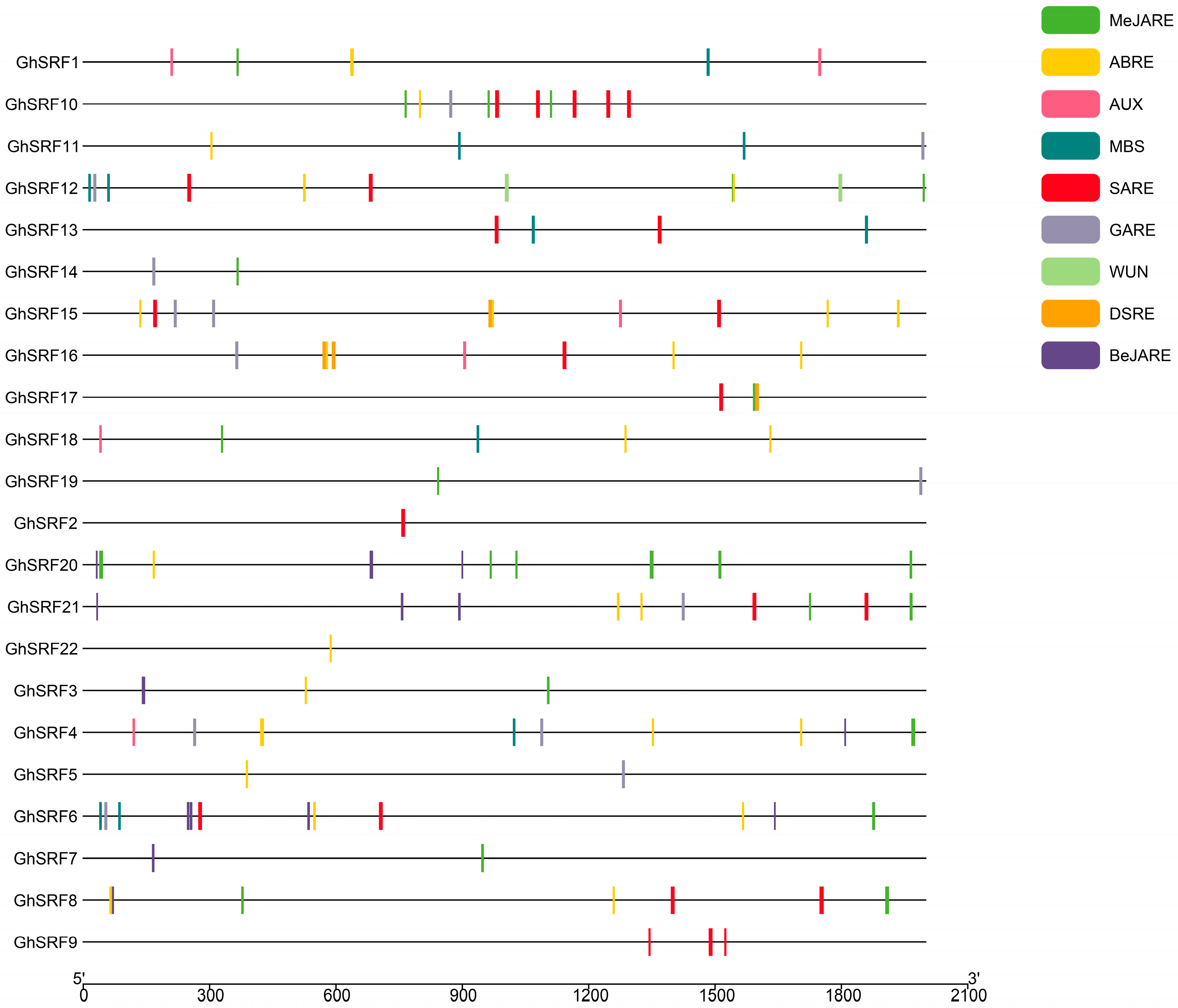
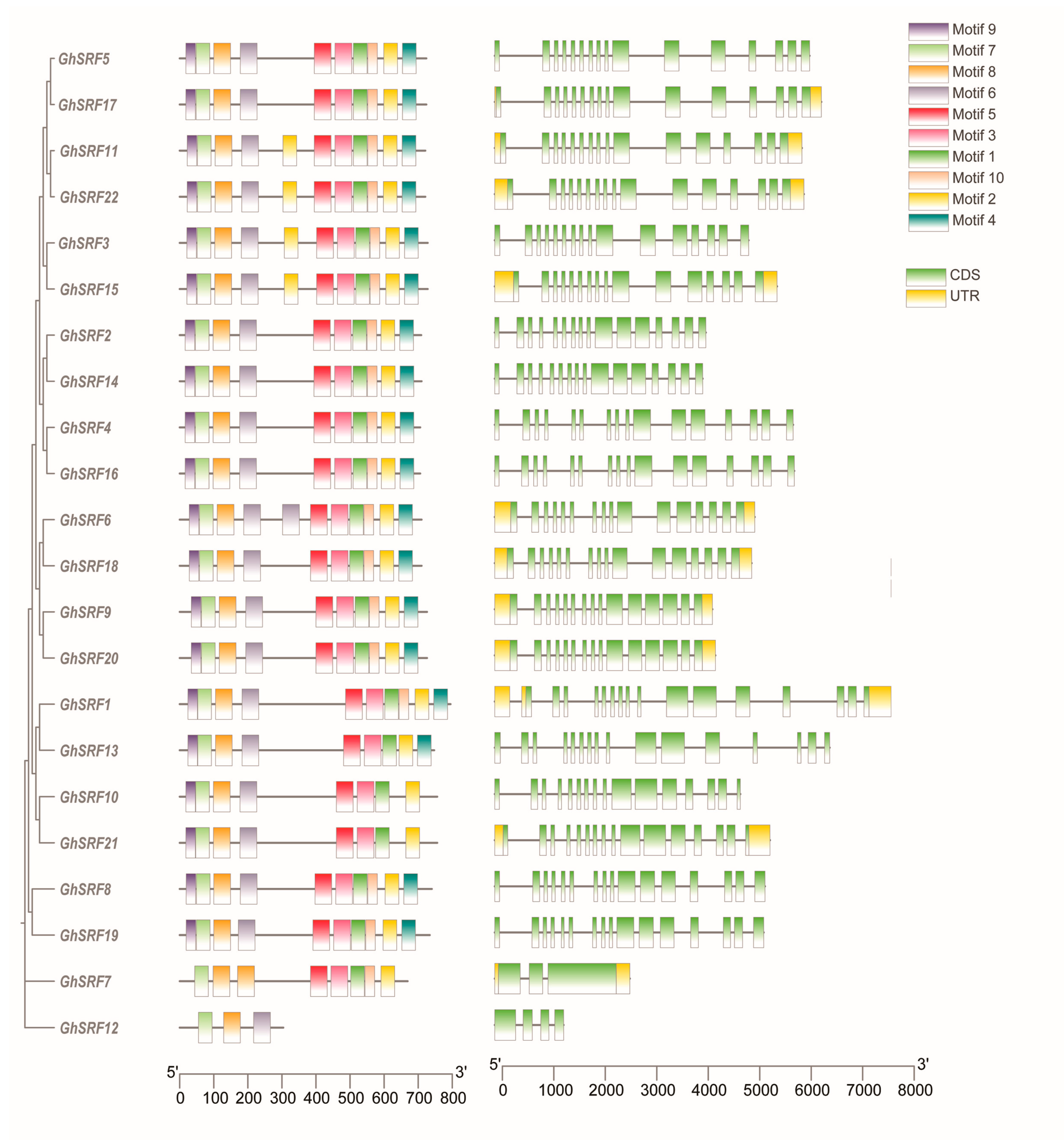
2.4. Promoter, Protein Motifs, and Gene Structure Analyses
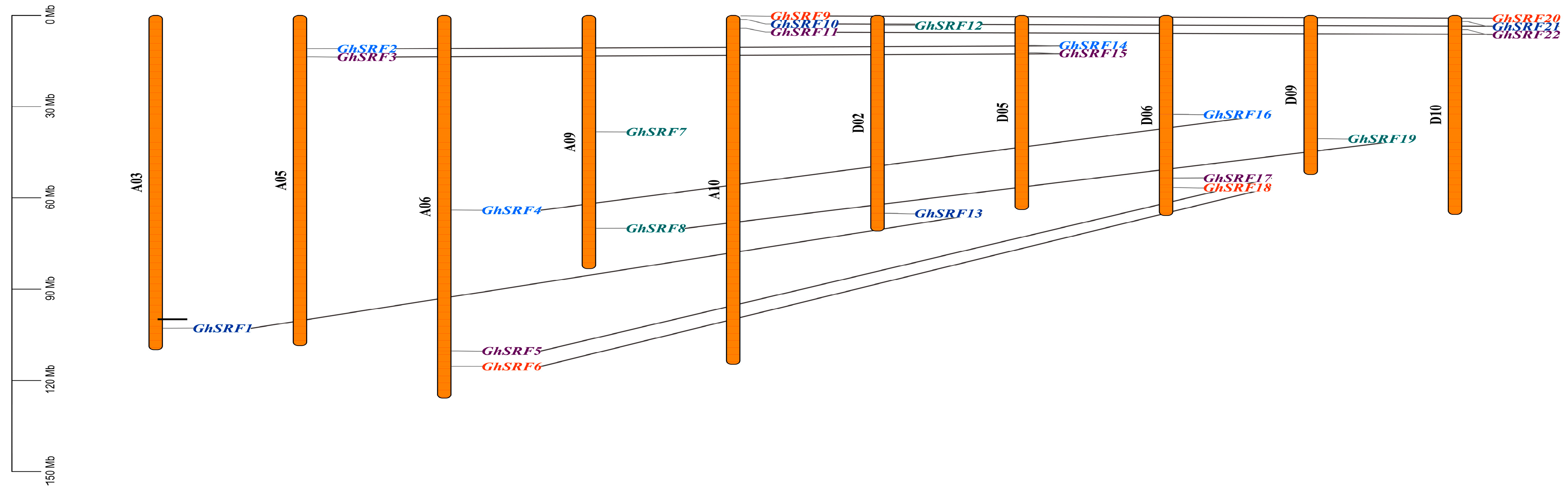
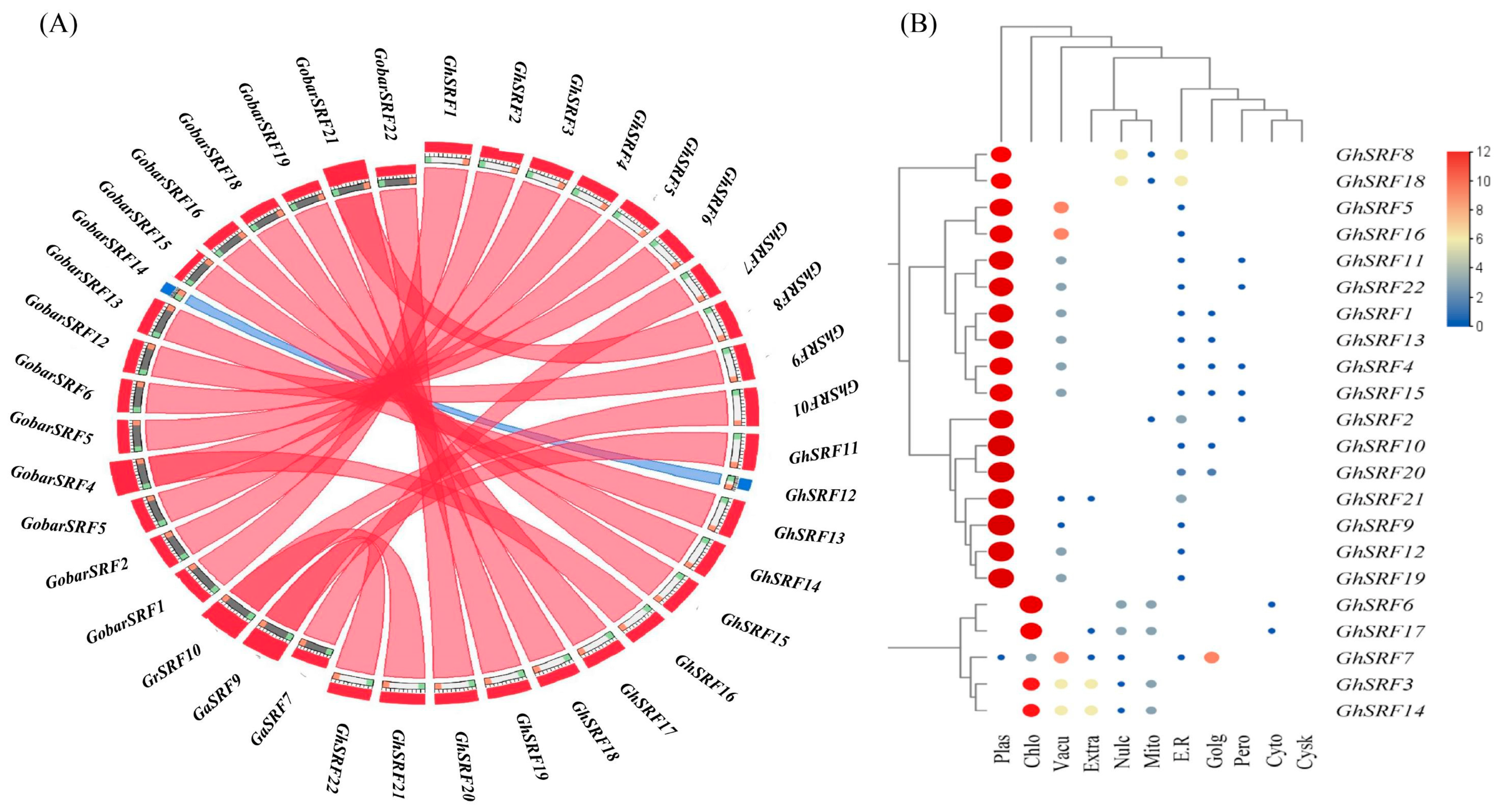
2.5. Sub-Cellular Localization, Gene Mapping on Chromosomes, Gene Duplication, Collinearity and Ratios of Ka/Ks
2.6. In Silico Expression Analysis of GhSRF Genes
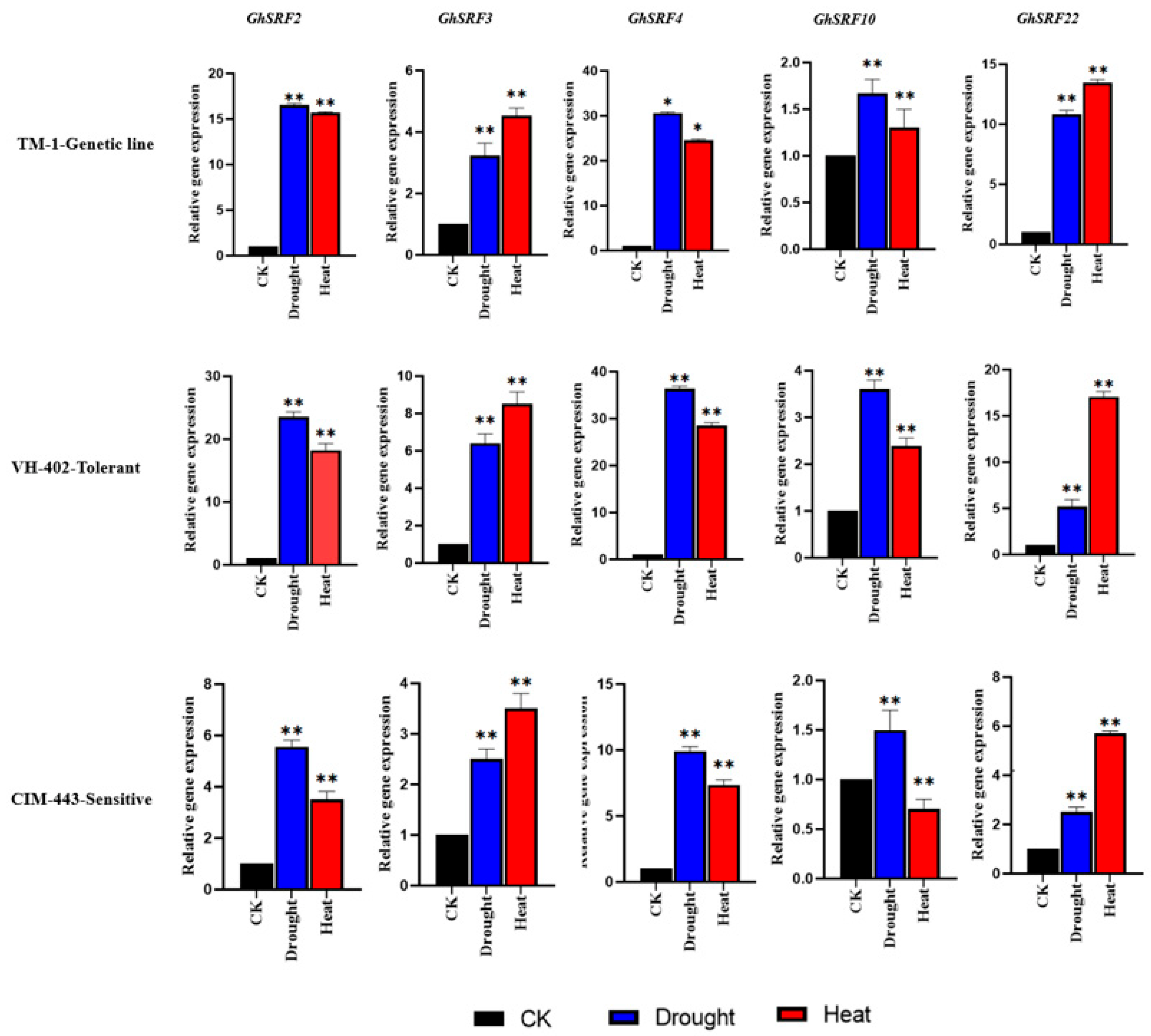
2.7. Expression Analysis and Validation of Candidate Genes
3. Results
3.1. Identification and Physiochemical Properties of SRF Genes in Cotton
3.2. Sequence Alignment, Phylostratum, and Phylogenetic Tree
3.3. Finding of Conserved Amino Acid Patterns
3.4. Analysis of Cis-Element in Promoter, Motif Finding and Gene Structure
3.5. Gene Mapping on Chromosomes, Ratio of Ka/Ks, Gene Duplication, and Sub-Cellular Localization
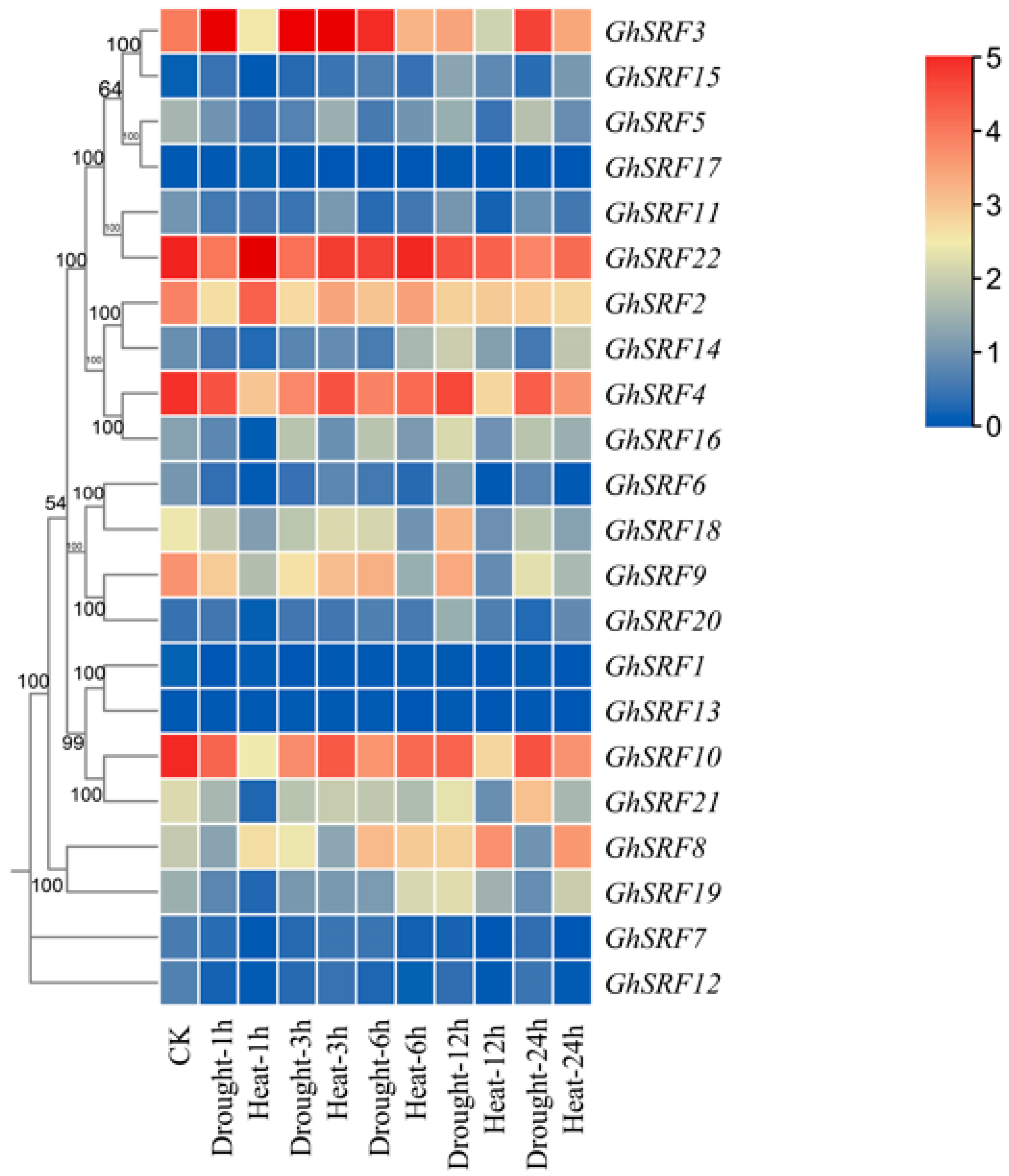
3.6. Gene Expression Analysis of GhSRF Genes Under Drought and Heat Stress Conditions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chattha, W.S.; Atif, R.M.; Iqbal, M.; Shafqat, W.; Farooq, M.A.; Shakeel, A. Genome-Wide Identification and Evolution of Dof Transcription Factor Family in Cultivated and Ancestral Cotton Species. Genomics 2020, 112, 4155–4170. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, M.; Ahmad, F.; Zang, Y.; Jin, S.; Ahmed, S.; Li, J.; Islam, F.; Ahmad, M.; Zhang, Y.; Hu, Y.; et al. HEAT RESPONSIVE PROTEIN Regulates Heat Stress via Fine-Tuning Ethylene/Auxin Signaling Pathways in Cotton. Plant Physiol. 2022, 191, 772–788. [Google Scholar] [CrossRef]
- Malhi, G.S.; Kaur, M.; Kaushik, P. Impact of Climate Change on Agriculture and Its Mitigation Strategies: A Review. Sustainability 2021, 13, 1318. [Google Scholar] [CrossRef]
- Zafar, M.M.; Chattha, W.S.; Khan, A.I.; Zafar, S.; Subhan, M.; Saleem, H.; Ali, A.; Ijaz, A.; Anwar, Z.; Qiao, F.; et al. Drought and Heat Stress on Cotton Genotypes Suggested Agro-Physiological and Biochemical Features for Climate Resilience. Front. Plant Sci. 2023, 14, 1265700. [Google Scholar] [CrossRef] [PubMed]
- Eckstein, D.; Künzel, V.; Schäfer, L. Global Climate Risk Index 2021; Germanwatch: Bonn, Germany, 2021; p. 28. [Google Scholar]
- Ali, M.M.; Ali, Z.; Ahmad, F.; Nawaz, F.; Shakil, Q.; Ahmad, S.; Khan, A.A. Transcript Abundance of Heat Shock Protein Genes Confer Heat Tolerance in Cotton (Gossypium hirsutum L.). Pak. J. Bot. 2022, 54, 65–71. [Google Scholar] [CrossRef]
- Raza, A.; Razzaq, A.; Mehmood, S.S.; Zou, X.; Zhang, X.; Lv, Y.; Xu, J. Impact of Climate Change on Crops Adaptation and Strategies to Tackle Its Outcome: A Review. Plants 2019, 8, 34. [Google Scholar] [CrossRef]
- Iqbal, M.; Ul-Allah, S.; Naeem, M.; Ijaz, M.; Sattar, A.; Sher, A. Response of Cotton Genotypes to Water and Heat Stress: From Field to Genes. Euphytica 2017, 213, 131. [Google Scholar] [CrossRef]
- Abro, A.A.; Anwar, M.; Javwad, M.U.; Zhang, M.; Liu, F.; Jiménez-Ballesta, R.; Salama, E.A.A.; Ahmed, M.A.A. Morphological and Physio-Biochemical Responses under Heat Stress in Cotton: Overview. Biotechnol. Rep. 2023, 40, e00813. [Google Scholar] [CrossRef]
- Provart, N.J.; Brady, S.M.; Parry, G.; Schmitz, R.J.; Queitsch, C.; Bonetta, D.; Waese, J.; Schneeberger, K.; Loraine, A.E. Anno Genominis XX: 20 Years of Arabidopsis Genomics. Plant Cell 2021, 33, 832–845. [Google Scholar] [CrossRef]
- Bhat, G.R.; Sethi, I.; Rah, B.; Kumar, R.; Afroze, D. Innovative in Silico Approaches for Characterization of Genes and Proteins. Front. Genet. 2022, 13, 865182. [Google Scholar] [CrossRef]
- Varshney, R.K.; Bohra, A.; Yu, J.; Graner, A.; Zhang, Q.; Sorrells, M.E. Designing Future Crops: Genomics-Assisted Breeding Comes of Age. Trends Plant Sci. 2021, 26, 631–649. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Gao, C.; Zhang, Y.; Yan, Q.; Hu, W.; Yang, L.; Wang, Z.; Li, F. Recent Progression and Future Perspectives in Cotton Genomic Breeding. J. Integr. Plant Biol. 2023, 65, 548–569. [Google Scholar] [CrossRef]
- Hou, S.; Zhu, G.; Li, Y.; Li, W.; Fu, J.; Niu, E.; Li, L.; Zhang, D.; Guo, W. Genome-Wide Association Studies Reveal Genetic Variation and Candidate Genes of Drought Stress Related Traits in Cotton (Gossypium hirsutum L.). Front. Plant Sci. 2018, 9, 1276. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Zhang, X.; Xu, X. Abundant Small Genetic Alterations after Upland Cotton Domestication. Biomed Res. Int. 2018, 2018, 9254302. [Google Scholar] [CrossRef] [PubMed]
- Eyüboglu, B.; Pfister, K.; Haberer, G.; Chevalier, D.; Fuchs, A.; Mayer, K.F.X.; Schneitz, K. Molecular Characterisation of the STRUBBELIG-RECEPTOR FAMILY of Genes Encoding Putative Leucine-Rich Repeat Receptor-like Kinases in Arabidopsis thaliana. BMC Plant Biol. 2007, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Yang, J.; Peng, M.; Liu, X.; He, H. Genome-Wide Characterization, Evolution, and Expression Analysis of the Leucine-Rich Repeat Receptor-like Protein Kinase (Lrr-Rlk) Gene Family in Medicago truncatula. Life 2020, 10, 176. [Google Scholar] [CrossRef]
- Bai, Y.; Vaddepalli, P.; Fulton, L.; Bhasin, H.; Hülskamp, M.; Schneitz, K. ANGUSTIFOLIA Is a Central Component of Tissue Morphogenesis Mediated by the Atypical Receptor-like Kinase STRUBBELIG. BMC Plant Biol. 2013, 13, 16. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Y.; Wu, X.; Long, Z.; Sun, C.; Wang, H.; Wang, S.; Birch, P.R.J.; Tian, Z. A Potato STRUBBELIG-RECEPTOR FAMILY Member, StLRPK1, Associates with StSERK3A/BAK1 and Activates Immunity. J. Exp. Bot. 2018, 69, 5573–5586. [Google Scholar] [CrossRef]
- Fulton, L.; Vaddepalli, P.; Yadav, R.K.; Batoux, M.; Schneitz, K. Inter-Cell-Layer Signalling during Arabidopsis Ovule Development Mediated by the Receptor-like Kinase STRUBBELIG. Biochem. Soc. Trans. 2010, 38, 583–587. [Google Scholar] [CrossRef]
- Fulton, L.; Batoux, M.; Vaddepalli, P.; Yadav, R.K.; Busch, W.; Andersen, S.U.; Jeong, S.; Lohmann, J.U.; Schneitz, K. DETORQUEO, QUIRKY, and ZERZAUST Represent Novel Components Involved in Organ Development Mediated by the Receptor-like Kinase STRUBBELIG in Arabidopsis thaliana. PLoS Genet. 2009, 5, e1000355. [Google Scholar] [CrossRef]
- Alcázar, R.; García, A.V.; Kronholm, I.; De Meaux, J.; Koornneef, M.; Parker, J.E.; Reymond, M. Natural Variation at Strubbelig Receptor Kinase 3 Drives Immune-Triggered Incompatibilities between Arabidopsis thaliana Accessions. Nat. Genet. 2010, 42, 1135–1139. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Zhong, S.H.; Cui, X.F.; Li, J.; He, Z.H. Characterization of temperature-sensitive mutants reveals a role for receptor-like kinase SCRAMBLED/STRUBBELIG in coordinating cell proliferation and differentiation during Arabidopsis leaf development. Plant J. 2012, 72, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yu, L.X.; Zheng, P.; Li, Y.; Rivera, M.; Main, D.; Greene, S.L. Identification of Loci Associated with Drought Resistance Traits in Heterozygous Autotetraploid Alfalfa (Medicago sativa L.) Using Genome-Wide Association Studies with Genotyping by Sequencing. PLoS ONE 2015, 10, e0138931. [Google Scholar] [CrossRef]
- Arifuzzaman, M.; Oladzadabbasabadi, A.; McClean, P.; Rahman, M. Shovelomics for Phenotyping Root Architectural Traits of Rapeseed/Canola (Brassica napus L.) and Genome-Wide Association Mapping. Mol. Genet. Genom. 2019, 294, 985–1000. [Google Scholar] [CrossRef]
- Chaudhary, A.; Chen, X.; Gao, J.; Leśniewska, B.; Hammerl, R.; Dawid, C.; Schneitz, K. The Arabidopsis Receptor Kinase STRUBBELIG Regulates the Response to Cellulose Deficiency. PLoS Genet. 2020, 16, e1008433. [Google Scholar] [CrossRef]
- Zhu, T.; Liang, C.; Meng, Z.; Sun, G.; Meng, Z.; Guo, S.; Zhang, R. CottonFGD: An Integrated Functional Genomics Database for Cotton. BMC Plant Biol. 2017, 17, 101. [Google Scholar] [CrossRef] [PubMed]
- Goodstein, D.M.; Shu, S.; Howson, R.; Neupane, R.; Hayes, R.D.; Fazo, J.; Mitros, T.; Dirks, W.; Hellsten, U.; Putnam, N.; et al. Phytozome: A Comparative Platform for Green Plant Genomics. Nucleic Acids Res. 2012, 40, 1178–1186. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Khedkar, S.; Bork, P. SMART: Recent Updates, New Developments and Status in 2020. Nucleic Acids Res. 2021, 49, D458–D460. [Google Scholar] [CrossRef]
- Jones, P.; Binns, D.; Chang, H.Y.; Fraser, M.; Li, W.; McAnulla, C.; McWilliam, H.; Maslen, J.; Mitchell, A.; Nuka, G.; et al. InterProScan 5: Genome-Scale Protein Function Classification. Bioinformatics 2014, 30, 1236–1240. [Google Scholar] [CrossRef] [PubMed]
- Martin, F.J.; Amode, M.R.; Aneja, A.; Austine-Orimoloye, O.; Azov, A.G.; Barnes, I.; Becker, A.; Bennett, R.; Berry, A.; Bhai, J.; et al. Ensembl 2023. Nucleic Acids Res. 2023, 51, D933–D941. [Google Scholar] [CrossRef]
- Gasteiger, E.; Gattiker, A.; Hoogland, C.; Ivanyi, I.; Appel, R.D.; Bairoch, A. ExPASy: The Proteomics Server for in-Depth Protein Knowledge and Analysis. Nucleic Acids Res. 2003, 31, 3784–3788. [Google Scholar] [CrossRef]
- Yu, C.-S.; Lin, C.-J.; Hwang, J.-K. Predicting Subcellular Localization of Proteins for Gram-Negative Bacteria by Support Vector Machines Based on n -Peptide Compositions. Protein Sci. 2004, 13, 1402–1406. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree of Life (ITOL) v6: Recent Updates to the Phylogenetic Tree Display and Annotation Tool. Nucleic Acids Res. 2024, 52, W78–W82. [Google Scholar] [CrossRef]
- Sharma, S.; Vakhlu, J. Evolution and Biology of CRISPR System: A New Era Tool for Genome Editing in Plants. Bot. Rev. 2021, 87, 496–517. [Google Scholar] [CrossRef]
- Crooks, G.E.; Hon, G.; Chandonia, J.M.; Brenner, S.E. WebLogo: A Sequence Logo Generator. Genome Res. 2004, 14, 1188–1190. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van De Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a Database of Plant Cis-Acting Regulatory Elements and a Portal to Tools for in Silico Analysis of Promoter Sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Nystrom, S.L.; McKay, D.J. Memes: A Motif Analysis Environment in R Using Tools from the MEME Suite. PLoS Comput. Biol. 2021, 17, e1008991. [Google Scholar] [CrossRef]
- Hu, B.; Jin, J.; Guo, A.Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An Upgraded Gene Feature Visualization Server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef]
- Yu, C.S.; Cheng, C.W.; Su, W.C.; Chang, K.C.; Huang, S.W.; Hwang, J.K.; Lu, C.H. CELLO2GO: A Web Server for Protein SubCELlular LOcalization Prediction with Functional Gene Ontology Annotation. PLoS ONE 2014, 9, e99368. [Google Scholar] [CrossRef] [PubMed]
- Horton, P.; Park, K.J.; Obayashi, T.; Fujita, N.; Harada, H.; Adams-Collier, C.J.; Nakai, K. WoLF PSORT: Protein Localization Predictor. Nucleic Acids Res. 2007, 35, 585–587. [Google Scholar] [CrossRef] [PubMed]
- Voorrips, R.E. Mapchart: Software for the Graphical Presentation of Linkage Maps and QTLs. J. Hered. 2002, 93, 77–78. [Google Scholar] [CrossRef] [PubMed]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; Mcgettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X Version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef]
- Suyama, M.; Torrents, D.; Bork, P. PAL2NAL: Robust Conversion of Protein Sequence Alignments into the Corresponding Codon Alignments. Nucleic Acids Res. 2006, 34, 609–612. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z. PAML 4: Phylogenetic Analysis by Maximum Likelihood. Mol. Biol. Evol. 2007, 24, 1586–1591. [Google Scholar] [CrossRef] [PubMed]
- Darzentas, N. Circoletto: Visualizing Sequence Similarity with Circos. Bioinformatics 2010, 26, 2620–2621. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing Real-Time PCR Data by the Comparative CT Method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Hong, K.; Radian, Y.; Manda, T.; Xu, H.; Luo, Y. The Development of Plant Genome Sequencing Technology and Its Conservation and Application in Endangered Gymnosperms. Plants 2023, 12, 4006. [Google Scholar] [CrossRef]
- Akram, U.; Song, Y.; Liang, C.; Abid, M.A.; Askari, M.; Myat, A.A.; Abbas, M.; Malik, W.; Ali, Z.; Guo, S.; et al. Genome-Wide Characterization and Expression Analysis of NHX Gene Family under Salinity Stress in Gossypium barbadense and Its Comparison with Gossypium hirsutum. Genes 2020, 11, 803. [Google Scholar] [CrossRef]
- Shahid, S.; Sher, M.A.; Ahmad, F.; ur Rehman, S.; Farid, B.; Raza, H.; Ali, Z.; Maqbool, A.; Alfarraj, S.; Ansari, M.J. Prediction of RNA Editing Sites and Genome-Wide Characterization of PERK Gene Family in Maize (Zea mays L.) in Response to Drought Stress. J. King Saud Univ.—Sci. 2022, 34, 102293. [Google Scholar] [CrossRef]
- Su, J.; Song, S.; Wang, Y.; Zeng, Y.; Dong, T.; Ge, X.; Duan, H. Genome-Wide Identification and Expression Analysis of DREB Family Genes in Cotton. BMC Plant Biol. 2023, 23, 169. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Xu, Y.; Yang, F.; Magwanga, R.O.; Cai, X.; Wang, X.; Wang, Y.; Hou, Y.; Wang, K.; Liu, F.; et al. Genome-Wide Identification of OSCA Gene Family and Their Potential Function in the Regulation of Dehydration and Salt Stress in Gossypium hirsutum. J. Cott. Res. 2019, 2, 11. [Google Scholar] [CrossRef]
- Liu, Z.; Ge, X.; Yang, Z.; Zhang, C.; Zhao, G.; Chen, E.; Liu, J.; Zhang, X.; Li, F. Genome-Wide Identification and Characterization of SnRK2 Gene Family in Cotton (Gossypium hirsutum L.). BMC Genet. 2017, 18, 54. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, Z.; Wei, Y.; Liu, Y.; Xing, L.; Liu, M.; Li, P.; Lu, Q.; Peng, R. Genome-Wide Identification of the MIOX Gene Family and Their Expression Profile in Cotton Development and Response to Abiotic Stress. PLoS ONE 2021, 16, e0254111. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, J.; Fang, L.; Zhang, Z.; Ma, W.; Niu, Y.; Ju, L.; Deng, J.; Zhao, T.; Lian, J.; et al. Gossypium barbadense and Gossypium hirsutum Genomes Provide Insights into the Origin and Evolution of Allotetraploid Cotton. Nat. Genet. 2019, 51, 739–748. [Google Scholar] [CrossRef]
- Peng, R.; Xu, Y.; Tian, S.; Unver, T.; Liu, Z.; Zhou, Z.; Cai, X.; Wang, K.; Wei, Y.; Liu, Y.; et al. Evolutionary Divergence of Duplicated Genomes in Newly Described Allotetraploid Cottons. Proc. Natl. Acad. Sci. USA 2022, 119, e2208496119. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Saxena, G.; Verma, R.K.; Asif, M.H.; Singh, V.P.; Sawant, S.V.; Singh, S.P. Genome-Wide Identification and Expression Analysis of Autophagy-Related Genes (ATG) in Gossypium spp. Reveals Their Crucial Role in Stress Tolerance. S. Afr. J. Bot. 2024, 167, 82–93. [Google Scholar] [CrossRef]
- Kilwake, J.W.; Umer, M.J.; Wei, Y.; Mehari, T.G.; Magwanga, R.O.; Xu, Y.; Hou, Y.; Wang, Y.; Shiraku, M.L.; Kirungu, J.N.; et al. Genome-Wide Characterization of the SAMS Gene Family in Cotton Unveils the Putative Role of GhSAMS2 in Enhancing Abiotic Stress Tolerance. Agronomy 2023, 13, 612. [Google Scholar] [CrossRef]
- Wei, W.; Ju, J.; Zhang, X.; Ling, P.; Luo, J.; Li, Y.; Xu, W.; Su, J.; Zhang, X.; Wang, C. GhBRX.1, GhBRX.2, and GhBRX4.3 Improve Resistance to Salt and Cold Stress in Upland Cotton. Front. Plant Sci. 2024, 15, 1353365. [Google Scholar] [CrossRef]
- Zhang, H.; Xiao, X.; Li, Z.; Chen, Y.; Li, P.; Peng, R.; Lu, Q.; Wang, Y. Exploring the Plasmodesmata Callose- Binding Protein Gene Family in Upland Cotton: Unraveling Insights for Enhancing Fiber Length. PeerJ 2024, 12, e17625. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, A.; Li, Z.; Wang, H.; Wang, J.; Dong, Z.; Yao, L.; Han, X.; Wei, F. Characterization and Gene Expression Analysis Reveal Universal Stress Proteins Respond to Abiotic Stress in Gossypium hirsutum. BMC Genom. 2024, 25, 98. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.; Qanmber, G.; Wei, Z.; Yu, D.; Li, Y.H.; Gan, L.; Li, F.; Wang, Z. Genome-Wide Characterization and Expression Analysis of Geranylgeranyl Diphosphate Synthase Genes in Cotton (Gossypium spp.) in Plant Development and Abiotic Stresses. BMC Genom. 2020, 21, 561. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, F.; Rehman, S.U.; Rahman, M.H.U.; Ahmad, S.; Khan, Z. Characterization of Strubbelig-Receptor Family (SRF) Related to Drought and Heat Stress Tolerance in Upland Cotton (Gossypium hirsutum L.). Agronomy 2024, 14, 1933. https://doi.org/10.3390/agronomy14091933
Ahmad F, Rehman SU, Rahman MHU, Ahmad S, Khan Z. Characterization of Strubbelig-Receptor Family (SRF) Related to Drought and Heat Stress Tolerance in Upland Cotton (Gossypium hirsutum L.). Agronomy. 2024; 14(9):1933. https://doi.org/10.3390/agronomy14091933
Chicago/Turabian StyleAhmad, Furqan, Shoaib Ur Rehman, Muhammad Habib Ur Rahman, Saghir Ahmad, and Zulqurnain Khan. 2024. "Characterization of Strubbelig-Receptor Family (SRF) Related to Drought and Heat Stress Tolerance in Upland Cotton (Gossypium hirsutum L.)" Agronomy 14, no. 9: 1933. https://doi.org/10.3390/agronomy14091933




