Automated Assessment of Wheat Leaf Disease Spore Concentration Using a Smart Microscopy Scanning System
Abstract
1. Introduction
2. Materials and Methods
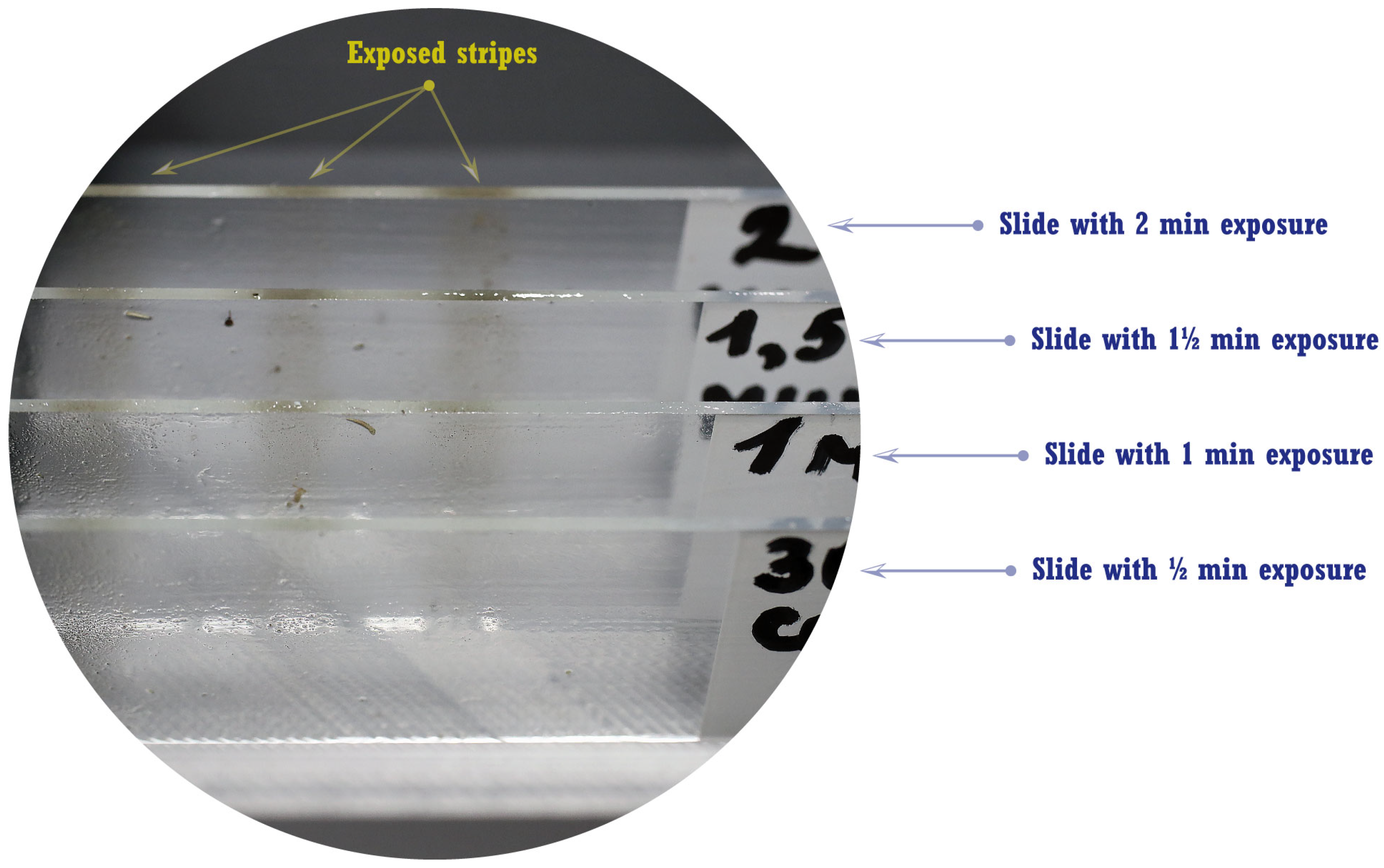
2.1. Airborne Spore Collection
2.2. Smart Microscopy Scanning System
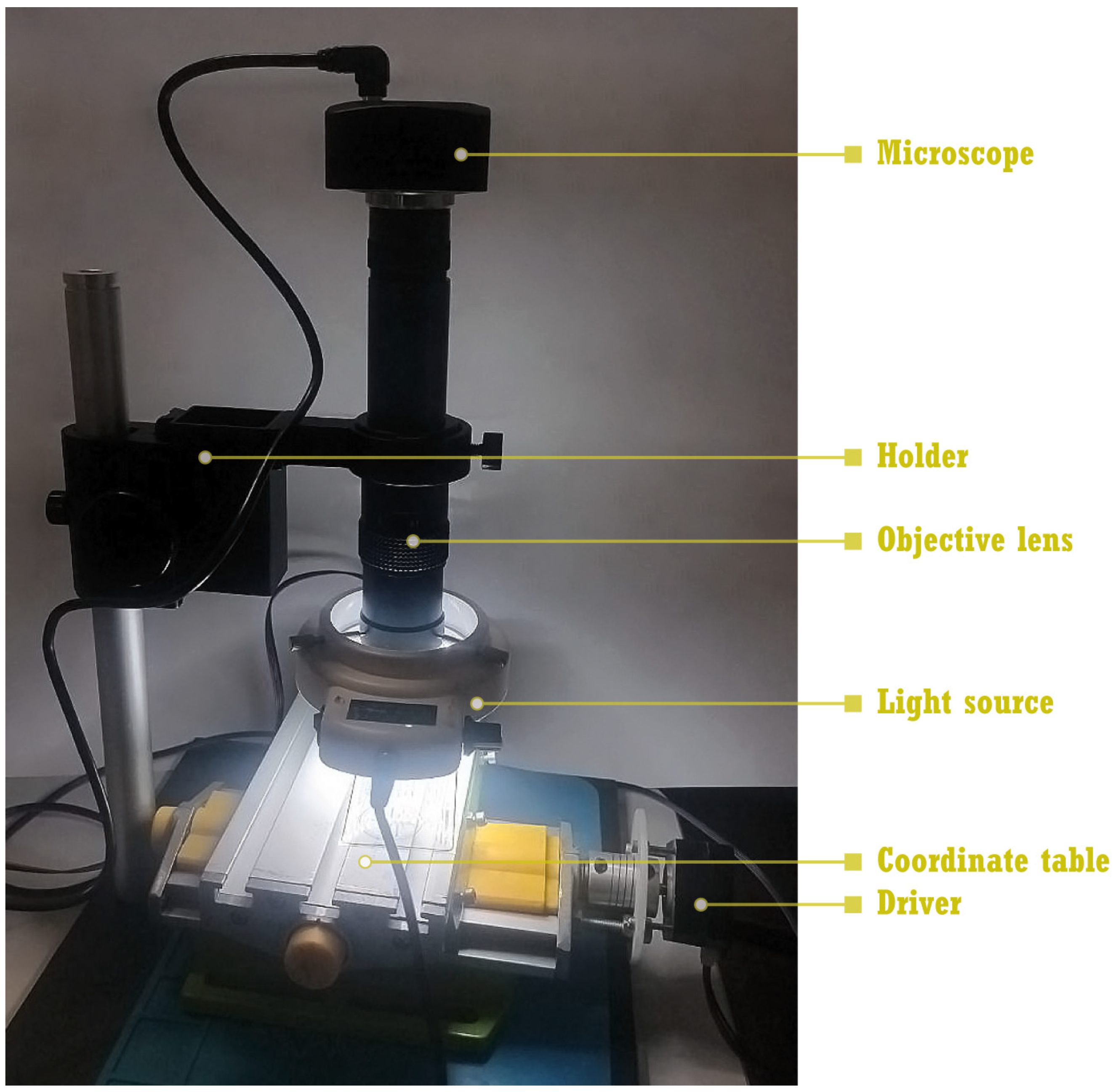
2.2.1. Design of the Device
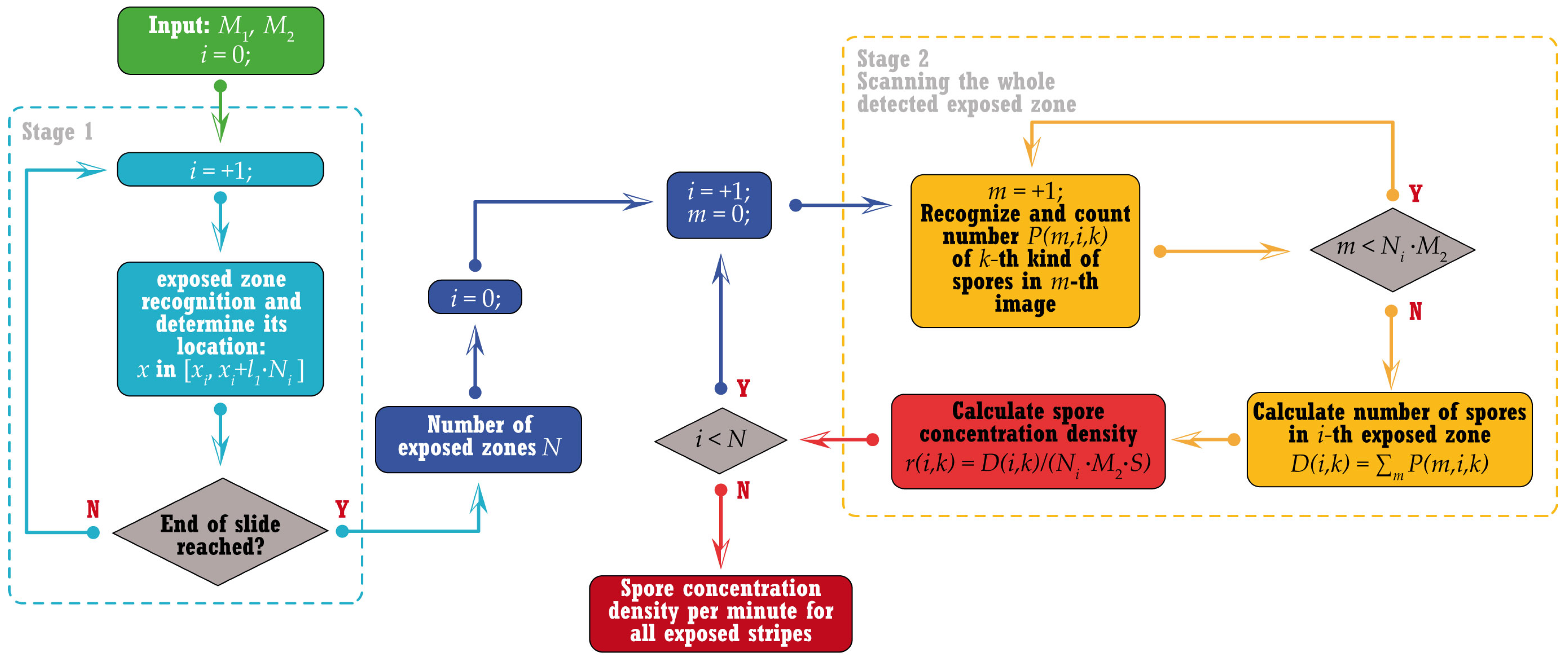
2.2.2. General Algorithm
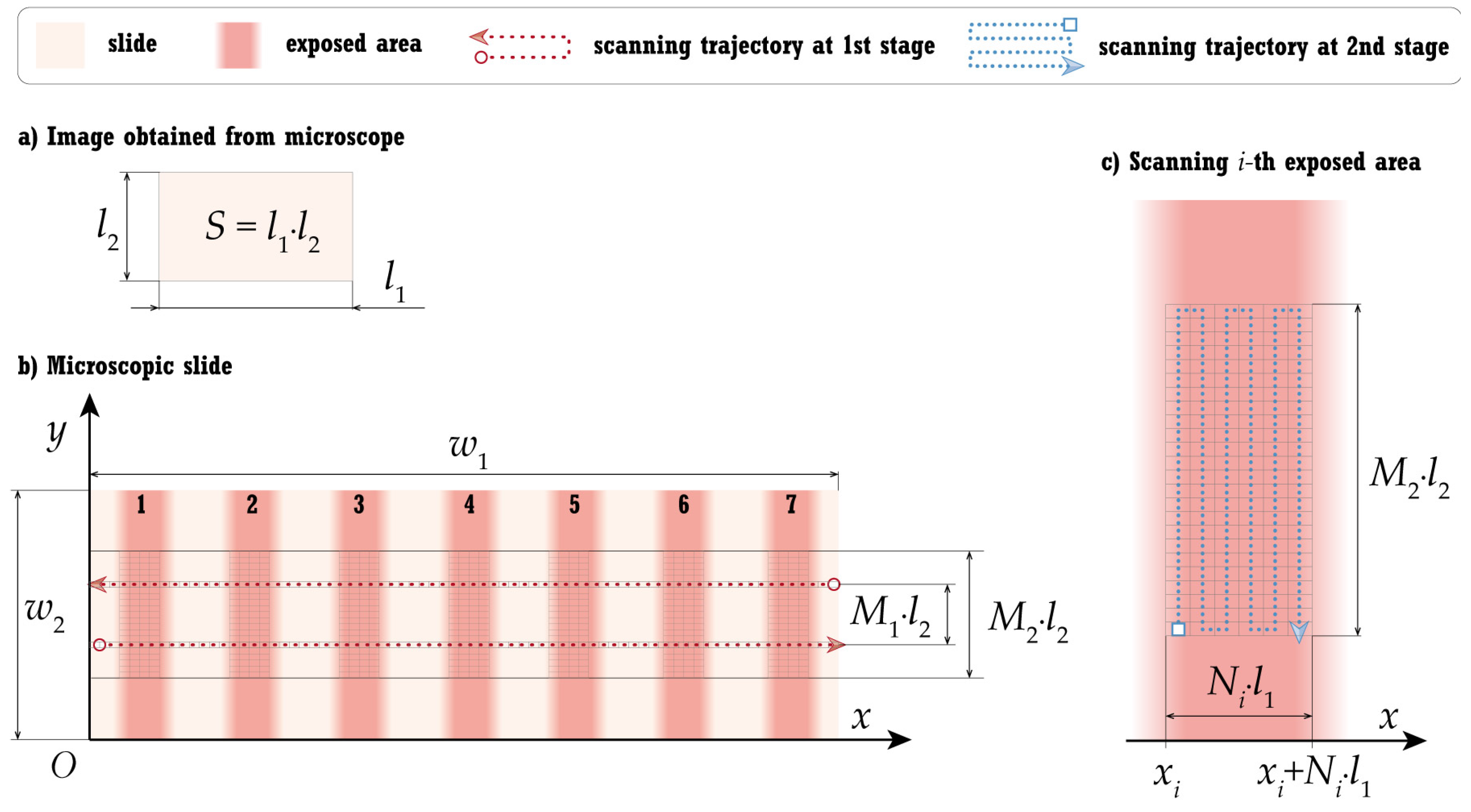
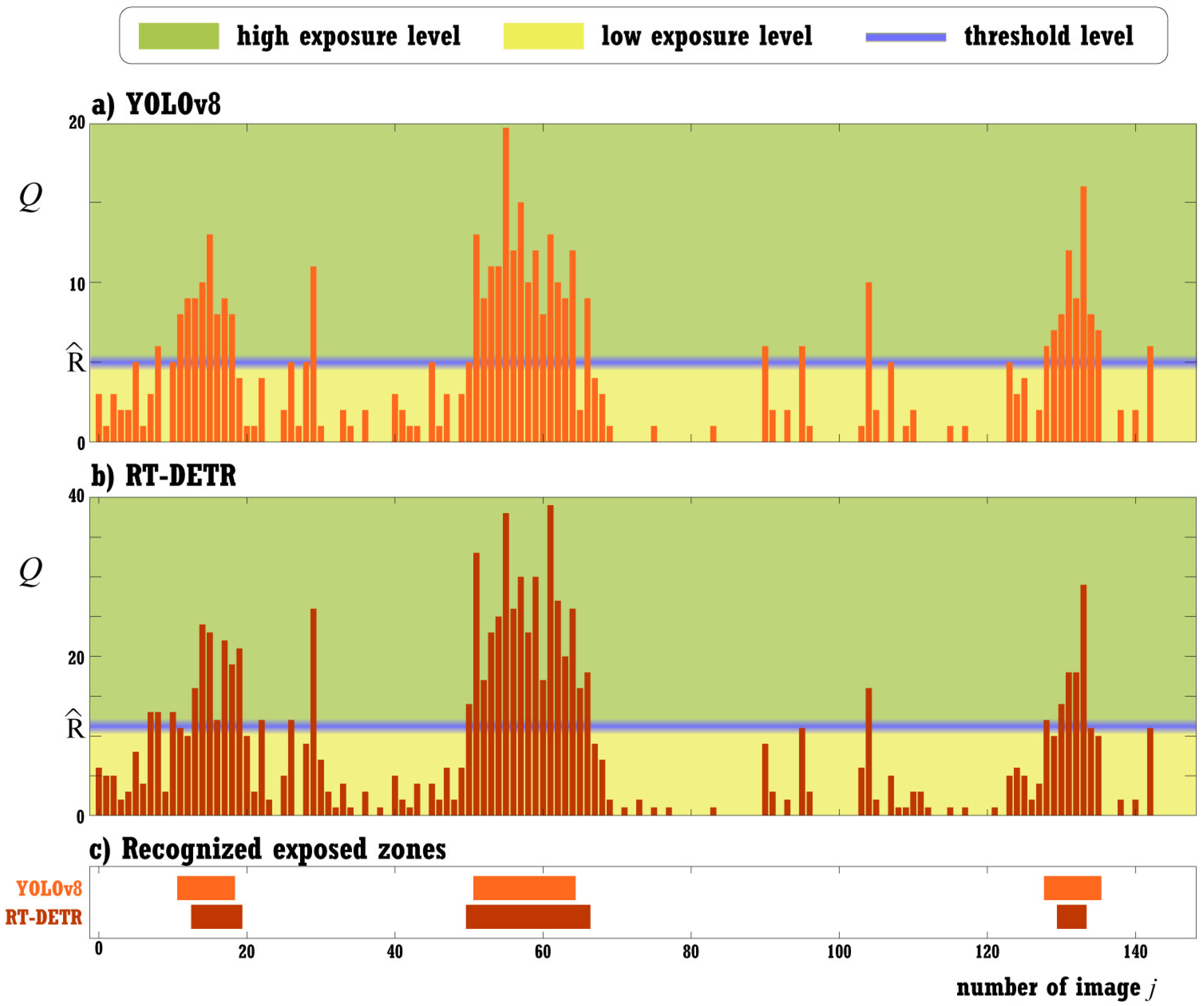
2.2.3. Stage 1: Exposed Zones Recognition
2.2.4. Stage 2: Pore Concentration Evaluation for Each Exposed Area
2.3. Proposed Methodology or Pipeline of the Automated Procedure
2.3.1. Dataset Creation
2.3.2. Spore Detection
2.3.3. Evaluation of the Number of Spores on a Micrograph of the Exposed Microscope Slide
3. Results
3.1. Spores Identification
3.2. Exposed Zones Recognition
3.3. Evaluation of Average Spore Concentration Density
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Singh, V.; Sharma, N.; Singh, S. A review of imaging techniques for plant disease detection. Artif. Intell. Agric. 2020, 4, 229–242. [Google Scholar] [CrossRef]
- Figueroa, M.; Hammond-Kosack, K.E.; Solomon, P.S. A review of wheat diseases—A field perspective. Mol. Plant Pathol. 2018, 19, 1523–1536. [Google Scholar] [CrossRef] [PubMed]
- Volkova, G.V.; Kudinova, O.A.; Matveeva, I.P. Virulence and diversity of Puccinia striiformis in South Russia. Phytopathol. Mediterr. 2021, 60, 119–127. [Google Scholar] [CrossRef]
- Kremneva, O.Y.; Mironenko, N.V.; Volkova, G.V.; Baranova, O.A.; Kim, Y.S.; Kovalenko, N.M. Resistance of winter wheat varieties to tan spot in the North Caucasus region of Russia. Saudi J. Biol. Sci. 2021, 28, 1787–1794. [Google Scholar] [CrossRef] [PubMed]
- Jha, K.; Doshi, A.; Patel, P.; Shah, M. A comprehensive review on automation in agriculture using artificial intelligence. Artif. Intell. Agric. 2019, 2, 1–12. [Google Scholar] [CrossRef]
- Demilie, W.B. Plant disease detection and classification techniques: A comparative study of the performances. J. Big Data 2024, 11, 5. [Google Scholar] [CrossRef]
- Adao, T.; Hruska, J.; Padua, L.; Bessa, J.; Peres, E.; Morais, R.; Sousa, J. Hyperspectral Imaging: A Review on UAV-Based Sensors, Data Processing and Applications for Agriculture and Forestry. Remote Sens. 2017, 9, 1110. [Google Scholar] [CrossRef]
- Terentev, A.; Dolzhenko, V.; Fedotov, A.; Eremenko, D. Current State of Hyperspectral Remote Sensing for Early Plant Disease Detection: A Review. Sensors 2022, 22, 757. [Google Scholar] [CrossRef]
- Khanal, S.; Fulton, J.; Shearer, S. An overview of current and potential applications of thermal remote sensing in precision agriculture. Comput. Electron. Agric. 2017, 139, 22–32. [Google Scholar] [CrossRef]
- Mahlein, A.K. Plant Disease Detection by Imaging Sensors - Parallels and Specific Demands for Precision Agriculture and Plant Phenotyping. Plant Dis. 2016, 100, 241–251. [Google Scholar] [CrossRef]
- Martinez-Bracero, M.; Markey, E.; Clancy, J.H.; McGillicuddy, E.J.; Sewell, G.; O’Connor, D.J. Airborne Fungal Spore Review, New Advances and Automatisation. Atmosphere 2022, 13, 398. [Google Scholar] [CrossRef]
- West, J.; Kimber, R. Innovations in air sampling to detect plant pathogens. Ann. Appl. Biol. 2015, 166, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Yuan, S.; Yang, N.; Wang, A.; Fordjour, A.; Chen, S. The Collection Method for Crop Fungal Spores Based on an Efficient Microfluidic Device. Aerosol Air Qual. Res. 2020, 20, 72–79. [Google Scholar] [CrossRef]
- Patel, R.; Mitra, B.; Vinchurkar, M.; Adami, A.; Patkar, R.; Giacomozzi, F.; Lorenzelli, L.; Baghini, M.S. A review of recent advances in plant-pathogen detection systems. Heliyon 2022, 8, e11855. [Google Scholar] [CrossRef]
- Wagner, J.; Macher, J. Automated spore measurements using microscopy, image analysis, and peak recognition of near-monodisperse aerosols. Aerosol Sci. Technol. 2012, 46, 862–873. [Google Scholar] [CrossRef]
- Lei, Y.; Yao, Z.; He, D. Automatic detection and counting of urediniospores of Puccinia striiformis f. sp. tritici using spore traps and image processing. Sci. Rep. 2018, 8, 13647. [Google Scholar] [CrossRef]
- Kubera, E.; Kubik-Komar, A.; Kurasinski, P.; Piotrowska-Weryszko, K.; Skrzypiec, M. Detection and Recognition of Pollen Grains in Multilabel Microscopic Images. Sensors 2022, 22, 2690. [Google Scholar] [CrossRef] [PubMed]
- Korsnes, R.; Westrum, K.; Fløistad, E.; Klingen, I. Computer-assisted image processing to detect spores from the fungus Pandora neoaphidis. MethodsX 2016, 3, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Biermann, R.; Niemeyer, L.; Rösner, L.; Ude, C.; Lindner, P.; Bice, I.; Beutel, S. Facilitated endospore detection for Bacillus spp. through automated algorithm-based image processing. Eng. Life Sci. 2022, 22, 299–307. [Google Scholar] [CrossRef]
- Danping, W.; Botao, W.; Yue, Y. The identification of powdery mildew spores image based on the integration of intelligent spore image sequence capture device. In Proceedings of the 2013 Ninth International Conference on Intelligent Information Hiding and Multimedia Signal Processing, Beijing, China, 16–18 October 2013; pp. 177–180. [Google Scholar] [CrossRef]
- Yang, N.; Yu, J.; Wang, A.; Tang, J.; Zhang, R.; Xie, L.; Shu, F.; Kwabena, O.P. A rapid rice blast detection and identification method based on crop disease spores’ diffraction fingerprint texture. J. Sci. Food Agric. 2020, 100, 3608–3621. [Google Scholar] [CrossRef]
- Javidan, S.M.; Banakar, A.; Vakilian, K.A.; Ampatzidis, Y.; Rahnama, K. Diagnosing the spores of tomato fungal diseases using microscopic image processing and machine learning. Multimed. Tools Appl. 2024, 83, 67283–67301. [Google Scholar] [CrossRef]
- Gao, W.; Li, M.; Wu, R.; Du, W.; Zhang, S.; Yin, S.; Chen, Z.; Huang, H. The design and application of an automated microscope developed based on deep learning for fungal detection in dermatology. Mycoses 2021, 64, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.Y.; Zhang, W.; Cheng, T.; Zhou, X.G.; Yan, Z.; Wu, Y.; Zhang, G.; Yang, X. Detection of wheat scab fungus spores utilizing the Yolov5-ECA-ASFF network structure. Comput. Electron. Agric. 2023, 210, 107953. [Google Scholar] [CrossRef]
- Zhou, H.; Lai, Q.; Huang, Q.; Cai, D.; Huang, D.; Wu, B. Automatic Detection of Rice Blast Fungus Spores by Deep Learning-Based Object Detection: Models, Benchmarks and Quantitative Analysis. Agriculture 2024, 14, 290. [Google Scholar] [CrossRef]
- Li, K.; Qiao, C.; Zhu, X.; Song, Y.; Zhang, L.; Gao, W.; Wang, Y. Lightweight fungal spore detection based on improved YOLOv5 in natural scenes. Int. J. Mach. Learn. Cybern. 2024, 15, 2247–2261. [Google Scholar] [CrossRef]
- Yao, C.; Yang, Z.; Li, P.; Liang, Y.; Fan, Y.; Luo, J.; Jiang, C.; Mu, J. Two-Stage Detection Algorithm for Plum Leaf Disease and Severity Assessment Based on Deep Learning. Agronomy 2024, 14, 1589. [Google Scholar] [CrossRef]
- Kremneva, O.; Danilov, R.; Gasiyan, K.; Ponomarev, A. Spore-Trapping Device: An Efficient Tool to Manage Fungal Diseases in Winter Wheat Crops. Plants 2023, 12, 391. [Google Scholar] [CrossRef]
- Kremneva, O.; Gasiyan, K.; Ponomarev, A. Detection of the causal agent of tan spot (Pyrenophora tritici-repentis) using spore-catching devices. Int. J. Ecosyst. Ecol. Sci. 2023, 13, 53–58. [Google Scholar] [CrossRef]
- Kremneva, O.Y.; Volkova, G.V.; Kovalenko, N.M. The dynamics of the race structure of Pyrenophora tritici-repentis in the North Caucasus region. Mikol. Fitopatol. 2019, 53, 246–253. [Google Scholar] [CrossRef]
- Jocher, G.; Chaurasia, A.; Qiu, J. Ultralytics YOLOv8. 2023. Available online: https://github.com/ultralytics/ultralytics (accessed on 15 April 2024).
- Lv, W.; Xu, S.; Zhao, Y.; Wang, G.; Wei, J.; Cui, C.; Du, Y.; Dang, Q.; Liu, Y. DETRs beat YOLOs on real-time object detection. arXiv 2023, arXiv:2304.08069. [Google Scholar]


| Magnification | Puccinia striiformis | Blumeria graminis | Pyrenophora tritici-repentis |
|---|---|---|---|
| 10× | 24 | 19 | 29 |
| 40× | 100 | 100 | 44 |
| Sample | Puccinia striiformis | Blumeria graminis | Pyrenophora tritici-repentis | Microparticle |
|---|---|---|---|---|
| train | 750 | 653 | 313 | 3626 |
| validation | 496 | 284 | 164 | 1231 |
| test | 241 | 265 | 85 | 721 |
| Model | Micro-Particles | Metrics | |||
|---|---|---|---|---|---|
| Precision (B) | Recall (B) | mAP50 (B) | mAP50-95 (B) | ||
| YOLOv8 | Y | 0.934 | 0.939 | 0.954 | 0.803 |
| N | 0.964 | 0.983 | 0.984 | 0.861 | |
| RT-DETR | Y | 0.978 | 0.984 | 0.942 | 0.791 |
| N | 0.961 | 0.977 | 0.982 | 0.857 | |
| Model | Micro-Particles | Type of Spores | TP | FN | FP | Recall | Precision |
|---|---|---|---|---|---|---|---|
| YOLOv8 | Y | P. striiformis | 486 | 10 | 4 | 0.9798 | 0.9918 |
| B. graminis | 279 | 5 | 25 | 0.9824 | 0.9148 | ||
| P. tritici-repentis | 162 | 2 | 3 | 0.9878 | 0.9818 | ||
| N | P. striiformis | 485 | 11 | 3 | 0.9778 | 0.9939 | |
| B. graminis | 277 | 7 | 24 | 0.9754 | 0.9203 | ||
| P. tritici-repentis | 161 | 3 | 3 | 0.9817 | 0.9817 | ||
| RT-DETR | Y | P. striiformis | 491 | 5 | 6 | 0.9899 | 0.9879 |
| B. graminis | 279 | 5 | 39 | 0.9936 | 0.8774 | ||
| P. tritici-repentis | 162 | 2 | 19 | 0.9878 | 0.895 | ||
| N | P. striiformis | 488 | 8 | 11 | 0.9839 | 0.978 | |
| B. graminis | 282 | 2 | 36 | 0.993 | 0.8868 | ||
| P. tritici-repentis | 162 | 2 | 20 | 0.9878 | 0.8901 |
| Model | Micro-Particles | Amount of Identified Spores | ||
|---|---|---|---|---|
| P. striiformis | B. graminis | P. tritici-repentis | ||
| YOLOv8 | Y | 242 | 267 | 85 |
| N | 241 | 261 | 84 | |
| RT-DETR | Y | 239 | 264 | 82 |
| N | 241 | 267 | 81 | |
| Amount | Exposure Time | RT-DETR, Y | Real Data |
|---|---|---|---|
| Spores per 1 mm2 | 1 min | 17.2 | 15.5 |
| 1.5 min | 39 | 44.5 | |
| 2 min | 52.2 | 59.2 | |
| Spores per 1 mm2 in a 1 min | 1 min | 17.2 | 15.5 |
| 1.5 min | 26 | 29.7 | |
| 2 min | 26.1 | 29.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doroshenko, O.V.; Golub, M.V.; Kremneva, O.Y.; Shcherban’, P.S.; Peklich, A.S.; Danilov, R.Y.; Gasiyan, K.E.; Ponomarev, A.V.; Lagutin, I.N.; Moroz, I.A.; et al. Automated Assessment of Wheat Leaf Disease Spore Concentration Using a Smart Microscopy Scanning System. Agronomy 2024, 14, 1945. https://doi.org/10.3390/agronomy14091945
Doroshenko OV, Golub MV, Kremneva OY, Shcherban’ PS, Peklich AS, Danilov RY, Gasiyan KE, Ponomarev AV, Lagutin IN, Moroz IA, et al. Automated Assessment of Wheat Leaf Disease Spore Concentration Using a Smart Microscopy Scanning System. Agronomy. 2024; 14(9):1945. https://doi.org/10.3390/agronomy14091945
Chicago/Turabian StyleDoroshenko, Olga V., Mikhail V. Golub, Oksana Yu. Kremneva, Pavel S. Shcherban’, Andrey S. Peklich, Roman Yu. Danilov, Ksenia E. Gasiyan, Artem V. Ponomarev, Ilya N. Lagutin, Ilya A. Moroz, and et al. 2024. "Automated Assessment of Wheat Leaf Disease Spore Concentration Using a Smart Microscopy Scanning System" Agronomy 14, no. 9: 1945. https://doi.org/10.3390/agronomy14091945
APA StyleDoroshenko, O. V., Golub, M. V., Kremneva, O. Y., Shcherban’, P. S., Peklich, A. S., Danilov, R. Y., Gasiyan, K. E., Ponomarev, A. V., Lagutin, I. N., Moroz, I. A., & Postovoy, V. K. (2024). Automated Assessment of Wheat Leaf Disease Spore Concentration Using a Smart Microscopy Scanning System. Agronomy, 14(9), 1945. https://doi.org/10.3390/agronomy14091945








