Effect of Ensiling Density on Fermentation Characteristics and Aerobic Stability of Pennisetum giganteum Silages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Silage Preparation
2.2. Chemical Composition and Microbial Count Analysis
2.3. Aerobic Stability Analysis
2.4. Statistical Analysis
3. Results
3.1. Chemical Compositions and Microbial Counts of Pennisetum giganteum
3.2. Fermentation Quality of Pennisetum giganteum
3.3. Chemical Compositions and Microbial Counts of Pennisetum giganteum Silage
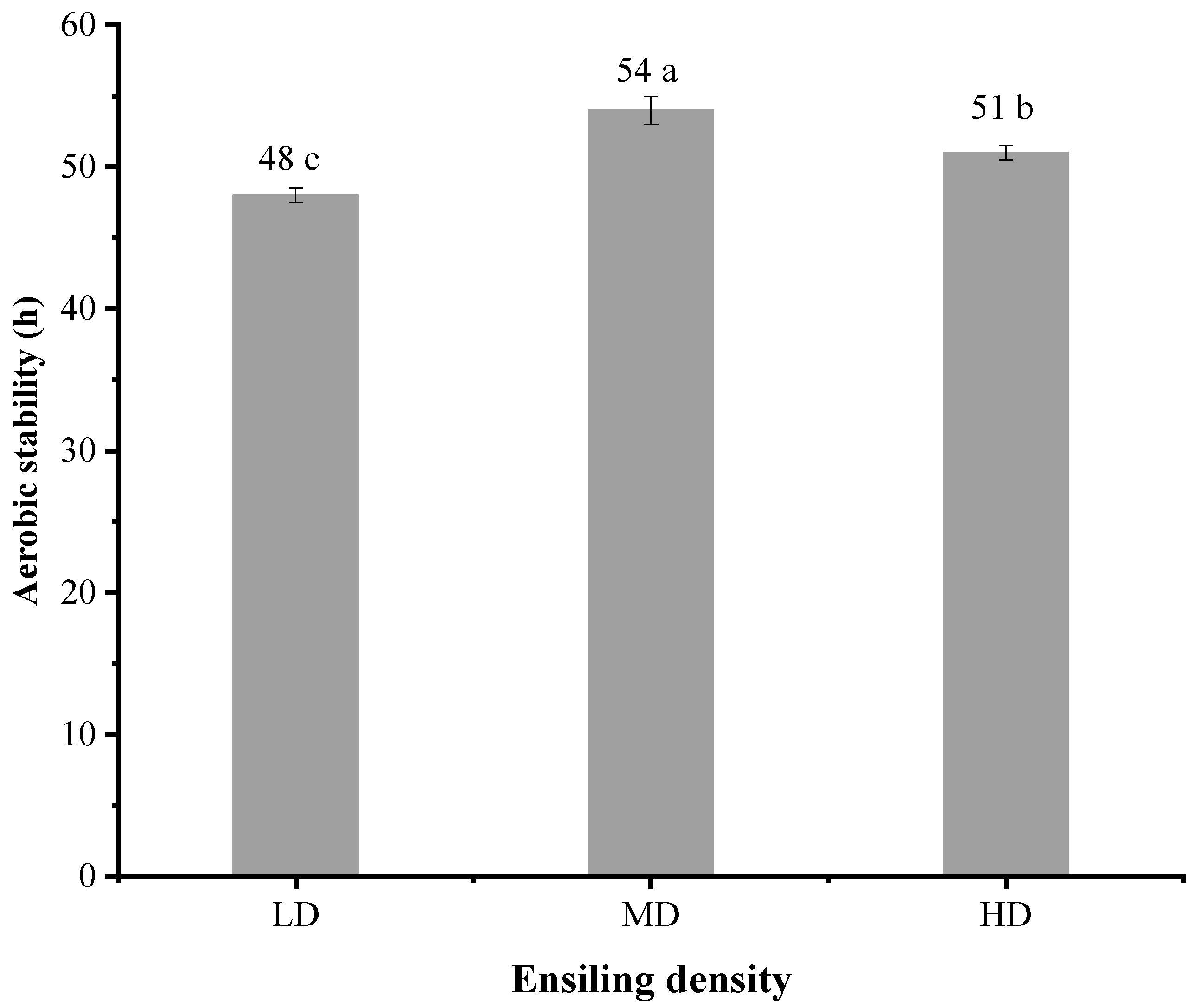
3.4. Aerobic Stability of Pennisetum giganteum Silage
4. Discussion
4.1. Fermentation Quality of Pennisetum giganteum
4.2. Chemical Compositions and Microbial Counts of Pennisetum giganteum Silages
4.3. Aerobic Stability of Pennisetum giganteum Silage
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jia, Y.; Liao, Z.; Chew, H.; Wang, L.; Lin, B.; Chen, C.; Lu, G.; Lin, Z. Effect of Pennisetum giganteum Z.X.Lin Mixed Nitrogen-Fixing Bacterial Fertilizer on the Growth, Quality, Soil Fertility and Bacterial Community of Pakchoi (Brassica chinensis L.). PLoS ONE 2020, 15, e0228709. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.D.; Su, P.; Shan, H. Mycotoxin Contamination of Rice in China. J. Food Sci. 2017, 82, 573–584. [Google Scholar] [CrossRef]
- Zhao, J.; Yin, X.; Dong, Z.; Wang, S.; Li, J.; Dong, D.; Shao, T. Using Gamma-Ray Irradiation and Epiphytic Microbiota Inoculation to Separate the Effects of Chemical and Microbial Factors on Fermentation Quality and Bacterial Community of Ensiled Pennisetum giganteum. J. Appl. Microbiol. 2022, 132, 1675–1686. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Guo, G.; Yu, C.; Zhang, J.; Shimojo, M.; Shao, T. The Effects of Replacement of Whole-Plant Corn with Oat and Common Vetch on the Fermentation Quality, Chemical Composition and Aerobic Stability of Total Mixed Ration Silage in Tibet. Anim. Sci. J. 2015, 86, 69–76. [Google Scholar] [CrossRef]
- Krishnamoorthy, U.; Muscato, T.; Sniffen, C.; Van Soest, P. Nitrogen Fractions in Selected Feedstuffs. J. Dairy Sci. 1982, 65, 217–225. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.A. An Automated Procedure for the Determination of Soluble Carbohydrates in Herbage. J. Sci. Food Agric. 1977, 28, 639–642. [Google Scholar] [CrossRef]
- Broderick, G.A.; Kang, J.H. Automated Simultaneous Determination of Ammonia and Total Amino Acids in Ruminal Fluid and In Vitro Media. J. Dairy Sci. 1980, 63, 64–75. [Google Scholar] [CrossRef]
- Kung, L., Jr.; Smith, M.L.; da Silva, E.B.; Windle, M.C.; da Silva, T.C.; Polukis, S.A. An Evaluation of the Effectiveness of a Chemical Additive Based on Sodium Benzoate, Potassium Sorbate, and Sodium Nitrite on the Fermentation and Aerobic Stability of Corn Silage. J. Dairy Sci. 2018, 101, 5949–5960. [Google Scholar] [CrossRef]
- Woolford, M.K. The Silage Fermentation; Springer: New York, NY, USA, 1984. [Google Scholar]
- Niu, D.Z.; Zheng, M.L.; Zuo, S.S.; Jiang, D.; Xu, C.C. Effects of Maize Meal and Limestone on the Fermentation Profile and Aerobic Stability of Smooth Bromegrass (Bromus inermis Leyss) Silage. Grass Forage Sci. 2018, 73, 622–629. [Google Scholar] [CrossRef]
- Kleinschmit, D.H.; Kung, L., Jr. A Meta-Analysis of the Effects of Lactobacillus buchneri on the Fermentation and Aerobic Stability of Corn and Grass and Small-Grain Silages. J. Dairy Sci. 2006, 89, 4005–4013. [Google Scholar] [CrossRef]
- McDonald, P.; Henderson, A.R.; Heron, S.J.E. The Biochemistry of Silage; Chalcombe Publications: Marlow, UK, 1991. [Google Scholar]
- Blajman, J.E.; Vinderola, G.; Páez, R.B.; Signorini, M.L. The Role of Homofermentative and Heterofermentative Lactic Acid Bacteria for Alfalfa Silage: A Meta-Analysis. J. Agric. Sci. 2020, 158, 107–118. [Google Scholar] [CrossRef]
- Muck, R. Factors Influencing Silage Quality and Their Implications for Management. J. Dairy Sci. 1988, 71, 2992–3002. [Google Scholar] [CrossRef]
- Hao, W.; Tian, P.; Zheng, M.; Wang, H.; Xu, C. Characteristics of Proteolytic Microorganisms and Their Effects on Proteolysis in Total Mixed Ration Silages of Soybean Curd Residue. Asian-Australas. J. Anim. Sci. 2019, 33, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, G.F.; Bélanger, G.; McRae, K.B.; Michaud, R. Proteolysis in Alfalfa Silages Made from Different Cultivars. Can. J. Plant Sci. 2001, 81, 685–692. [Google Scholar] [CrossRef]
- Winters, A.; Cockburn, J.; Dhanoa, M.; Merry, R. Effects of Lactic acid Bacteria in Inoculants on Changes in Amino Acid Composition during Ensilage of Sterile and Nonsterile Ryegrass. J. Appl. Microbiol. 2000, 89, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Li, X.; Guan, H.; Huang, L.; Ma, X.; Peng, Y.; Li, Z.; Nie, G.; Zhou, J.; Yang, W.; et al. Microbial Community and Fermentation Characteristic of Italian Ryegrass Silage Prepared with Corn Stover and Lactic Acid Bacteria. Bioresour. Technol. 2019, 279, 166–173. [Google Scholar] [CrossRef]
- Dong, M.; Li, Q.; Xu, F.; Wang, S.; Chen, J.; Li, W. Effects of Microbial Inoculants on the Fermentation Characteristics and Microbial Communities of Sweet Sorghum Bagasse Silage. Sci. Rep. 2020, 10, 837. [Google Scholar] [CrossRef]
- Ferrero, F.; Tabacco, E.; Piano, S.; Casale, M.; Borreani, G. Temperature During Conservation in Laboratory Silos Affects Fermentation Profile and Aerobic Stability of Corn Silage Treated with Lactobacillus buchneri, Lactobacillus hilgardii, and Their Combination. J. Dairy Sci. 2021, 104, 1696–1713. [Google Scholar] [CrossRef] [PubMed]
- Danner, H.; Holzer, M.; Mayrhuber, E.; Braun, R. Acetic Acid Increases Stability of Silage under Aerobic Conditions. Appl. Environ. Microbiol. 2003, 69, 562–567. [Google Scholar] [CrossRef]
- Woolford, M. The Detrimental Effects of Air on Silage. J. Appl. Bacteriol. 1990, 68, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Nishino, N.; Harada, H.; Sakaguchi, E. Evaluation of Fermentation and Aerobic Stability of Wet Brewers’ Grains Ensiled Alone or in Combination with Various Feeds as a Total Mixed Ration. J. Sci. Food Agric. 2003, 83, 557–563. [Google Scholar] [CrossRef]
- Miah, R.; Nina, S.; Murate, T.; Kataoka, N.; Matsutani, M.; Matsushita, K.; Yakushi, T. Major Aldehyde Dehydrogenase AldFGH of Gluconacetobacter Diazotrophicus is Independent of Pyrroloquinoline Quinone but Dependent on Molybdopterin for Acetic Acid Fermentation. Appl. Microbiol. Biotechnol. 2021, 105, 2341–2350. [Google Scholar] [CrossRef]
- Bernardes, T.F.; De Oliveira, I.L.; Lara, M.A.S.; Casagrande, D.R.; Ávila, C.L.S.; Pereira, O.G. Effects of Potassium Sorbate and Sodium Benzoate at Two Application Rates on Fermentation and Aerobic Stability of Maize Silage. Grass Forage Sci. 2015, 70, 491–498. [Google Scholar] [CrossRef]
| Items 1 | Pennisetum giganteum 2 |
|---|---|
| Chemical compositions | |
| DM (g/kg FW) | 199 ± 15.25 |
| CP (g/kg DM) | 108 ± 6.76 |
| aNDF (g/kg DM) | 586 ± 16.80 |
| ADF (g/kg DM) | 324 ± 8.52 |
| Hemicellulose (g/kg DM) | 262 ± 10.49 |
| WSC (g/kg DM) | 61.0 ± 8.14 |
| pH | 5.85 ± 0.14 |
| Buffering capacity (mEq/kg DM) | 19.6 ± 2.48 |
| Microbial compositions | |
| LAB (log10 cfu/g FW) | 3.08 ± 0.30 |
| Yeast (log10 cfu/g FW) | 5.62 ± 0.47 |
| Aerobic bacteria (log10 cfu/g FW) | 4.93 ± 0.43 |
| Items 1 | Treatments 2 | Ensiling Days | Means | SEM 3 | p-Value 4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 | 6 | 8 | 14 | 30 | 45 | T | D | T × D | T-L | T-Q | ||||
| pH | LD | 4.15 Aa | 3.94 Aa | 3.79 ab | 3.34 b | 3.74 ab | 3.74 ab | 3.78 A | 0.030 | <0.001 | <0.001 | 0.188 | <0.001 | 0.113 |
| MD | 3.81 Ba | 3.70 ABa | 3.69 a | 3.18 b | 3.63 a | 3.63 a | 3.61 AB | |||||||
| HD | 3.67 Ba | 3.50 Ba | 3.69 a | 3.22 b | 3.62 a | 3.62 a | 3.55 B | |||||||
| LA (g/kg DM) | LD | 22.8 Bb | 25.5 b | 39.3 ab | 50.8 a | 30.2 b | 35.4 ab | 34.0 | 2.452 | 0.361 | 0.001 | 0.933 | 0.216 | 0.482 |
| MD | 22.7 B | 27.4 | 31.6 | 44.6 | 34.4 | 43.6 | 34.1 | |||||||
| HD | 34.0 A | 32.6 | 35.0 | 58.9 | 33.4 | 39.8 | 39.0 | |||||||
| AA (g/kg DM) | LD | 14.1 b | 14.0 b | 18.6 ab | 23.8 a | 19.5 ab | 18.8 ab | 18.1 | 0.601 | 0.316 | <0.001 | 0.123 | 0.132 | 0.929 |
| MD | 11.9 b | 13.8 b | 16.4 b | 18.1 b | 18.3 ab | 24.9 a | 17.2 | |||||||
| HD | 15.1 | 15.7 | 16.0 | 17.8 | 17.7 | 18.9 | 16.5 | |||||||
| LA/AA | LD | 1.62 B | 1.83 | 2.07 | 2.14 | 1.62 | 1.93 | 1.87 B | 0.056 | 0.001 | 0.072 | 0.939 | <0.001 | 0.235 |
| MD | 1.9 AB | 1.98 | 1.94 | 2.42 | 1.88 | 1.71 | 1.97 B | |||||||
| HD | 2.29 A | 2.39 | 2.25 | 2.61 | 2.28 | 2.12 | 2.32 A | |||||||
| PA (g/kg DM) | LD | 8.72 A | 7.61 | 7.94 | 9.33 | 8.68 | 9.11 | 8.57 A | 0.105 | 0.002 | <0.001 | 0.319 | <0.001 | 0.302 |
| MD | 7.81 Bab | 7.39 b | 8.50 ab | 8.55 ab | 9.17 a | 8.93 a | 8.39 AB | |||||||
| HD | 7.56 B | 7.43 | 7.81 | 8.06 | 7.95 | 8.39 | 7.87 B | |||||||
| Ethanol (g/kg DM) | LD | 11.1 Aab | 10.1 b | 11.1 ab | 16.9 Aab | 11.3 ab | 17.5 a | 13.0 | 0.424 | 0.034 | <0.001 | 0.686 | 0.010 | 0.980 |
| MD | 10.21 AB | 10.1 | 11.0 | 12.4 B | 12.2 | 15.2 | 11.9 | |||||||
| HD | 9.75 B | 9.92 | 10.1 | 11.1 B | 10.1 | 13.6 | 10.7 | |||||||
| Items 1 | Treatments 2 | Ensiling Days | Means | SEM 3 | p-Value 4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 | 6 | 8 | 14 | 30 | 45 | T | D | T × D | T-L | T-Q | ||||
| DM (g/kg FW) | LD | 181 B | 206 | 188 | 174 B | 187 | 180 | 186 B | 2.029 | <0.001 | 0.018 | 0.630 | <0.001 | 0.055 |
| MD | 196 A | 206 | 188 | 188 AB | 175 | 183 | 189 B | |||||||
| HD | 209 A | 213 | 209 | 209 A | 206 | 198 | 207 A | |||||||
| WSC (g/kg DM) | LD | 39.0 | 29.7 | 26.3 | 27.7 | 30.2 | 27.2 | 30.0 A | 0.996 | 0.788 | <0.001 | 0.952 | 0.645 | 0.611 |
| MD | 43.06 a | 28.2 b | 26.1 b | 28.9 b | 32.5 b | 29.1 b | 31.3 A | |||||||
| HD | 41.73 a | 33.2 ab | 21.2 b | 29.0 ab | 30.7 ab | 29.5 b | 30.9 A | |||||||
| NH3-N (g/kg TN) | LD | 46.2 c | 62.4 abc | 53.9 bc | 101 Aa | 96.0 ab | 76.8 abc | 72.8 A | 2.608 | <0.001 | <0.001 | 0.227 | <0.001 | 0.744 |
| MD | 49.1 c | 51.2 bc | 57.0 abc | 76.3 Ba | 73.9 ab | 69.9 abc | 62.9 AB | |||||||
| HD | 43.3 b | 49.3 ab | 45.7 b | 66.0 Ba | 59.3 ab | 68.2 a | 55.3 B | |||||||
| LAB (log10 cfu/g FW) | LD | 7.00 c | 8.03 b | 8.97 Ba | 9.11 a | 8.38 ab | 8.53 Aab | 8.34 | 0.104 | 0.080 | <0.001 | 0.003 | 0.129 | 0.092 |
| MD | 7.39 c | 8.51 b | 9.60 Aa | 9.44 a | 8.41 b | 8.03 Bb | 8.56 | |||||||
| HD | 7.75 c | 8.66 abc | 9.51 Aa | 9.05 ab | 8.24 bc | 7.74 Cc | 8.49 | |||||||
| Yeast (log10 cfu/g FW) | LD | 7.03 Ad | 9.23 Aa | 8.73 Aab | 8.59 Abc | 8.32 Abc | 8.03 Ac | 8.32 A | 0.096 | <0.001 | <0.001 | 0.415 | <0.001 | 0.197 |
| MD | 6.78 ABb | 8.46 Ba | 8.12 ABa | 7.76 ABa | 7.90 ABa | 7.78 ABa | 7.80 B | |||||||
| HD | 6.45 Bb | 7.96 Ba | 7.72 Ba | 7.68 Ba | 7.60 Ba | 7.45 Ba | 7.48 B | |||||||
| Aerobic bacteria (log10 cfu/g FW) | LD | 9.63 Aa | 8.64 ab | 8.27 b | 8.35 b | 7.70 b | 7.87 ABb | 8.41 | 0.915 | 0.017 | <0.001 | 0.043 | 0.006 | 0.470 |
| MD | 8.86 Ba | 8.53 ab | 8.33 ab | 8.31 ab | 7.73 b | 8.10 Aab | 8.31 | |||||||
| HD | 8.84 Ba | 8.78 a | 8.39 a | 8.13 ab | 7.23 bc | 7.01 Bc | 8.06 | |||||||
| Items 1 | Treatments 2 | Aerobic Exposure Days | Means | SEM 3 | p-Value 4 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | T | D | T × D | T-L | T-Q | ||||
| pH | LD | 3.74 c | 3.82 Ac | 4.45 Ab | 6.69 Aa | 4.67 A | 0.142 | <0.001 | <0.001 | <0.001 | <0.001 | 0.100 |
| MD | 3.63 b | 3.56 Bb | 3.77 Bab | 3.94 Ba | 3.73 B | |||||||
| HD | 3.62 ab | 3.58 Bb | 3.78 Ba | 3.80 Bab | 3.70 B | |||||||
| LA (g/kg DM) | LD | 35.4 ab | 47.8 Aa | 36.4 ab | 23.3 b | 35.7 | 1.619 | 0.748 | 0.081 | 0.055 | 0.454 | 0.930 |
| MD | 43.6 a | 28.0 Bab | 35.4 ab | 29.5 b | 34.1 | |||||||
| HD | 39.8 | 29.0 B | 30.1 | 33.5 | 33.1 | |||||||
| AA (g/kg DM) | LD | 18.8 | 22.6 A | 23.8 A | 15.6 A | 20.2 A | 0.959 | <0.001 | <0.001 | 0.004 | <0.001 | 0.423 |
| MD | 24.9 a | 22.5 Aab | 16.7 ABbc | 12.7 Bc | 19.2 AB | |||||||
| HD | 18.8 a | 14.6 Bb | 14.5 Bb | 12.2 Bb | 15.0 B | |||||||
| PA (g/kg DM) | LD | 9.11 | 9.68 A | 10.4 | 9.13 A | 9.57 A | 0.197 | <0.001 | 0.558 | 0.218 | <0.001 | 0.134 |
| MD | 8.93 a | 8.00 Bab | 7.63 b | 8.47 Bab | 8.26 B | |||||||
| HD | 8.40 a | 6.98 Cb | 8.24 a | 8.31 Ba | 8.00 B | |||||||
| Ethanol (g/kg DM) | LD | 17.5 a | 23.1 Aab | 12.9 b | 11.3 Ab | 16.2 A | 0.777 | 0.006 | 0.001 | 0.217 | 0.002 | 0.379 |
| MD | 15.2 | 15.4 AB | 10.1 | 10.4 B | 12.8 AB | |||||||
| HD | 13.6 | 11.7 B | 10.6 | 10.2 B | 11.5 B | |||||||
| Items 1 | Treatments 2 | Aerobic Exposure Days | Means | SEM 3 | p-Value 4 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | T | D | T × D | T-L | T-Q | ||||
| DM (g/kg FW) | LD | 180 | 180 | 192 B | 201 | 188 B | 2.346 | <0.001 | <0.001 | 0.833 | <0.001 | 0.244 |
| MD | 183 | 192 | 195 AB | 205 | 194 B | |||||||
| HD | 198 b | 201 b | 203 Ab | 224 a | 207 A | |||||||
| NH3-N (g/kg TN) | LD | 75.8 c | 89.3 Ac | 127 Ab | 181 Aa | 119 A | 6.285 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| MD | 69.9 ab | 59.6 Bab | 50.2 Bb | 79.0 Ba | 64.7 B | |||||||
| HD | 68.2 b | 63.0 Bb | 59.0 Bb | 75.6 Ba | 66.4 B | |||||||
| LAB (log10 cfu/g FW) | LD | 8.53 Ac | 9.85 Ab | 10.9 Aa | 11.1 Aa | 10.1 A | 0.187 | <0.001 | <0.001 | 0.005 | <0.001 | 0.397 |
| MD | 8.03 Bc | 8.86 Bb | 10.2 Ba | 10.8 Aa | 9.49 AB | |||||||
| HD | 7.74 Cc | 8.72 Bb | 9.88 Ba | 9.70 Ba | 9.01 B | |||||||
| Yeast (log10 cfu/g FW) | LD | 8.03 Ad | 8.79 c | 10.1 Ab | 10.8 Aa | 9.43 | 0.174 | <0.001 | <0.001 | 0.004 | <0.001 | 0.147 |
| MD | 7.78 ABd | 8.47 c | 9.16 Bb | 10.5 Aa | 8.96 | |||||||
| HD | 7.45 Bc | 8.68 b | 9.04 Bb | 9.77 Ba | 8.74 | |||||||
| Aerobic bacteria (log10 cfu/g FW) | LD | 7.87 ABb | 8.94 b | 11.8 Aa | 11.5 a | 10.0 | 0.282 | <0.001 | <0.001 | 0.542 | <0.001 | 0.785 |
| MD | 8.10 Ab | 8.38 b | 11.0 Ba | 11.1 a | 9.64 | |||||||
| HD | 7.01 Bc | 8.09 b | 10.9 Ba | 10.6 a | 9.15 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, G.; Yang, F.; Hu, J.; Wang, Y.; Dong, D.; Dong, Z.; Li, J.; Shao, T. Effect of Ensiling Density on Fermentation Characteristics and Aerobic Stability of Pennisetum giganteum Silages. Agronomy 2024, 14, 1990. https://doi.org/10.3390/agronomy14091990
Xu G, Yang F, Hu J, Wang Y, Dong D, Dong Z, Li J, Shao T. Effect of Ensiling Density on Fermentation Characteristics and Aerobic Stability of Pennisetum giganteum Silages. Agronomy. 2024; 14(9):1990. https://doi.org/10.3390/agronomy14091990
Chicago/Turabian StyleXu, Guofeng, Feifei Yang, Junfeng Hu, Yanjie Wang, Dong Dong, Zhihao Dong, Junfeng Li, and Tao Shao. 2024. "Effect of Ensiling Density on Fermentation Characteristics and Aerobic Stability of Pennisetum giganteum Silages" Agronomy 14, no. 9: 1990. https://doi.org/10.3390/agronomy14091990




