Soil Characteristics and Response Mechanism of the Microbial Community in a Coal–Grain Compound Area with High Groundwater Levels
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Soil Sampling
2.3. Determination of Soil Characteristics
2.4. DNA Extraction and PCR Amplification
2.5. Statistical Analysis and Processing
3. Results
3.1. Temporal and Spatial Changes in Ecosystem Services
3.2. Differences in Microbial Species Diversity
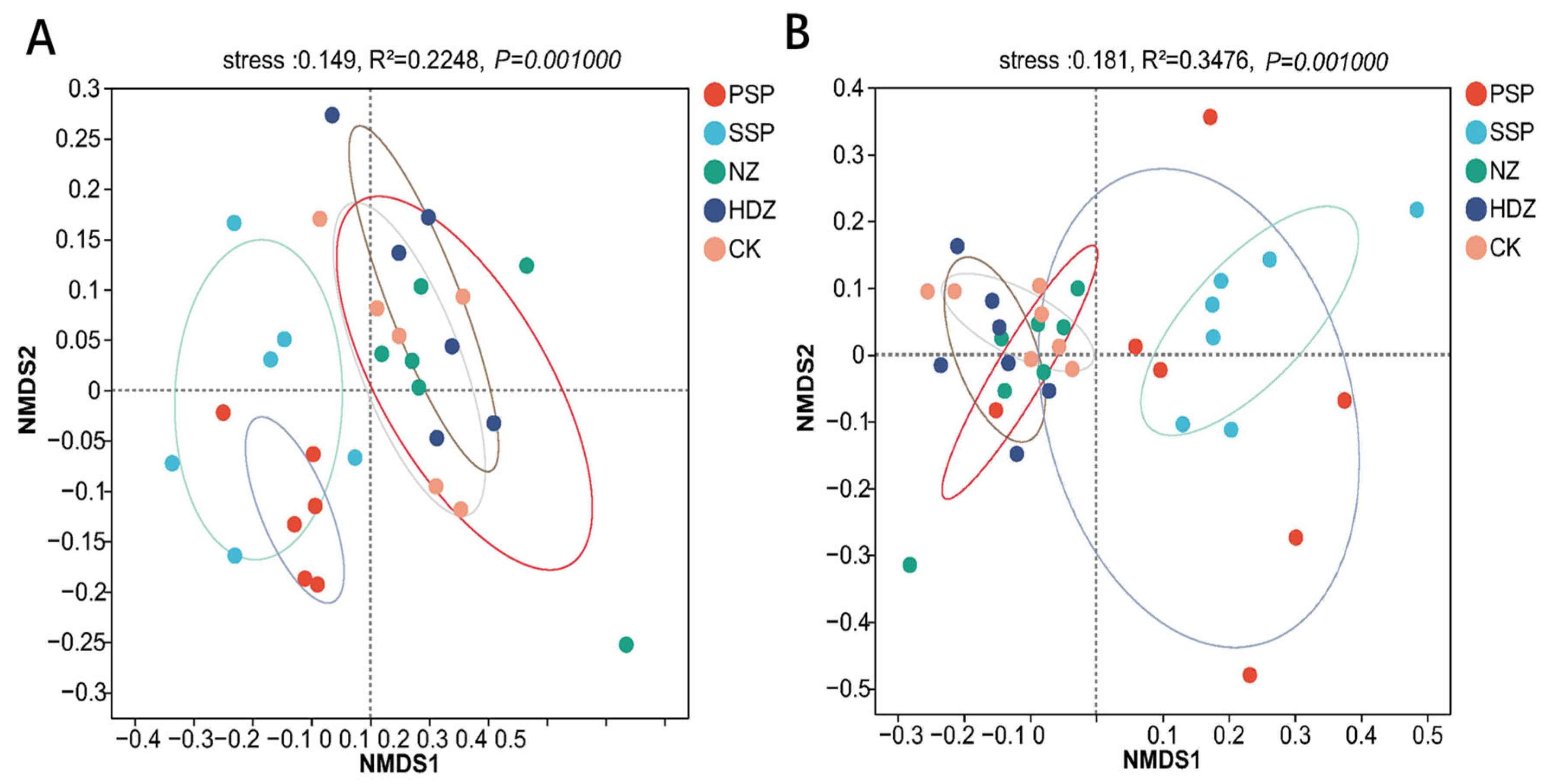
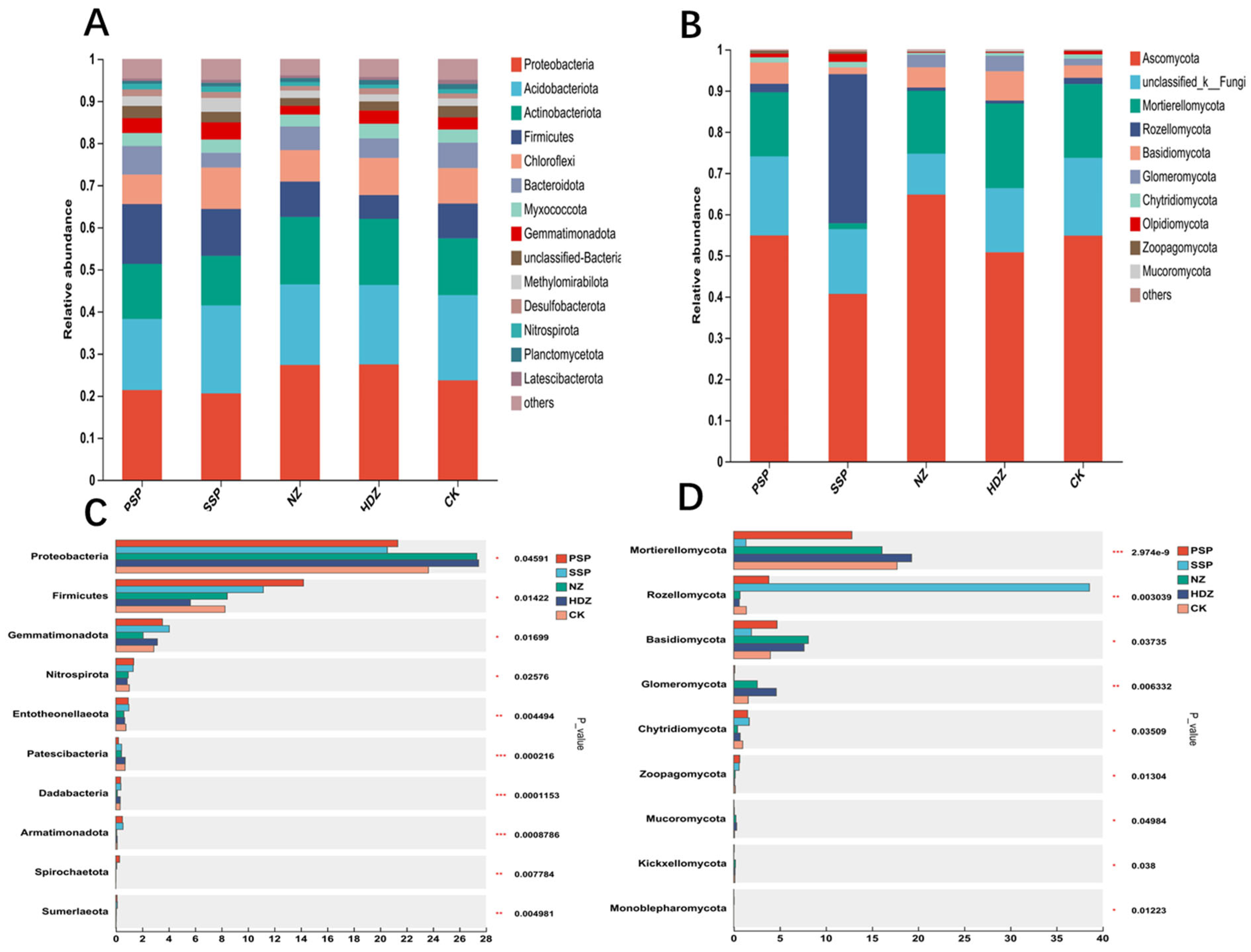
3.3. Differences in Soil Microbial Community Composition
3.4. The Relationship between Soil Microbial Community and Environmental Factors
4. Discussion
4.1. Subsidence and Water Accumulation Impact on Soil Properties and Microbial Community Composition
4.2. Soil Properties Impact on Microbial Communities Composition in Subsidence Areas
4.3. Soil Properties Impact on Microbial Dominant Population in Subsidence Areas
4.4. Inspiration for Precise Reclamation of Mineral-Grain Composite Area
5. Conclusions
- (1)
- Soil properties and microbial community structure exhibit significant variations across different regions in coal mining subsidence areas with high levels of disturbance. The levels of TN, TP, SOM, and bacterial and fungal diversity in the soil all decline to different extents in areas affected by coal mining subsidence. The distance from the water logging area follows a pattern of initially decreasing and then increasing, with the lowest levels seen in SSP. The soil in the coal mining subsidence area with a high water table is degraded by groundwater and lateral seepage, whereas the soil surrounding the subsidence water area is located at the base of the subsidence slope and is influenced by sedimentation. The presence of coal mining subsidence and water accumulation greatly affects the properties of the surrounding soil.
- (2)
- There are significant disparities in soil properties and microbial community composition between non-waterlogged and waterlogged areas in coal mining subsidence zones with a high water table. The nutrient level and stability of the microbial community structure in coal mining subsidence areas are generally low. However, the SSP shows the lowest level, while the PSP exhibits some improvement. This improvement helps to maintain a high level of soil nutrients and microorganisms, enabling the establishment of a stable wetland habitat. Developing biodiversity and enhancing soil ecosystem stability in areas affected by high-intensity coal mining subsidence is an effective land use strategy.
- (3)
- The presence of Proteobacteria and Firmicutes in bacteria, and Mortierellomycota, Rozellomycota, and Basidiomycota in fungi, differed significantly from the control group (CK) in the coal mining subsidence area. This suggests that the soil microbial communities in the subsidence area were significantly disrupted. It is important to take into account the introduction of these microorganisms during the later reclamation.
- (4)
- The correlation between soil bacterial and fungal diversity, as well as their important populations, and soil properties varied to different extents. The alteration of the microbial community was mostly influenced by soil nutrients and soil water content (SWC).
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jie, D.; Xu, X.; Guo, F. The future of coal supply in China based on non-fossil energy development and carbon price strategies. Energy 2021, 220, 119644. [Google Scholar] [CrossRef]
- Qiao, Q.; Shi, G.; Yang, D.; Wang, L.; Zhang, X.; Li, S.; Bai, X.-E. Study on the destruction law of physical and shear properties of soil in mining disturbance. Sci. Rep. 2023, 13, 17751. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhu, B.; Zhang, Y. A meta-analysis on the responses of soil microbial biomass and community structure to antibiotics. Appl. Soil Ecol. 2023, 184, 104786. [Google Scholar] [CrossRef]
- Feng, Z.; Hu, Z.; Li, G.; Zhang, Y.; Zhang, X.; Zhang, H. Improving mine reclamation efficiency for farmland sustainable use: Insights from optimizing mining scheme. J. Clean. Prod. 2022, 379, 134615. [Google Scholar] [CrossRef]
- Behera, A.; Singh Rawat, K. A brief review paper on mining subsidence and its geo-environmental impact. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, M.; Su, L.; Chen, X.; Liu, C.; Sun, A. A boundary model of terrain reconstruction in a coal-mining subsidence waterlogged area. Environ. Earth Sci. 2021, 80, 187. [Google Scholar] [CrossRef]
- Jiang, C.; Liu, D.; Jiang, C.; Wang, Q.; Sadat-Noori, M.; Li, H. Tracing groundwater discharge into a coal mining subsidence lake in eastern China: Observations from water stable (δD and δ18O) and radon (222Rn) isotopes. Appl. Geochem. 2023, 156, 105757. [Google Scholar] [CrossRef]
- Wang, Z.H.; Wu, S.X.; Li, J.L.; Sun, W.C.; Wang, Z.F.; Liu, P.J. Surface subsidence and its reclamation of a coal mine locating at the high groundwater table, China. Int. J. Environ. Sci. Technol. 2023, 20, 13635–13654. [Google Scholar] [CrossRef]
- Xie, J.; Gao, J.; Cao, H.; Li, J.; Wang, X.; Zhang, J.; Meng, H.; Hong, J.; Li, T.; Xu, M. Calcium carbonate promotes the formation and stability of soil macroaggregates in mining areas of China. J. Integr. Agric. 2024, 23, 1034–1047. [Google Scholar] [CrossRef]
- Punia, A. Carbon dioxide sequestration by mines: Implications for climate change. Clim. Chang. 2021, 165, 10. [Google Scholar] [CrossRef]
- Gayan, A.; Borah, P.; Nath, D.; Kataki, R. Chapter 4—Soil microbial diversity, soil health and agricultural sustainability. In Sustainable Agriculture and the Environment; Farooq, M., Gogoi, N., Pisante, M., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 107–126. [Google Scholar] [CrossRef]
- Xu, Y.; Ge, X.; Gao, G.; Yang, Y.; Hu, Y.; Li, Z.; Zhou, B. Microbial pathways driving stable soil organic carbon change in abandoned Moso bamboo forests in southeast China. J. Environ. Manag. 2023, 345, 118890. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zeng, Z.; Song, Z.; Wang, F.; Tian, D.; Mi, W.; Huang, X.; Wang, J.; Song, L.; Yang, Z.; et al. Vital roles of soil microbes in driving terrestrial nitrogen immobilization. Glob. Change Biol. 2021, 27, 1848–1858. [Google Scholar] [CrossRef] [PubMed]
- Abou-Shanab, R.A.I.; El-sheekh, M.; Sadowsky, M.J. Role of Rhizobacteria in Phytoremediation of Metal-Impacted Sites. In Emerging and Eco-Friendly Approaches for Waste Management; Springer: Singapore, 2019. [Google Scholar]
- Zhai, C.; Han, L.; Xiong, C.; Ge, A.; Yue, X.; Li, Y.; Zhou, Z.; Feng, J.; Ru, J.; Song, J.; et al. Soil microbial diversity and network complexity drive the ecosystem multifunctionality of temperate grasslands under changing precipitation. Sci. Total Environ. 2024, 906, 167217. [Google Scholar] [CrossRef]
- Das, P.P.; Singh, K.R.B.; Nagpure, G.; Mansoori, A.; Singh, R.P.; Ghazi, I.A.; Kumar, A.; Singh, J. Plant-soil-microbes: A tripartite interaction for nutrient acquisition and better plant growth for sustainable agricultural practices. Environ. Res. 2022, 214, 113821. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Sheng, H.; Zhang, L.; Zhang, L.; Pan, B.; Zhou, P. How does land-use change alter soil microbial diversity, composition, and network in subtropical China? Catena 2023, 231, 107335. [Google Scholar] [CrossRef]
- Liu, S.; Sun, Y.; Shi, F.; Liu, Y.; Wang, F.; Dong, S.; Li, M. Composition and Diversity of Soil Microbial Community Associated With Land Use Types in the Agro-Pastoral Area in the Upper Yellow River Basin. Front. Plant Sci. 2022, 13, 819661. [Google Scholar] [CrossRef]
- Guo, Y.; Wu, J.; Yu, Y. Differential Response of Soil Microbial Community Structure in Coal Mining Areas during Different Ecological Restoration Processes. Processes 2022, 10, 2013. [Google Scholar] [CrossRef]
- Song, W.; Li, J.; Li, X.; Xu, D.; Min, X. Effects of land reclamation on soil organic carbon and its components in reclaimed coal mining subsidence areas. Sci. Total Environ. 2024, 908, 168523. [Google Scholar] [CrossRef]
- Yang, Y.; Chai, Y.; Xie, H.; Zhang, L.; Zhang, Z.; Yang, X.; Hao, S.; Gai, J.; Chen, Y. Responses of soil microbial diversity, network complexity and multifunctionality to three land-use changes. Sci. Total Environ. 2023, 859, 160255. [Google Scholar] [CrossRef]
- Lu, D.; Mao, Z.; Tang, Y.; Feng, B.; Xu, L. Driving Factors Influencing Soil Microbial Community Succession of Coal Mining Subsidence Areas during Natural Recovery in Inner Mongolia Grasslands. Microorganisms 2024, 12, 87. [Google Scholar] [CrossRef]
- Song, Z.; Liu, G.; Sun, L.; Wang, F.; Guo, J. Influence of coal mining disturbance on soil physiochemical properties and the response mechanism of microbial community. Coal Geol. Explor. 2023, 51, 95–102. [Google Scholar] [CrossRef]
- Zhao, J.; Ma, J.; Yang, Y.; Yu, H.; Zhang, S.; Chen, F. Response of Soil Microbial Community to Vegetation Reconstruction Modes in Mining Areas of the Loess Plateau, China. Front. Microbiol. 2021, 12, 714967. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ma, J.; Yang, Y.; Luo, Z.; Wang, Y.; Chen, F. Mining Subsidence-Induced Microtopographic Effects Alter the Interaction of Soil Bacteria in the Sandy Pasture, China. Front. Environ. Sci. 2021, 9, 656708. [Google Scholar] [CrossRef]
- Mickan, B.S.; Abbott, L.K.; Solaiman, Z.M.; Mathes, F.; Siddique, K.H.M.; Jenkins, S.N. Soil disturbance and water stress interact to influence arbuscular mycorrhizal fungi, rhizosphere bacteria and potential for N and C cycling in an agricultural soil. Biol. Fertil. Soils 2019, 55, 53–66. [Google Scholar] [CrossRef]
- Gao, P.; Fan, K.; Zhang, G.; Yin, X.; Jia, C.; Tian, H. Coal-mining subsidence changed distribution of the microbiomes and their functional genes in a farmland. J. Basic Microbiol. 2023, 63, 542–557. [Google Scholar] [CrossRef]
- Medriano, C.A.; Chan, A.; De Sotto, R.; Bae, S. Different types of land use influence soil physiochemical properties, the abundance of nitrifying bacteria, and microbial interactions in tropical urban soil. Sci. Total Environ. 2023, 869, 161722. [Google Scholar] [CrossRef]
- Tian, Q.; Taniguchi, T.; Shi, W.-Y.; Li, G.; Yamanaka, N.; Du, S. Land-use types and soil chemical properties influence soil microbial communities in the semiarid Loess Plateau region in China. Sci. Rep. 2017, 7, 45289. [Google Scholar] [CrossRef]
- Wu, Q.; Tang, Y.; Chen, R.; Xu, F.; Wu, Q.; He, Y.; Xiao, W.; Li, J.; Liu, Z.; Chen, Y. Metabolism characteristics of nitrogen functional microorganisms in bioretention system under multiple dry-wet alternation. J. Water Process Eng. 2023, 53, 103685. [Google Scholar] [CrossRef]
- Tian, W.; Li, Q.; Luo, Z.; Wu, C.; Sun, B.; Zhao, D.; Chi, S.; Cui, Z.; Xu, A.; Song, Z. Microbial community structure in a constructed wetland based on a recirculating aquaculture system: Exploring spatio-temporal variations and assembly mechanisms. Mar. Environ. Res. 2024, 197, 106413. [Google Scholar] [CrossRef]
- Wang, M.; Sun, M.; Zhao, Y.; Shi, Y.; Sun, S.; Wang, S.; Zhou, Y.; Chen, L. Seasonal changes of soil microbiota and its association with environmental factors in coal mining subsidence area. AMB Express 2023, 13, 147. [Google Scholar] [CrossRef]
- Guo, R.; Chen, Y.; Xiang, M.; Yang, S.; Wang, F.; Cao, W.; Yue, H.; Peng, S. Soil nutrients drive changes in the structure and functions of soil bacterial communities in a restored forest soil chronosequence. Appl. Soil Ecol. 2024, 195, 105247. [Google Scholar] [CrossRef]
- Toledo, S.; Bondaruk, V.F.; Yahdjian, L.; Oñatibia, G.R.; Loydi, A.; Alberti, J.; Bruschetti, M.; Pascual, J.; Peter, G.; Agüero, W.D.; et al. Environmental factors regulate soil microbial attributes and their response to drought in rangeland ecosystems. Sci. Total Environ. 2023, 892, 164406. [Google Scholar] [CrossRef] [PubMed]
- Séneca, J.; Söllinger, A.; Herbold, C.W.; Pjevac, P.; Prommer, J.; Verbruggen, E.; Sigurdsson, B.D.; Peñuelas, J.; Janssens, I.A.; Urich, T.; et al. Increased microbial expression of organic nitrogen cycling genes in long-term warmed grassland soils. ISME Commun. 2021, 1, 69. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhang, Y.; Huang, H.; Yang, F. Deciphering soil bacterial community structure in subsidence area caused by underground coal mining in arid and semiarid area. Appl. Soil Ecol. 2021, 163, 103916. [Google Scholar] [CrossRef]
- Tang, Y.; Che, Y.-J.; Bai, X.-Y.; Wang, Z.-Y.; Gu, S.-Y. Effects of application of phosphate and phosphate-solubilizing bacteria on bacterial diversity and phosphorus fractions in a Phaeozems. Heliyon 2023, 9, e22937. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Huang, L.; Chen, J.; Zhou, H.; Wan, Y.; Qu, Q.; Wang, M.; Xue, S. Changes in soil microbial activity and their linkages with soil carbon under global warming. Catena 2023, 232, 107419. [Google Scholar] [CrossRef]
- Meng, Q.-J.; Feng, Q.-Y.; Wu, Q.-Q.; Meng, L.; Cao, Z.-Y. Distribution characteristics of nitrogen and phosphorus in mining induced subsidence wetland in Panbei coal mine, China. Procedia Earth Planet. Sci. 2009, 1, 1237–1241. [Google Scholar] [CrossRef]
- Zhu, M.; Bazai, N.; Li, X.; Huang, M.; Muhammad, T.; Lei, M.; Wang, H. Research on wetland ecological restoration of coal mining subsidence area in Suzhou, China. Fresenius Environ. Bull. 2017, 26, 5177–5183. [Google Scholar]
- Wang, C.; Fu, B.; Zhang, L.; Xu, Z. Soil moisture–plant interactions: An ecohydrological review. J. Soils Sediments 2019, 19, 1–9. [Google Scholar] [CrossRef]
- Długosz, J.; Piotrowska-Długosz, A.; Breza-Boruta, B. The effect of differences in soil water content on microbial and enzymatic properties across the soil profiles. Ecohydrol. Hydrobiol. 2023, 24, 547–556. [Google Scholar] [CrossRef]
- Pedrinho, A.; Mendes, L.W.; de Araujo Pereira, A.P.; Araujo, A.S.F.; Vaishnav, A.; Karpouzas, D.G.; Singh, B.K. Soil microbial diversity plays an important role in resisting and restoring degraded ecosystems. Plant Soil 2024, 500, 325–349. [Google Scholar] [CrossRef]
- Philippot, L.; Chenu, C.; Kappler, A.; Rillig, M.C.; Fierer, N. The interplay between microbial communities and soil properties. Nat. Rev. Microbiol. 2024, 22, 226–239. [Google Scholar] [CrossRef] [PubMed]
- Xun, W.; Huang, T.; Zhao, J.; Ran, W.; Wang, B.-R.; Shen, Q.; Zhang, R. Environmental conditions rather than microbial inoculum composition determine the bacterial composition, microbial biomass and enzymatic activity of reconstructed soil microbial communities. Soil Biol. Biochem. 2015, 90, 10–18. [Google Scholar] [CrossRef]
- Guo, J.; Wu, Y.; Wu, X.; Ren, Z.; Wang, G. Soil bacterial community composition and diversity response to land conversion is depth-dependent. Glob. Ecol. Conserv. 2021, 32, e01923. [Google Scholar] [CrossRef]
- Du, H.-D.; Wang, S.-M.; Nie, W.-J.; Song, S.-J. Soil Properties and Bacterial Community Dynamics in a Coal Mining Subsidence Area: Active Versus Passive Revegetation. J. Soil Sci. Plant Nutr. 2021, 21, 2573–2585. [Google Scholar] [CrossRef]
- Mhete, M.; Eze, P.N.; Rahube, T.O.; Akinyemi, F.O. Soil properties influence bacterial abundance and diversity under different land-use regimes in semi-arid environments. Sci. Afr. 2020, 7, e00246. [Google Scholar] [CrossRef]
- Zhang, M.; Dang, P.; Haegeman, B.; Han, X.; Wang, X.; Pu, X.; Qin, X.; Siddique, K.H.M. The effects of straw return on soil bacterial diversity and functional profiles: A meta-analysis. Soil Biol. Biochem. 2024, 195, 109484. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, S.H.; Jo, H.Y.; Finneran, K.T.; Kwon, M.J. Diversity and composition of soil Acidobacteria and Proteobacteria communities as a bacterial indicator of past land-use change from forest to farmland. Sci. Total Environ. 2021, 797, 148944. [Google Scholar] [CrossRef]
- Cleveland, C.C.; Nemergut, D.R.; Schmidt, S.K.; Townsend, A.R. Increases in soil respiration following labile carbon additions linked to rapid shifts in soil microbial community composition. Biogeochemistry 2007, 82, 229–240. [Google Scholar] [CrossRef]
- Jenkins, S.N.; Rushton, S.P.; Lanyon, C.; Whiteley, A.S.; Waite, I.S.; Brookes, P.C.; Kemmitt, S.J.; Evershed, R.P.; O’donnell, A.G. Taxon-specific responses of soil bacteria to the addition of low level C inputs. Soil Biol. Biochem. 2010, 42, 1624–1631. [Google Scholar] [CrossRef]
- Yang, N.; Li, X.; Liu, D.; Zhang, Y.; Chen, Y.; Wang, B.; Hua, J.; Zhang, J.; Peng, S.; Ge, Z.; et al. Diversity patterns and drivers of soil bacterial and fungal communities along elevational gradients in the Southern Himalayas, China. Appl. Soil Ecol. 2022, 178, 104563. [Google Scholar] [CrossRef]
- Manici, L.M.; Caputo, F.; De Sabata, D.; Fornasier, F. The enzyme patterns of Ascomycota and Basidiomycota fungi reveal their different functions in soil. Appl. Soil Ecol. 2024, 196, 105323. [Google Scholar] [CrossRef]
- Li, W.; Li, Z.; Liu, Y.; Nie, X.; Zheng, H.; Zhang, G.; Wang, S.; Ma, Y. Soil nutrients shape the composition and function of fungal communities in abandoned ancient rice terraces. J. Environ. Manag. 2023, 329, 117064. [Google Scholar] [CrossRef]
- Zhenqi, H.U. Theory and method of soil reconstruction of reclaimed mined land. J. China Coal Soc. 2022, 47, 2499–2515. [Google Scholar]
- Mustafa, G.; Hayat, N.; Alotaibi, B.A. Chapter fifteen—How and why to prevent over fertilization to get sustainable crop production. In Sustainable Plant Nutrition; Aftab, T., Hakeem, K.R., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 339–354. [Google Scholar] [CrossRef]




| Sampling Area | TN (g kg−1) | TP (g kg−1) | SOM (g kg−1) | AN (mg kg−1) | AP (mg kg−1) |
|---|---|---|---|---|---|
| CK | 1.368 ± 0.222 a | 1.473 ± 0.548 a | 19.6 ± 4.114 ab | 102.889 ± 21.991 a | 42.667 ± 31.312 a |
| PWSA | 1.319 ± 0.136 ab | 1.12 ± 0.114 b | 21.356 ± 1.994 a | 69.444 ± 14.8 b | 19.511 ± 5.212 bc |
| SSWA | 1.143 ± 0.242 b | 0.992 ± 0.167 b | 17.778 ± 1.884 b | 82.333 ± 21.407 ab | 14.978 ± 8.579 c |
| NA | 0.976 ± 0.202 b | 1.002 ± 0.096 b | 17.178 ± 2.786 b | 86.444 ± 27.668 ab | 16.433 ± 4.955 bc |
| TCA | 0.967 ± 0.204 b | 1.026 ± 0.242 b | 16.2 ± 2.147 b | 92 ± 25.51 ab | 22.589 ± 9.916 b |
| F | 2.745 | 4.731 | 7.645 | 5.161 | 7.549 |
| p | <0.001 | <0.01 | <0.001 | <0.01 | <0.001 |
| Sampling area | pH | SWC (%) | C/N | C/P | N/P |
| CK | 7.773 ± 0.27 a | 14.8 ± 18.3 ab | 14.255 ± 1.349 b | 14.133 ± 3.763 b | 0.986 ± 0.213 a |
| PWSA | 7.727 ± 0.068 ab | 18.9 ± 1.6 a | 16.234 ± 1.143 ab | 19.304 ± 3.091 a | 1.19 ± 0.183 a |
| SSWA | 7.721 ± 0.043 ab | 18 ± 0.8 a | 15.992 ± 2.859 ab | 18.194 ± 2.317 a | 1.153 ± 0.153 a |
| NA | 7.59 ± 0.16 b | 12.5 ± 5.2 b | 18.328 ± 5.306 a | 17.115 ± 2.153 a | 0.99 ± 0.26 a |
| TCA | 7.58 ± 0.111 b | 9.8 ± 2.8 b | 17.293 ± 3.924 ab | 16.768 ± 2.528 ab | 1.023 ± 0.304 a |
| F | 4.198 | 1.593 | 2.94 | 1.749 | 1.908 |
| p | <0.01 | 0.195 | <0.01 | 0.158 | 0.128 |
| Diversity Index | TN (g kg−1) | TP (g kg−1) | SOM (g kg−1) | AN (g kg−1) | AP (g kg−1) |
|---|---|---|---|---|---|
| Chao | 0.011 (0.953) | 0.422 (0.020 **) | −0.014 (0.940) | 0.227 (0.228) | 0.591 (0.001 ***) |
| Shannon | −0.146 (0.441) | 0.199 (0.293) | −0.298 (0.110) | 0.333 (0.072 *) | 0.463 (0.010 **) |
| Simpson | 0.111 (0.560) | −0.044 (0.816) | 0.347 (0.060 *) | −0.364 (0.048 **) | −0.393 (0.032 **) |
| diversity index | pH | SWC (%) | C/N | C/P | N/P |
| Chao | 0.366 (0.046 **) | −0.597 (0.000 ***) | −0.152 (0.421) | −0.527 (0.003 ***) | −0.507 (0.004 ***) |
| Shannon | 0.221 (0.240) | −0.62 (0.000 ***) | −0.337 (0.068 *) | −0.463 (0.010 ***) | −0.355 (0.054 *) |
| Simpson | −0.009 (0.962) | 0.497 (0.005 ***) | 0.406 (0.026 **) | 0.281 (0.133) | 0.092 (0.628) |
| Diversity Index | TN (g kg−1) | TP (g kg−1) | SOM (g kg−1) | AN (g kg−1) | AP (g kg−1) |
|---|---|---|---|---|---|
| Chao | 0.129 (0.399) | −0.148 (0.333) | −0.124 (0.416) | 0.068 (0.656) | −0.044 (0.776) |
| Shannon | −0.034 (0.826) | −0.108 (0.481) | −0.258 (0.087 *) | 0.151 (0.323) | 0.005 (0.973) |
| Simpson | 0.075 (0.623) | 0.046 (0.765) | 0.248 (0.100) | −0.201 (0.185) | −0.103 (0.501) |
| diversity index | PH | WATER | C/N | C/P | N/P |
| Chao | −0.154 (0.311) | −0.331 (0.026 **) | −0.261 (0.083 *) | −0.039 (0.797) | 0.167 (0.273) |
| Shannon | −0.221 (0.144) | −0.323 (0.031 **) | −0.191 (0.208) | −0.095 (0.535) | 0.108 (0.482) |
| Simpson | 0.337 (0.024 **) | 0.414 (0.005 ***) | 0.134 (0.380) | 0.134 (0.379) | −0.039 (0.802) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Luo, J.; Jiao, Y.; Lyu, X.; Wang, S.; Zhang, H. Soil Characteristics and Response Mechanism of the Microbial Community in a Coal–Grain Compound Area with High Groundwater Levels. Agronomy 2024, 14, 1993. https://doi.org/10.3390/agronomy14091993
Chen Z, Luo J, Jiao Y, Lyu X, Wang S, Zhang H. Soil Characteristics and Response Mechanism of the Microbial Community in a Coal–Grain Compound Area with High Groundwater Levels. Agronomy. 2024; 14(9):1993. https://doi.org/10.3390/agronomy14091993
Chicago/Turabian StyleChen, Zhichao, Jialiang Luo, Yiheng Jiao, Xiaoxuan Lyu, Shidong Wang, and Hebing Zhang. 2024. "Soil Characteristics and Response Mechanism of the Microbial Community in a Coal–Grain Compound Area with High Groundwater Levels" Agronomy 14, no. 9: 1993. https://doi.org/10.3390/agronomy14091993
APA StyleChen, Z., Luo, J., Jiao, Y., Lyu, X., Wang, S., & Zhang, H. (2024). Soil Characteristics and Response Mechanism of the Microbial Community in a Coal–Grain Compound Area with High Groundwater Levels. Agronomy, 14(9), 1993. https://doi.org/10.3390/agronomy14091993





