Exploring the Molecular Landscape of Nitrogen Use Efficiency in Potato (Solanum tuberosum L.) under Low Nitrogen Stress: A Transcriptomic and Metabolomic Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Treatment
2.2. Analysis of Growth and Yield Parameters
2.3. RNA Extraction, Library Construction, RNA Sequencing, and Data Analysis
2.4. Metabolomic Analysis
2.5. Quantitative Real-Time PCR (qRT-PCR)
2.6. Statistical Analysis
3. Results
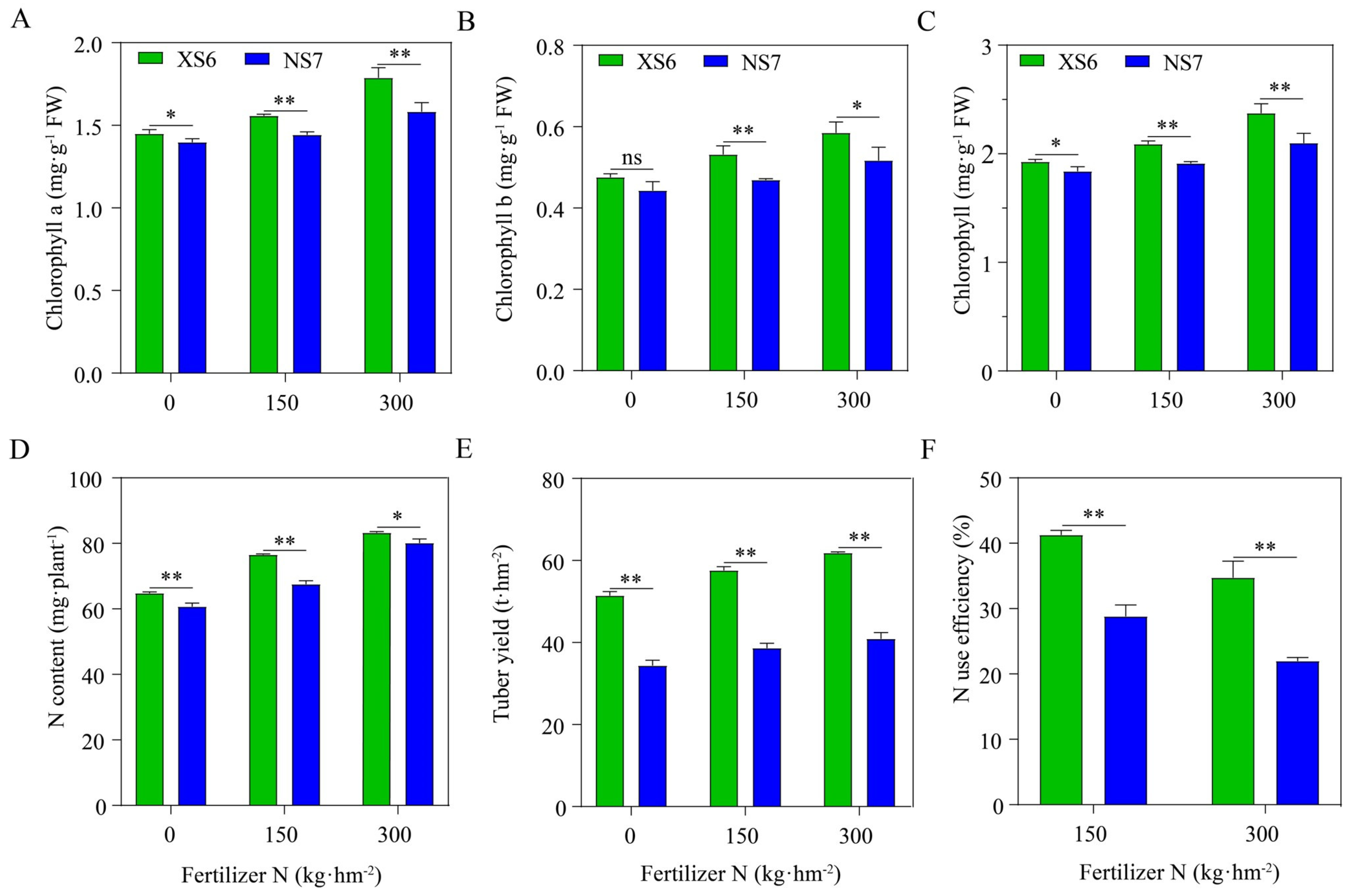
3.1. Evaluation of Nitrogen Efficiency-Related Indicators in Two Potato Varieties
3.2. Physiological Responses of Two Potato Varieties to Nitrogen Supply
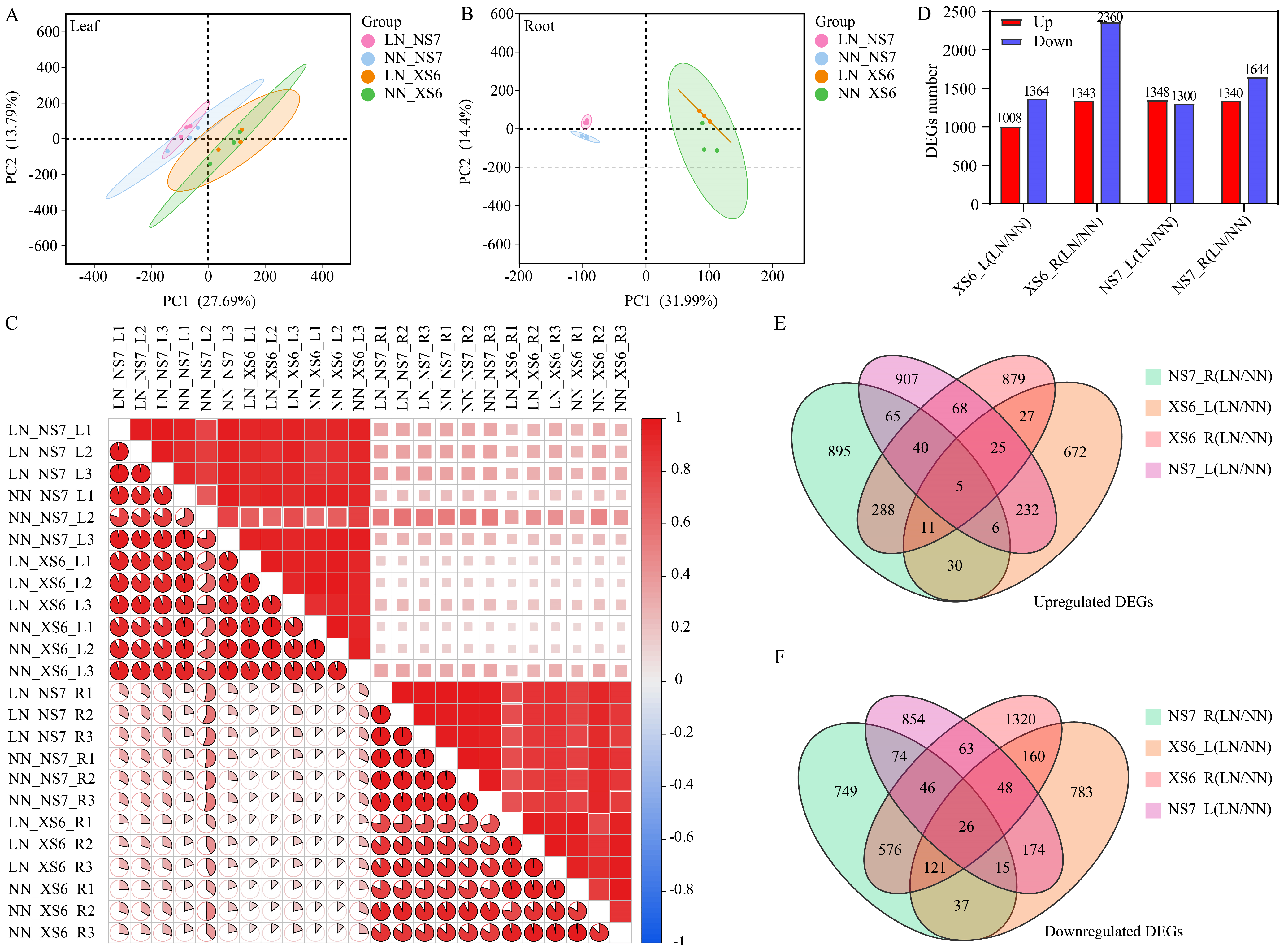
3.3. Transcriptomic Analysis
3.3.1. Sequencing and Identification of Differentially Expressed Genes (DEGs)
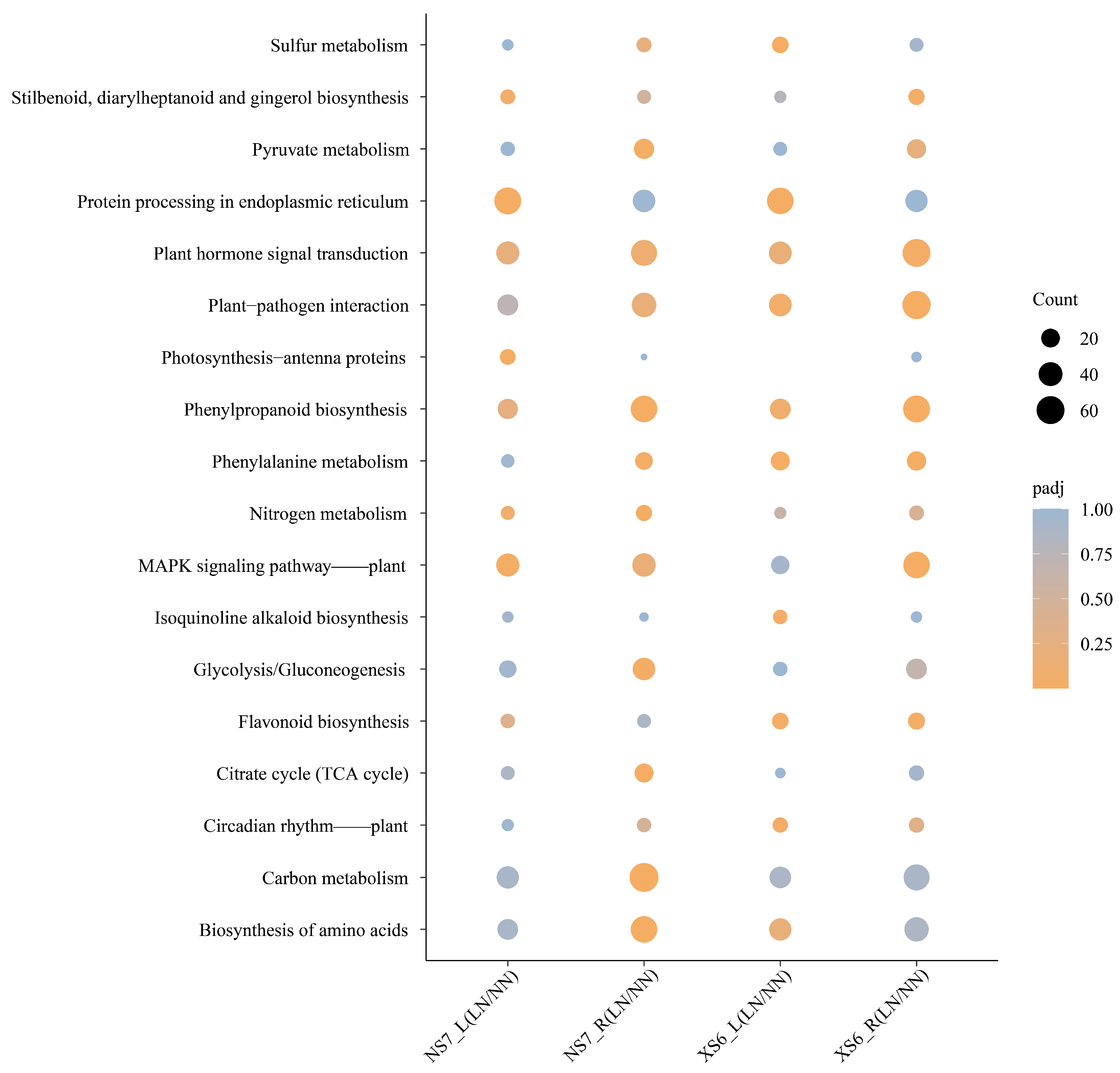
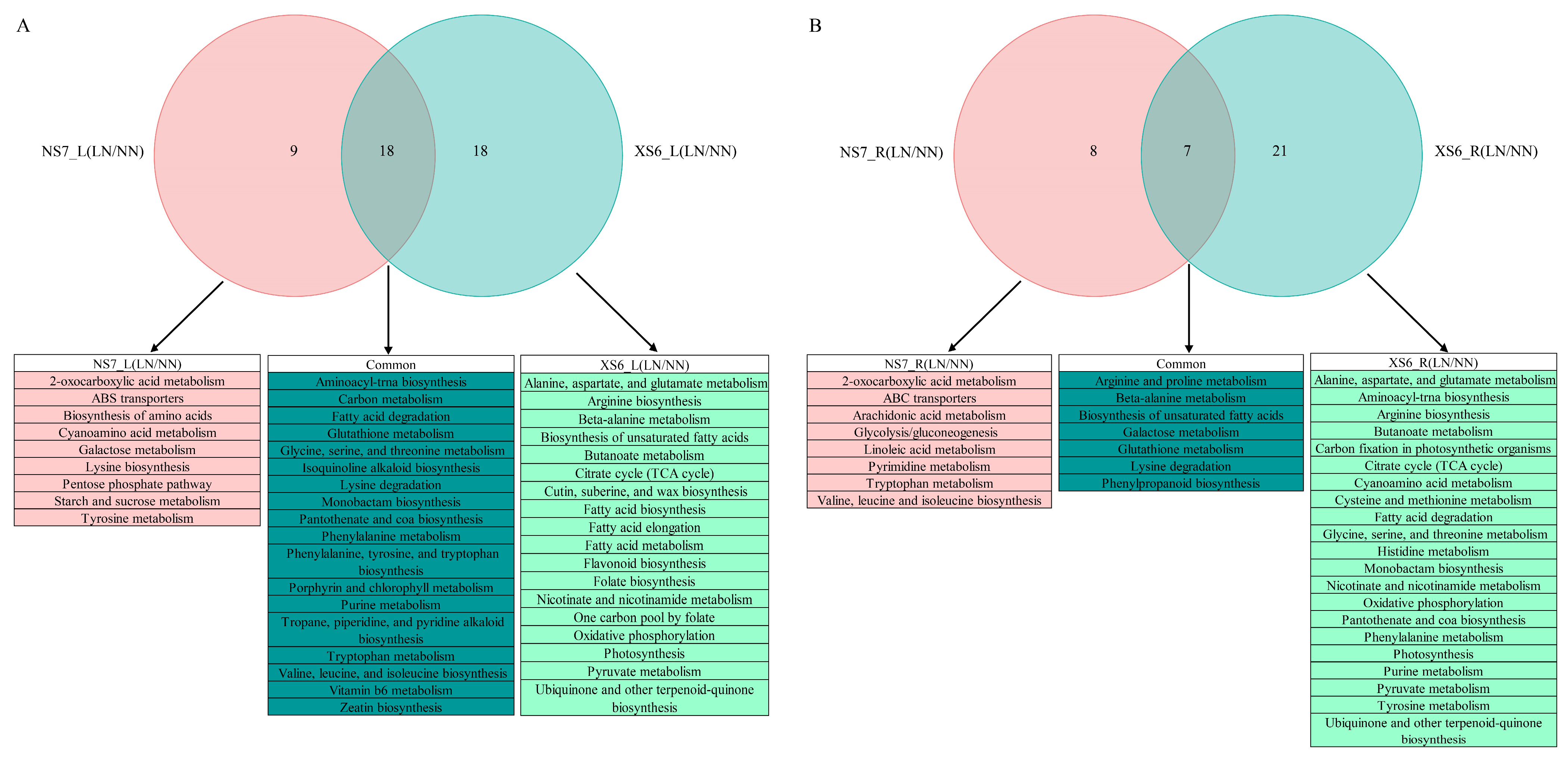
3.3.2. Enrichment Analysis of DEGs: GO and KEGG Pathways
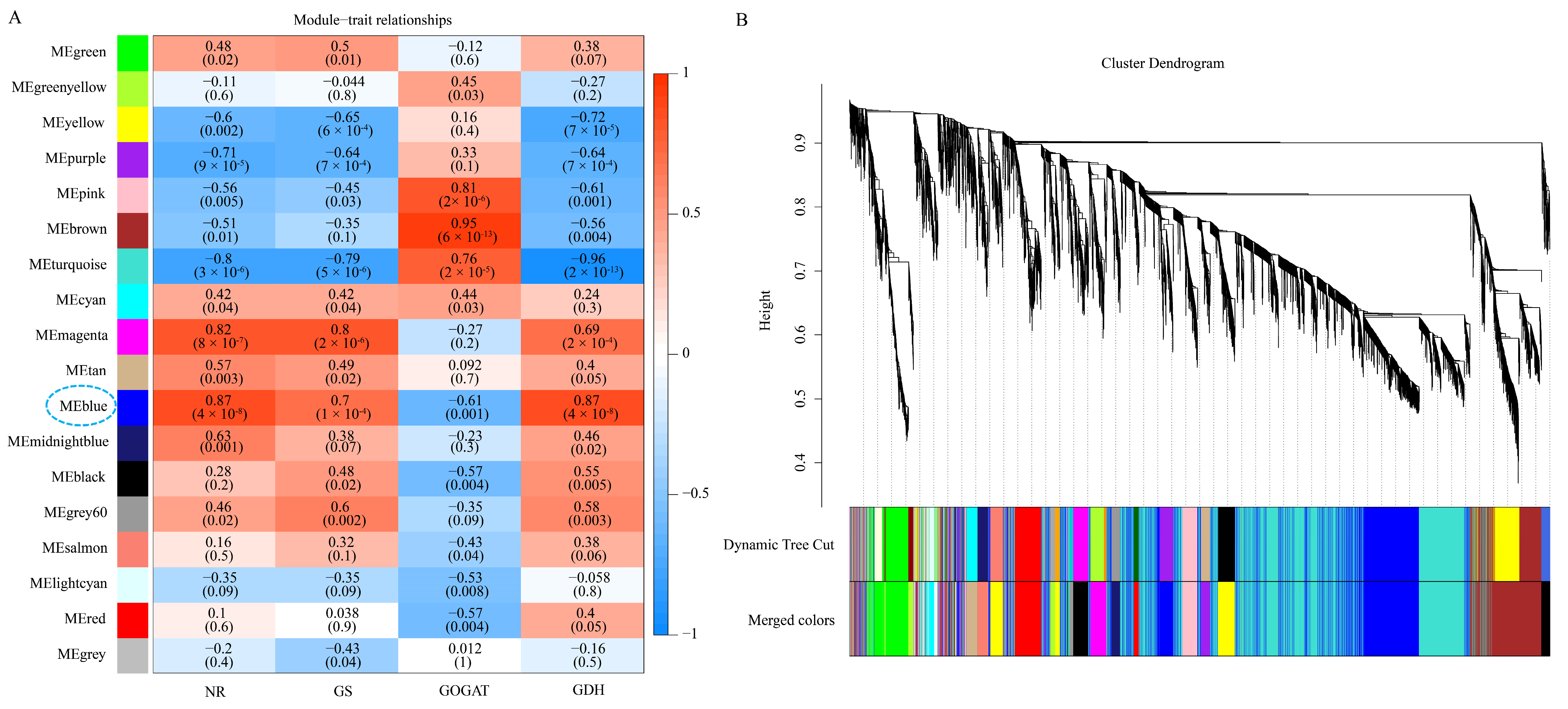
3.4. Weighted Gene Co-Expression Network Analysis (WGCNA) of DEGs
3.5. Metabolomic Profiling under LN Stress
3.5.1. Metabolomic Data Quality Control
3.5.2. Identification and Analysis of Differentially Accumulated Metabolites (DAMs)
3.5.3. Pathway Enrichment Analysis of Differentially Accumulated Metabolites
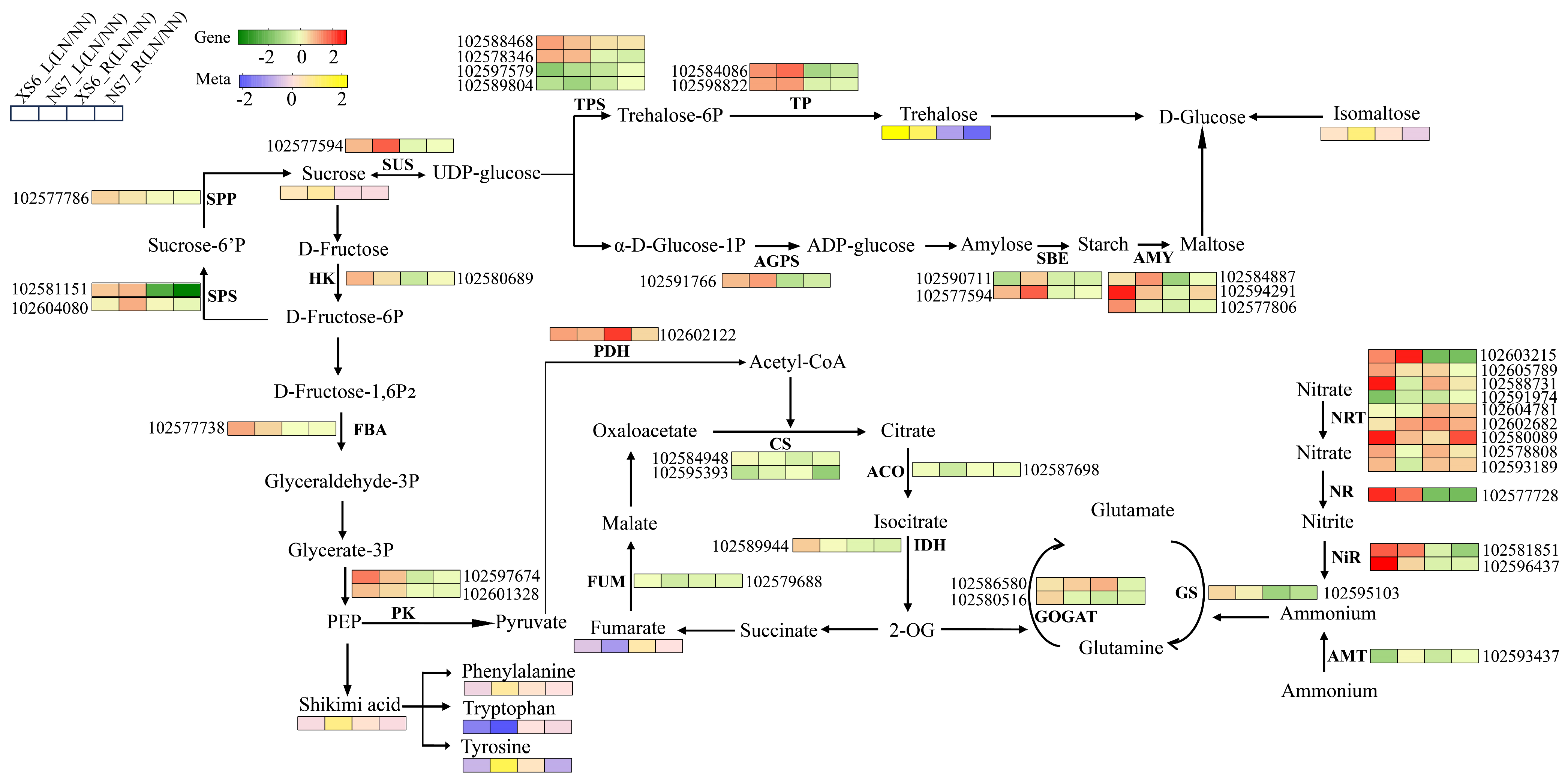
3.6. Joint Transcriptomic and Metabolomic Pathway Analysis
3.7. Examination of Key Metabolic Pathways under LN Stress
3.8. Validation of RNA-Seq Results through qRT-PCR
4. Discussion
4.1. Physiological Characteristics That Contribute to High NUE in Potato
4.2. Molecular Mechanism and Regulation of Carbon and Nitrogen Metabolism
4.3. Hypothesis
- (1)
- Enhanced photosynthesis: XS6 exhibits a higher chlorophyll content than NS7, laying a robust foundation for improved photosynthesis and increased organic matter production.
- (2)
- Efficient nitrogen uptake and accumulation: During the critical tuber expansion stage, XS6 demonstrates remarkable N uptake and accumulation capability. This efficiency is underpinned by the sustained high expression levels of NRT and nitrate reductase NR genes, even under N-deficient conditions. Such molecular adaptation facilitates greater N uptake from the soil and its accumulation in the plant’s aboveground tissues, effectively reducing N leakage into the groundwater.
- (3)
- Role of trehalose in alleviating nitrogen deficiency: The observed upregulation of trehalose in both XS6 and NS7 under LN conditions suggests a crucial role for this sugar in mitigating the effects of N deficiency. Furthermore, N stored in aboveground tissues is potentially mobilized and redirected to the roots and tubers, supporting sustained development despite periods of N scarcity.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Han, X.; Wu, K.; Fu, X.; Liu, Q. Improving Coordination of Plant Growth and Nitrogen Metabolism for Sustainable Agriculture. Abiotech 2020, 1, 255–275. [Google Scholar] [CrossRef]
- Li, C.; Aluko, O.O.; Yuan, G.; Li, J.; Liu, H. The Responses of Soil Organic Carbon and Total Nitrogen to Chemical Nitrogen Fertilizers Reduction Base on a Meta-Analysis. Sci. Rep. 2022, 12, 16326. [Google Scholar] [CrossRef]
- Makino, A. Photosynthesis, Grain Yield, and Nitrogen Utilization in Rice and Wheat. Plant Physiol. 2011, 155, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Ågren, G.I.; Weih, M. Plant Stoichiometry at Different Scales: Element Concentration Patterns Reflect Environment More than Genotype. New Phytol. 2012, 194, 944–952. [Google Scholar] [CrossRef]
- Kiba, T.; Krapp, A. Plant Nitrogen Acquisition Under Low Availability: Regulation of Uptake and Root Architecture. Plant Cell Physiol. 2016, 57, 707–714. [Google Scholar] [CrossRef]
- Li, Q.; Ding, G.; Yang, N.; White, P.J.; Ye, X.; Cai, H.; Lu, J.; Shi, L.; Xu, F. Comparative Genome and Transcriptome Analysis Unravels Key Factors of Nitrogen Use Efficiency in Brassica napus L. Plant Cell Environ. 2020, 43, 712–731. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.L.; Kielland, K. Amino Acid, Peptide and Protein Mineralization Dynamics in a Taiga Forest Soil. Soil Biol. Biochem. 2012, 55, 60–69. [Google Scholar] [CrossRef]
- McAllister, C.H.; Beatty, P.H.; Good, A.G. Engineering Nitrogen Use Efficient Crop Plants: The Current Status. Plant Biotechnol. J. 2012, 10, 1011–1025. [Google Scholar] [CrossRef]
- Walter, K.; Don, A.; Fuß, R.; Kern, J.; Drewer, J.; Flessa, H. Direct Nitrous Oxide Emissions from Oilseed Rape Cropping—a Meta-Analysis. GCB Bioenergy 2015, 7, 1260–1271. [Google Scholar] [CrossRef]
- Teshager, A.D.; Gassman, P.W.; Secchi, S.; Schoof, J.T. Simulation of Targeted Pollutant-Mitigation-Strategies to Reduce Nitrate and Sediment Hotspots in Agricultural Watershed. Sci. Total Environ. 2017, 607–608, 1188–1200. [Google Scholar] [CrossRef]
- Zhao, J.; Yao, Y.; Li, D.; Zhu, W.; Xiao, H.; Xie, M.; Xiong, Y.; Wu, J.; Ni, Q.; Zhang, M.; et al. Metagenome and Metabolome Insights into the Energy Compensation and Exogenous Toxin Degradation of Gut Microbiota in High-Altitude Rhesus Macaques (Macaca Mulatta). NPJ Biofilms Microbiomes 2023, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Kant, S.; Bi, Y.-M.; Rothstein, S.J. Understanding Plant Response to Nitrogen Limitation for the Improvement of Crop Nitrogen Use Efficiency. J. Exp. Bot. 2011, 62, 1499–1509. [Google Scholar] [CrossRef]
- White, P.; George, T.; Dupuy, L.; Karley, A.; Valentine, T.; Wiesel, L.; Wishart, J. Root Traits for Infertile Soils. Front. Plant Sci. 2013, 4, 193. [Google Scholar] [CrossRef]
- Xu, G.; Fan, X.; Miller, A.J. Plant Nitrogen Assimilation and Use Efficiency. Annu. Rev. Plant Biol. 2012, 63, 153–182. [Google Scholar] [CrossRef]
- Moll, R.H.; Kamprath, E.J.; Jackson, W.A. Analysis and Interpretation of Factors Which Contribute to Efficiency of Nitrogen Utilization1. Agron. J. 1982, 74, 562–564. [Google Scholar] [CrossRef]
- Han, M.; Okamoto, M.; Beatty, P.H.; Rothstein, S.J.; Good, A.G. The Genetics of Nitrogen Use Efficiency in Crop Plants. Annu. Rev. Genet. 2015, 49, 269–289. [Google Scholar] [CrossRef]
- Rakotoson, T.; Dusserre, J.; Letourmy, P.; Ramonta, I.R.; Cao, T.-V.; Ramanantsoanirina, A.; Roumet, P.; Ahmadi, N.; Raboin, L.-M. Genetic Variability of Nitrogen Use Efficiency in Rainfed Upland Rice. Field Crops Res. 2017, 213, 194–203. [Google Scholar] [CrossRef]
- Chen, K.-E.; Chen, H.-Y.; Tseng, C.-S.; Tsay, Y.-F. Improving Nitrogen Use Efficiency by Manipulating Nitrate Remobilization in Plants. Nat. Plants 2020, 6, 1126–1135. [Google Scholar] [CrossRef] [PubMed]
- Xin, W.; Zhang, L.; Zhang, W.; Gao, J.; Yi, J.; Zhen, X.; Du, M.; Zhao, Y.; Chen, L. An Integrated Analysis of the Rice Transcriptome and Metabolome Reveals Root Growth Regulation Mechanisms in Response to Nitrogen Availability. Int. J. Mol. Sci. 2019, 20, 5893. [Google Scholar] [CrossRef]
- Yu, J.; Xuan, W.; Tian, Y.; Fan, L.; Sun, J.; Tang, W.; Chen, G.; Wang, B.; Liu, Y.; Wu, W.; et al. Enhanced OsNLP4-OsNiR Cascade Confers Nitrogen Use Efficiency by Promoting Tiller Number in Rice. Plant Biotechnol. J. 2021, 19, 167–176. [Google Scholar] [CrossRef]
- Iqbal, A.; Dong, Q.; Wang, X.; Gui, H.; Zhang, H.; Zhang, X.; Song, M. Transcriptome Analysis Reveals Differences in Key Genes and Pathways Regulating Carbon and Nitrogen Metabolism in Cotton Genotypes under N Starvation and Resupply. Int. J. Mol. Sci. 2020, 21, 1500. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Yang, J.; Syed Muhammad Sadiq, S.; Yang, R.; Yu, J.; Li, W. Comparative Transcriptome Analysis of Different Nitrogen Responses in Low-Nitrogen Sensitive and Tolerant Maize Genotypes. J. Integr. Agric. 2021, 20, 2043–2055. [Google Scholar] [CrossRef]
- Luo, L.; Zhang, Y.; Xu, G. How Does Nitrogen Shape Plant Architecture? J. Exp. Bot. 2020, 71, 4415–4427. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; von Wirén, N. Signaling Pathways Underlying Nitrogen-Dependent Changes in Root System Architecture: From Model to Crop Species. J. Exp. Bot. 2020, 71, 4393–4404. [Google Scholar] [CrossRef] [PubMed]
- Spooner, D.M.; Gavrilenko, T.; Jansky, S.H.; Ovchinnikova, A.; Krylova, E.; Knapp, S.; Simon, R. Ecogeography of Ploidy Variation in Cultivated Potato (Solanum Sect. Petota). Am. J. Bot. 2010, 97, 2049–2060. [Google Scholar] [CrossRef] [PubMed]
- Haverkort, A.J.; Struik, P.C. Yield Levels of Potato Crops: Recent Achievements and Future Prospects. Field Crops Res. 2015, 182, 76–85. [Google Scholar] [CrossRef]
- Makani, M.; Zotarelli, L.; Sargent, S.; Huber, D.; Sims, C. Nitrogen Fertilizer Rate Affects Yield and Tuber Quality of Drip-Irrigated Tablestock Potatoes (Solanum tuberosum L.) Grown under Subtropical Conditions. Am. J. Potato Res. 2020, 97, 605–614. [Google Scholar] [CrossRef]
- Mohamed, E.; Rosen, C.J.; Lauer, F.I.; Martin, M.W.; Bamberg, J.B.; Birong, D.E. Screening of Exotic Potato Germplasm for Nitrogen Uptake and Biomass Production. Am. J. Pot. Res. 1998, 75, 93–100. [Google Scholar] [CrossRef]
- Errebhi, M.; Rosen, C.J.; Lauer, F.I.; Martin, M.W.; Bamberg, J.B. Evaluation of Tuber-bearingSolanum Species for Nitrogen Use Efficiency and Biomass Partitioning. Am. J. Pot. Res. 1999, 76, 143–151. [Google Scholar] [CrossRef]
- Zebarth, B.; Tai, G.; Tarn, R.; De JONG, H.; Milburn, P. Nitrogen Use Efficiency Characteristics of Commercial Potato Cultivars. Can. J. Plant Sci. 2004, 84, 589–598. [Google Scholar] [CrossRef]
- Getahun, B.B.; Kassie, M.M.; Visser, R.G.F.; van der Linden, C.G. Genetic Diversity of Potato Cultivars for Nitrogen Use Efficiency Under Contrasting Nitrogen Regimes. Potato Res. 2020, 63, 267–290. [Google Scholar] [CrossRef]
- Tiwari, J.K.; Buckseth, T.; Singh, R.K.; Kumar, M.; Kant, S. Prospects of Improving Nitrogen Use Efficiency in Potato: Lessons From Transgenics to Genome Editing Strategies in Plants. Front. Plant Sci. 2020, 11, 597481. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Yin, Y.; Zhao, J.; An, W.; Fan, Y.; Liang, X.; Cao, Y. Metabolomic and Transcriptomic Analysis of Lycium Chinese and L. Ruthenicum under Salinity Stress. BMC Plant Biol. 2022, 22, 8. [Google Scholar] [CrossRef]
- Tiedge, K.; Li, X.; Merrill, A.T.; Davisson, D.; Chen, Y.; Yu, P.; Tantillo, D.J.; Last, R.L.; Zerbe, P. Comparative Transcriptomics and Metabolomics Reveal Specialized Metabolite Drought Stress Responses in Switchgrass (Panicum virgatum). New Phytol. 2022, 236, 1393–1408. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Li, B.; Tian, H.; Li, X.; Ren, H.; Zhou, Q. Metabolome and Transcriptome Analysis Predicts Metabolism of Violet-Red Color Change in Lilium Bulbs. J. Sci. Food Agric. 2022, 102, 2903–2915. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Li, L.; Ma, Z.; Fan, M. Improving Potato Yield, Water Productivity and Nitrogen Use Efficiency by Managing Irrigation Based on Potato Root Distribution. Int. J. Plant Prod. 2022, 16, 547–555. [Google Scholar] [CrossRef]
- Thomas, R.L.; Sheard, R.W.; Moyer, J.R. Comparison of Conventional and Automated Procedures for Nitrogen, Phosphorus, and Potassium Analysis of Plant Material Using a Single Digestion1. Agron. J. 1967, 59, 240–243. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Want, E.J.; Masson, P.; Michopoulos, F.; Wilson, I.D.; Theodoridis, G.; Plumb, R.S.; Shockcor, J.; Loftus, N.; Holmes, E.; Nicholson, J.K. Global Metabolic Profiling of Animal and Human Tissues via UPLC-MS. Nat. Protoc. 2013, 8, 17–32. [Google Scholar] [CrossRef]
- Zhang, X.; Ding, Y.; Ma, Q.; Li, F.; Tao, R.; Li, T.; Zhu, M.; Ding, J.; Li, C.; Guo, W.; et al. Comparative Transcriptomic and Metabolomic Analysis Revealed Molecular Mechanism of Two Wheat Near-Isogenic Lines Response to Nitrogen Application. Plant Physiol. Biochem. 2023, 195, 47–57. [Google Scholar] [CrossRef]
- Wen, B.; Mei, Z.; Zeng, C.; Liu, S. metaX: A Flexible and Comprehensive Software for Processing Metabolomics Data. BMC Bioinform. 2017, 18, 183. [Google Scholar] [CrossRef]
- Wu, J.; Ma, Y.; Xie, R.; Zhang, Z.; Zhang, S.; Wu, X.; Wang, P.; Wang, D.; Lu, C. Integrated Metabolomics and Transcriptomics Reveals Difference Involved in Flavonoid and Indole Alkaloids Biosynthesis in Potato Tuber Flesh. Sci. Hortic. 2024, 324, 112630. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Adnan, M.; Morton, G.; Hadi, S. Analysis of rpoS and bolA Gene Expression under Various Stress-Induced Environments in Planktonic and Biofilm Phase Using 2(-ΔΔCT) Method. Mol. Cell Biochem. 2011, 357, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, J.K.; Buckseth, T.; Devi, S.; Varshney, S.; Sahu, S.; Patil, V.U.; Zinta, R.; Ali, N.; Moudgil, V.; Singh, R.K.; et al. Physiological and Genome-Wide RNA-Sequencing Analyses Identify Candidate Genes in a Nitrogen-Use Efficient Potato Cv. Kufri Gaurav. Plant Physiol. Biochem. 2020, 154, 171–183. [Google Scholar] [CrossRef]
- Tiwari, J.K.; Buckseth, T.; Zinta, R.; Saraswati, A.; Singh, R.K.; Rawat, S.; Dua, V.K.; Chakrabarti, S.K. Transcriptome Analysis of Potato Shoots, Roots and Stolons under Nitrogen Stress. Sci. Rep. 2020, 10, 1152. [Google Scholar] [CrossRef]
- Zinta, R.; Tiwari, J.K.; Buckseth, T.; Thakur, K.; Goutam, U.; Kumar, D.; Challam, C.; Bhatia, N.; Poonia, A.K.; Naik, S.; et al. Root System Architecture for Abiotic Stress Tolerance in Potato: Lessons from Plants. Front. Plant Sci. 2022, 13, 926214. [Google Scholar] [CrossRef]
- Sun, T.; Zhang, J.; Zhang, Q.; Li, X.; Li, M.; Yang, Y.; Zhou, J.; Wei, Q.; Zhou, B. Integrative Physiological, Transcriptome, and Metabolome Analysis Reveals the Effects of Nitrogen Sufficiency and Deficiency Conditions in Apple Leaves and Roots. Environ. Exp. Bot. 2021, 192, 104633. [Google Scholar] [CrossRef]
- Sharma, N.; Kumari, S.; Jaiswal, D.K.; Raghuram, N. Comparative Transcriptomic Analyses of Nitrate-Response in Rice Genotypes With Contrasting Nitrogen Use Efficiency Reveals Common and Genotype-Specific Processes, Molecular Targets and Nitrogen Use Efficiency-Candidates. Front. Plant Sci. 2022, 13, 881204. [Google Scholar] [CrossRef]
- Tegeder, M.; Masclaux-Daubresse, C. Source and Sink Mechanisms of Nitrogen Transport and Use. New Phytol. 2018, 217, 35–53. [Google Scholar] [CrossRef]
- Tilsner, J.; Kassner, N.; Struck, C.; Lohaus, G. Amino Acid Contents and Transport in Oilseed Rape (Brassica napus L.) under Different Nitrogen Conditions. Planta 2005, 221, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xu, J.; Wang, X.; Fu, H.; Zhao, M.; Wang, H.; Shi, L. Photosynthetic Characteristics and Metabolic Analyses of Two Soybean Genotypes Revealed Adaptive Strategies to Low-Nitrogen Stress. J. Plant Physiol. 2018, 229, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Ren, Y.; Fu, H.; Li, Z.; Kong, F.; Yuan, J. Cultivar Differences in Carbon and Nitrogen Accumulation, Balance, and Grain Yield in Maize. Front. Plant Sci. 2022, 13, 992041. [Google Scholar] [CrossRef] [PubMed]
- Boussadia, O.; Steppe, K.; Zgallai, H.; Ben El Hadj, S.; Braham, M.; Lemeur, R.; Van Labeke, M.C. Effects of Nitrogen Deficiency on Leaf Photosynthesis, Carbohydrate Status and Biomass Production in Two Olive Cultivars ‘Meski’ and ‘Koroneiki. ’ Sci. Hortic. 2010, 123, 336–342. [Google Scholar] [CrossRef]
- Duan, Y.; Yang, H.; Yang, H.; Wei, Z.; Che, J.; Wu, W.; Lyu, L.; Li, W. Physiological and Morphological Responses of Blackberry Seedlings to Different Nitrogen Forms. Plants 2023, 12, 1480. [Google Scholar] [CrossRef] [PubMed]
- Krapp, A.; Berthomé, R.; Orsel, M.; Mercey-Boutet, S.; Yu, A.; Castaings, L.; Elftieh, S.; Major, H.; Renou, J.-P.; Daniel-Vedele, F. Arabidopsis Roots and Shoots Show Distinct Temporal Adaptation Patterns toward Nitrogen Starvation. Plant Physiol. 2011, 157, 1255–1282. [Google Scholar] [CrossRef]
- Iordachescu, M.; Imai, R. Trehalose Biosynthesis in Response to Abiotic Stresses. J. Integr. Plant Biol. 2008, 50, 1223–1229. [Google Scholar] [CrossRef]
- Yang, L.; Zhao, X.; Zhu, H.; Paul, M.; Zu, Y.; Tang, Z. Exogenous Trehalose Largely Alleviates Ionic Unbalance, ROS Burst, and PCD Occurrence Induced by High Salinity in Arabidopsis Seedlings. Front. Plant Sci. 2014, 5, 570. [Google Scholar] [CrossRef]
- Loka, D.A.; Oosterhuis, D.M.; Baxevanos, D.; Noulas, C.; Hu, W. Single and Combined Effects of Heat and Water Stress and Recovery on Cotton (Gossypium hirsutum L.) Leaf Physiology and Sucrose Metabolism. Plant Physiol. Biochem. 2020, 148, 166–179. [Google Scholar] [CrossRef]
- Hu, H.; Liu, Y.; He, B.; Chen, X.; Ma, L.; Luo, Y.; Fei, X.; Wei, A. Integrative Physiological, Transcriptome, and Metabolome Analysis Uncovers the Drought Responses of Two Zanthoxylum bungeanum Cultivars. Ind. Crops Prod. 2022, 189, 115812. [Google Scholar] [CrossRef]
- Martínez-Reyes, I.; Chandel, N.S. Mitochondrial TCA Cycle Metabolites Control Physiology and Disease. Nat. Commun. 2020, 11, 102. [Google Scholar] [CrossRef]
- Liu, D.; Li, M.; Liu, Y.; Shi, L. Integration of the Metabolome and Transcriptome Reveals the Resistance Mechanism to Low Nitrogen in Wild Soybean Seedling Roots. Environ. Exp. Bot. 2020, 175, 104043. [Google Scholar] [CrossRef]
- Li, D.; Liu, J.; Zong, J.; Guo, H.; Li, J.; Wang, J.; Wang, H.; Li, L.; Chen, J. Integration of the Metabolome and Transcriptome Reveals the Mechanism of Resistance to Low Nitrogen Supply in Wild Bermudagrass (Cynodon dactylon (L.) Pers.) Roots. BMC Plant Biol. 2021, 21, 480. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Zhou, J.; Li, H.; Shi, W.; Polle, A.; Lu, M.; Sun, X.; Luo, Z.-B. Global Poplar Root and Leaf Transcriptomes Reveal Links between Growth and Stress Responses under Nitrogen Starvation and Excess. Tree Physiol. 2015, 35, 1283–1302. [Google Scholar] [CrossRef]
- Wany, A.; Pathak, P.K.; Gupta, K.J. Methods for Measuring Nitrate Reductase, Nitrite Levels, and Nitric Oxide from Plant Tissues. Methods Mol. Biol. 2020, 2057, 15–26. [Google Scholar] [CrossRef]
- Hu, M.; Zhao, X.; Liu, Q.; Hong, X.; Zhang, W.; Zhang, Y.; Sun, L.; Li, H.; Tong, Y. Transgenic Expression of Plastidic Glutamine Synthetase Increases Nitrogen Uptake and Yield in Wheat. Plant Biotechnol. J. 2018, 16, 1858–1867. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; de Bang, T.C.; Schjoerring, J.K. Cisgenic Overexpression of Cytosolic Glutamine Synthetase Improves Nitrogen Utilization Efficiency in Barley and Prevents Grain Protein Decline under Elevated CO2. Plant Biotechnol. J. 2019, 17, 1209–1221. [Google Scholar] [CrossRef]
- Martin, A.; Lee, J.; Kichey, T.; Gerentes, D.; Zivy, M.; Tatout, C.; Dubois, F.; Balliau, T.; Valot, B.; Davanture, M.; et al. Two Cytosolic Glutamine Synthetase Isoforms of Maize Are Specifically Involved in the Control of Grain Production. Plant Cell 2006, 18, 3252–3274. [Google Scholar] [CrossRef]
- Lee, S.; Marmagne, A.; Park, J.; Fabien, C.; Yim, Y.; Kim, S.; Kim, T.-H.; Lim, P.O.; Masclaux-Daubresse, C.; Nam, H.G. Concurrent Activation of OsAMT1;2 and OsGOGAT1 in Rice Leads to Enhanced Nitrogen Use Efficiency under Nitrogen Limitation. Plant J. 2020, 103, 7–20. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, R.; Jin, X.; Fang, J.; Wei, S.; Ma, J.; Liu, Y.; Cheng, Y.; Chen, L.; Liu, J.; Liu, Y.; et al. Exploring the Molecular Landscape of Nitrogen Use Efficiency in Potato (Solanum tuberosum L.) under Low Nitrogen Stress: A Transcriptomic and Metabolomic Approach. Agronomy 2024, 14, 2000. https://doi.org/10.3390/agronomy14092000
Xie R, Jin X, Fang J, Wei S, Ma J, Liu Y, Cheng Y, Chen L, Liu J, Liu Y, et al. Exploring the Molecular Landscape of Nitrogen Use Efficiency in Potato (Solanum tuberosum L.) under Low Nitrogen Stress: A Transcriptomic and Metabolomic Approach. Agronomy. 2024; 14(9):2000. https://doi.org/10.3390/agronomy14092000
Chicago/Turabian StyleXie, Rui, Xiaolei Jin, Jing Fang, Shuli Wei, Jie Ma, Ying Liu, Yuchen Cheng, Liyu Chen, Jiawei Liu, Yanan Liu, and et al. 2024. "Exploring the Molecular Landscape of Nitrogen Use Efficiency in Potato (Solanum tuberosum L.) under Low Nitrogen Stress: A Transcriptomic and Metabolomic Approach" Agronomy 14, no. 9: 2000. https://doi.org/10.3390/agronomy14092000





