Abstract
The initial outbreak of Xylella fastidiosa subsp. pauca (Xfp) on olive groves in Salento (Apulia, Italy) dates back to the years 2008 and 2009 when extensive twig and branch diebacks were observed in the area of Gallipoli area (province of Lecce). Subsequently, the bacterium also spread northwards to other areas of Apulia. In many cases, entire olive groves, also including the centennial ones, died. After the crown collapse, in many cases, it has been observed that the suckers are resprouting at the base of the trunk. After two to three years, such suckers usually died as well. However, during the last four to five years, in the first Xfp outbreak area, a complete restoration of the crown of the Xfp-susceptible cultivars Ogliarola salentina and Cellina di Nardò has been noticed. Such trees or olive groves also started to yield again. To monitor this tree resilience phenomenon, together with local non-profit organizations, a survey in the province of Lecce has been carried out to find olive groves for which any curative or agronomical practices have been applied since the bacterium outbreak. Resilient olive groves are scattered in many municipalities all over the province of Lecce. The phenomenon regards both young and adult olive groves and also includes some centennial trees. In many cases, the trees are yielding fruits, and farmers started to cultivate them again. Olive resilience in Salento is already being studied and can represent a significant opportunity to restore the local and valuable olive germplasm.
Keywords:
“olive quick decline syndrome”; resilience; resprouting; drought; waterlogging; herbicides 1. Introduction
Xylella fastidiosa subsp. pauca (Xfp) was reported in association with olive trees that showed extensive declining symptoms in Salento, in October 2013 [1]. The area (i.e., about 8–10.000 hectares) where it was first observed was the one surrounding the municipality of Gallipoli (Lecce province). The main symptoms included leaf and twig dieback, and branch wilting followed by plant death. Some phytopathogenic fungi, such as Phaeoacremonium spp. and Phaeomoniella sp., were also frequently found associated with symptomatic olive trees [2]. The disease was named “olive quick decline syndrome” (OQDS), and one year after its first report, a value was found of about 23.000 [3], mainly on the two local cultivars Ogliarola salentina and Cellina di Nardò. During the following years, the disease spread to a vast area of the province of Lecce, and, subsequently, it also reached the surrounding provinces of Taranto and Brindisi. Currently, OQDS occurs in the whole area of the province of Lecce, and relevant parts of Taranto and Brindisi provinces. It has also reached the “Piana degli Olivi monumentali” (Carovigno, Ostuni, Fasano, Cisternino, and Monopoli municipalities) which is characterized by a high number of centennial and millennial olive trees, and the province of Bari [4].
Until some years ago, the olive trees that had not completely wilted as a result of the Xfp attack often had numerous suckers growing at the base of the tree that usually survived a few years, and, subsequently, they died as well [5,6,7]. By contrast, during the last four to five years it has been noted that, in some olive groves located in the areas of the first Xfp outbreaks in Salento, including the Gallipoli area, the suckers formed a crown, and, in some circumstances, the grower has pruned them to restore the shape of the tree as before the Xfp outbreak. The phenomenon occurred either in young (i.e., up to 30–35 years old) or adult groves (i.e., more than 35 years old), also including centennial trees belonging to the susceptible cultivars Ogliarola salentina and Cellina di Nardò. Moreover, the trees are flowering and yielding fruits. It should be stressed that such trees did not receive any care upon the occurrence of the disease, so they were completely abandoned [8]. This phenomenon shows the characteristics of “resilience”.
Resilience is described in humans, plants, and the environment. In humans, resilience represents the strength to handle and deal with a problem, and to continue normally through life [9]. Resilience refers to “the ability to cope with difficult, stressful situations while maintaining or restoring normal functioning”. In ecosystem ecology, resilience is defined as a “measure of the persistence of systems and of their ability to absorb change and disturbance and reorganize while undergoing change so as to still retain essentially the same function, structure, identity, and feedback” [10,11]. The phenomenon observed on the susceptible olive cultivars previously infected by Xfp was totally unexpected since many trees of Ogliarola salentina and Cellina di Nardò resulted in being completely desiccated in the past [12], which prompted us to perform field surveys to ascertain its extension. A recent article [13] describes the relationships between a resilience phenomenon observed in an olive grove of the Lecce province and the re-programming of some tree lipid pathways upon the first outbreak of Xfp that occurred in the area around 2009–2010 [8]. Jasmonic acid and salicylic acid are involved in the resprouting of resilient trees. The resilience of the site was ascertained through satellite imagery and the normalized difference vegetation index (NDVI) (Figure 1) [13]. In this report, we show where the resilience of olive trees previously severely damaged by Xfp is currently occurring in the province of Lecce.
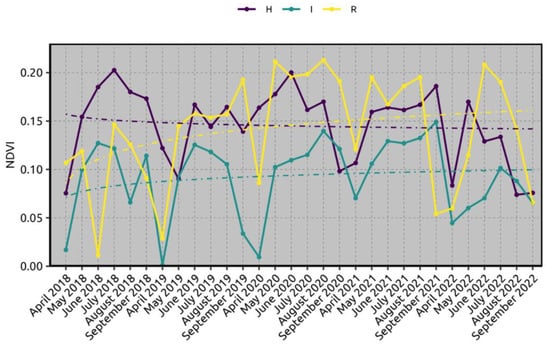
Figure 1.
Normalized difference vegetation index (NDVI) trend assessed through satellite imagery from April 2018 to September 2022 for a resilient olive grove located in Parabita (Lecce province). The data have been compared with nearby visibly infected trees and with healthy ones located in Taranto province. H: healthy olive trees; I: infected olive trees; and R: resilient olive trees. For each condition of state health, an average logarithmic trendline was drawn to show the trend. Notice that the NDVI value of the resilient trees is higher than that of the healthy ones during the last two years. Reproduced from [13].
2. Survey for the Observation of Resilience in Olive Groves in Salento
The criteria for defining an olive grove that is currently showing resilience towards OQDS can be summarized as follows: (a) the olive grove must be planted with the local susceptible cultivars Ogliarola salentina and/or Cellina di Nardò; (b) extensive symptoms of OQDS (i.e., severe branch diebacks and collapse of the most of the canopy) were previously observed in the olive grove; (c) all the agronomical practices (i.e., pruning, herbicide distribution, and soil tillage) performed before the occurrence of OQDS were suspended upon the appearance of symptoms; (d) no cure to the tree (i.e., soil or crown fertilization or spray treatments to the canopy with bactericidal compounds) was carried out during the tree resprouting; and (e) the farmer is currently performing the renovation of the grove through the sucker or canopy training. Such information was directly obtained by us, local farmers, and by many associations of farmers and/or citizens who observed and claimed the revitalization of olive trees during the last four to five years. This survey concerns a vast portion of the province of Lecce. A map of Salento illustrating the municipalities where the resilience of olive groves is currently occurring is shown in Figure 2. Such a distribution should not be considered definitive since other examples of resilience could have been unnoticed. Some examples of resilient olive groves observed in Salento are shown in Figure 3, Figure 4, Figure 5 and Figure 6. Such resilient olive groves where the farmer is newly training the crop are neighbors to completely wilted and abandoned trees and sometimes represent an oasis within a completely withered territory. In addition, there are examples of resprouting trees still to restore a productive grove.
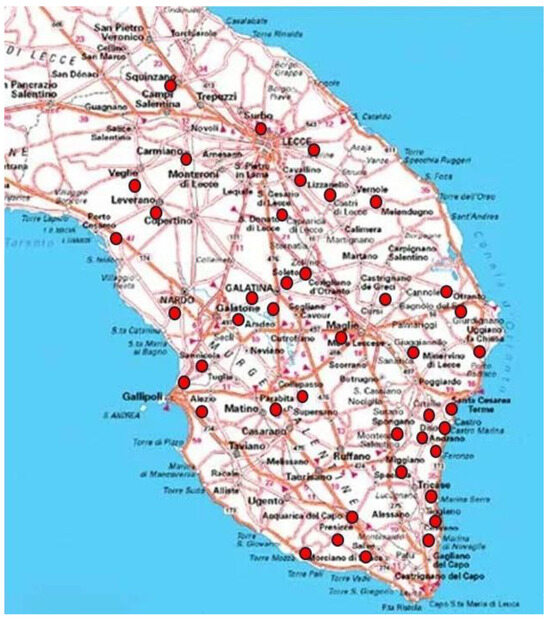
Figure 2.
Mapof Lecce province that shows the occurrence of resilient olive groves. Red circles indicate the municipality where these olive groves were noticed.

Figure 3.
Resilient olive groves planted with Cellina di Nardò and Ogliarola salentina as observed in Lecce province in November 2023. (A) Parabita; (B) Alezio; (C) Tiggiano; and (D) Sannicola. The olive grove of Parabita has been assessed for the first study on olive tree resilience in Salento upon Xylella fastidiosa subsp. pauca outbreak [13]. In panels (A,D), the resprouting of new vegetation along wilted branches is evident. Panel A refers to the study of Scala et al. [13].

Figure 4.
Resilient olive groves planted with Cellina di Nardò and Ogliarola salentina as observed in Lecce province in November 2023. (A) Nardò; (B) Maglie; (C) Diso; and (D) Spongano. In panels (A,D), it is evident that the new crown was obtained upon the cutting of the main tree branches that wilted upon Xylella fastidiosa subsp. pauca infection and the sucker resprouting.
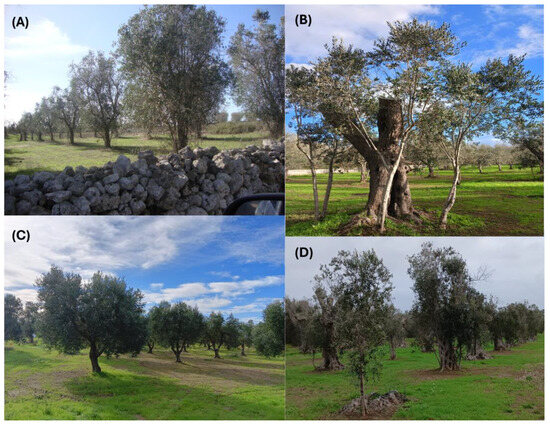
Figure 5.
Resilient olive groves planted with Cellina di Nardò and Ogliarola salentina as observed in Lecce province in November 2023. (A) Giuggianello; (B) Vernole; (C) Corsano; and (D) Giurdignano. In panels (B,D), it is evident that the new crown was obtained upon the cutting of the main tree branches that wilted upon Xylella fastidiosa subsp. pauca infection and the sucker resprouting.

Figure 6.
Fruit setting in resilient olive groves planted with Cellina di Nardò and Ogliarola salentina in Lecce province as observed in June 2024. (A) Nardò; (B) Galatone; (C) Giurdignano; and (D) Vernole.
From this survey, resilience is quite present in olive groves of Salento previously showing extensive symptoms of OQDS. This phenomenon is very important for trying to restore residual olive groves in the area heavily damaged by the disease, and to partially save the typical olive agro-ecosystem. Of note, some of these resilient olive groves started to yield fruits. The production ranges from a few fruits per tree to some kg (i.e., 5–15 kg) per tree. Some olive groves are just resprouting without fruiting.
3. Consideration of the Resilience of Olive Groves
The resilience of olive groves is a direct consequence of tree resprouting, a key response to stress and disturbance widely recognized in woody plant species [14,15]. Upon stress, biotic or abiotic, plants can react by activating internal physiological mechanisms enabling them to maintain both plant viability and functions [16]. The resprouting occurs in the meristematic tissues of apical, basal, and below-ground buds [14]. Relevant resprouting has also been recently found in California in oak trees affected by the “sudden oak death” disease, caused by Phytophthora ramorum, and such a survival strategy has remained fundamental for the conservation of trees affected by the pathogen [17]. Resprouting has also remained crucial in a global warming context since plant species capable of reacting to severe stress, such as prolonged drought, show more possibilities of recovering when compared with non-resprouting species [18].
Resilience found in olive groves previously severely attacked by Xfp deserves further in-depth studies aimed at investigating both the plant molecular and physiological/anatomical changes in progress that are in relationship with the phenomenon. Of note, preliminary observations carried out on some resilient Ogliarola salentina and Cellina di Nardò adult trees of Salento indicated that Xfp is still present within the canopy and does not show any visible sign of infection (i.e., no apparent OQDS symptoms). The average Xfp concentration, indeed, ranged from 104 to 107 CFU/mL [13,19] (Figure 7), with the latter concentration usually related to symptomatic trees [20]. So far, we can only discuss the predisposing factors that may have incited the severity of the infection and, after the abandoning of the groves, the potential causes that have induced the resilience. In Figure 8, some aspects have been summarized that could have had a role in promoting a high incidence and severity of OQDS in olive groves in Salento.
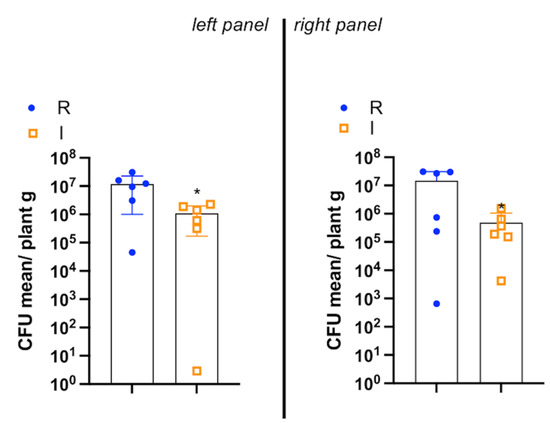
Figure 7.
Boxplot representation of quantitative PCR quantification of Xylella fastidiosa subsp. pauca within the crown of infected (I) and resilient (R) olive trees. In the left panel are the reported results of six trees of cultivar Cellina di Nardò; in the right panel are the reported results of six trees of cultivar Ogliarola salentina. Data are expressed as CFU mean ± S.D. (* p < 0.02, R vs. I). I: indicates olive trees symptomatic and positive to X. fastidiosa subsp. pauca; R indicates positive to X. fastidiosa subsp. pauca and resprouting olive tree. Reproduced from [13]. Notice that the cell concentration of the bacterium is even higher in the resilient trees than in the infected ones.
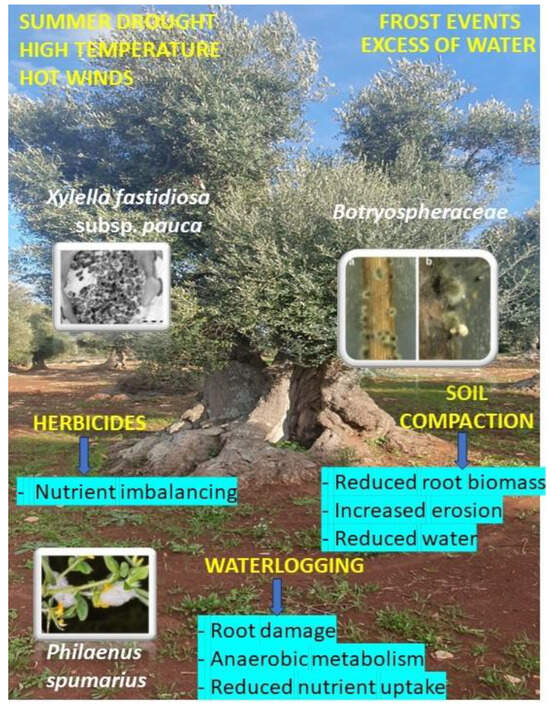
Figure 8.
An overview that illustrates some predisposing factors, phytopathogens, vectors of Xylella fastidiosa subsp. pauca, and environmental consequences all acting on olive groves of Salento before and during the decline syndrome of olive trees. a: immature conidiomata of Neofusicoccum mediterraneum; b: mature conidiomata of N. mediterraneum excluding masses of conidia.
Among abiotic factors, repeated summer droughts accompanied by high air temperature and hot winds, frost events, and excess precipitation that occurred over a short time on a limited surface are all present during the last decades in the Salento area and may have contributed to promoting physiological stress to the olive groves [21,22,23], so that the trees may have resulted in becoming weaker to the bacterium attack. Soil waterlogging for some consecutive days caused by an excess of rain establishes a condition of hypoxia in the soil, and, consequently, in the roots. Hypoxia causes a switch from aerobic metabolism to anaerobic metabolism, which induces fermentation to satisfy energy requests. Prolonged waterlogging causes the accumulation of toxic compounds such as lactic acid, alcohols, aldehydes, and other anaerobic metabolites, which can also lead to plant death [24]. In addition, the microbiota composition of the soil shifts towards detrimental anaerobic microflora [25]. Drought causes a decrease in leaf water content and an associated reduction in photosynthesis [26]. Moreover, an increase in xylem cavitation can be observed, which can cause embolism and the interruption of water transport from the root to the canopy [27]. During drought, there is a marked reduction in nutrient uptake within the plant, resulting in reduced yield, fruit dry mass, and oil accumulation [28,29]. Ethylene might inhibit plant growth under drought conditions, as it is involved in leaf abscission [30].
During the last four to five years, such stress events have not disappeared (Figure 9) [31], and, consequently, the resilient trees were capable of facing them and re-starting growth, as well. The insect vector that is transmitting Xfp, namely Philaenus spumarius, is still spread in the whole Salento territory, and, according to the Xfp cell concentration found in the resilient trees [13,19], it can suck the bacterium from the olive foliage, other than from the wild flora, and continue to transmit the inoculum in the areas where the resilient trees are located. However, the abandoning of the olive groves resulted in a higher density of wild vegetation during the whole season. This change might have attracted the vector in the cover crops more than the olive leaves, thus reducing the times that the bacterium has been introduced into the leaves.
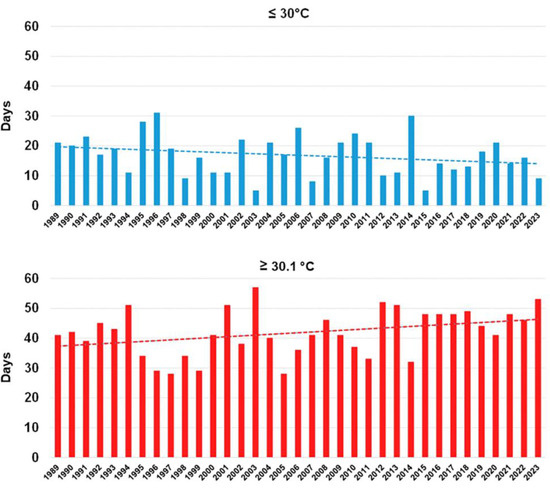
Figure 9.
Number of days in which maximum temperatures reached values ≤ 30 °C and ≥30.1 °C in the period of July–August in Galatina (Lecce province) and in the time span of 1989–2023. Dashed lines are the trend lines. Reproduced from [31].
Recently, the occurrence of some Botryospheraceae fungi has been observed, namely Neofusicoccum spp., and Diplodia seriata widely spread in Salento, frequently associated with the bacterium in the same olive tree and showing a relevant aggressiveness to olive trees [31,32,33]. The occurrence of such fungi, which cause symptoms very similar to those incited by Xfp, could have further caused twig and branch wilting in the trees already infected by the bacterium [34]. This additional biotic stress factor is also present in the area. The occurrence of such fungi also in the resilient olive groves is under study. So far, we can say that such fungi are favored by drought and high temperatures during summer and that they are very frequently found in olive trees of the Salento area also infected by Xfp [31,32,33]. Resilient trees have also faced such stress in a way that is still to be fully understood.
Among the agronomic practices that have been abandoned upon the Xfp outbreaks and spread in the olive groves in Salento, there is the distribution to the soil of herbicides. This practice was very frequent in the territory during the last decades [35], with an average of three distributions per year [36]. The repeated distribution of herbicides can cause some modification in the macro- and microelement content of the soil [37]. A study, performed in an olive grove of Salento, aimed at verifying the trend of soil macro- and microelement content upon two consecutive years of glyphosate distribution, ascertained a statistically significant increase in phosphorous content, and a statistically significant decrease in magnesium and nickel content [38].
The increase in phosphorous content upon glyphosate distribution, indeed, is well known [39] since glyphosate–phosphorous is converted in the soil to phosphoric acid or phosphate ions [40]. The regular utilization of glyphosate for weed control over many years can pose some risks for the olive groves, especially in sandy soils that generally show a lower capacity for buffering the herbicide degradation, to potentially induce phytotoxicity [41]. In addition, the increase in phosphorous content can go unnoticed by the grower that, by adding the required amount of this macroelement for the yield through the yearly fertilization, could incite some toxicity. Of note, indeed, the addition of phosphorous could re-mobilize residues of glyphosate; this potentially induces significant phytotoxicity [42]. An excess of phosphorous in olive, indeed, can cause disturbance in the absorption of zinc, copper, manganese, and iron [43,44]. High levels of phosphorous can also negatively interfere with the normal mycorrhizal growth [45].
The decrease in magnesium content in the soil that follows the glyphosate distribution has been already observed [46]. This can lead to a significant reduction in absorption and translocation of this macroelement within the plant [47], to a consequent reduction in growth [48], and to an increase in disease susceptibility of the crop [49]. Such a content reduction has been observed in tissues of different plant species [50,51]. A deficiency of magnesium in the plant can lead to a reduction in chlorophyll biosynthesis, inhibition of sucrose transport, alteration of nutrient uptake, and secondary metabolism [52,53]. The glyphosate distribution can also cause a reduction in nickel content in the soil [54] due to the well-known chelating activity of this herbicide [55] which also concerns this microelement [56]. Nickel is an activator of metalloenzymes such as urease which hydrolyzes urea in plant tissue, and it is also involved in the degradation of methylglyoxal, a potent cytotoxic compound [57,58]. The depletion of nickel, by reducing the antioxidant activity of the plant cell, could reduce the resistance to stressful situations [58].
Nickel is also fundamental for nitrogen assimilation, and its depletion can cause reduced plant growth and senescence [59]. Nickel deficiency can also cause a reduction in the symbiotic hydrogenase activity in Rhizobium leguminosarum that can, consequently, negatively affect the symbiotic nitrogen fixation [60,61].
The distribution of herbicides for killing the weeds and for preparing the olive groves for the harvest could have also favored soil compaction through the repeated passage of machinery. Soil compaction reduces soil porosity and aeration, and soil hydraulic properties; this leads to reduced water availability for the root system and a higher possibility of soil erosion [62]. Such a scenario can ultimately lead to a decrease in nutrient availability, water uptake, root growth, and rooting depth [62].
These features might have had some implication for the tree resilience since after some years of herbicide disposal and the abandoning of any soil agronomical practice (i.e., tillage); the soil could have naturally increased the organic matter content and the total nitrogen content [63], thus promoting the tree resprouting. In addition, repeated herbicide distribution significantly altered the composition of soil microbiota [64]. Moreover, it has been observed that over a period of 17 years, the soil of an olive grove cultivated according to a sustainable approach, which does not include herbicide treatments, is characterized by beneficial bacterial communities that colonize the xylem and promote protective functions for the tree [65]. The abandonment of any cultural practice also implies that local wild flora can grow for years without any disturbance. Such conditions can promote high bacterial biomass with a vast array of different functions beneficial to the tree [66].
The herein-discussed abiotic factors that currently act on the resilient olive groves can be framed within the climate change scenario for which the resilience of plants should be enhanced [67]. Among the general negative effects that climate change (i.e., drought, hot temperatures, and excess of water) can impose on plants, there are physiological alterations (i.e., increased transpiration rate and reduced net photosynthesis rate), morphological alterations (i.e., shoot growth inhibition, leaf senescence and abscission, and inhibited growth root), metabolic alterations (i.e., alteration in nitrogen and carbohydrate metabolism and production of reactive oxygen species), and reproductive alterations (reduced crop growth rate and fruit set) [68]. Some potential proposed solutions to achieve resilience in crops could be a better understanding of agronomical techniques to conserve soil water, organic matter, and beneficial microorganisms, providing specific crop microbiota to promote tolerance to stress, improving phenotyping for the identification of genes responsible for resilient traits, and improving research to understand signaling in molecular pathways related to resilient traits [68].
Resilience in the Salento area is a remarkable phenomenon that is currently characterizing olive groves previously severely attacked by Xfp. In this area, many municipalities host olive groves that show a good vegetative status by showing no or very little sign of infection (i.e., leaf and twig dieback). This phenomenon was not predicted upon the occurrence and spread of the bacterium in the area but now deserves further analyses to find out the reasons for such unexpected resilience. Resilience can be a strong factor that can allow for the recovery of the local olive industry.
Author Contributions
M.S. and D.R.: writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
The authors are grateful to the many local associations that contributed to observing and reporting the resilience in the olive groves of Salento.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Saponari, M.; Boscia, D.; Nigro, F.; Martelli, G.P. Identification of DNA sequences related to Xylella fastidiosa in oleander, almond and olive trees exhibiting leaf scorch symptoms in Apulia (Southern Italy). J. Plant Pathol. 2013, 95, 688. [Google Scholar]
- Nigro, F.; Boscia, D.; Antelmi, I.; Ippolito, A. Fungal species associated with a severe decline of olive in southern Italy. J. Plant Pathol. 2013, 95, 668. [Google Scholar]
- Stokstad, E. Italy’s olives under siege. Science 2015, 348, 620. [Google Scholar] [CrossRef] [PubMed]
- Ciervo, M.; Scortichini, M. A decade of monitoring surveys for Xylella fastidiosa subsp. pauca in olive groves of Apulia (Italy) reveals a low incidence of the bacterium in the demarcated area. J. Phytopathol. 2024, 172, e13272. [Google Scholar] [CrossRef]
- Martelli, G.P. The current status of the quick decline syndrome of olive in southern Italy. Phytoparasitica 2016, 44, 1–10. [Google Scholar] [CrossRef]
- Semeraro, T.; Buccolieri, R.; Vergine, M.; De Bellis, L.; Luvisi, A.; Emmanuel, R.; Marwan, R. Analysis of olive grove destruction by Xylella fastidiosa bacterium on the land surface temperature in Salento detected using satellite images. Forests 2021, 12, 1266. [Google Scholar] [CrossRef]
- Camposeo, S.; Vivaldi, G.A.; Saponari, M. Attempts to reduce the systemic spread of Xylella fastidiosa in olive trees by pruning. Agronomy 2022, 12, 2917. [Google Scholar] [CrossRef]
- Scortichini, M. The epidemiology and control of “olive quick decline syndrome” in Salento (Apulia, Italy). Agronomy 2022, 12, 2475. [Google Scholar] [CrossRef]
- Babić, R.; Babić, M.; Rastović, P.; Ćurlin, M.; Šimić, J.; Mandić, K.; Pavlović, K. Resilience in health and illness. Psychiatr. Danub. 2020, 32 (Suppl. S2), 226–232. [Google Scholar] [PubMed]
- Walker, B.; Holling, C.S.; Carpenter, S.R.; Kinzig, A. Resilience, adaptability and transformability in social–ecological systems. Ecol. Soc. 2004, 9, 5. [Google Scholar] [CrossRef]
- Crespi, E.; Burnap, R.; Chen, J.; Das, M.; Gassman, N.; Rosa, E., Jr.; Simmons, R.; Wada, H.; Wang, Z.Q.; Xiao, J.; et al. Resolving the rules of robustness and resilience in biology across scale. Integr. Comp. Biol. 2021, 61, 2163–2179. [Google Scholar] [CrossRef]
- Saponari, M.; Giampetruzzi, A.; Loconsole, G.; Boscia, D.; Saldarelli, P. Xylella fastidiosa in olive in Apulia: Where we stand. Phytopathology 2019, 109, 175–186. [Google Scholar] [CrossRef]
- Scala, V.; Scortichini, M.; Marini, F.; La Montagna, D.; Beccaccioli, M.; Micalizzi, K.; Cacciotti, A.; Pucci, N.; Tatulli, G.; Fiorani, R.; et al. Assessment of fatty acid and oxylipin profile of resprouting olive trees positive to Xylella fastidiosa subsp. pauca in Salento (Apulia, Italy). Plants 2024, 13, 2186. [Google Scholar] [CrossRef]
- Clarke, P.J.; Lawes, M.J.; Midgley, J.J.; Ojeda, F.; Burrows, G.E.; Enright, N.J.; Knox, K.J.E. Resprouting as a key functional trait: How buds, protection and resources drive persistence after fire. New Phytol. 2013, 197, 19–35. [Google Scholar] [CrossRef]
- Bendall, E.R.; Bedward, M.; Boer, M.; Clarke, H.; Collins, L.; Leigh, A.; Bradstock, R.A. Changes in the resilience of resprouting juvenile tree populations in temperate forests due to coupled severe drought and fire. Plant Ecol. 2022, 223, 907–923. [Google Scholar] [CrossRef]
- Bussotti, F.; Pollastrini, M. Revisiting the concept of stress in forest trees at the time of global change and issues for stress monitoring. Plant Stress 2021, 2, 100013. [Google Scholar] [CrossRef]
- Simler, A.B.; Metz, M.R.; Frangioso, K.M.; Meentemeyer, R.K.; Rizzo, D.M. Novel disturbance interactions between fire and an emerging disease impact survival and growth of resprouting trees. Ecology 2018, 99, 2217–2229. [Google Scholar] [CrossRef]
- Zeppel, M.J.B.; Harrison, S.P.; Adams, H.D.; Kelley, D.I.; Li, G.; Tissue, D.T.; Dawson, T.E.; Fensham, R.; Medlyn, B.E.; Palmer, A.; et al. Drought and resprouting plants. New Phytol. 2015, 206, 583–589. [Google Scholar] [CrossRef]
- Loreti, S.; Scala, V.; Pucci, N.; L’Aurora, A.; Tatulli, G.; Fiorani, R.; Lucchesi, S.; Scortichini, M. Preliminary observations on “resilient” olive trees infected by Xylella fastidiosa in the Salento area (Apulia, Italy). J. Plant Pathol. 2023, 105, 1279. [Google Scholar]
- Tatulli, G.; Modesti, V.; Pucci, N.; Scala, V.; L’Aurora, A.; Lucchesi, S.; Salustri, M.; Scortichini, M.; Loreti, S. Further in vitro assessment and mid-term evaluation of control strategy of Xylella fastidiosa subsp. pauca in olive groves of Salento (Apulia, Italy). Pathogens 2021, 10, 85. [Google Scholar] [CrossRef]
- De Pascali, M.; Vergine, M.; Sabella, E.; Aprile, A.; Nutricati, E.; Nicoli, F.; Buja, I.; Negro, C.; Miceli, A.; Rampino, P.; et al. Molecular effects of Xylella fastidiosa and drought combine stress in olive trees. Plants 2019, 8, 437. [Google Scholar] [CrossRef] [PubMed]
- Navarro, C.; Parra, M.A. Plantacíon. In El Cultivo del Olivo; Barranco, D., Fernández-Escobar, R., Rallo, L., Eds.; Junta de Andalucía y Mundi-Prensa: Madrid, Spain, 2008; pp. 163–195. [Google Scholar]
- Scortichini, M. Predisposing factors for “olive quick decline syndrome” in Salento (Apulia, Italy). Agronomy 2020, 10, 1445. [Google Scholar] [CrossRef]
- Loreti, E.; Perata, P. The many facets of hypoxia in plants. Plants 2020, 9, 745. [Google Scholar] [CrossRef]
- Gschwend, F.; Aregger, K.; Gramlich, A.; Walter, T.; Widmer, F. Periodic waterlogging consistently shapes agricultural soil microbiomes by promoting specific taxa. Appl. Soil Ecol. 2020, 155, 103623. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Plant Physiology, 4th ed.; Sinauer Associates, Inc.: Sunderland, MA, USA, 2006. [Google Scholar]
- Fernández, J.E.; Diaz-Espejo, A.; Infante, J.M.; Durán, P.; Palomo, M.J.; Chamorro, V.; Girón, I.F.; Villagarcía, L. Water relations and gas exchange in olive trees under regulated deficit irrigation and partial rootzone drying. Plant Soil 2006, 84, 273–291. [Google Scholar] [CrossRef]
- Stefanoudaki, E.; Chartzoulakis, K.; Koutsaftakis, A.; Kotsifaki, F. Effect of drought stress on qualitative characteristics of olive oil of cv Koroneiki. Grasas Aceites 2001, 52, 202–206. [Google Scholar] [CrossRef]
- Tognetti, R.; D’Andria, R.; Sacchi, R.; Lavini, A.; Morelli, G.; Alvino, A. Deficit irrigation affects seasonal changes in leaf physiology and oil quality of Olea europaea (cultivars Frantoio and Leccino). Ann. Appl. Biol. 2007, 150, 169–186. [Google Scholar] [CrossRef]
- Gomez-Cadenas, A.; Tadeo, F.R.; Talon, M.; Primo Millo, E. Leaf abscission induced by ethylene in water-stressed intact seedlings of Cleopatra mandarin requires previous abscisic acid accumulation in roots. Plant Physiol. 1996, 112, 401–408. [Google Scholar] [CrossRef]
- Manetti, G.; Brunetti, A.; Sciarroni, L.; Lumia, V.; Bechini, S.; Marangi, P.; Reverberi, M.; Scortichini, M.; Pilotti, M. Diplodia seriata isolated from declining olive trees in Salento (Apulia, Italy): Pathogenicity trials give a glimpse that it is more virulent to drought-stressed olive trees and in the warmth-conditioned environment. Plants 2014, 13, 2245. [Google Scholar] [CrossRef]
- Brunetti, A.; Matere, A.; Lumia, V.; Pasciuta, V.; Fusco, V.; Sansone, D.; Marangi, P.; Cristella, N.; Faggioli, F.; Scortichini, M.; et al. Neofusicoccum mediterraneum is involved in a twig and branch dieback of olive trees observed in Salento (Apulia, Italy). Pathogens 2022, 11, 53. [Google Scholar] [CrossRef]
- Manetti, G.; Brunetti, A.; Lumia, V.; Sciarroni, L.; Marangi, P.; Cristella, N.; Faggioli, F.; Reverberi, M.; Scortichini, M.; Pilotti, M. Identification and characterization of Neofusicoccum stellenboschiana in branch and twig dieback-affected olive trees in Italy and comparative pathogenicity with N. mediterraneum. J. Fungi 2023, 9, 292. [Google Scholar] [CrossRef]
- Scortichini, M.; Manetti, G.; Brunetti, A.; Lumia, V.; Sciarroni, L.; Pilotti, M. Xylella fastidiosa subsp. pauca, Neofusicoccum spp., and the decline of olive trees in Salento (Apulia, Italy): Comparison of symptoms, possible interactions, certainties and doubts. Plants 2023, 12, 3593. [Google Scholar] [CrossRef]
- Ciervo, M. The olive quick decline syndrome (OQDS) diffusion in Apulia region: An apparent contradiction according to the agricultural model. Belgeo 2016, 4. [Google Scholar] [CrossRef]
- Collavo, A. Resistenza al Glifosate nelle Colture Arboree. In Veneto Agricoltura, Forum Fitoiatrico 19 Gennaio; CNR-Istituto di Biologia Agro-ambientale e Forestale: Legnaro, Italy, 2012; Available online: https://www.venetoagricoltura.org/upload/pubblicazioni/Forum%20Fito%20023/COLLAVO.pdf (accessed on 6 August 2024).
- Ferreira, I.Q.; Arrobas, M.; Claro, A.M.; Rodrigues, M.A. Soil management in rainfed olive orchards may result in conflicting effects on olive production and soil fertility. Span. J. Agric. Res. 2013, 2, 472–480. [Google Scholar] [CrossRef]
- Fanizzi, F.P. (University, “Unisalento”, Monteroni-Lecce, Italy). Personal Communication. 2024. Available online: https://www.unisalento.it/scheda-utente/-/people/luigi.patrono (accessed on 6 August 2024).
- Hébert, M.P.; Fugère, V.; Gonzalez, A. The overlooked impact of rising glyphosate use on phosphorous loading in agricultural watersheds. Front. Ecol. Environ. 2019, 17, 48–56. [Google Scholar] [CrossRef]
- Hove-Jensen, B.; Zechel, D.L.; Jochimsen, B. Utilization of glyphosate as phosphate source: Biochemistry and genetics of bacterial carbon-phosphorous lyase. Microbiol. Mol. Biol. Rev. 2014, 78, 176–197. [Google Scholar] [CrossRef]
- Rose, T.J.; Van Zwieten, L.; Claassens, A.; Scanlon, C.; Rose, M.T. Phytotoxicity of soilborne glyphosate residues is influenced by the method of phosphorous fertiliser application. Plant Soil 2017, 422, 455–465. [Google Scholar] [CrossRef]
- Bott, S.; Tesfamariam, T.; Kania, A.; Eman, B.; Aslan, N.; Römheld, V.; Neumann, G. Phytotoxicity of glyphosate soil residues re-mobilised by phosphate fertilization. Plant Soil 2011, 342, 249–263. [Google Scholar] [CrossRef]
- Sanzani, S.M.; Schena, L.; Nigro, F.; Sergeeva, V.; Ippolito, A.; Salerno, M.G. Abiotic diseases of olive. J. Plant Pathol. 2012, 94, 469–491. [Google Scholar]
- Hussein, H.A.Z.; Awad, A.A.M.; Beheiry, H.R. Improving nutrients uptake and productivity of stressed olive trees with mono-ammonium phosphate and urea phosphate application. Agronomy 2022, 12, 2390. [Google Scholar] [CrossRef]
- Jiménez-Moreno, M.J.; Del Carmen Moreno-Marquez, M.; Moreno-Alias, I.; Rapoport, H.; Escobar, R. Interaction between mycorrhization with Glomus intraradices and phosphorus in nursery olive plants. Sci. Hortic. 2018, 233, 249–255. [Google Scholar] [CrossRef]
- Okada, E.; Costa, J.L.; Bedmar, F. Adsorption and mobility of glyphosate in different soils under no-till and conventional tillage. Geoderma 2016, 263, 78–85. [Google Scholar] [CrossRef]
- Duke, S.O. Glyphosate. In Herbicides: Chemistry, degradation, and mode of action; Kearney, P.C., Kaufman, D.D., Eds.; Marcel Dekker Inc.: New York, NY, USA, 1988; Volume 3, pp. 1–70. [Google Scholar]
- Sundaram, A.; Sundaram, K.M.S. Solubility products of six metal-glyphosate complexes in water and forestry soils, and their influence on glyphosate toxicity to plants. J. Environ. Sci. Health Part B 1997, 32, 583–598. [Google Scholar] [CrossRef]
- Huber, D.M.; Jones, J.B. The role of magnesium in plant disease. Plant Soil 2013, 368, 73–85. [Google Scholar] [CrossRef]
- Cakmak, I.; Yazici, A.; Tutus, Y.; Ozturk, L. Glyphosate reduced seed and leaf concentration of calcium, magnesium, manganese and iron in non-glyphosate resistant soybean. Eur. J. Agron. 2009, 31, 114–119. [Google Scholar] [CrossRef]
- Zobiole, L.H.S.; Oliveira, R.S., Jr.; Huber, D.M.; Constantin, J.; Castro, C.; Oliveira, F.A.; Oliveira, A., Jr. Glyphosate reduces shoot concentrations of mineral nutrients in glyphosate resistant soybeans. Plant Soil 2010, 328, 57–69. [Google Scholar] [CrossRef]
- Guo, W.; Nazim, H.; Liang, Z.; Yang, D. Magnesium deficiency in plants: An urgent problem. Crop J. 2016, 4, 83–91. [Google Scholar] [CrossRef]
- Chaudry, A.H.; Nayab, S.; Hussain, S.B.; Ali, M.; Pan, Z. Current understanding on magnesium deficiency and future outlooks for sustainable agriculture. Int. J. Mol. Sci. 2021, 22, 1819. [Google Scholar] [CrossRef]
- Yamada, T.; Kremer, R.J.; Castro, P.R.C.; Wood, B.W. Glyphosate interactions with physiology, nutrition, and diseases of plants: Threat to agricultural sustainability? Eur. J. Agron. 2009, 31, 111–113. [Google Scholar] [CrossRef]
- Mertens, M.; Höss, S.; Neumann, G.; Afzal, J.; Reichenbecher, W. Glyphosate, a chelating agent-relevant for ecological risk assessment? Environ. Sci. Pollut. Res. Int. 2018, 25, 5298–5317. [Google Scholar] [CrossRef]
- Romero-Natale, A.; Palchetti, I.; Avelar, M.; Gonzalez-Vergara, E.; Garate-Morales, J.L.; Torres, E. Spectrophotometric detection of glyphosate in water by complex formation between bis-5-phenyldipyrrinate of nickel (II) and glyphosate. Water 2019, 11, 719. [Google Scholar] [CrossRef]
- Wood, B.W.; Reilly, C.C. Interaction of nickel and plant disease. In Mineral Nutrition and Plant Disease; Datnoff, L.E., Elmer, W.H., Huber, D., Eds.; American Phytopathological Society Press: Minneapolis, MN, USA, 2007; pp. 217–247. [Google Scholar]
- Fabiano, C.C.; Tezotto, T.; Favarin, J.L.; Polacco, J.C.; Mazzafera, P. Essentiality of nickel in plants: A role in plant stresses. Front. Plant Sci. 2015, 6, 754. [Google Scholar] [CrossRef]
- Shahzad, B.; Tanveer, M.; Rehman, A.; Cheema, S.A.; Fahad, S.; Rehman, S.; Sharma, A. Nickel: Whether toxic or essential for plants and environment. A review. Plant Physiol. Biochem. 2018, 132, 641–651. [Google Scholar] [CrossRef]
- Ureta, A.C.; Imperial, J.; Ruiz-Argueso, T.; Palacios, J.M. Rhizobium leguminosarum biovar viciae symbiotic hydrogenase activity and processing are limited by the level of nickel in agricultural soils. Appl. Environ. Microbiol. 2005, 71, 7603–7606. [Google Scholar] [CrossRef]
- Zobiole, L.H.S.; Oliveira, R.S., Jr.; Kremer, R.J.; Constantin, J.; Yamada, T.; Castro, C.; Oliveira, F.A.; Oliveira, A., Jr. Effect of glyphosate on symbiotic N2 fixation and nickel concentration in glyphosate-resistant soybeans. Appl. Soil Ecol. 2009, 44, 176–180. [Google Scholar] [CrossRef]
- Shaheb, M.R.; Venkatesh, R.; Shearer, S.A. A review on the effect of soil compaction and its management for sustainable crop production. J. Biosyst. Eng. 2021, 46, 417–439. [Google Scholar] [CrossRef]
- Palese, A.M.; Magno, R.; Casacchia, T.; Curci, M.; Baronti, S.; Miglietta, F.; Crecchio, C.; Xiloyannis, C.; Sofo, A. Chemical, biochemical and microbiological properties of soil from abandoned and extensively cultivated olive orchards. Sci. World J. 2013, 2013, 496278. [Google Scholar] [CrossRef]
- Bórtoli, P.V.; Verdenelli, R.A.; Conforto, C.; Vargas, S.G.; Meriles, J.M. Efectos del herbicida glifosato sobre la estructura y el funcionamiento de comunidades microbianas de dos suelos de plantaciones de olivo. Ecol. Austral 2012, 22, 33–42. [Google Scholar]
- Fausto, C.; Mininni, A.N.; Sofo, A.; Crecchio, C.; Scagliola, M.; Dichio, B.; Xiloyannis, C. Olive orchard microbiome: Characterisation of bacterial communities in soil-plant compartments and their comparison between sustainable and conventional soil management systems. Plant Ecol. Div. 2018, 11, 597–610. [Google Scholar] [CrossRef]
- Moreno, B.; Garcia-Rodriguez, S.; Cañizares, R.; Castro, J.; Benítez, E. Rainfed olive farming in south-eastern Spain: Long-term effect of soil management on biological indicators of soil quality. Agric. Ecosyst. Environ. 2009, 131, 333–339. [Google Scholar] [CrossRef]
- Braun, D.M.; Washburn, J.D.; Wood, J.D. Enanching the resilience of plant systems to climate change. J. Exp. Bot. 2023, 74, 2787–2789. [Google Scholar] [CrossRef]
- Benitez-Alfonso, Y.; Soanes, B.K.; Zimba, S.; Sinanaj, B.; German, L.; Sharma, V.; Bohra, A.; Kolesnikova, A.; Dunn, J.A.; Martin, A.C.; et al. Enhancing climate change resilience in agricultral crops. Curr. Biol. 2023, 33, R1246–R1261. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).