Growth Stimulation of Tropical Grass (Megathyrsus maximus Jacq.) by Humic Substances and Herbaspirillum seropedicae
Abstract
:1. Introduction
2. Materials and Methods
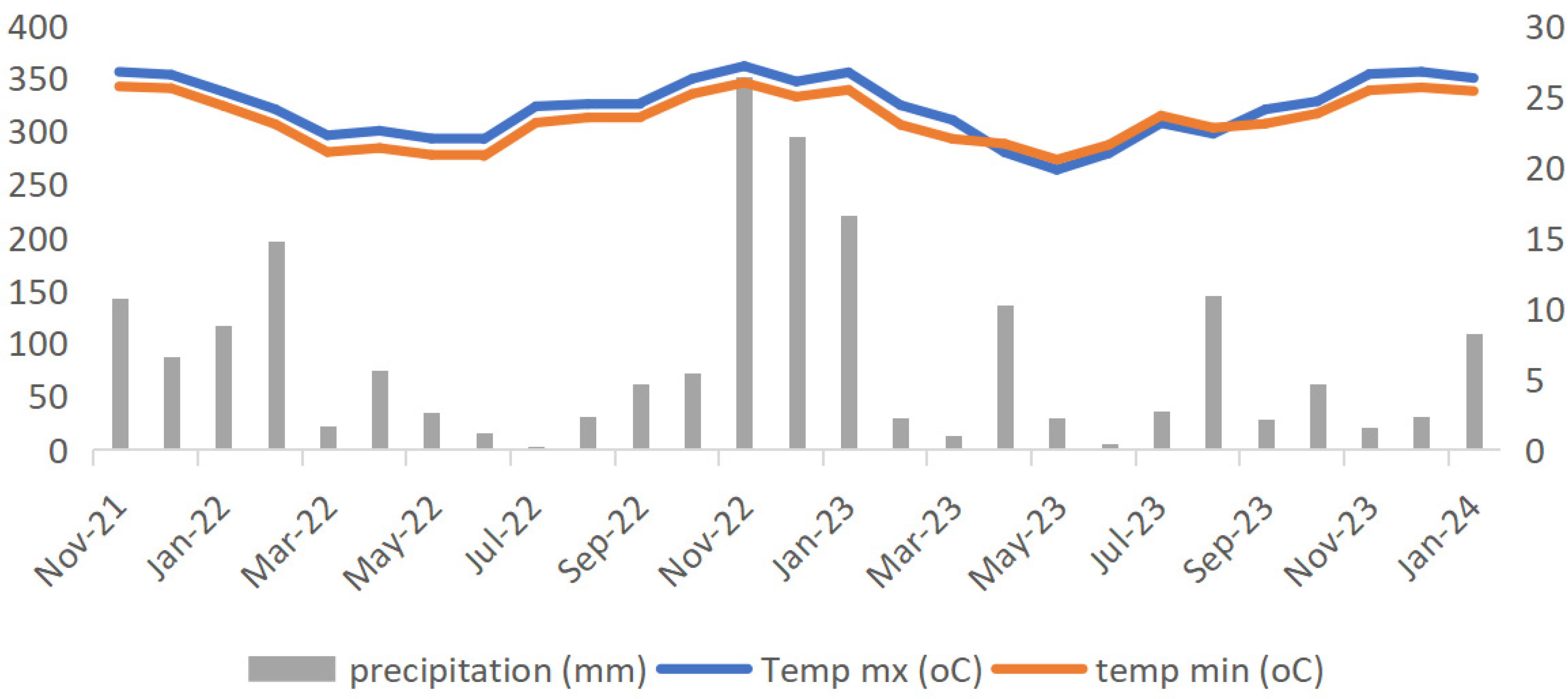
Field Experiment
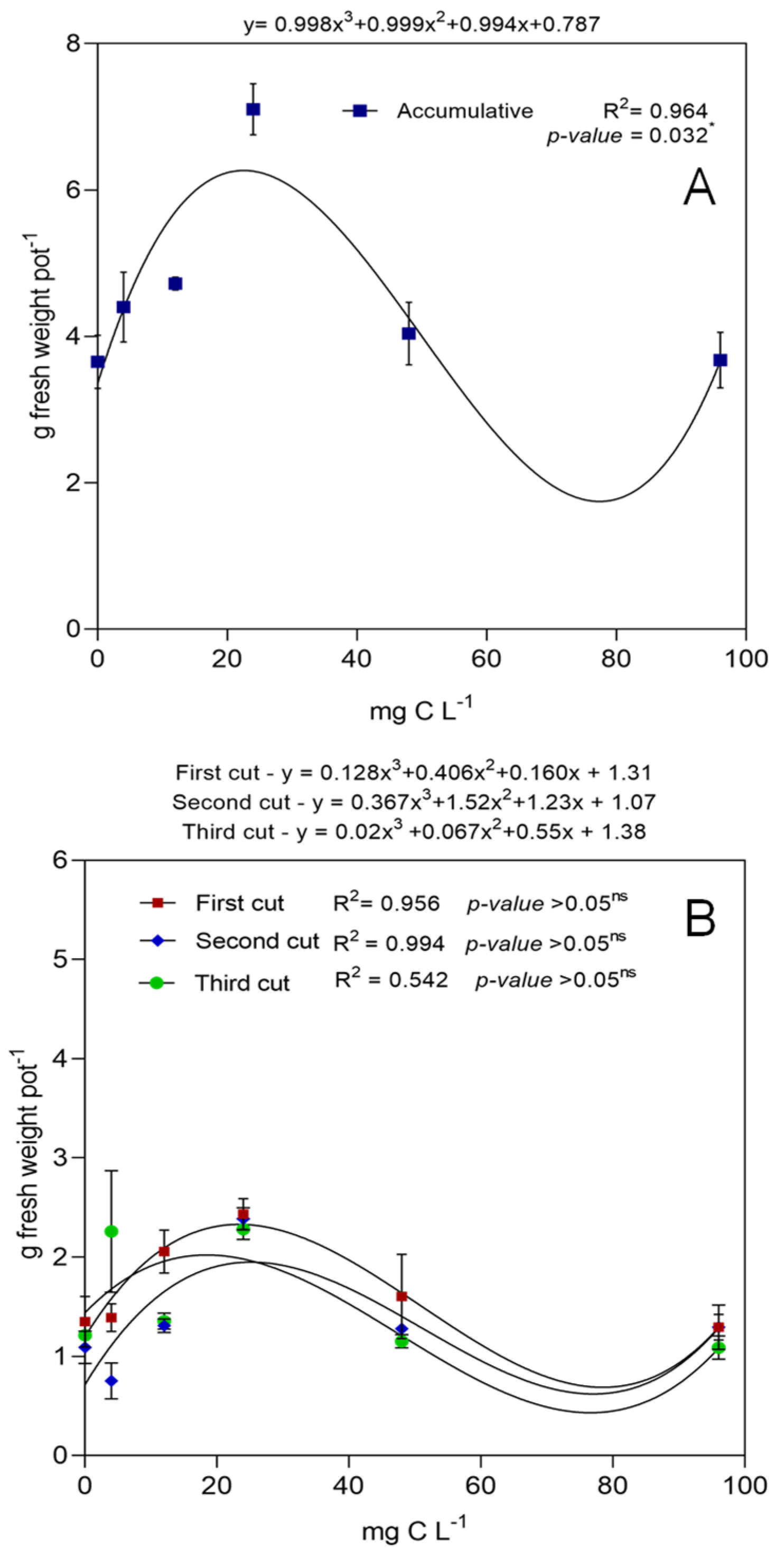
3. Results
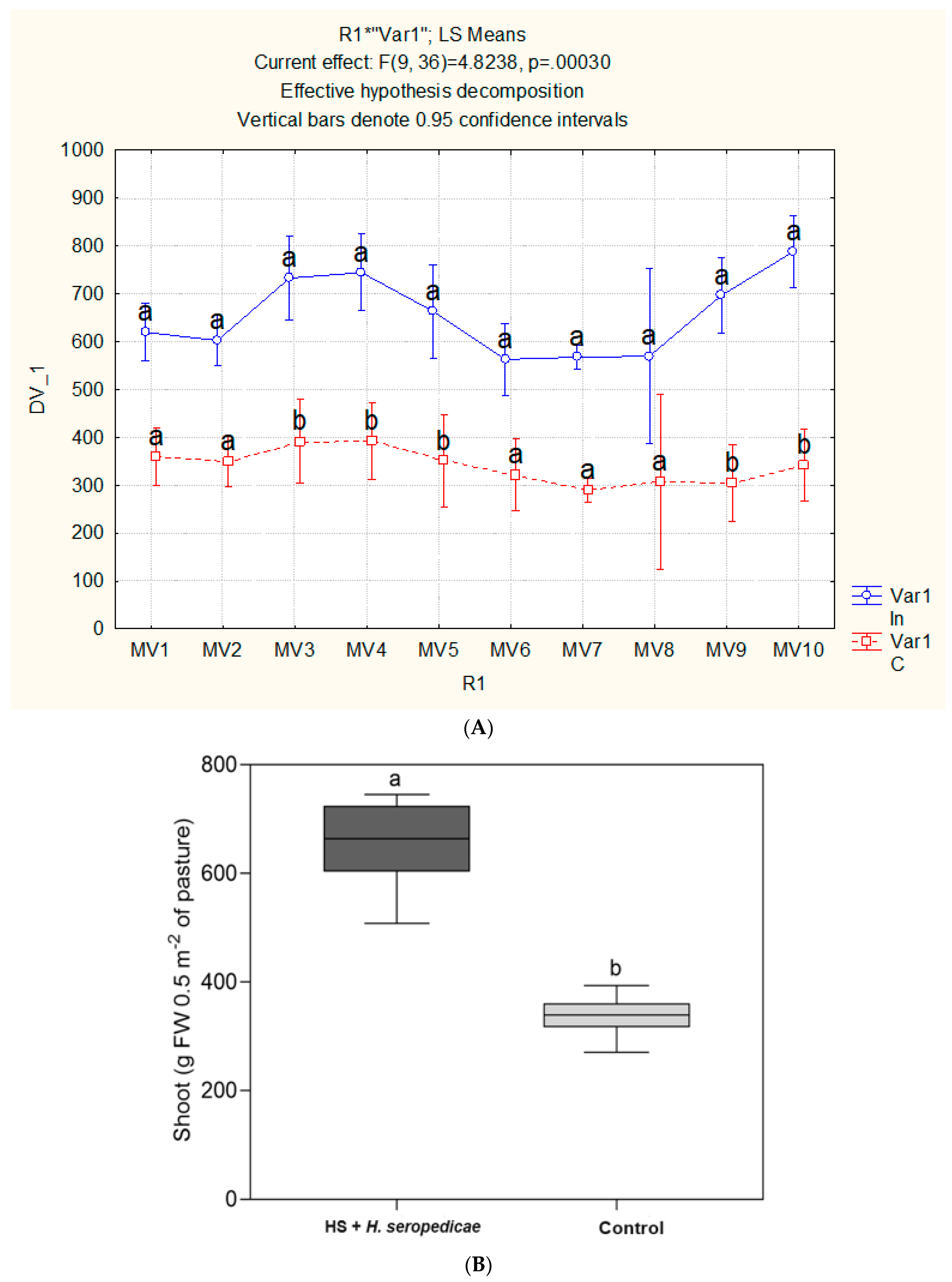
Field Trial
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Piccolo, A. The supramolecular structure of humic substances: A novel understanding of humus chemistry and implications in soil science. Adv. Agron. 2002, 75, 57–134. [Google Scholar]
- Nardi, S.; Carletti, P.; Pizzeghello, D.; Muscolo, A. Biological activities of humic substances. In Biophysico-Chemical Processes Involving Natural Nonliving Organic Matter in Environmental Systems; Part 1. Fundamentals and impact of mineral-organicbiota interactions on the formation, transformation, turnover, and storage of natural nonliving organic matter (NOM); Senesi, N., Xing, B., Huang, P.M., Eds.; Wiley: Hoboken, NJ, USA, 2009; Volume 2, pp. 305–339. [Google Scholar]
- Baldani, J.I.; Baldani, V.L.D.; Seldin, L.; Döbereiner, J. Characterization of Herbaspirillum seropedicae gen. nov., sp. nov., a root-associated nitrogen-fixing bacterium. Int. J. Syst. Evol. Microbiol. 1986, 36, 86–93. [Google Scholar] [CrossRef]
- Estrada, G.A.; Baldani, V.L.D.; De Oliveira, D.M.; Urquiaga, S.; Baldani, J.I. Selection of phosphate-solubilizing diazotrophic Herbaspirillum and Burkholderia strains and their effect on rice crop yield and nutrient uptake. Plant Soil 2013, 369, 115–129. [Google Scholar] [CrossRef]
- Canellas, L.P.; Olivares, F.L.; Canellas, N.A.; Jindo, K.; Rosa, R.C.C.; Piccolo, A. Challenge of transition: The history of a case study involving tropical fruits polyculture stimulated by humic acids and plant-growth promoting bacteria. Chem. Biol. Technol. Agric. 2022, 9, 76. [Google Scholar] [CrossRef]
- Pinheiro, P.L.; Passos, R.R.; Peçanha, A.L.; Canellas, L.P.; Olivares, F.L.; Mendonça, E.S. Promoting the growth of Brachiaria decumbens by humic acids (HAs). Austr. J. Crop Sci. 2018, 12, 1114–1121. [Google Scholar]
- Gerk, J.; Meyer, U.; Römer, W. Phosphate, Fe and Mn uptake of N2 fixing red clover and ryegrass from an Oxisol as affected by P and model humic substances application. 1. Plant parameters and soil solution composition. J. Plant Nut. Plant Sci. 1995, 158, 261–268. [Google Scholar] [CrossRef]
- Verlinden, G.; Coussens, T.; De Vliegher, A.; Baert, G.; Haesaert, G. Effect of humic substances on nutrient uptake by herbage and on production and nutritive value of herbage from sown grass pastures. Grass For. Sci. 2010, 65, 133–144. [Google Scholar] [CrossRef]
- Little, K.R.; Rose, M.T.; Jackson, W.R.; Cavagnaro, T.R.; Patti, A.F. Do lignite-derived organic amendments improve early-stage pasture growth and key soil biological and physicochemical properties? Crop Past. Sci. 2014, 65, 899–910. [Google Scholar] [CrossRef]
- Espie, P.; Ridgway, H. Bioactive carbon improves nitrogen fertiliser efficiency and ecological sustainability. Sci. Rep. 2020, 10, 3227. [Google Scholar] [CrossRef]
- IBGE—Instituto Brasileiro de Geografia e Estatística. Produção de Leite no Brasil. Available online: https://www.ibge.gov.br/explica/producao-agropecuaria/leite/br2022 (accessed on 12 January 2024).
- Sales, M.F.L.; Valentim, J.F. Capim Mombaça—Formação E Manejo de Pastagens No Acre; Embrapa: Rio Branco, Brazil, 2002. [Google Scholar]
- Olivares, F.L.; James, E.K.; Baldani, J.I.; Dobereiner, J. Infection of mottled stripe disease-susceptible and resistant sugar cane varieties by the endophytic diazotroph Herbaspirilium. New Phytol. 1997, 135, 723–737. [Google Scholar] [CrossRef]
- Pankievicz, V.C.S.; Amaral, F.P.; Santos, K.F.D.N.; Agtuca, B.; Xu, Y.; Schueller, M.J.; Arisi, A.C.M.; Steffens, M.B.R.; de Souza, E.M.; Pedrosa, F.O.; et al. Robust biological nitrogen fixation in a model grass–bacterial association. Plant J. 2015, 81, 907–919. [Google Scholar] [CrossRef]
- Olivares, F.L.; Busato, J.G.; Paula, A.M.; Lima, L.S.; Aguiar, N.O.; Canellas, L.P. Plant growth promoting bacteria and humic substances: Crop promotion and mechanisms of action. Chem. Biol. Technol. Agric. 2017, 4, 30. [Google Scholar] [CrossRef]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in Plant Science: A Global Perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef] [PubMed]
- EMBRAPA—Empresa Brasileira de Pesquisa Agropecuária. Manual de Métodos de Análises de Solo, 2nd ed.; Centro Nacional de Pesquisa de Solos: Rio de Janeiro, Brazil, 1997; 212p. [Google Scholar]
- Nelson, J.T.; Adjuik, T.A.; Moore, E.B.; VanLoocke, A.D.; Ramirez Reyes, A.; McDaniel, M.D. A Simple, Affordable, Do-It-Yourself Method for Measuring Soil Maximum Water Holding Capacity. Commun. Soil Sci. Plant Anal. 2024, 55, 1190–1204. [Google Scholar] [CrossRef]
- Oliveira, P.A.; Bernardi, A.C.C.; Alves, T.C.; Pedroso, A.F. Evolução na recomendação de fertilização de solos sob pastagens: Eficiência e sustentabilldade na pecuária. In Embrapa Southeast Livestock; Empresa Brasileira de Pesquisa Agropecuária—Embrapa: São Carlos, Brazil, 2015. [Google Scholar]
- Siri-Prieto, G.; Bustamante, M.; Picasso, V. Impact of nitrogen and phosphorous on biomass yield, nitrogen efficiency, and nutrient removal of perennial grasses for bioenergy. Biom. Bioenergy 2020, 136, 105526. [Google Scholar] [CrossRef]
- Asli, S.; Neumann, P. Rhizosphere humic acid interacts with root cell walls to reduce hydraulic conductivity and plant development. Plant Soil 2010, 336, 313–322. [Google Scholar] [CrossRef]
- Olaetxea, M.; Mora, V.; Bacaicoa, E.; Baigorri, R.; Garnica, M.; Fuentes, M.; Casanova, E.; Zamarreño, A.M.; Iriarte, J.C.; Etayo, D.; et al. ABA-regulation of root hydraulic conductivity and aquaporin gene- expression is crucial to the plant shoot rise caused by rhizosphere humic acids. Plant Physiol. 2015, 16, 2587–2596. [Google Scholar] [CrossRef]
- Olaetxea, M.; Mora, V.; Baigorri, R.; Zamarreño, A.M.; García-Mina, J.M. The Singular molecular conformation of humic acids in solution influences their ability to enhance root hydraulic conductivity and plant growth. Molecules 2021, 26, 3. [Google Scholar] [CrossRef]
- Trevisan, S.; Botton, A.; Vaccaro, S.; Vezzaroa, A.; Quaggiotti, S.; Nardi, S. Humic substances affect Arabidopsis physiology by altering the expression of genes involved in primary metabolism, growth and development. Environ. Exp. Bot. 2011, 74, 45–55. [Google Scholar] [CrossRef]
- Silva, R.M.; Peres, A.N.A.; Peres, L.E.P.; Olivares, F.L.; Sangi, S.M.; Canellas, N.A.; Spaccini, R.; Cangemi, S.; Canellas, L.P. Humic substances isolated from recycled biomass trigger jasmonic acid biosynthesis and signalling. Plants 2023, 12, 3148. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Eiroa, B.; Fernán Fernández, E.; Méndez-Martínez, G.; Soto-Oñate, D. Operational principles of circular economy for sustainable development: Linking theory and practice. J. Clean. Prod. 2019, 214, 952–961. [Google Scholar] [CrossRef]
- Little, K.R. Commercial Lignite Coal-Derived Amendments for Improved Pasture Growth and Soil Health. Ph.D. Thesis, Monash University, Melbourne, VIC, Australia, 2015. [Google Scholar]
- Matteoli, F.P.; Olivares, F.L.; Venancio, T.M.; Rocha, L.O.; Irineu, L.E.S.S.; Canellas, L.P. Herbaspirillum. In Beneficial Microbes in Agro-Ecology: Bacteria and Fungi; Amaresan, N., Kumar, M.S., Annapurna, K., Kumar, K., Sankaranarayanan, A., Eds.; Academic Press: Cambridge, MA, USA; Elsevier: London, UK, 2020; pp. 493–505. [Google Scholar]


| pH * | P | K+ | Na+ | Al3 | H+ + Al3+ | Ca2+ | Mg2+ | CEC |
|---|---|---|---|---|---|---|---|---|
| g kg−1 | cmolc dm−3 | |||||||
| 4.6 | 4.3 | 15.0 | 0.2 | 0.1 | 3.2 | 0.8 | 1.04 | 5.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canellas, L.P.; Canellas, N.A.; Silva, R.M.; Novotny, E.H.; Olivares, F.L. Growth Stimulation of Tropical Grass (Megathyrsus maximus Jacq.) by Humic Substances and Herbaspirillum seropedicae. Agronomy 2024, 14, 2006. https://doi.org/10.3390/agronomy14092006
Canellas LP, Canellas NA, Silva RM, Novotny EH, Olivares FL. Growth Stimulation of Tropical Grass (Megathyrsus maximus Jacq.) by Humic Substances and Herbaspirillum seropedicae. Agronomy. 2024; 14(9):2006. https://doi.org/10.3390/agronomy14092006
Chicago/Turabian StyleCanellas, Luciano P., Natália A. Canellas, Rakiely M. Silva, Etelvino H. Novotny, and Fabio L. Olivares. 2024. "Growth Stimulation of Tropical Grass (Megathyrsus maximus Jacq.) by Humic Substances and Herbaspirillum seropedicae" Agronomy 14, no. 9: 2006. https://doi.org/10.3390/agronomy14092006






