The Application of Biochar Derived from Rice Husk Enhanced the Bioremediation of Petroleum-Contaminated Soil in Semi-Arid Areas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Contaminated Soil and Chemicals
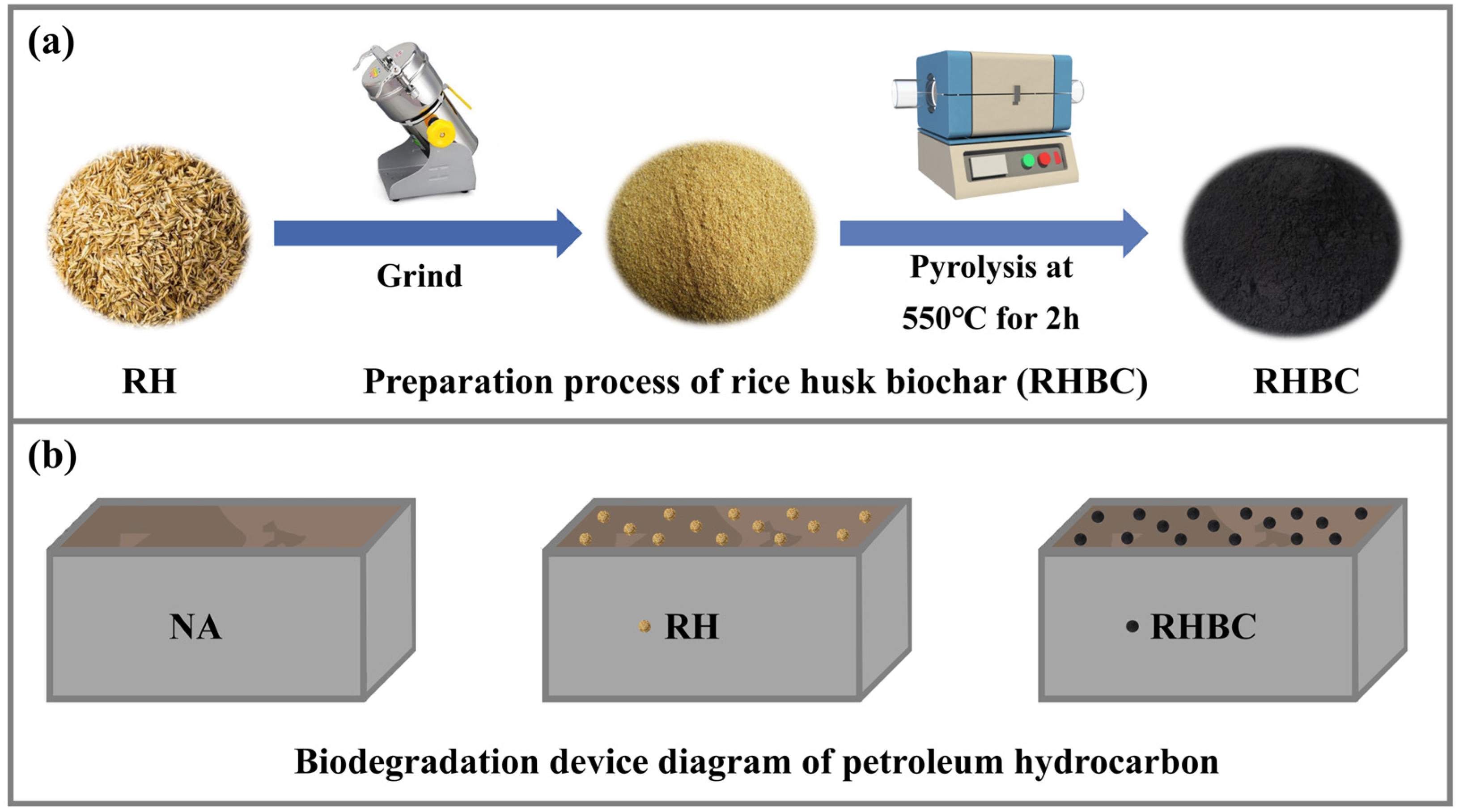
2.2. Preparation and Characterization of RHBC
2.3. Experimental Design
2.4. Extraction and Determination of Petroleum Hydrocarbons
2.5. Measurement of Dehydrogenase Activity
2.6. Count of Petroleum-Degrading Bacteria
2.7. TPHs Degradation Kinetics
2.8. Statistical Analysis
3. Results and Discussion
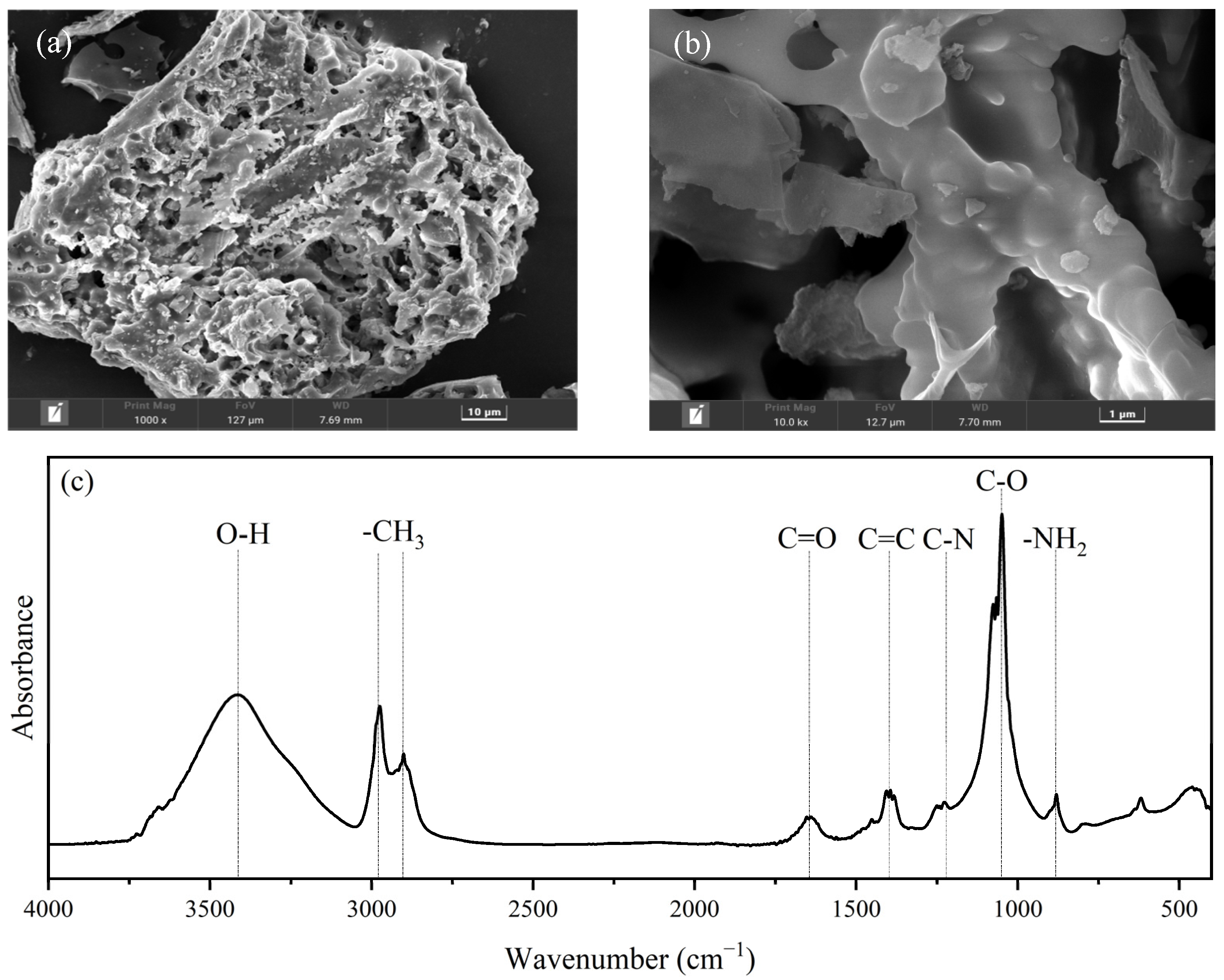
3.1. Characterization of RHBC Particles
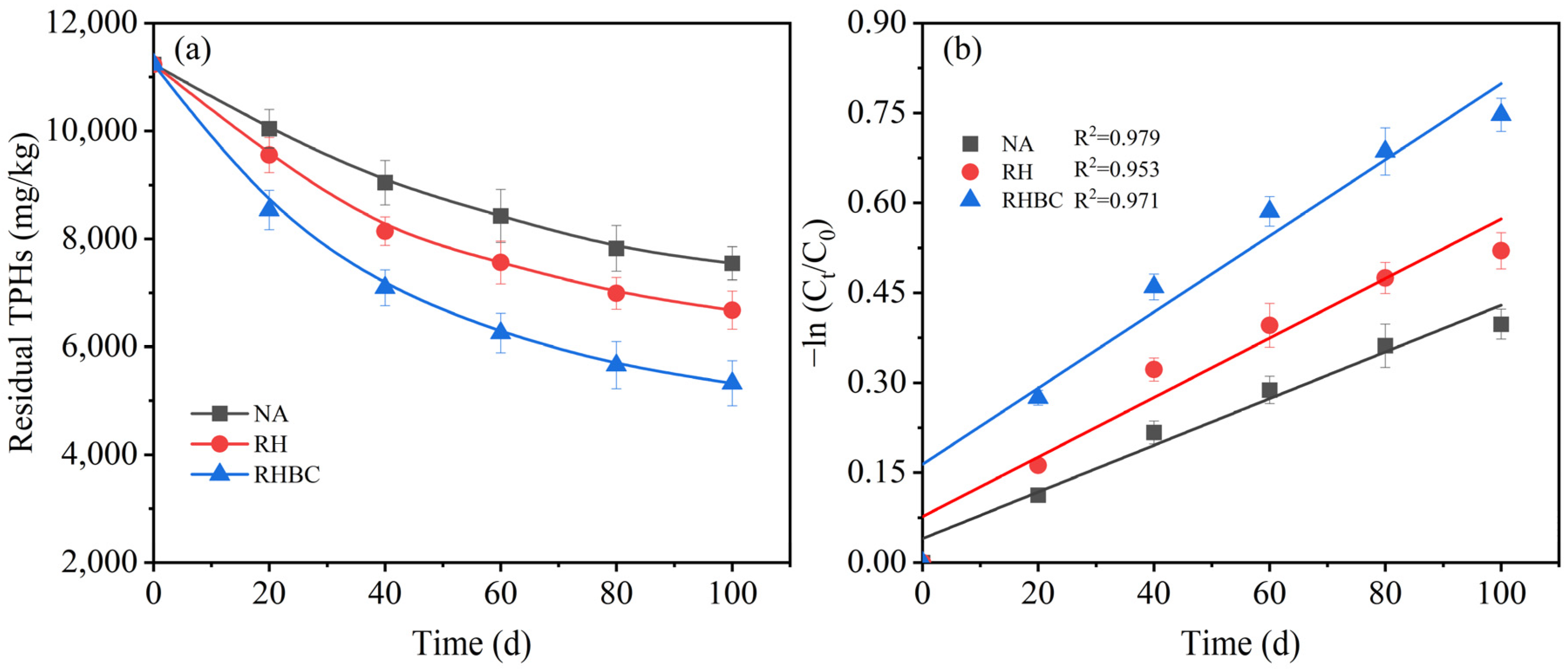
3.2. Analysis of TPHs Degradation Kinetics
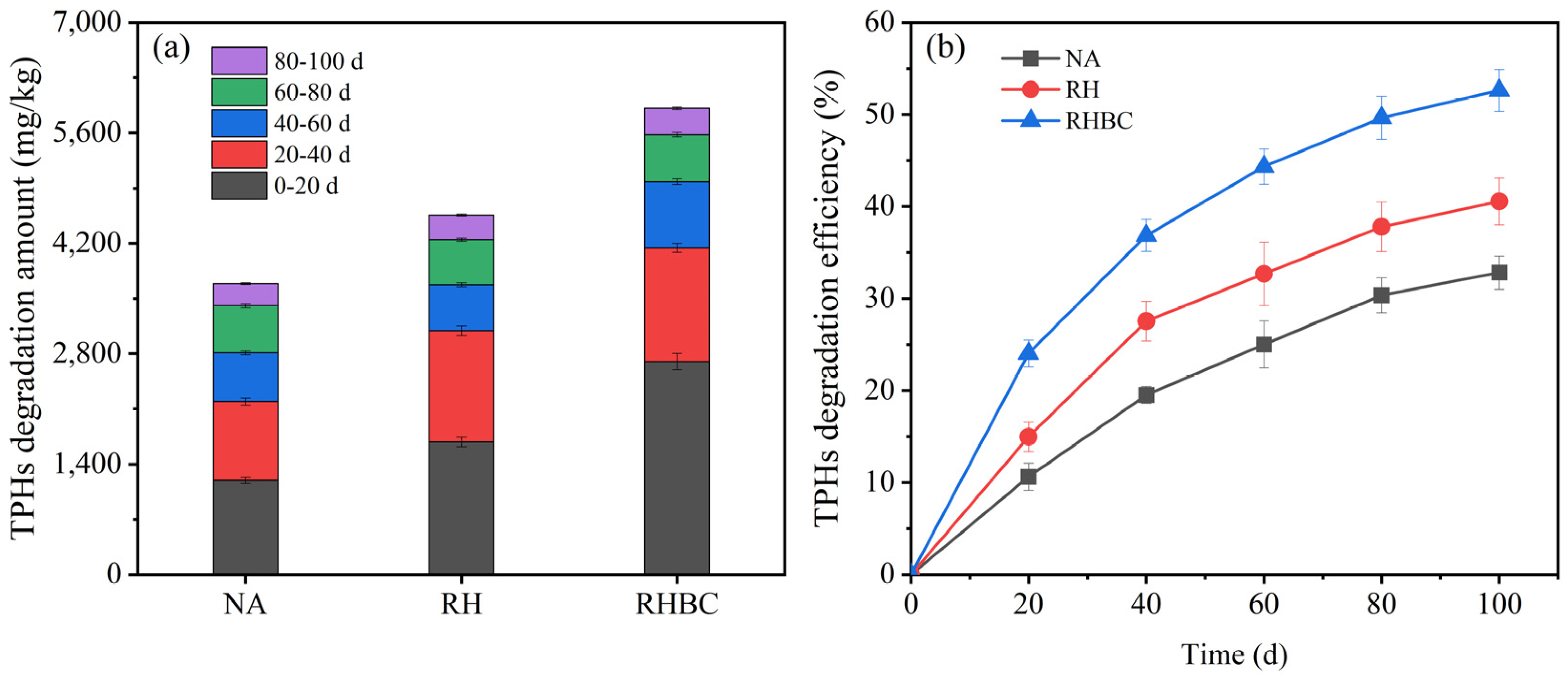
3.3. TPHs Degradation in Different Stages
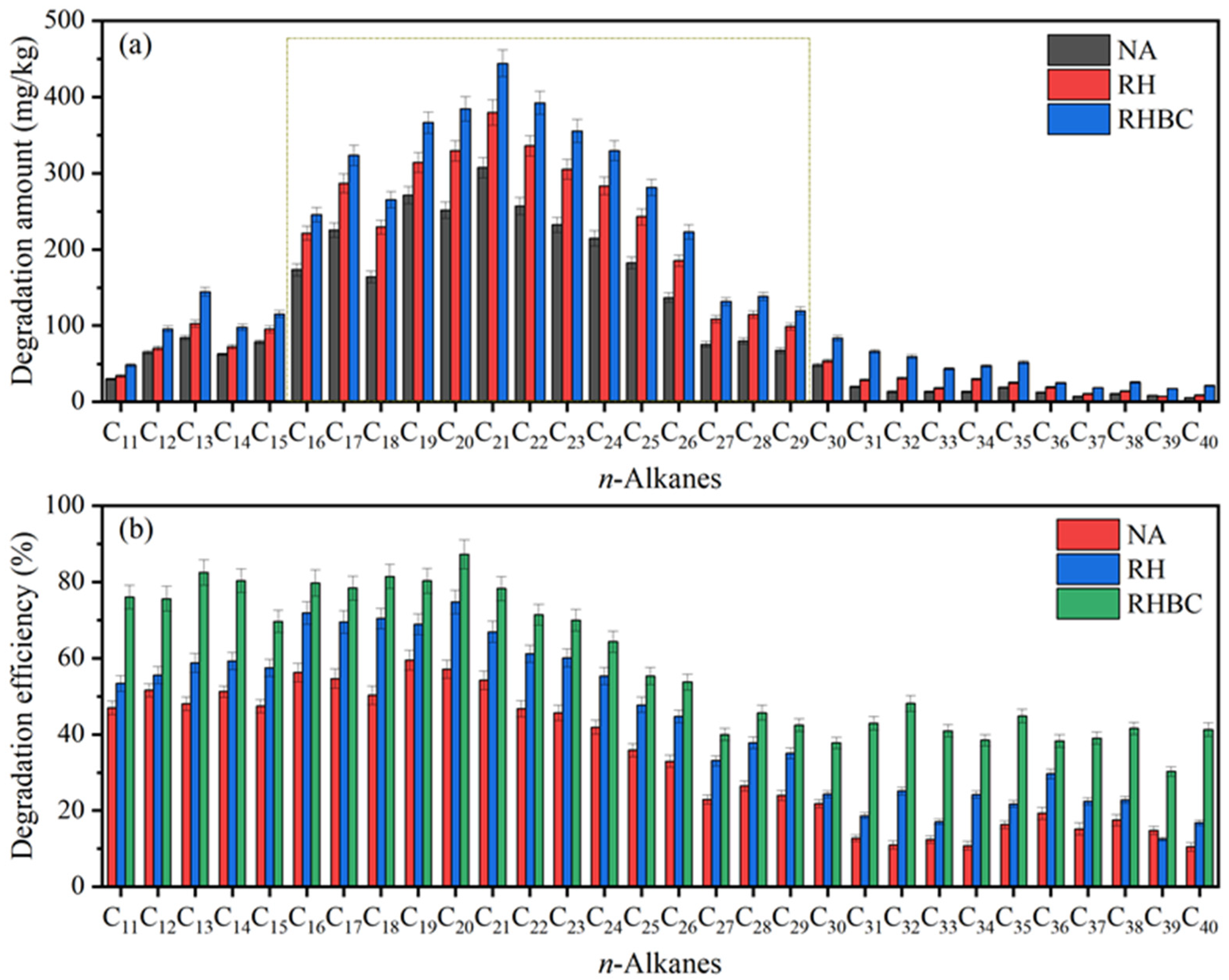
3.4. Degradation Amount of Individual n-Alkanes after Biodegradation
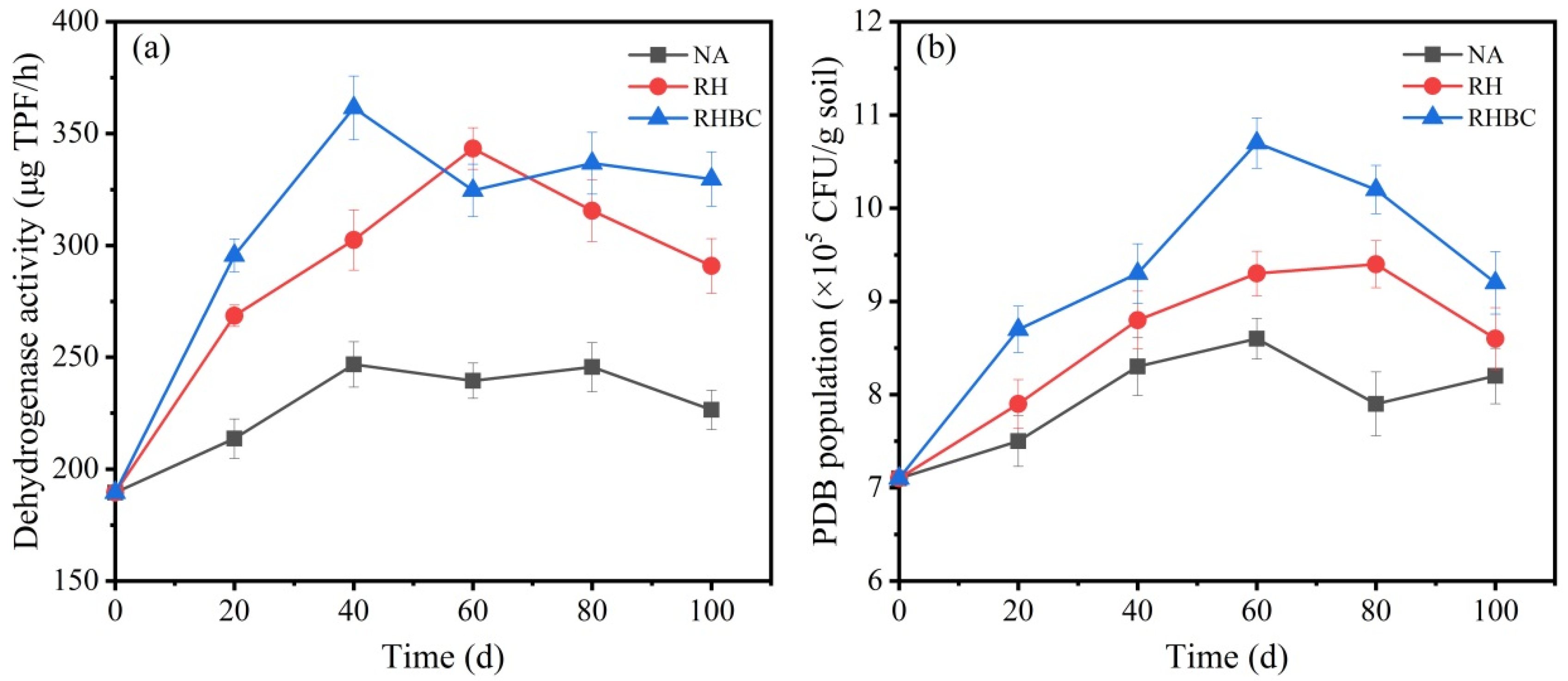
3.5. Dynamic Changes in Enzyme Activity and Microbial Number
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, M.; Ma, C.; Wang, D.; Liu, H.; Zhu, C.C.; Xu, H.N. Nutrient drip irrigation for refractory hydrocarbon removal and microbial community shift in a historically petroleum-contaminated soil. Sci. Total Environ. 2020, 713, 136331. [Google Scholar] [CrossRef] [PubMed]
- Varjani, S.; Pandey, A.; Upasani, V.N. Petroleum sludge polluted soil remediation: Integrated approach involving novel bacterial consortium and nutrient application. Sci. Total Environ. 2021, 763, 142934. [Google Scholar] [CrossRef]
- Ali, M.; Song, X.; Wang, Q.; Zhang, Z.X.; Che, J.L.; Chen, X.; Tang, Z.W.; Liu, X. Mechanisms of biostimulant-enhanced biodegradation of PAHs and BTEX mixed contaminants in soil by native microbial consortium. Environ. Pollut. 2023, 318, 120831. [Google Scholar] [CrossRef]
- Rong, L.; Zheng, X.; Oba, B.T.; Shen, C.B.; Wang, X.Y.; Wang, H.; Luo, Q.; Sun, L.N. Activating soil microbial community using bacillus and rhamnolipid to remediate TPH contaminated soil. Chemosphere 2021, 275, 130062. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Teng, Y.; Ren, W.; Han, Y.; Wang, X.; Li, X. Soil bacterial diversity and functionality are driven by plant species for enhancing polycyclic aromatic hydrocarbons dissipation in soils. Sci. Total Environ. 2021, 797, 149204. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, J.; Yang, Z. Efficient oriented interfacial oxidation of petroleum hydrocarbons by functionalized Fe/N co-doped biochar-mediated heterogeneous Fenton for heavily contaminated soil remediation. Chem. Eng. J. 2022, 450, 138466. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Q.; Wang, X.; Li, Y.T.; Zhou, Q.X. Surfactants selectively reallocated the bacterial distribution in soil bioelectrochemical remediation of petroleum hydrocarbons. J. Hazard. Mater. 2018, 344, 23–32. [Google Scholar] [CrossRef]
- Ma, M.; Chen, Y.; Su, R.; Liu, Z.; He, J.K.; Zhou, W.Z.; Gu, M.X.; Yan, M.L.; Li, Q. In situ synthesis of Fe-N co-doped carbonaceous nanocomposites using biogas residue as an effective persulfate activator for remediation of aged petroleum contaminated soils. J. Hazard. Mater. 2022, 435, 128963. [Google Scholar] [CrossRef]
- Chen, B.; Xu, J.; Lu, H.; Zhu, L.Z. Remediation of benzo[a]pyrene contaminated soils by moderate chemical oxidation coupled with microbial degradation. Sci. Total Environ. 2023, 871, 161801. [Google Scholar] [CrossRef]
- Wang, S.; Cheng, F.; Shao, Z.; Wu, B.; Guo, S.H. Effects of thermal desorption on ecotoxicological characteristics of heavy petroleum-contaminated soil. Sci. Total Environ. 2023, 857, 159405. [Google Scholar] [CrossRef]
- Li, X.; Xu, J.; Yang, Z. Insight on efficiently oriented oxidation of petroleum hydrocarbons by redistribution of oxidant through inactivation of soil organic matter coupled with passivation of manganese minerals. J. Hazard. Mater. 2023, 443, 130192. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, L.; Zhang, X. Bioremediation of petroleum hydrocarbon-contaminated soil by petroleum-degrading bacteria immobilized on biochar. RSC Adv. 2019, 9, 35304–35311. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Huang, Y.; Wen, D.; Fu, R.B.; Feng, L.Y. Application of alkyl polyglycosides for enhanced bioremediation of petroleum hydrocarbon-contaminated soil using Sphingomonas changbaiensis and Pseudomonas stutzeri. Sci. Total Environ. 2020, 719, 137456. [Google Scholar] [CrossRef] [PubMed]
- Banet, G.; Turaani, A.K.; Farber, R.; Armoza-Zvuloni, R.; Rotem, N.; Stavi, I.; Cahan, R. The effects of biostimulation and bioaugmentation on crude oil biodegradation in two adjacent terrestrial oil spills of different age, in a hyper-arid region. J. Environ. Manag. 2021, 286, 112248. [Google Scholar] [CrossRef] [PubMed]
- Shahi, A.; Aydin, S.; Ince, B.; Ince, O. Reconstruction of bacterial community structure and variation for enhanced petroleum hydrocarbons degradation through biostimulation of oil contaminated soil. Chem. Eng. J. 2016, 306, 60–66. [Google Scholar] [CrossRef]
- Safdari, M.; Kariminia, H.; Rahmati, M.; Fazlollahi, F.; Polasko, A.; Mahendra, S.; Wilding, W.; Fletcher, T. Development of bioreactors for comparative study of natural attenuation, biostimulation, and bioaugmentation of petroleum-hydrocarbon contaminated soil. J. Hazard. Mater. 2018, 342, 270–278. [Google Scholar] [CrossRef]
- Sutton, N.B.; Grotenhuis, T.; Rijnaarts, H.H.M. Impact of organic carbon and nutrients mobilized during chemical oxidation on subsequent bioremediation of a diesel-contaminated soil. Chemosphere 2014, 97, 64–70. [Google Scholar] [CrossRef]
- Liu, X.; Li, Z.; Zhang, C.; Tan, X.J.; Yang, X.; Wan, C.L.; Lee, D. Enhancement of anaerobic degradation of petroleum hydrocarbons by electron intermediate: Performance and mechanism. Bioresour. Technol. 2020, 295, 122305. [Google Scholar] [CrossRef]
- Dike, C.C.; Khudur, L.S.; Hakeem, I.G.; Rani, A.; Shahsavari, E.; Surapaneni, A.; Shah, K.; Ball, A.S. Biosolids-derived biochar enhances the bioremediation of diesel-contaminated soil. J. Environ. Chem. Eng. 2022, 10, 108633. [Google Scholar]
- Ambaye, T.G.; Formicola, F.; Sbaffoni, S.; Milanese, C.; Franzetti, A.; Vaccari, M. Effect of biochar on petroleum hydrocarbon degradation and energy production in microbial electrochemical treatment. J. Environ. Chem. Eng. 2023, 11, 110817. [Google Scholar] [CrossRef]
- Zhang, H.; Xiao, R.; Li, R.; Ali, A.; Chen, A.L.; Zhang, Z.Q. Enhanced aqueous Cr(VI) removal using chitosan-modified magnetic biochars derived from bamboo residues. Chemosphere 2020, 261, 127694. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Xu, J.; Zhang, Y.; Li, B.; Fan, S.S.; Xu, H.C. Effect of Fe-N modification on the properties of biochars and their adsorption behavior on tetracycline removal from aqueous solution. Bioresour. Technol. 2021, 325, 124732. [Google Scholar] [CrossRef]
- Wang, Y.; Wan, S.; Yu, W.; Yuan, D.; Sun, L. The role of Fe3O4@biochar as electron shuttle in enhancing the biodegradation of gaseous para-xylene by aerobic surfactant secreted strains. J. Hazard. Mater. 2022, 438, 129475. [Google Scholar] [CrossRef] [PubMed]
- Zhen, L.; Hu, T.; Lv, R.; Wu, Y.C.; Chang, F.; Jia, F.A.; Gu, J. Succession of microbial communities and synergetic effects during bioremediation of petroleum hydrocarbon-contaminated soil enhanced by chemical oxidation. J. Hazard. Mater. 2021, 410, 124869. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, Y.; Li, C.; Li, P.F.; Zhang, A.N.; Liu, Z.; Wang, Z.; Wei, C.X.; Yang, Z.Z.; Li, Z.H. Optimizing carbon sources regulation in the biochemical treatment systems for coal chemical wastewater: Aromatic compounds biodegradation and microbial response strategies. Water Res. 2024, 256, 121627. [Google Scholar] [CrossRef]
- Xia, C.; Liu, Q.; Zhao, L.; Wang, L.; Tang, J.C. Enhanced degradation of petroleum hydrocarbons in soil by FeS@BC activated persulfate and its mechanism. Sep. Purif. Technol. 2022, 282, 120060. [Google Scholar] [CrossRef]
- Song, L.; Niu, X.; Zhang, N.; Li, T. Effect of biochar-immobilized Sphingomonas sp. PJ2 on bioremediation of PAHs and bacterial community composition in saline soil. Chemosphere 2021, 279, 130427. [Google Scholar] [CrossRef]
- Wang, G.; Gao, X.; Li, Q.; Zhao, H.; Liu, Y.; Wang, X.C.; Chen, R. Redox-based electron exchange capacity of biowaste-derived biochar accelerates syntrophic phenol oxidation for methanogenesis via direct interspecies electron transfer. J. Hazard. Mater. 2020, 390, 121726. [Google Scholar] [CrossRef]
- Dike, C.C.; Hakeem, I.G.; Rani, A.; Surapaneni, A.; Khudur, L.; Shah, K.; Ball, A.S. The co-application of biochar with bioremediation for the removal of petroleum hydrocarbons from contaminated soil. Sci. Total Environ. 2022, 849, 157753. [Google Scholar] [CrossRef]
- Bushnaf, K.M.; Puricelli, S.; Saponaro, S.; Werner, D. Effect of biochar on the fate of volatile petroleum hydrocarbons in an aerobic sandy soil. J. Contam. Hydrol. 2011, 126, 208–215. [Google Scholar] [CrossRef]
- Ni, N.; Wang, F.; Song, Y.; Bian, Y.; Shi, R.; Yang, X.; Gu, C.; Jiang, X. Mechanisms of biochar reducing the bioaccumulation of PAHs in rice from soil: Degradation stimulation vs. immobilization. Chemosphere 2018, 196, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Durenkamp, M.; De Nobili, M.; Lin, Q.; Brookes, P.C. Short term soil priming effects and the mineralisation of biochar following its incorporation to soils of different pH. Soil Biol. Biochem. 2011, 43, 2304–2314. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, P.; Yuan, X.; Li, Y.; Han, L. Effect of pyrolysis temperature and correlation analysis on the yield and physicochemical properties of crop residue biochar. Bioresour. Technol. 2020, 296, 122318. [Google Scholar] [CrossRef]
- Feng, L.; Jiang, X.; Huang, Y.; Wen, D.D.; Fu, T.Y.; Fu, R.B. Petroleum hydrocarbon-contaminated soil bioremediation assisted by isolated bacterial consortium and sophorolipid. Environ. Pollut. 2021, 273, 116476. [Google Scholar] [CrossRef]
- Guo, S.; Liu, X.; Tang, J. Enhanced degradation of petroleum hydrocarbons by immobilizing multiple bacteria on wheat bran biochar and its effect on greenhouse gas emission in saline-alkali soil. Chemosphere 2022, 286, 131663. [Google Scholar] [CrossRef]
- Varjani, S.; Kumar, G.; Rene, E.R. Developments in biochar application for pesticide remediation: Current knowledge and future research directions. J. Environ. Manag. 2019, 232, 505–513. [Google Scholar] [CrossRef]
- Wei, Z.; Wei, Y.; Liu, Y.; Niu, S.; Xu, Y.; Park, J.; Wang, J.J. Biochar-based materials as remediation strategy in petroleum hydrocarbon-contaminated soil and water: Performances, mechanisms, and environmental impact. J. Environ. Sci. 2024, 138, 350–372. [Google Scholar] [CrossRef] [PubMed]
- Sutton, N.B.; Langenhoff, A.A.M.; Lasso, D.H.; van der Zaan, B.; van Gaans, P.; Maphosa, F.; Smidt, H.; Grotenhuis, T.; Rijnaarts, H.H.M. Recovery of microbial diversity and activity during bioremediation following chemical oxidation of diesel contaminated soils. Appl. Microbiol. Biotechnol. 2014, 98, 2751–2764. [Google Scholar] [CrossRef]
- Jiang, C.; Bo, J.; Xiao, X.; Zhang, S.; Wang, Z.; Yan, G.; Wu, Y.; Wong, C.; He, H. Converting waste lignin into nano-biochar as a renewable substitute of carbon black for reinforcing styrene-butadiene rubber. Waste Manag. 2020, 102, 732–742. [Google Scholar] [CrossRef]
- Yang, X.; Chen, Z.; Wu, Q.; Xu, M. Enhanced phenanthrene degradation in river sediments using a combination of biochar and nitrate. Sci. Total Environ. 2018, 619–620, 600–605. [Google Scholar] [CrossRef]
- Kołtowski, M.; Hilber, I.; Bucheli, T.D.; Oleszczuk, P. Effect of steam activated biochar application to industrially contaminated soils on bioavailability of polycyclic aromatic hydrocarbons and ecotoxicity of soils. Sci. Total Environ. 2016, 566–567, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, Z.; Chen, S.; Zhou, W. Enhanced phytoremediation of petroleum-contaminated soil by biochar and urea. J. Hazard. Mater. 2023, 453, 131404. [Google Scholar] [CrossRef] [PubMed]
- Faheem, D.J.; Kim, S.H.; Hassan, M.A.; Irshad, S.; Bao, J. Application of biochar in advanced oxidation processes: Supportive, adsorptive, and catalytic role. Environ. Sci. Pollut. Res. 2020, 27, 37286–37312. [Google Scholar] [CrossRef]
- Margesin, R.; Zimmerbauer, A.; Schinner, F. Monitoring of bioremediation by soil biological activities. Chemosphere 2000, 40, 339–346. [Google Scholar] [CrossRef]
- Hoang, S.A.; Lamb, D.; Sarkar, B.; Seshadri, B.; Yu, R.; Tran, T.; O’Connor, J.; Rinklebe, J.; Kirkham, M.B.; Vo, H.; et al. Phosphorus application enhances alkane hydroxylase gene abundance in the rhizosphere of wild plants grown in petroleum-hydrocarbon-contaminated soil. Environ. Res. 2022, 204, 111924. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhu, Z.; Shen, B.; Liu, L. Insights into biochar and hydrochar production and applications: A review. Energy 2019, 171, 581–598. [Google Scholar] [CrossRef]
- Fu, H.; Wei, C.; Qu, X.; Li, H.; Zhu, D. Strong binding of apolar hydrophobic organic contaminants by dissolved black carbon released from biochar: A mechanism of pseudomicelle partition and environmental implications. Environ. Pollut. 2018, 232, 402–410. [Google Scholar] [CrossRef]
- Silva-Castro, G.A.; Rodelas, B.; Perucha, C.; Laguna, J.; González-López, J.; Calvo, C. Bioremediation of diesel-polluted soil using biostimulation as post-treatment after oxidation with Fenton-like reagents: Assays in a pilot plant. Sci. Total Environ. 2013, 445–446, 347–355. [Google Scholar] [CrossRef]
- Chen, K.F.; Chang, Y.C.; Chiou, W.T. Remediation of diesel-contaminated soil using in situ chemical oxidation (ISCO) and the effects of common oxidants on the indigenous microbial community: A comparison study. J. Chem. Technol. Biotechnol. 2016, 91, 1877–1888. [Google Scholar] [CrossRef]
- Xu, J.; Deng, X.; Cui, Y.; Kong, F.X. Impact of chemical oxidation on indigenous bacteria and mobilization of nutrients and subsequent bioremediation of crude oil-contaminated soil. J. Hazard. Mater. 2016, 320, 160–168. [Google Scholar] [CrossRef]
- Liu, L.; Chen, P.; Sun, M.; Shen, G.; Shang, G. Effect of biochar amendment on PAH dissipation and indigenous degradation bacteria in contaminated soil. J. Soils Sediments 2015, 15, 313–322. [Google Scholar] [CrossRef]
- Kong, L.; Gao, Y.; Zhou, Q.; Zhao, X.; Sun, Z. Biochar accelerates PAHs biodegradation in petroleum-polluted soil by biostimulation strategy. J. Hazard. Mater. 2018, 343, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Zeng, G.; Wu, H.; Liang, J.; Zhang, C.; Dai, J.; Xiong, W.; Song, B.; Wu, S.; Yu, J. The effects of activated biochar addition on remediation efficiency of co-composting with contaminated wetland soil. Resour. Conserv. Recycl. 2019, 140, 278–285. [Google Scholar] [CrossRef]
| pH | Sand (%) | Silt (%) | Clay (%) | DOC (mg/kg) | NH4+-N (mg/kg) | TPHs (mg/kg) |
|---|---|---|---|---|---|---|
| 7.9 ± 0.3 | 46.3 ± 1.5 | 38.5 ± 1.2 | 15.2 ± 0.5 | 483.7 ± 14.5 | 6.5 ± 0.2 | 11236.8 ± 637.1 |
| Treatment | Setup Composition | Dynamics Equation | kobs (d−1) | Half-Life (d) |
|---|---|---|---|---|
| NA | Soil | ln (Ct/C0) = −0.0039t − 0.04 | 0.0039 | 167.5 |
| RH | Soil + 0.05 g/g RS | ln (Ct/C0) = −0.0050t − 0.08 | 0.0050 | 122.6 |
| RHBC | Soil + 0.05 g/g RSBC | ln (Ct/C0) = −0.0064t − 0.16 | 0.0064 | 83.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Zhang, Y.; Li, X.; Sun, Z.; Zhang, R.; Li, X.; Du, Y. The Application of Biochar Derived from Rice Husk Enhanced the Bioremediation of Petroleum-Contaminated Soil in Semi-Arid Areas. Agronomy 2024, 14, 2015. https://doi.org/10.3390/agronomy14092015
Liu Z, Zhang Y, Li X, Sun Z, Zhang R, Li X, Du Y. The Application of Biochar Derived from Rice Husk Enhanced the Bioremediation of Petroleum-Contaminated Soil in Semi-Arid Areas. Agronomy. 2024; 14(9):2015. https://doi.org/10.3390/agronomy14092015
Chicago/Turabian StyleLiu, Zhe, Yang Zhang, Xiumin Li, Zenghui Sun, Ruiqing Zhang, Xuxiang Li, and Yichun Du. 2024. "The Application of Biochar Derived from Rice Husk Enhanced the Bioremediation of Petroleum-Contaminated Soil in Semi-Arid Areas" Agronomy 14, no. 9: 2015. https://doi.org/10.3390/agronomy14092015





