Integrating Heterosis for Root Architecture and Nitrogen Use Efficiency of Maize: A Comparison between Hybrids from Different Decades
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Experimental Design
2.3. Sampling and Measurements
2.3.1. Root Architecture
2.3.2. Nitrogen and Heterosis Indices
2.3.3. Yield and Yield Components
2.4. Statistical Analysis
3. Results
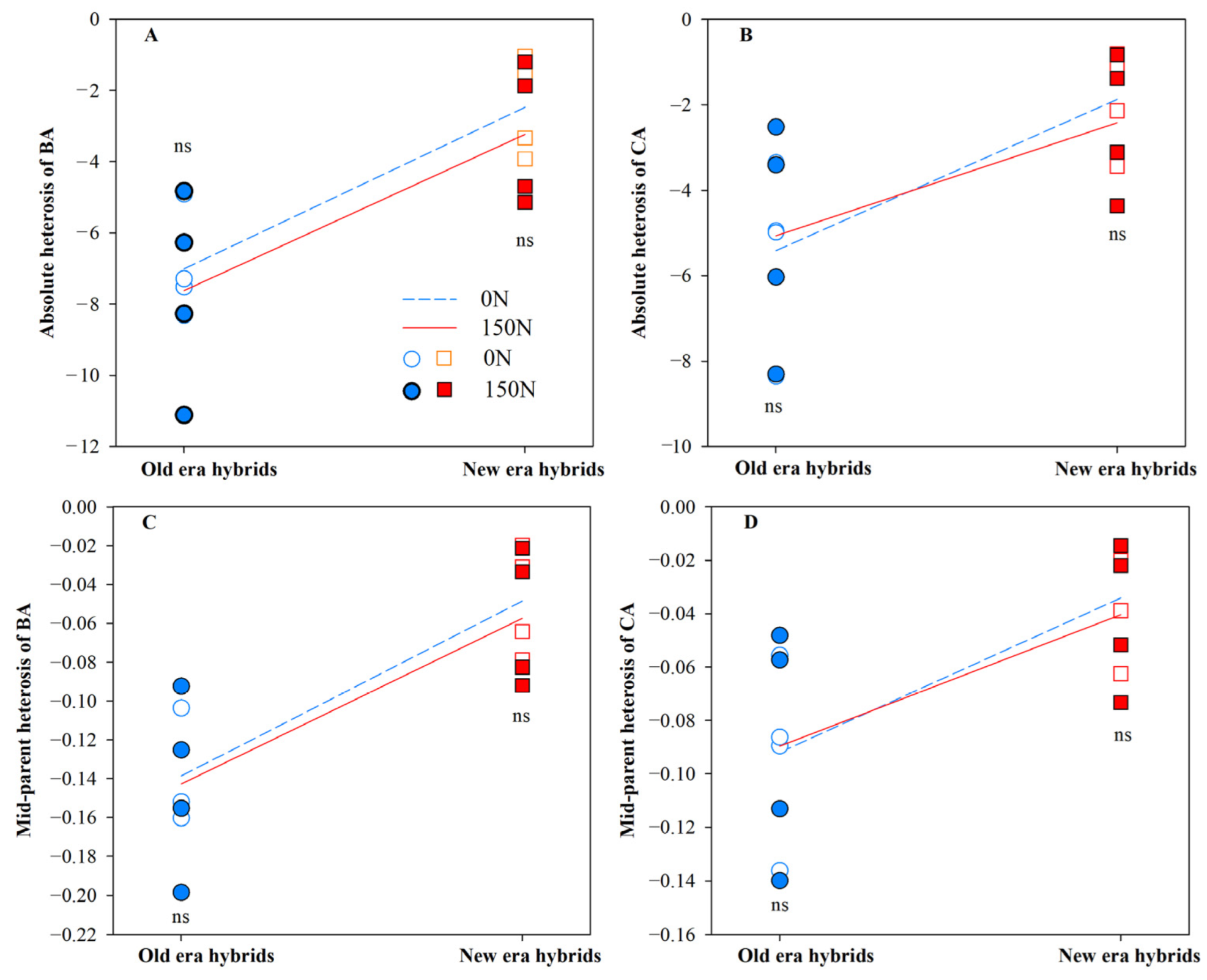
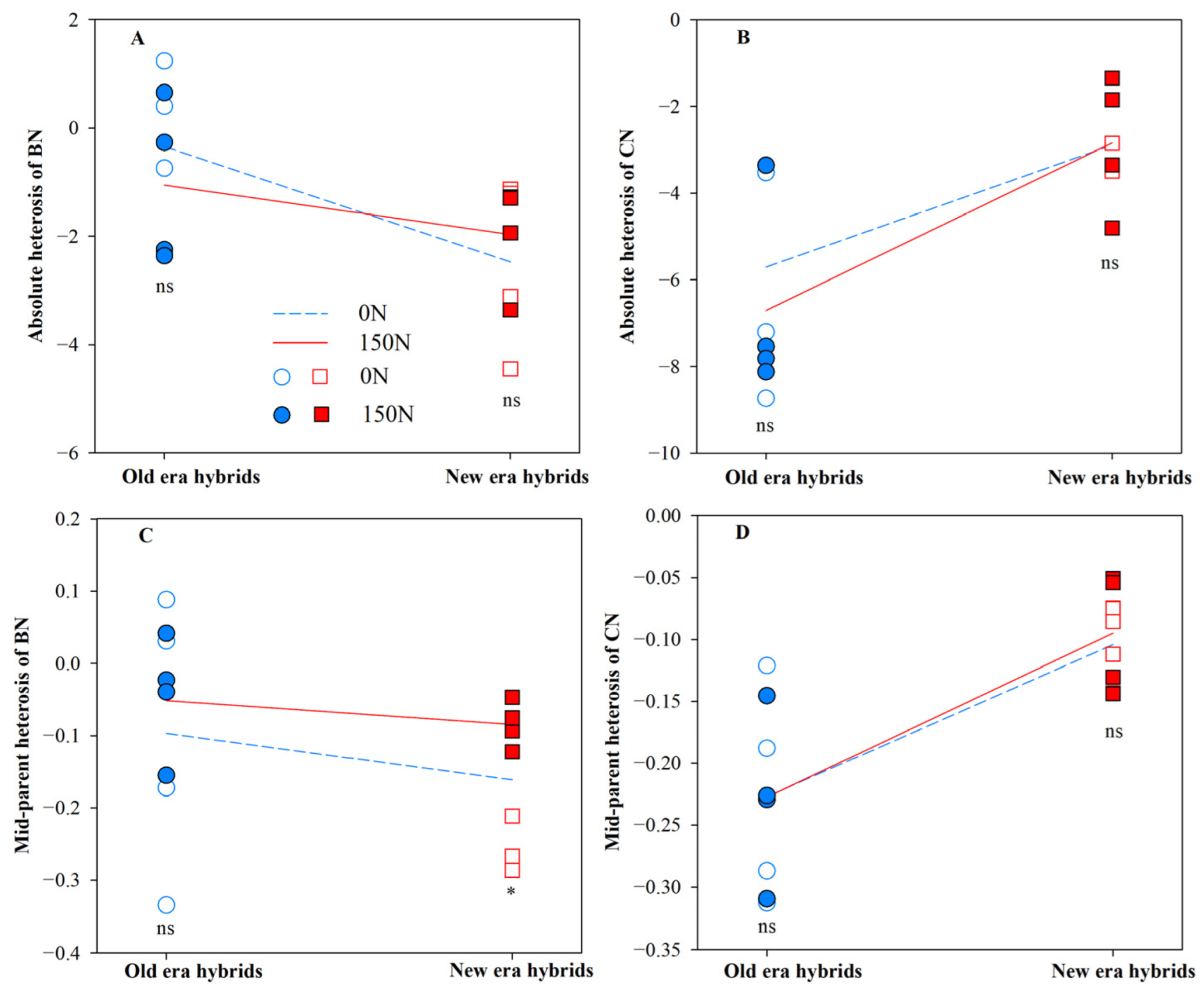
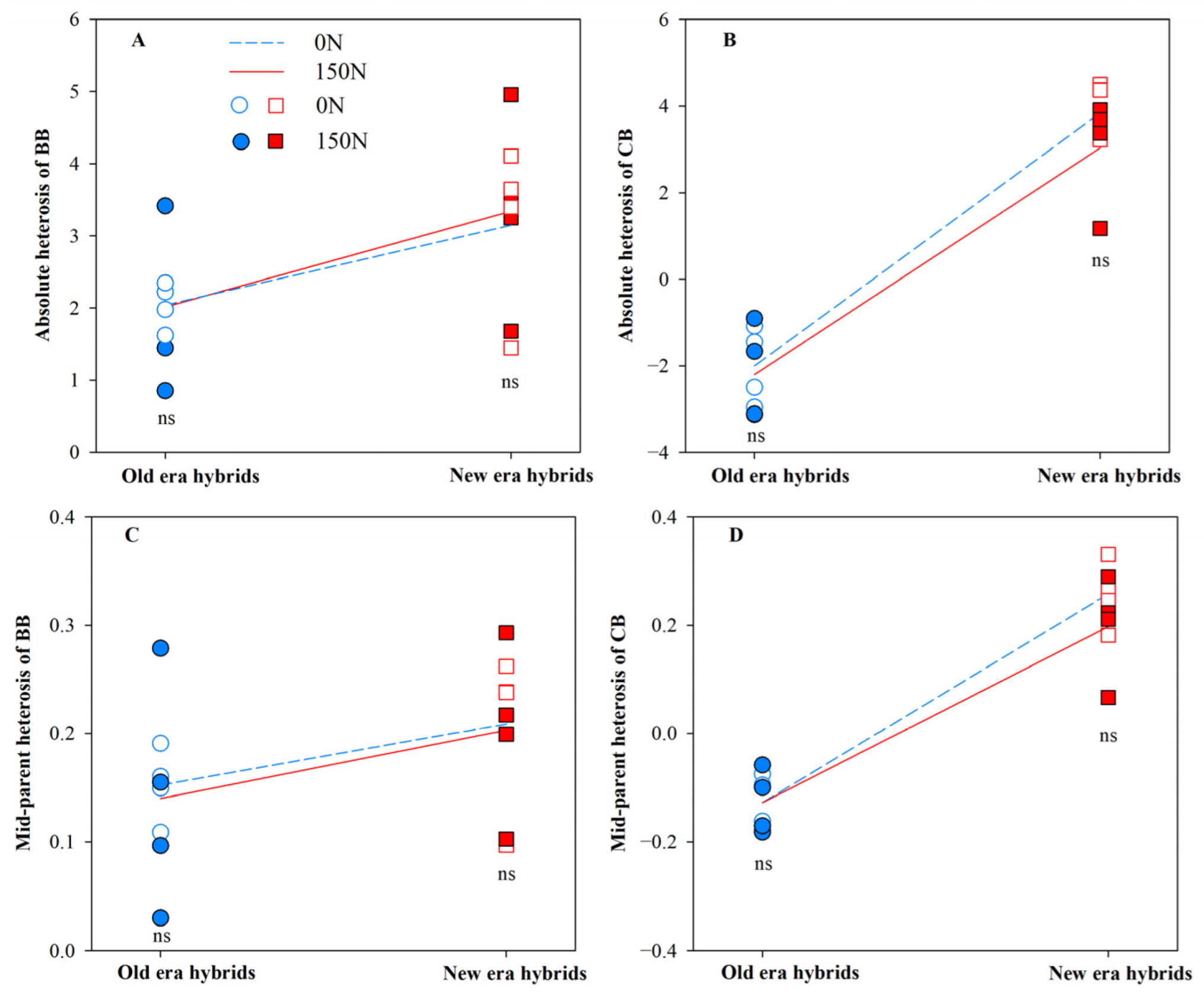
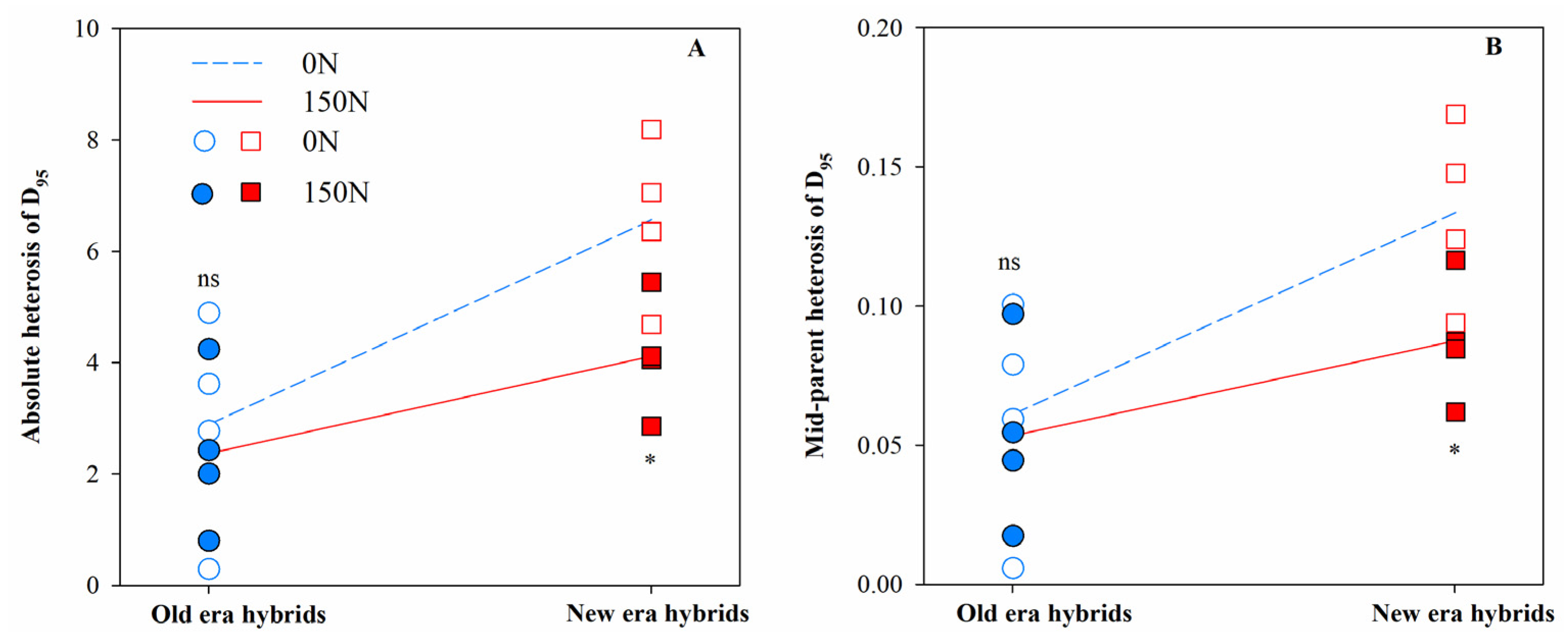
3.1. Heterosis for Root Architecture and Its Component Processes
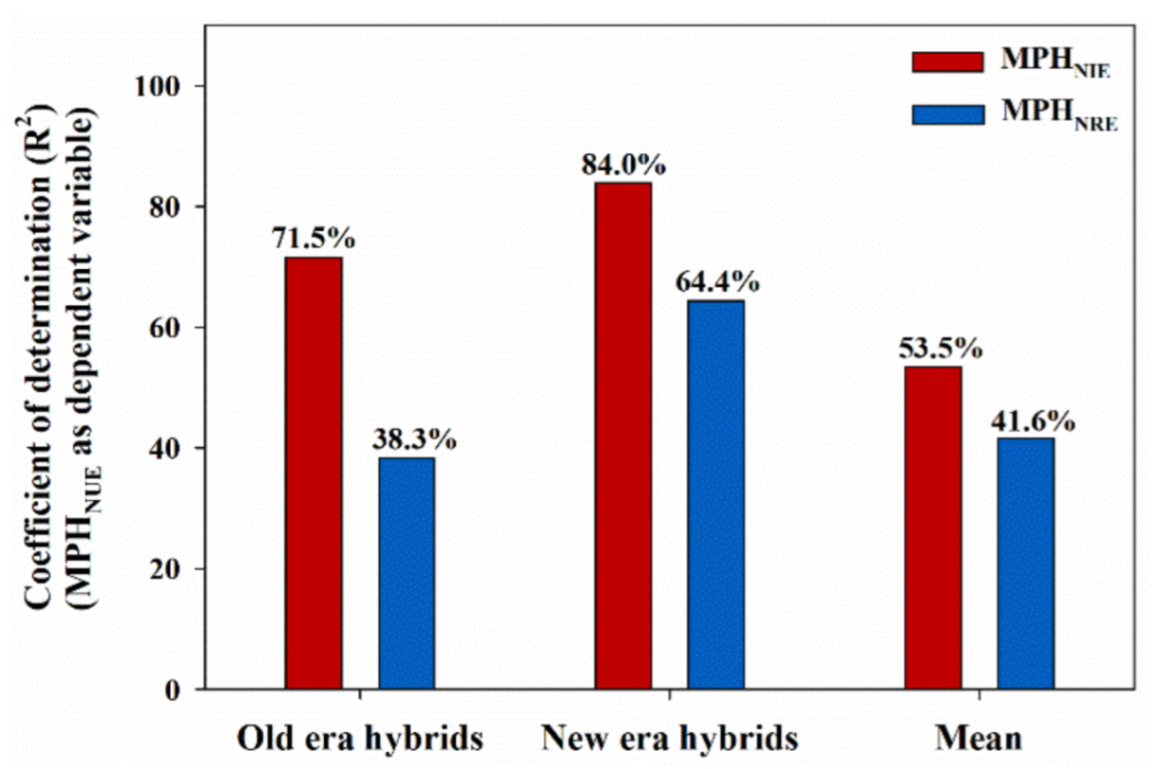
3.2. Heterosis for Nitrogen Use Efficiency and Its Component Processes
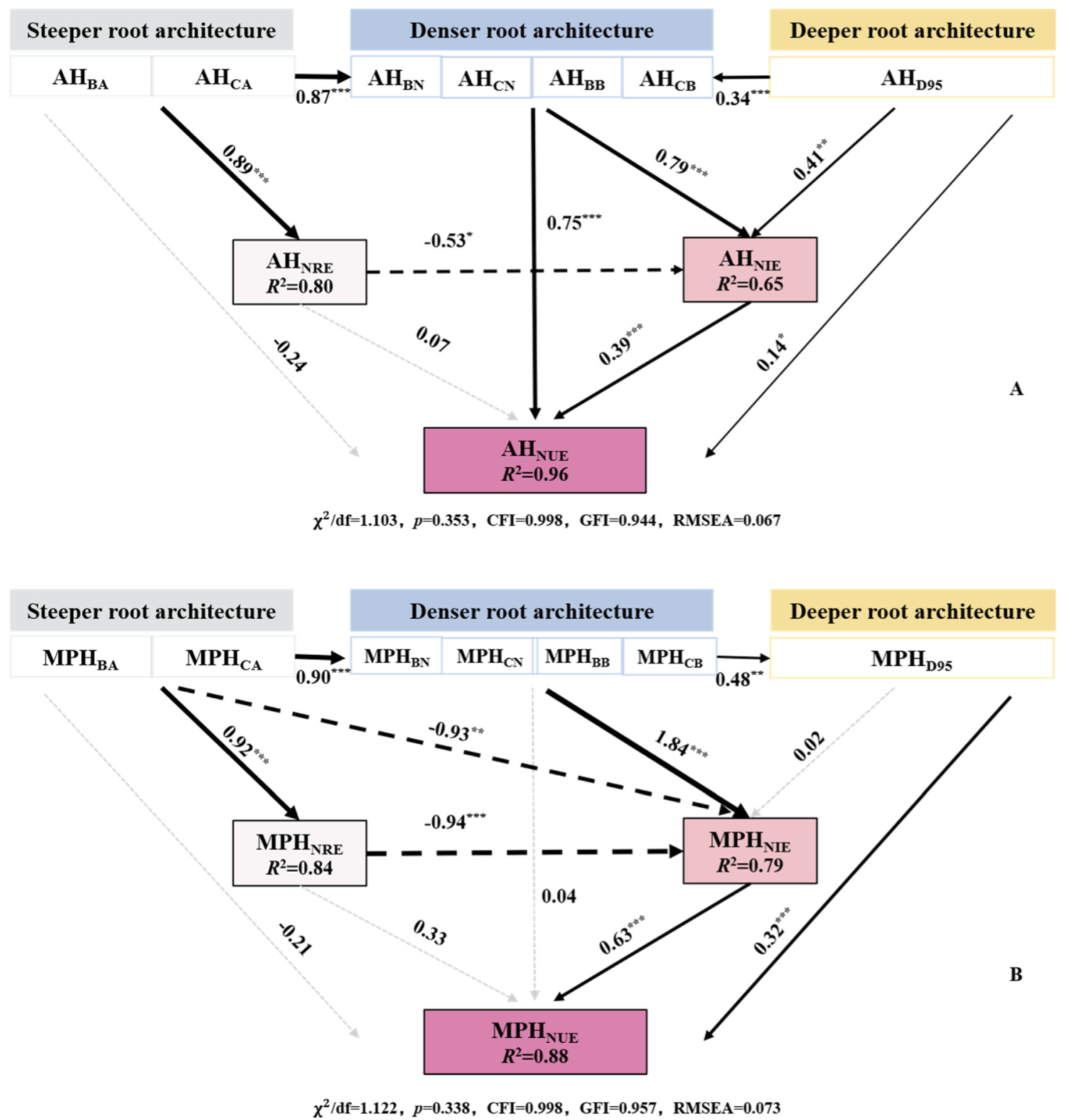
3.3. Correlation between Root Architecture Heterosis and Nitrogen Use Efficiency Heterosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAO. FAOStat; Food and Agriculture Organization of the United Nations: Rome, Italy, 2021. [Google Scholar]
- Zhang, P.; Wang, Y.Y.; Sheng, D.C.; Zhang, S.A.; Gu, S.C.; Yan, Y.; Zhao, F.C.; Wang, P.; Huang, S.B. Optimizing root system architecture to improve root anchorage strength and nitrogen absorption capacity under high plant density in maize. Field Crops Res. 2023, 303, 109109. [Google Scholar] [CrossRef]
- Wang, B.B.; Hou, M.; Shi, J.P.; Ku, L.X.; Song, W.; Li, C.H.; Ning, Q.; Li, X.; Li, C.Y.; Zhao, B.B.; et al. De novo genome assembly and analyses of 12 founder inbred lines provide insights into maize heterosis. Nat. Genet. 2023, 55, 312–323. [Google Scholar] [CrossRef]
- Li, Y.X.; Li, C.H.; Yang, J.P.; Yang, H.; Cheng, W.D.; Wang, L.M.; Li, F.Y.; Li, H.Y.; Wang, Y.B.; Li, S.H.; et al. Genetic dissection of heterosis for Huangzaosi, a foundation parental inbred line of maize in China. Sci. Agric. Sin. 2020, 53, 4113–4126. (In Chinese) [Google Scholar]
- Wang, Z.G.; Ma, B.L.; Yu, X.F.; Gao, J.L.; Sun, J.Y.; Su, Z.J.; Yu, S.B. Physiological Basis of Heterosis for Nitrogen Use Efficiency of Maize. Sci. Rep. 2019, 9, 18708. [Google Scholar] [CrossRef] [PubMed]
- Cassman, K.G.; Dobermann, A.; Walters, D.T. Agroecosystems, nitrogen-use efficiency, and nitrogen management. Ambio 2002, 31, 132–140. [Google Scholar] [CrossRef]
- Linkohr, B.I.; Williamson, L.C.; Fitter, A.H.; Ottoline Leyser, H.M. Nitrate and phosphate availability and distribution have different effects on root system architecture of Arabidopsis. Plant J. 2002, 29, 751–760. [Google Scholar] [CrossRef]
- Ren, W.; Zhao, L.F.; Liang, J.X.; Wang, L.F.; Chen, L.M.; Li, P.C.; Liu, Z.G.; Li, X.J.; Zhang, Z.H.; Li, J.P.; et al. Genome-wide dissection of changes in maize root system architecture during modern breeding. Nat. Plants 2022, 8, 1408–1422. [Google Scholar] [CrossRef]
- Lai, Z.L.; Zhang, H.; Ding, X.H.; Liao, Z.Q.; Zhang, C.; Yu, J.; Pei, S.Z.; Dou, Z.Y.; Li, Z.J.; Fan, J.L. Ridge-furrow film mulch with nitrogen fertilization improves grain yield of dryland maize by promoting root growth, plant nitrogen uptake and remobilization. Soil Tillage Res. 2024, 241, 106118. [Google Scholar] [CrossRef]
- Pace, J.; Gardner, C.; Romay, C.; Ganapathysubramanian, B.; LÜbberstedt, T. Genome-wide association analysis of seedling root development in maize (Zea mays L.). BMC Genom. 2015, 16, 47. [Google Scholar] [CrossRef]
- Lynch, J.P. Steep, cheap and deep: An ideotype to optimize water and N acquisition by maize root systems. Ann. Bot. 2013, 112, 347–357. [Google Scholar] [CrossRef]
- Trachsel, S.; Kaeppler, S.M.; Brown, K.M.; Lynch, J.P. Maize root growth angles become steeper under low N conditions. Field Crops Res. 2013, 140, 18–31. [Google Scholar] [CrossRef]
- Dathe, A.; Postma, J.A.; Postma-Blaauw, M.B.; Lynch, J.P. Impact of axial root growth angles on nitrogen acquisition in maize depends on environmental conditions. Ann. Bot. 2016, 118, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Saengwilai, P.; Nord, E.A.; Chimungu, J.; Brown, K.M.; Lynch, J.P. Root cortical aerenchyma enhances nitrogen acquisition from low nitrogen soils in maize. Plant Physiol. 2014, 166, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Saengwilai, P.; Tian, X.; Lynch, J. Low crown root number enhances nitrogen acquisition from low-nitrogen soils in maize. Plant Physiol. 2014, 166, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Postma, J.A.; Dathe, A.; Lynch, J.P. The optimal lateral root branching density for maize depends on nitrogen and phosphorous availability. Plant Physiol. 2014, 166, 590–602. [Google Scholar] [CrossRef]
- Zhang, A.; Lynch, J.P. Reduced frequency of lateral root branching improves N capture from low-N soils in maize. J. Exp. Bot. 2015, 66, 2055–2065. [Google Scholar] [CrossRef]
- Lynch, J.P. Root phenotypes for improved nutrient capture: An underexploited opportunity for global agriculture. New Phytol. 2019, 223, 548–564. [Google Scholar] [CrossRef]
- Wang, K.J.; Zheng, H.J.; Liu, K.C.; Zhang, J.W.; Dong, S.T.; Hu, C.H. Evolution of maize root distribution in space-time during maize varieties replacing in China. Acta Phytoecol. Sin. 2001, 25, 472–475. (In Chinese) [Google Scholar]
- Chen, F.J.; Fang, Z.G.; Gao, Q.; Ye, Y.L.; Jia, L.L.; Yuan, L.X.; Mi, G.H.; Zhang, F.S. Evaluation of the yield and nitrogen use efficiency of the dominant maize hybrids grown in North and Northeast China. Sci. Sin. Vitae 2013, 43, 342–350. (In Chinese) [Google Scholar] [CrossRef]
- Liu, M.; Wu, G.J.; Lu, D.X.; Xu, Z.H.; Dong, S.T.; Zhang, J.W.; Zhao, B.; Li, G.; Liu, P. Improvement of nitrogen use efficiency and the relationship with root system characters of maize cultivars in different years. J. Plant Nutr. Fertil. 2017, 23, 71–82. (In Chinese) [Google Scholar]
- Asibi, A.E.; Chai, Q.; Coulter, J.A. Mechanisms of Nitrogen Use in Maize. Agronomy 2019, 9, 775. [Google Scholar] [CrossRef]
- Garnett, T.; Conn, V.; Kaiser, B.N. Root based approaches to improving nitrogen use efficiency in plants. Plant Cell Environ. 2009, 32, 1272–1283. [Google Scholar] [CrossRef] [PubMed]
- Sciarresi, C.; Thies, A.; Topp, C.; Eudy, D.; Trifunovic, S.; Ruiz, A.; Dixon, P.M.; Miguez, F.; Burras, L.C.; Archontoulis, S.V. Do newer maize hybrids grow roots faster and deeper? Crop Sci. 2024, 64, 1559–1576. [Google Scholar] [CrossRef]
- Wang, Z.G.; Ma, B.L.; Li, Y.J.; Li, R.F.; Jia, Q.; Yu, X.F.; Sun, J.Y.; Hu, S.P.; Gao, J.L. Concurrent improvement in maize grain yield and nitrogen use efficiency by enhancing inherent soil productivity. Front. Plant Sci. 2022, 13, 790188. [Google Scholar] [CrossRef] [PubMed]
- Wani, S.H.; Vijayan, R.; Choudhary, M.; Kumar, A.; Zaid, A.; Singh, V.; Kumar, P.; Yasin, J.K. Nitrogen use efficiency (NUE): Elucidated mechanisms, mapped genes and gene networks in maize (Zea mays L.). Physiol. Mol. Biol. Plants 2021, 27, 2875–2891. [Google Scholar] [CrossRef]
- Mueller, S.M.; Messina, C.D.; Vyn, T.J. Simultaneous gains in grain yield and nitrogen efficiency over 70 years of maize genetic improvement. Sci. Rep. 2019, 9, 9095. [Google Scholar] [CrossRef]
- Wang, H.B.; Han, T.T.; Bai, A.M.; Xu, H.H.; Wang, J.J.; Hou, X.L.; Li, Y. Potential regulatory networks and heterosis for flavonoid and terpenoid contents in Pak Choi: Metabolomic and transcriptome analyses. Int. J. Mol. Sci. 2024, 25, 3587. [Google Scholar] [CrossRef]
- Duvick, D.N. The contribution of breeding to yield advances in maize (Zea mays L.). Adv. Agron. 2005, 86, 83–145. [Google Scholar]
- Tollenaar, M.; Ahmadzadeh, A.; Lee, E.A. Physiological basis of heterosis for grain yield in maize. Crop Sci. 2004, 44, 2086–2094. [Google Scholar] [CrossRef]
- Tollenaar, M.; Deen, W.; Echarte, L.; Liu, W. Effect of crowding stress on dry matter accumulation and harvest index in maize. Agron. J. 2006, 98, 930–937. [Google Scholar] [CrossRef]
- Liu, W.D.; Tollenaar, M. Response of yield heterosis to increasing plant density in maize. Crop Sci. 2009, 49, 1807–1816. [Google Scholar] [CrossRef]
- Li, R.F.; Gao, J.L.; Li, Y.Y.; Yu, S.B.; Wang, Z.G. Heterosis for nitrogen use efficiency of maize hybrids enhanced over decades in China. Agriculture 2022, 12, 764. [Google Scholar] [CrossRef]
- Hund, A.; Reimer, R.; Stamp, P.; Walter, A. Can we improve heterosis for root growth of maize by selecting parental inbred lines with different temperature behaviour? Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 1580–1588. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.R.; Tang, C.; Salvato, F.; Clouse, K.M.; Bartlett, A.; Vintila, S.; Phillips, L.; Sermons, S.; Hoffmann, M.; Balint-Kurti, P.J.; et al. Microbe-dependent heterosis in maize. Proc. Natl. Acad. Sci. USA 2021, 118, e2021965118. [Google Scholar] [CrossRef]
- Haegele, J.W.; Cook, K.A.; Nichols, D.M.; Below, F.E. Changes in nitrogen use traits associated with genetic improvement for grain yield of maize hybrids released in different decades. Crop Sci. 2013, 53, 1256–1268. [Google Scholar] [CrossRef]
- Hoecker, N.; Keller, B.; Piepho, H.P.; Hochholdinger, F. Manifestation of heterosis during early maize (Zea mays L.) root development. Theor. Appl. Genet. 2005, 112, 421–429. [Google Scholar] [CrossRef]
- Xu, M.M.; Lu, X.M.; Sun, X.J.; Yang, H.L.; Yan, P.S.; Du, H.W.; Chen, X.Y.; Tang, J.H. Heterotic loci analysis for root traits of maize seedlings using an SSSL test population under different nitrogen conditions. Mol. Breed. 2020, 40, 34. [Google Scholar] [CrossRef]
- Liu, X.; He, K.; Ali, F.; Li, D.; Cai, H.; Zhang, H.; Yuan, L.; Liu, W.; Mi, G.; Chen, F.; et al. Genetic dissection of N use efficiency using maize inbred lines and testcrosses. Crop J. 2023, 11, 1242–1250. [Google Scholar] [CrossRef]
- Wang, H.; Wu, Y.J.; An, T.T.; Chen, Y.L. Lateral root elongation enhances nitrogen-use efficiency in maize genotypes at the seedling stage. J. Sci. Food Agric. 2022, 102, 5389–5398. [Google Scholar] [CrossRef]
- Liu, W.D.; Tollenaar, M. Physiological mechanisms underlying heterosis for shade tolerance in maize. Crop Sci. 2009, 49, 1817–1826. [Google Scholar] [CrossRef]
- Tollenaar, M.T.; Lee, E.A. Strategies for enhancing grain yield in maize. Plant Breed. Rev. 2011, 34, 37–82. [Google Scholar]
- Chen, K.; Vyn, T.J. Post-silking Factor Consequences for N Efficiency Changes Over 38 Years of Commercial Maize Hybrids. Front. Plant Sci. 2017, 8, 1737–1755. [Google Scholar] [CrossRef] [PubMed]
- Mastrodomenico, A.T.; Hendrix, C.C.; Below, F.E. Nitrogen use efficiency and the genetic variation of maize expired plant variety protection germplasm. Agriculture 2018, 8, 3–20. [Google Scholar] [CrossRef]
- Trachsel, S.; Kaeppler, S.M.; Brown, K.M.; Lynch, J.P. Shovelomics: High throughput phenotyping of maize (Zea mays, L.) root architecture in the field. Plant Soil 2011, 341, 75–87. [Google Scholar] [CrossRef]
- Ma, D.L.; Li, S.K.; Zhai, L.C.; Yu, X.F.; Xie, R.Z.; Gao, J.L. Response of maize barrenness to density and nitrogen increases in Chinese cultivars released from the 1950s to 2010s. Field Crops Res. 2020, 250, 107766. [Google Scholar] [CrossRef]
- Ma, D.L.; Yu, X.F.; Ming, B.; Li, S.K.; Xie, R.Z.; Gao, J.L. Maize nitrogen use efficiency improved due to density tolerance increase since the 1950s. Agronomy 2020, 112, 1537–1548. [Google Scholar] [CrossRef]
- Cai, H.G.; Chen, F.J.; Mi, G.H.; Zhang, F.S.; Maurer, H.P.; Liu, W.X.; Reif, J.C.; Yuan, L.X. Mapping QTLs for root system architecture of maize (Zea mays L.) in the field at different developmental stages. Theor. Appl. Genet. 2012, 125, 1313–1324. [Google Scholar] [CrossRef]
- Cassman, K.G.; Dobermann, A.; Walters, D.T.; Yang, H.S. Meeting cereal demand while protecting natural resources and improving environmental quality. Annu. Rev. Environ. Resour. 2003, 28, 315–358. [Google Scholar] [CrossRef]
- Ciampitti, I.A.; Vyn, T.J. A comprehensive study of plant density consequences on nitrogen uptake dynamics of maize plants from vegetative to reproductive stages. Field Crops Res. 2011, 121, 2–18. [Google Scholar] [CrossRef]
- Ciampitti, I.A.; Murrell, S.T.; Camberato, J.J.; Tuinstra, M.; Xia, Y.B.; Friedemann, P.; Vyn, T.J. Physiological dynamics of maize nitrogen uptake and partitioning in response to plant density and nitrogen stress factors: II. Reproductive phase. Crop Sci. 2013, 53, 2588–2602. [Google Scholar] [CrossRef]
- Chun, L.; Chen, F.J.; Zhang, F.S.; Mi, G.H. Root growth, nitrogen uptake and yield formation of hybrid maize with different N efficiency. J. Plant Nutr. Fertil. 2005, 11, 615–619. (In Chinese) [Google Scholar]
- Peng, Y.F.; Niu, J.F.; Peng, Z.P.; Zhang, F.S.; Li, C.J. Shoot growth potential drives N uptake in maize plants and correlates with root growth in the soil. Field Crops Res. 2010, 115, 85–93. [Google Scholar] [CrossRef]
- Mackay, A.D.; Barber, S.A. Effect of Nitrogen on Root Growth of Two Corn Genotypes in the Field. Agron. J. 1986, 78, 699–703. [Google Scholar] [CrossRef]
- Coque, M.; Gallais, A. Genomic regions involved in response to grain yield selection at high and low nitrogen fertilization in maize. Theor. Appl. Genet. 2006, 112, 1205–1220. [Google Scholar] [CrossRef]
- Sha, Y.; Liu, Z.; Hao, Z.H.; Huang, Y.W.; Shao, H.; Feng, G.Z.; Chen, F.J.; Mi, G.H. Root growth, root senescence and root system architecture in maize under conservative strip tillage system. Plant Soil 2024, 495, 253–269. [Google Scholar] [CrossRef]
- Sattelmacher, B.; Gerendas, J.; Thoms, K.; BrÜck, H.; Bagdady, N.H. Interaction between root growth and mineral nutrition. Environ. Exp. Bot. 1993, 33, 63–73. [Google Scholar] [CrossRef]
- Dwyer, L.M.; Ma, B.L.; Stewart, D.W.; Hayhoe, H.N.; Balchin, D.; Culley, J.L.B. Root mass distribution under conventional and conservation tillage. Can. J. Soil Sci. 1996, 76, 23–28. [Google Scholar] [CrossRef]
- Guo, S.; Liu, Z.G.; Zhou, Z.J.; Lu, T.Q.; Chen, S.H.; He, M.J.; Zeng, X.Z.; Chen, K.; Yu, H.; Shangguan, Y.X.; et al. Root system architecture differences of maize cultivars affect yield and nitrogen accumulation in Southwest China. Agriculture 2022, 12, 209. [Google Scholar] [CrossRef]
- Wiesler, F.; Horst, W.J. Differences between Maize Cultivars in Yield Formation, Nitrogen Uptake and associated Depletion of Soil Nitrate. J. Agron. Crop Sci. 1992, 168, 226–237. [Google Scholar] [CrossRef]
- Wiesler, F.; Horst, W.J. Differences among maize cultivars in the utilization of soil nitrate and the related losses of nitrate through leaching. Plant Soil 1993, 151, 193–203. [Google Scholar] [CrossRef]
- Wiesler, F.; Horst, W.J. Root growth and nitrate utilization of maize cultivars under field conditions. Plant Soil 1994, 163, 267–277. [Google Scholar] [CrossRef]
- Liu, J.X.; Chen, F.J.; Olokhnuud, C.; Glass, A.D.M.; Tong, Y.P.; Zhang, F.S.; Mi, G.H. Root size and nitrogen-uptake activity in two maize (Zea mays) inbred lines differing in nitrogen-use efficiency. J. Plant Nutr. Soil Sci. 2009, 172, 230–236. [Google Scholar] [CrossRef]
- Chen, X.C.; Zhang, J.; Chen, Y.L.; Li, Q.; Chen, F.J.; Yuan, L.X.; Mi, G.H. Changes in root size and distribution in relation to nitrogen accumulation during maize breeding in China. Plant Soil 2014, 374, 121–130. [Google Scholar] [CrossRef]
- York, L.M.; Galindo-Castaneda, E.T.; Schussler, J.R.; Lynch, J.P. Evolution of US maize (Zea mays L.) root architectural and anatomical phenes over the past 100 years corresponds to increased tolerance of nitrogen stress. J. Exp. Bot. 2015, 66, 2347–2358. [Google Scholar] [CrossRef] [PubMed]
- Hund, A. Genetic variation in the gravitropic response of maize roots to low temperatures. Plant Root 2010, 4, 22–30. [Google Scholar] [CrossRef]
- Chen, X.C.; Chen, F.J.; Chen, Y.L.; Gao, Q.; Yang, X.L.; Yuan, L.X.; Zhang, F.S.; Mi, G.H. Modern maize hybrids in Northeast China exhibit increased yield potential and resource use efficiency despite adverse climate change. Glob. Chang. Biol. 2013, 19, 923–936. [Google Scholar] [CrossRef] [PubMed]
- Ciampitii, I.A.; Vyn, T.J. Physiological perspectives of changes over time in maize yield dependency on nitrogen uptake and associated nitrogen efficiencies: A review. Field Crops Res. 2012, 133, 48–67. [Google Scholar] [CrossRef]
- Fan, P.P.; Ming, B.; Evers, J.B.; Li, Y.Y.; Li, S.K.; Xie, R.Z.; Anten, N.P.R. Nitrogen availability determines the vertical patterns of accumulation, partitioning, and reallocation of dry matter and nitrogen in maize. Field Crops Res. 2023, 297, 108927. [Google Scholar] [CrossRef]
- Rinehart, B.; Borras, L.; Salmeron, M.; McNear, D.H.; Poffenbarger, H. Commercial maize hybrids have smaller root systems after 80 years of breeding. Rhizosphere 2024, 30, 100915. [Google Scholar] [CrossRef]
- Yu, P.; Li, X.X.; White, P.J.; Li, C.J. A large and deep root system underlies high nitrogen-use efficiency in maize production. PLoS ONE 2015, 10, e0126293. [Google Scholar] [CrossRef]
- Chen, F.J.; Liu, J.C.; Liu, Z.G.; Chen, Z.; Ren, W.; Gong, X.P.; Wang, L.F.; Cai, H.G.; Pan, Q.C.; Yuan, L.X.; et al. Breeding for high-yield and nitrogen use efficiency in maize: Lessons from comparison between Chinese and US cultivars. Agronomy 2021, 166, 251–275. [Google Scholar]
- Shao, H.; Xia, T.T.; Wu, D.L.; Chen, F.J.; Mi, G.H. Root growth and root system architecture of field-grown maize in response to high planting density. Plant Soil 2018, 430, 395–411. [Google Scholar] [CrossRef]
- Wen, G.Q.; Ma, B.L. Chapter six—Optimizing crop nitrogen use efficiency: Integrating root performance and machine learning into nutrient management. Agronomy 2024, 187, 311–363. [Google Scholar] [CrossRef]

| Era of Hybrids | Hybrids | Parental Combination | Year of Hybrid Release | Institution That Developed the Cultivar |
|---|---|---|---|---|
| Old-era hybrids | Zhongdan 2 (ZD2) | Mo17 × Zi 330 | 1972 [46,47] | Chinese AAS, Beijing, China |
| Danyu 13 (DY13) | Mo17 × E 28 | 1981 [46,47] | Dandong AAS of Liaoning Province, Dandong, China | |
| New-era hybrids | Zhengdan 958 (ZD958) | Zheng 58 × Chang 7-2 | 2000 [46,47] | Luohe AAS of Henan Province, Luohe, China |
| Xianyu 335 (XY335) | PH6WC × PH4CV | 2004 [46,47] | The Tieling Pioneer limited company, Tieling, China |
| Index | BA (°) | CA (°) | BN | CN | BB | CB | D95 (cm) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 N | 150 N | 0 N | 150 N | 0 N | 150 N | 0 N | 150 N | 0 N | 150 N | 0 N | 150 N | 0 N | 150 N | |
| Old-era hybrids | 45.0c | 43.1c | 53.8b | 51.6b | 12.2c | 14.3b | 19.7c | 22.9c | 15.6b | 17.1b | 13.4c | 14.7c | 50.6b | 47.1b |
| New-era hybrids | 53.7b | 51.2b | 58.1a | 55.7a | 13.1bc | 15.2b | 25.7b | 27.8b | 18.2a | 19.8a | 19.0a | 19.0a | 55.9a | 51.1a |
| Old-era inbred lines | 52.3b | 50.4b | 59.2a | 56.6a | 13.7b | 15.1b | 25.3b | 29.6a | 13.6c | 15.6c | 15.4b | 16.9b | 47.8c | 44.8c |
| New-era inbred lines | 56.2a | 54.3a | 59.9a | 58.1a | 15.6a | 16.5a | 28.6a | 30.6a | 15.0bc | 16.4c | 15.2b | 15.9b | 49.3bc | 47.0b |
| Source of variation | ||||||||||||||
| Hybrids (H) | ** | ** | ** | ** | ns | ns | ** | ** | ** | ** | ** | ** | ** | ** |
| Inbred lines (I) | * | * | ns | ns | * | * | * | ns | ns | ns | ns | ns | ns | * |
| N rates (N) | * | * | * | * | * | ns | ** | |||||||
| H × N | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| I × N | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| Heterotic Index | Source of Variation | ||
|---|---|---|---|
| Eras (E) | N Rates (N) | (E × N) | |
| Absolute heterosis (AH) | |||
| BA | 20.14 ** | 0.09 | 0.07 |
| CA | 9.94 ** | 0.01 | 0.21 |
| BB | 4.98 * | 0.04 | 0.25 |
| CB | 121.44 ** | 1.01 | 0.33 |
| BN | 0.23 | 7.95 * | 1.51 |
| CN | 11.69 ** | 0.24 | 0.29 |
| D95 | 11.32 * | 1.03 | 0.43 |
| Mid-parent heterosis (MPH) | |||
| BA | 27.75 ** | 0.34 | 0.01 |
| CA | 10.77 ** | 0.02 | 0.07 |
| BB | 6.38 * | 0.34 | 0.36 |
| CB | 108.60 ** | 0.79 | 0.78 |
| BN | 1.07 | 8.16 * | 1.16 |
| CN | 16.65 ** | 0.02 | 0.02 |
| D95 | 13.10 * | 0.08 | 0.56 |
| Index | GY (Mg ha−1) | VegN (kg ha−1) | RepN (kg ha−1) | Plant N (kg ha−1) | NUE (kg kg−1) | NRE (kg kg−1) | NIE (kg kg−1) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 N | 150 N | 0 N | 150 N | 0 N | 150 N | 0 N | 150 N | 150 N | 150 N | 150 N | |
| Old-era hybrids | 10.8a | 12.8b | 115.6b | 153.3b | 40.3b | 66.4a | 155.9b | 219.7b | 13.5b | 0.43c | 31.7b |
| New-era hybrids | 11.2a | 14.7a | 137.9a | 193.5a | 39.5b | 53.7b | 177.4a | 247.2a | 23.1a | 0.47c | 50.6a |
| Old-era inbred lines | 6.7b | 8.2c | 61.1c | 125.7c | 41.1a | 68.8a | 102.2c | 194.5c | 9.5c | 0.62a | 14.5d |
| New-era inbred lines | 7.4b | 9.0c | 69.9c | 130.9c | 42.9a | 60.9a | 112.8c | 191.8c | 10.5c | 0.53b | 21.2c |
| Source of variation | |||||||||||
| Hybrids (H) | ns | ** | ** | ** | ns | ** | ** | ** | ** | ns | * |
| Inbred lines (I) | ns | ns | ns | ns | ns | ns | ns | ns | ns | * | * |
| N rates (N) | ** | ** | ** | ** | - | - | - | ||||
| H × N | ns | ns | ns | ns | ns | ns | ns | ns | - | - | - |
| I × N | ns | ns | ns | ns | ns | ns | ns | ns | - | - | - |
| Index | AH | MPH (%) | ||
|---|---|---|---|---|
| Old-Era Hybrids | New-Era Hybrids | Old-Era Hybrids | New-Era Hybrids | |
| GY | 4.1a | 3.8a | 55.4a | 61.7a |
| VegN | 27.6b | 62.6a | 21.9b | 47.8a |
| RepN | −2.4a | −7.2a | −3.5a | −11.8a |
| Plant N | 25.2b | 55.4a | 13.1b | 28.9a |
| NUE | 4.0b | 12.6a | 42.1b | 120.0a |
| NRE | −0.2b | −0.1a | −22.3a | −11.3a |
| NIE | 17.2b | 30.4a | 118.4b | 143.6a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Bai, L.; Wei, S.; Wu, H.; Li, R.; Wang, Y.; Wang, Z. Integrating Heterosis for Root Architecture and Nitrogen Use Efficiency of Maize: A Comparison between Hybrids from Different Decades. Agronomy 2024, 14, 2018. https://doi.org/10.3390/agronomy14092018
Li Y, Bai L, Wei S, Wu H, Li R, Wang Y, Wang Z. Integrating Heterosis for Root Architecture and Nitrogen Use Efficiency of Maize: A Comparison between Hybrids from Different Decades. Agronomy. 2024; 14(9):2018. https://doi.org/10.3390/agronomy14092018
Chicago/Turabian StyleLi, Yuanyuan, Lanfang Bai, Shuli Wei, Hao Wu, Rongfa Li, Yongqiang Wang, and Zhigang Wang. 2024. "Integrating Heterosis for Root Architecture and Nitrogen Use Efficiency of Maize: A Comparison between Hybrids from Different Decades" Agronomy 14, no. 9: 2018. https://doi.org/10.3390/agronomy14092018







