Community Diversity of Endophytic Bacteria in the Leaves and Roots of Pea Seedlings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Bacterial Genomic DNA Extraction and Illumina MiSeq Sequencing
2.3. Bioinformatic and Statistical Analyses
2.4. Isolation of Endophytes from the Leaves and Roots of Pea Plants
2.5. Characterization of Endophytic Bacteria for Plant Growth-Promoting (PGP) Traits
2.5.1. Solubilization of Phosphorus and Potassium
2.5.2. Production of IAA and ACC Deaminase
2.5.3. Determination of Enzyme Activities
2.5.4. PCR Identification of Endophytic Bacterial Isolates
2.5.5. Alignment of Sequences and Construction of Phylogenetic Trees
3. Results
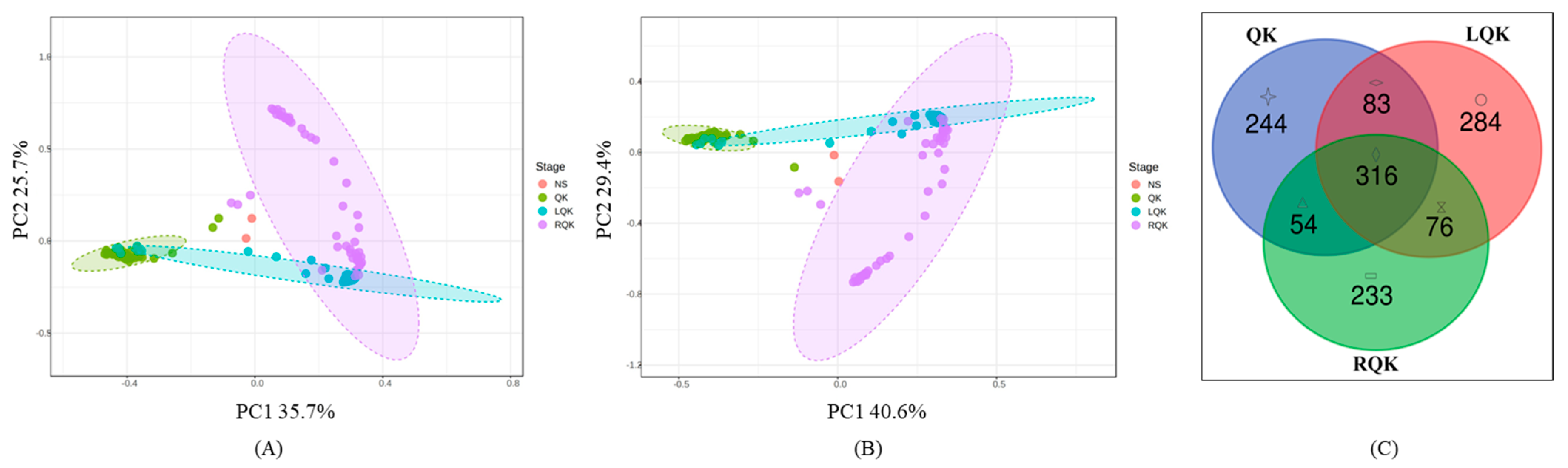
3.1. Microbial Community Structure and Distribution
3.2. OTU Cluster Analysis of Different Kinds of Samples
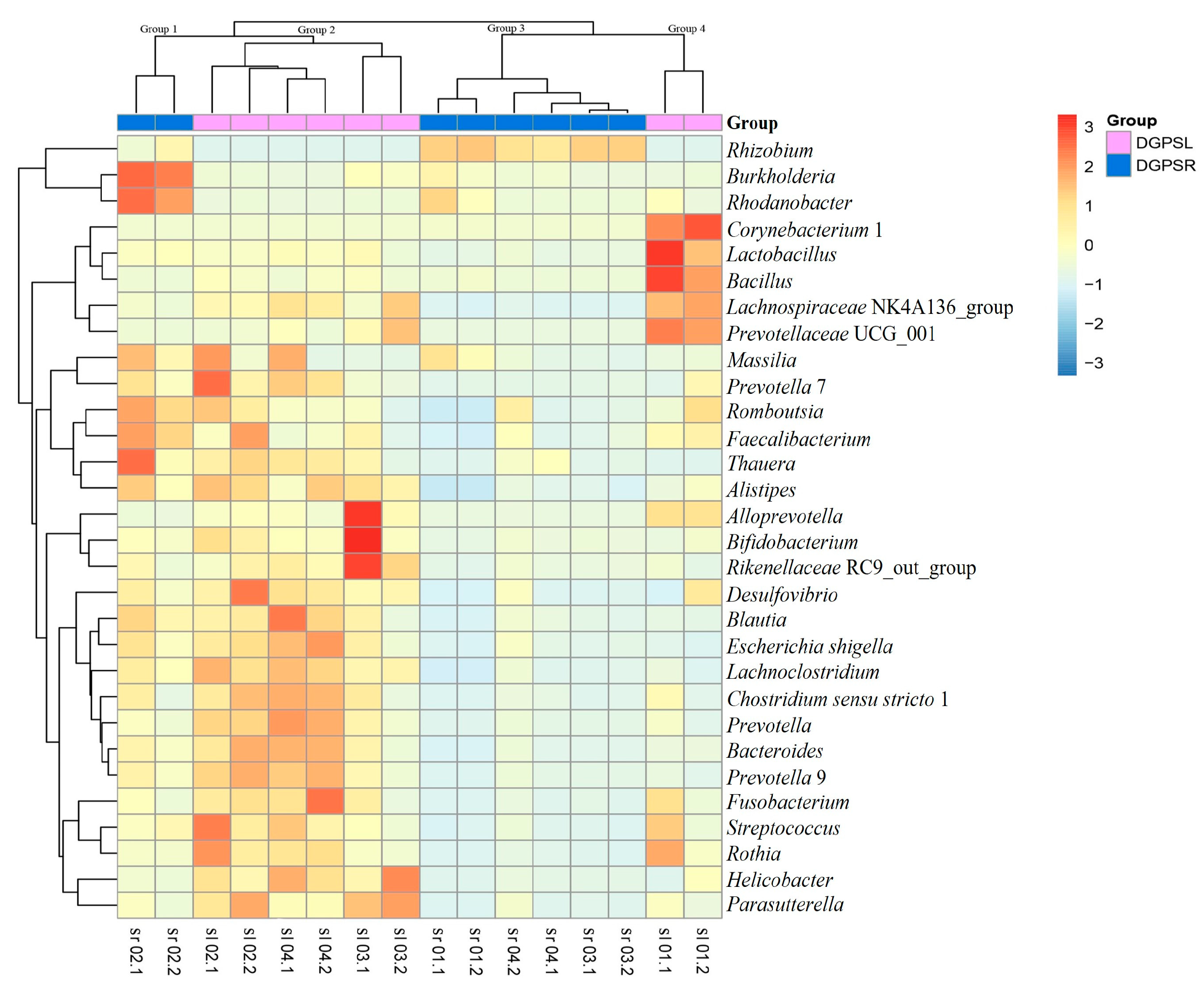
3.3. Phylogenetic Tree and Heatmap
3.4. Isolation and Identification of Endophytes from the Leaves and Roots of Pea Plants
3.5. Solubilization of Phosphorus and Potassium of Endophytic Isolates
3.6. Production of IAA and ACC Deaminase of Isolates
3.7. Determination of Nitrogenase, Cellulase, and Pectinase Activities of Endophytic Isolates
3.8. Phylogenetic Analysis
4. Discussion
4.1. Diversity of Endophytic Communities in Leaves and Roots
4.2. Niche Clustering Analysis at Seedling Stage
4.3. Phylogenetic Analysis and Potential Plant Growth Promotion of Endophytic Isolates
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kraiser, T.; Gras, D.E.; Gutiérrez, A.G.; González, B.; Gutiérrez, R.A. A holistic view of nitrogen acquisition in plants. J. Exp. Bot. 2011, 62, 1455–1466. [Google Scholar] [CrossRef]
- Reinhold-Hurek, B.; Hurek, T. Living inside plants: Bacterial endophytes. Curr. Opin. Plant Biol. 2011, 14, 435–443. [Google Scholar] [CrossRef]
- Ali, S.; Charles, T.C.; Glick, B.R. Delay of flower senescence by bacterial endophytes expressing 1-aminocyclopropane-1-carboxylate deaminase. J. Appl. Microbiol. 2012, 113, 1139–1144. [Google Scholar] [CrossRef]
- Ali, S.; Charles, T.C.; Glick, B.R. Amelioration of high salinity stress damage by plant growth-promoting bacterial endophytes that contain ACC deaminase. Plant Physiol. Biochem. 2014, 80, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, R.L.; Pieterse, C.M.; Bakker, P.A. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Lau, J.A.; Lennon, J.T. Evolutionary ecology of plant-microbe interactions: Soil microbial structure alters selection on plant traits. New Phytol. 2011, 192, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.R.; Lundberg, D.S.; Rio, T.G.D.; Tringe, S.G.; Dangl, J.L.; Mitchell-Olds, T. Host genotype and age shape the leaf and root microbiomes of a wild perennial plant. Nat. Commun. 2016, 7, 12151. [Google Scholar] [CrossRef]
- Guo, J.J.; Ling, N.; Li, Y.; Li, K.; Ning, H.L.; Shen, Q.R.; Guo, S.W.; Vandenkoornhuyse, P. Seed-borne, endospheric and rhizospheric core microbiota as predictors of plant functional traits across rice cultivars are dominated by deterministic processes. New Phyt. 2021, 230, 2047–2060. [Google Scholar] [CrossRef]
- Ku, Y.L.; Han, X.T.; Lei, Y.T.; Zhang, M.; Zhao, Z. Different sensitivities and assembly mechanisms of the root-associated microbial communities of Robinia pseudoacacia to spatial variation at the regional scale. Plant Soil 2023, 486, 621–637. [Google Scholar] [CrossRef]
- Agler, M.T.; Ruhe, J.; Kroll, S.; Morhenn, C.; Kim, S.T.; Weigel, D.; Kemen, E.M. Microbial Hub Taxa Link Host and Abiotic Factors to Plant Microbiome Variation. PLoS Biol. 2016, 14, e1002352. [Google Scholar] [CrossRef]
- Nelson, E.B. The seed microbiome: Origins, interactions, and impacts. Plant Soil 2018, 422, 7–34. [Google Scholar] [CrossRef]
- Aziz, U.; Rehmania, M.S.; Wang, L.; Luo, X.F.; Xian, B.S.; Wei, S.W.; Wang, G.D.; Shu, K. Toward a Molecular Un-derstanding of Rhizosphere, Phyllosphere, and Spermosphere Interactions in Plant Growth and Stress Response. Crit. Rev. Plant Sci. 2021, 40, 479–500. [Google Scholar] [CrossRef]
- Burbano, J.L.; Hurek, C.S.; Reinhold-Hurek, B. Isolation and characterization of root-associated bacteria from agricultural crops in the Kavango region of Namibia. Plant Soil. 2012, 356, 67–82. [Google Scholar]
- Jorquera, M.A.; Shaharoona, B.; Nadeem, S.M.; Mora, M.D.L.L.; Crowley, D.E. Plant Growth-Promoting Rhizobacteria Associated with Ancient Clones of Creosote Bush (Larrea tridentata). Microb. Ecol. 2012, 64, 1008–1017. [Google Scholar] [CrossRef]
- Bulgarelli, D.; Schlaeppi, K.; Spaepen, S.; Themaat, E.V.L.V.; Schulze-Lefert, P. Structure and functions of the bacterial microbiota of plants. Annu. Rev. Plant Biol. 2013, 64, 807–838. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Growth of the plant cell wall. Nat. Rev. Mol. Cell Biol. 2005, 6, 850–861. [Google Scholar] [CrossRef]
- Mirabet, V.; Das, P.; Boudaoud, A.; Hamant, O. The role of mechanical forces in plant morphogenesis. Annu. Rev. Plant Biol. 2011, 62, 365–385. [Google Scholar] [CrossRef] [PubMed]
- Haas, K.T.; Wightman, R.; Peaucelle, A.; Hofte, H. The role of pectin phase separation in plant cell wall assembly and growth. Cell Surf. 2021, 7, 100054. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, D.J. Structure and growth of plant cell walls. Nat. Rev. Mol. Cell Biol. 2023, 25, 340–358. [Google Scholar] [CrossRef]
- Ofek, M.; Hadar, Y.; Minz, D. Colonization of cucumber seeds by bacteria during germination. Environ. Microbiol. 2011, 13, 2794–2807. [Google Scholar] [CrossRef]
- Wu, X.; Li, N.; Hao, J.; Hu, J.; Zhang, X.; Blair, M.W. Genetic diversity of Chinese and global pea (Pisum sativum L.) collections. Crop Sci. 2016, 57, 1574–1584. [Google Scholar] [CrossRef]
- Nossa, C.W.; Oberdorf, W.E.; Aas, J.A.; Paster, B.J.; DeSantis, T.Z.; Brodie, E.L.; Malamud, D.; Poles, M.A.; Pei, Z. Design of 16s rRNA gene primers for 454 pyrosequencing of the human foregut microbiome. World J. Gastroenterol. 2010, 16, 4135. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Reyon, D.; Tsai, S.Q.; Khayter, C.; Foden, J.A.; Sander, J.D.; Joung, J.K. FLASH assembly of TALENs for high-throughput genome editing. Nat. Biotechnol. 2012, 30, 460–465. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of r RNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2017, 73, 5261–5267. [Google Scholar] [CrossRef]
- Kõljalg, U.; Larsson, K.H.; Abarenkov, K.; Nilsson, R.H.; Alexander, I.J.; Eberhardt, U.; Erland, S.; Høiland, K.; Kjøller, R.; Larsson, E.; et al. UNITE: A database providing web-based methods for the molecular identification of ectomycorrhizal fungi. New Phyt. 2005, 166, 1063–1068. [Google Scholar] [CrossRef]
- Lv, X.; Wang, Q.; Zhang, X.; Hao, J.; Li, L.; Chen, W.; Li, H.; Wang, Y.; Ma, C.; Wang, J.; et al. Community structure and associated networks of endophytic bacteria in pea roots throughout plant life cycle. Plant Soil 2021, 468, 225–238. [Google Scholar] [CrossRef]
- Dhariwal, A.; Chong, J.; Habib, S.; King, I.L.; Agellon, L.B.; Xia, J. MicrobiomeAnalyst: A web-based tool for compre-hensive statistical, visual and meta-analysis of microbiome data. Nucleic Acids Res. 2017, 45, W180–W188. [Google Scholar] [CrossRef]
- Barter, R.L.; Yu, B. Superheat: An R package for creating beautiful and extendable heatmaps for visualizing complex data. J. Comput. Graph. Stat. 2018, 27, 910–922. [Google Scholar] [CrossRef]
- Bai, W.; Hu, R.; Zhang, J.; Feng, L.; Xu, H. A study on the isolation, screening and phosphate solubilizing capacity of phosphate solubilizing bacteria in the rhizosphere of corn. J. South China Agric. Univ. 2013, 34, 167–176. [Google Scholar]
- Borah, A.R.; Das, R.; Mazumdar Thakur, D. Culturable endophytic bacteria of Camellia species endowed with plant growth promoting characteristics. J. Appl. Microbiol. 2019, 127, 825–844. [Google Scholar] [CrossRef]
- Etesami, H.; Emami, S.; Alikhani, H.A. Potassium solubilizing bacteria (KSB): Mechanisms, promotion of plant growth, and future prospects A review. J. Soil Sci. Plant Nut. 2017, 17, 897–911. [Google Scholar] [CrossRef]
- Gordon, S.A.; Weber, R.P. Colorimetric estimation of indoleacetic acid. Plant Physiol. 1951, 26, 192–195. [Google Scholar] [CrossRef] [PubMed]
- Penrose, D.M.; Glick, B.R. Methods for isolating and characterizing ACC deaminase-containing plant growth-promoting rhizobacteria. Physiol. Plant. 2003, 118, 10–15. [Google Scholar] [CrossRef]
- Chimwamurombe, P.M.; Grönemeyer, J.L.; Reinhold-Hurek, B. Isolation and characterization of culturable seed-associated bacterial endophytes from gnotobiotically grown Marama bean seedlings. FEMS Microbiol. Ecol. 2016, 92, 1574–6941. [Google Scholar] [CrossRef]
- Jacobson, C.B.; Pasternak, J.J.; Glick, B.R. Partial purification and characterization of 1-aminocyclopropane-1-carboxylate de-aminase from the plant growth promoting rhizobacterium Pseudomonas putida GR12-2. Can. J. Microbiol. 1994, 40, 1019–1025. [Google Scholar] [CrossRef]
- Dai, Z.G. Colorimetric determination of nitrogenase activity in rhizobia. Ningxia Agric. Sci. Technol. 1981, 3, 43–44. [Google Scholar]
- Tariq, M.; Hameed, S.; Yasmeen, T.; Zahid, M.; Zafar, M. Molecular characterization and identification of plant growth promoting endophytic bacteria isolated from the root nodules of pea (Pisum sativum L.). World J. Microb. Biot. 2014, 30, 719–725. [Google Scholar] [CrossRef]
- Wang, X.N.; Luo, J.H.; Hao, J.J.; Zhang, X.Y.; Liu, L.; Fu, L.P.; Wang, J.L.; Han, Y.H.; Liu, Q.L. Plant growth-promoting activities of endophytic nitrogen -fixing bacteria isolated from seedings of faba bean (Vicia faba L.). J. Agric. Sci. 2020, 22, 33–39. [Google Scholar]
- Da Silva, A.C.; Rachid, C.T.C.D.C.; De Jesus, H.E.; Rosado, A.S.; Peixoto, R.S. Predicting the biotechnological potential of bacteria isolated from Antarctic soils, including the rhizosphere of vascular plants. Polar Biol. 2017, 40, 1393–1407. [Google Scholar] [CrossRef]
- Grönemeyer, J.L.; Kulkarni, A.; Berkelmann, D.; Hurek, T.; Reinhold-Hurek, B. Identification and characterization of rhizobia indigenous to the Okavango region in Sub-Saharan Africa. Appl. Environ. Microb. 2014, 80, AEM-02417. [Google Scholar] [CrossRef]
- Drancourt, M.; Bollet, C.; Carlioz, A.; Martelin, R.; Gayra, J.P.; Raoult, D. 16S Ribosomal DNA sequence analysis of a large collection of environmental and clinical unidentifiable bacterial isolates. J. Clin. Microbiol. 2000, 38, 3623–3630. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. 1999, 41, 95–98. [Google Scholar]
- Langenwalder, D.B.; Schmidt, S.; Gilli, U.; Pantchev, N.; Ganter, M.; Silaghi, C.; von Loewenich, F.D. Genetic characterization of Anaplasma phagocytophilum strains from goats (Capra aegagrus hircus) and water buffalo (Bubalus bubalis) by 16S rRNA gene, ankA gene and multilocus sequence typing. Ticks Tick-Borne Dis 2009, 10, 101267. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Tamura, K.; Nei, M.; Kumar, S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc. Natl. Acad. Sci. USA 2004, 101, 11030–11035. [Google Scholar] [CrossRef]
- Kwak, M.J.; Kong, H.G.; Choi, K.; Kwon, S.K.; Song, J.Y.; Lee, J.; Lee, P.A.; Choi, S.Y.; Seo, M.; Lee, H.J.; et al. Rhizosphere microbiome structure alters to enable wilt resistance in tomato. Nat. Biotechnol. 2018, 36, 1117. [Google Scholar] [CrossRef]
- Zuo, X.A.; Zhao, H.L.; Zhao, X.Y.; Guo, Y.R.; Yun, J.Y.; Wang, S.K.; Miyasaka, T.J.E.G. Vegetation pattern variation, soil degradation and their relationship along a grassland desertification gradient in Horqin Sandy Land, northern China. Environ. Geol. 2009, 58, 1227–1237. [Google Scholar] [CrossRef]
- Luo, J.; Tao, Q.; Jupa, R.; Liu, Y.; Li, T. The role of vertical transmission of shoot endophytes in root-associated microbiome assembly and heavy metal hyperaccumulation in sedum alfredii. Environ. Sci. Technol. 2019, 53, 6954–6963. [Google Scholar] [CrossRef]
- Bai, Y.; Müller, D.B.; Srinivas, G.; Garrido-Oter, R.; Potthoff, E.; Rott, M.; Dombrowski, N.; Münch, P.C.; Spaepen, S.; Remus-Emsermann, M.; et al. Functional overlap of the Arabidopsis leaf and root microbiota. Nature 2015, 528, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, C.L.; Voerman, S.E.; Lang, J.M.; Stachowicz, J.J.; Eisen, J.A. Microbial communities in sediment from Zostera marina patches, but not the Z. marina leaf or root microbiomes, vary in relation to distance from patch edge. PeerJ 2017, 5, e3246. [Google Scholar] [CrossRef] [PubMed]
- Wei, N.; Ashman, T.L. The effects of host species and sexual dimorphism differ among root, leaf and flower microbiomes of wild strawberries in situ. Sci. Rep. 2018, 8, 5195. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Liu, P.; Ye, W.X. Illumina-based analysis of endophytic bacterial diversity of tree peony (Paeonia Sect. Moutan) roots and leaves. Braz. J. Microbiol. 2017, 48, 695–705. [Google Scholar] [CrossRef]
- Cai, S.; Zhao, X. Responses of bacterial communities and nitrogen-cycling microbiomes to the conversion from cereals to legumes in the rice-based system. Appl. Soil Ecol. 2024, 195, 105241. [Google Scholar] [CrossRef]
- Adnan, M.; Wahid, F.; Fahad, S.; Arif, M.; Shi, S.M.; He, X.H. Mycorrhizae and soil health. In Improving Soil Health; Burleigh Dodds Science Publishing Limited: Cambridge, UK, 2023; pp. 5–83. [Google Scholar]
- Sasse, J.; Martinoia, E.; Northen, T. Feed your friends: Do plant exudates shape the root microbiome? Trends Plant Sci. 2017, 23, 25–41. [Google Scholar] [CrossRef]
- Degli Esposti, M.; Martinez Romero, E. A survey of the energy metabolism of nodulating symbionts reveals a new form of respiratory complex I. FEMS Microbiol. Ecol. 2016, 92, fiw084. [Google Scholar] [CrossRef]
- Mukhopadhya, I.; Hansen, R.; El-Omar, E.M.; Hold, G.L. IBD—What role do Proteobacteria play? Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 219–230. [Google Scholar] [CrossRef]
- Liu, A.; Contador, C.A.; Fan, K.; Lam, H.M. Interaction and regulation of carbon, nitrogen, and phosphorus metabolisms in root nodules of legumes. Front. Plant Sci. 2018, 9, 426283. [Google Scholar] [CrossRef]
- Kemen, A.C.; Agler, M.T.; Kemen, E. Host–microbe and microbe–microbe interactions in the evolution of obligate plant parasitism. New Phyt. 2015, 206, 1207–1228. [Google Scholar] [CrossRef] [PubMed]
- Truyens, S.; Weyens, N.; Cuypers, A.; Vangronsveld, J. Bacterial seed endophytes: Genera, vertical transmission and interaction with plants. Environ. Microbiol. Rep. 2015, 7, 40–50. [Google Scholar] [CrossRef]
- He, R.; Hu, S.; Li, Q.; Zhao, D.; Wu, Q.L.; Zeng, J. Greater transmission capacities and small-world characteristics of bacterial communities in the above-than those in the below-ground niches of a typical submerged macrophyte, Vallisneria natans. Sci. Total Environ. 2023, 903, 166229. [Google Scholar] [CrossRef] [PubMed]
- Braybrook, S.A.; JöNsson, H. Shifting foundations: The mechanical cell wall and development. Curr. Opin. Plant Biol. 2016, 29, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Moreno, Z.R.; Caballero-Mellado, J.; Coutinho, B.G.; Mendonça-Previato, L.; James, E.K.; Venturi, V. Common features of environmental and potentially beneficial plant-associated Burkholderia. Microb. Ecol. 2012, 63, 249–266. [Google Scholar] [CrossRef]
- İnceoğlu, Ö.; Al-Soud, W.A.; Salles, J.F.; Semenov, A.V.; Elsas, J.D.V. Comparative analysis of bacterial communities in a potato field as determined by pyrosequencing. PLoS ONE 2011, 6, 1932–6203. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Lou, K.; Li, C. Growth promotion effects of the endophyte Acinetobacter johnsonii strain 3-1 on sugar beet. Symbiosis 2011, 54, 159–166. [Google Scholar] [CrossRef]
- Cherif-Silini, H.; Silini, A.; Yahiaoui, B.; Ouzari, I.; Boudabous, A. Phylogenetic and plant-growth-promoting characteristics of Bacillus isolated from the wheat rhizosphere. Ann. Microbiol. 2016, 66, 1087–1097. [Google Scholar] [CrossRef]



| No. | Phylotype | Candidate ID. | Accession Number | Inorganic Phosphorus | Organic Phosphorus | Potassium | Cellulase | Pectinase | IAA | ACC | Nitrogenase Activity |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | LZM1 | Acinetobacter johnsonii | MN709198 | - | + | - | - | - | + | - | + |
| 2 | LZM2 | Acinetobacter johnsonii | MN709199 | + | + | - | - | - | + | + | + |
| 3 | LZM3 | Bacillus australimaris | MN709173 | - | - | - | + | + | + | + | + |
| 4 | LZM4 | Bacillus aerius | MN709174 | - | + | - | + | + | + | + | + |
| 5 | LZM5 | Acinetobacter johnsonii | MN709200 | - | - | - | + | + | + | - | + |
| 6 | LZM6 | Acinetobacter johnsonii | MN709201 | - | + | - | - | - | + | + | + |
| 7 | LZM7 | Acinetobacter johnsonii | MN709202 | + | + | - | + | - | + | - | + |
| 8 | LZM8 | Rahnella sp. | MN709205 | - | + | - | + | - | - | + | + |
| 9 | LZM9 | Bacillus australimaris | MN709182 | - | - | - | + | + | - | + | + |
| 10 | LZM10 | Enterobacter roggenkampii | MN709209 | - | - | - | - | - | + | + | + |
| 11 | LZM11 | Paenibacillus tundrae | MN709193 | - | - | - | + | - | - | + | - |
| 12 | LZM12 | Acinetobacter oryzae | MN709203 | + | - | - | + | + | + | + | + |
| 13 | LZM13 | Paenibacillus tundrae | MN709194 | - | - | - | + | + | + | + | + |
| 14 | LZM14 | Bacillus australimaris | MN709183 | + | + | - | - | - | + | + | + |
| 15 | LZM15 | Pantoea agglomerans | MN709214 | + | + | - | + | + | + | + | + |
| 16 | LZM16 | Bacillus safensis | MN709187 | - | - | - | - | - | - | + | + |
| 17 | LZM17 | Bacillus australimaris | MN709181 | + | - | + | + | + | + | + | + |
| 18 | LZM18 | Acinetobacter lwoffii | MN709204 | - | - | - | - | - | + | + | + |
| 19 | LZM19 | Enterobacter ludwigii | MN709211 | + | - | - | - | - | - | - | + |
| 20 | LZM20 | Bacillus xiamenensis | MN709188 | - | - | - | + | - | + | + | + |
| 21 | LZM21 | Rahnella aquatilis | MN709206 | + | - | + | - | - | + | + | + |
| 22 | LZM22 | Bacillus aerius | MN709179 | - | + | - | - | - | + | + | + |
| 23 | LZM23 | Rahnella aquatilis | MN709207 | + | - | + | + | + | + | + | + |
| 24 | LZM24 | Bacillus australimaris | MN709184 | - | - | - | - | - | + | + | + |
| 25 | LZM25 | Bacillus pumilus | MN709185 | + | + | + | + | + | + | - | + |
| 26 | LZM26 | Pantoea brenneri | MN709213 | - | + | - | + | - | - | + | + |
| 27 | LZM27 | Microbacterium hydrocar | MN709195 | - | - | - | - | + | - | - | + |
| 28 | LZM28 | Paenibacillus taichungensis | MN712330 | + | - | + | + | - | + | + | + |
| 29 | LZM29 | Pantoea agglomerans | MN712331 | + | - | - | + | - | + | + | + |
| 30 | LZM30 | Pantoea agglomerans | MN709215 | + | - | - | - | - | + | + | + |
| 31 | LZM31 | Pantoea agglomerans | MN709216 | - | - | - | + | + | + | + | + |
| 32 | LZM32 | Bacillus pumilus | MN709186 | + | - | - | - | - | + | + | + |
| 33 | RZM1 | Bacillus megaterium | MN709191 | - | - | - | - | - | + | + | + |
| 34 | RZM2 | Enterobacter ludwigii | MN709212 | - | - | - | + | + | - | - | - |
| 35 | RZM3 | Bacillus thuringiensis | MN709190 | + | - | - | - | - | + | + | - |
| 36 | RZM4 | Pseudomonas baetica | MN709197 | - | - | - | - | - | + | - | + |
| 37 | RZM5 | Pantoea vagans | MN709217 | + | - | + | - | - | + | + | + |
| 38 | RZM6 | Bacillus subtilis | MN709178 | - | - | - | + | + | + | + | + |
| 39 | RZM7 | Bacillus halotolerans | MN709176 | - | - | - | + | - | + | - | - |
| 40 | RZM8 | Enterobacter roggenkampii | MN709210 | - | - | - | - | - | - | + | - |
| 41 | RZM9 | Bacillus sp. | MN709177 | + | - | - | + | + | + | + | + |
| 42 | RZM10 | Leifsonia shinshuensis | MN709196 | - | - | - | - | - | - | - | - |
| 43 | RZM11 | Bacillus mojavensis | MN709175 | + | - | - | + | + | + | - | - |
| 44 | RZM12 | Burkholderia sp. | MN712332 | + | - | - | - | - | + | + | - |
| 45 | RZM13 | Bacillus cereus | MN709189 | + | + | - | + | - | + | - | + |
| 46 | RZM14 | Bacillus aerius | MN709180 | + | - | - | - | - | + | + | + |
| 47 | RZM15 | Rahnella aquatilis | MN709208 | + | - | - | - | - | + | + | - |
| 48 | RZM16 | Bacillus megaterium | MN709192 | + | - | + | - | - | - | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, J.; Liu, Q.; Song, F.; Cui, X.; Liu, L.; Fu, L.; Zhang, S.; Wu, X.; Zhang, X. Community Diversity of Endophytic Bacteria in the Leaves and Roots of Pea Seedlings. Agronomy 2024, 14, 2030. https://doi.org/10.3390/agronomy14092030
Hao J, Liu Q, Song F, Cui X, Liu L, Fu L, Zhang S, Wu X, Zhang X. Community Diversity of Endophytic Bacteria in the Leaves and Roots of Pea Seedlings. Agronomy. 2024; 14(9):2030. https://doi.org/10.3390/agronomy14092030
Chicago/Turabian StyleHao, Junjie, Quanlan Liu, Fengjing Song, Xiao Cui, Lu Liu, Liping Fu, Shouan Zhang, Xingbo Wu, and Xiaoyan Zhang. 2024. "Community Diversity of Endophytic Bacteria in the Leaves and Roots of Pea Seedlings" Agronomy 14, no. 9: 2030. https://doi.org/10.3390/agronomy14092030





