Genome-Wide Identification of CAMTA Gene Family in Oat (Avena sativa) and Expression Analysis under Various Abiotic Stresses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Identification of CAMTA Genes
2.2. Gene Structure, Motif Composition, and Chromosomal Localization of the AsCAMTA Gene Family
2.3. Collinearity and Phylogenetic Analysis of CAMTA Genes
2.4. Plant Material and Stress Treatments
2.5. Analysis of Cis-Acting Elements in the AsCAMTA Gene Family
2.6. RNA Isolation, cDNA Synthesis, and Quantitative Real-Time PCR Expression Analyses
3. Results
3.1. Identification of CAMTA Family Numbers in A. sativa
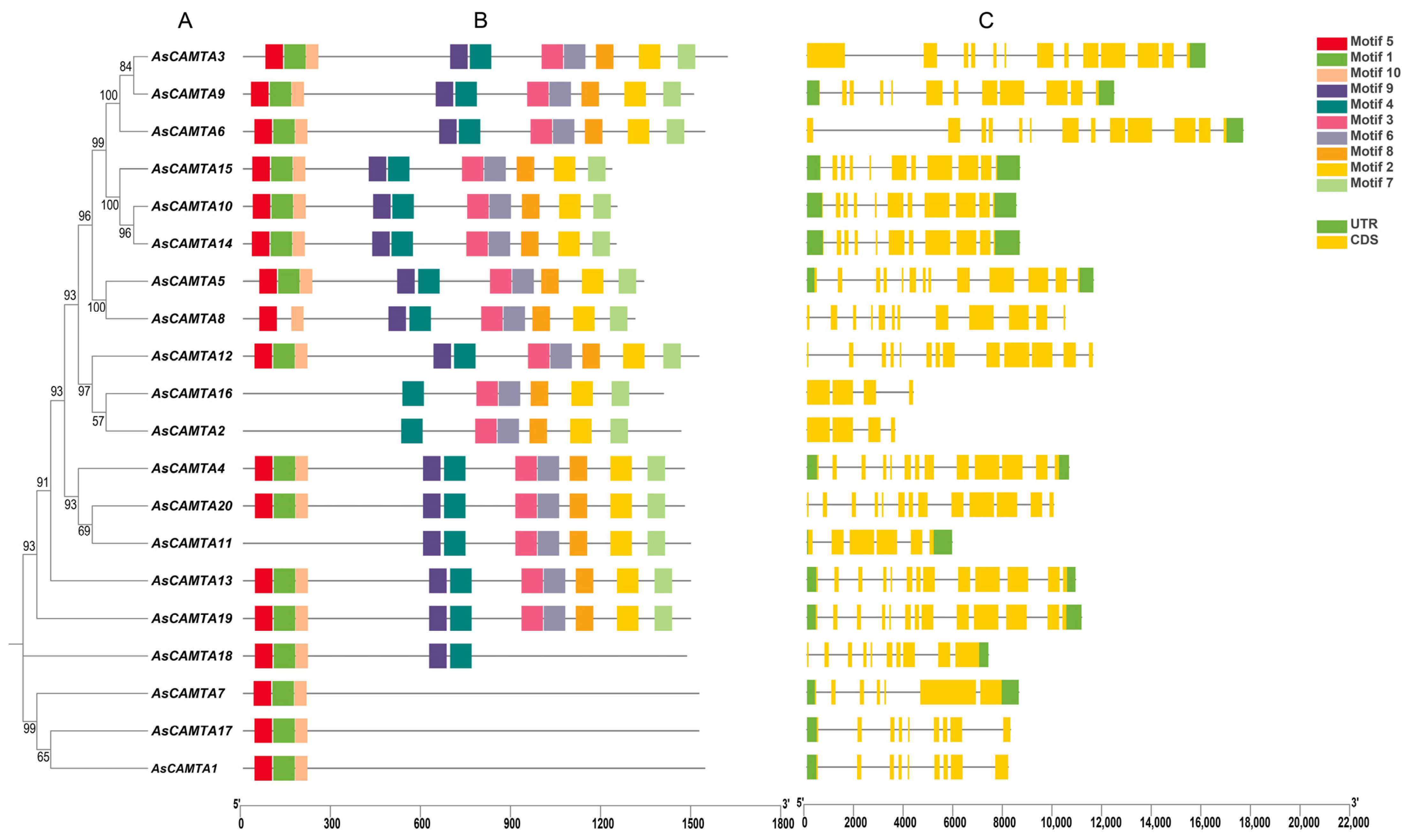
3.2. Gene Structure and Motif Composition of the AsCAMTA Gene Family
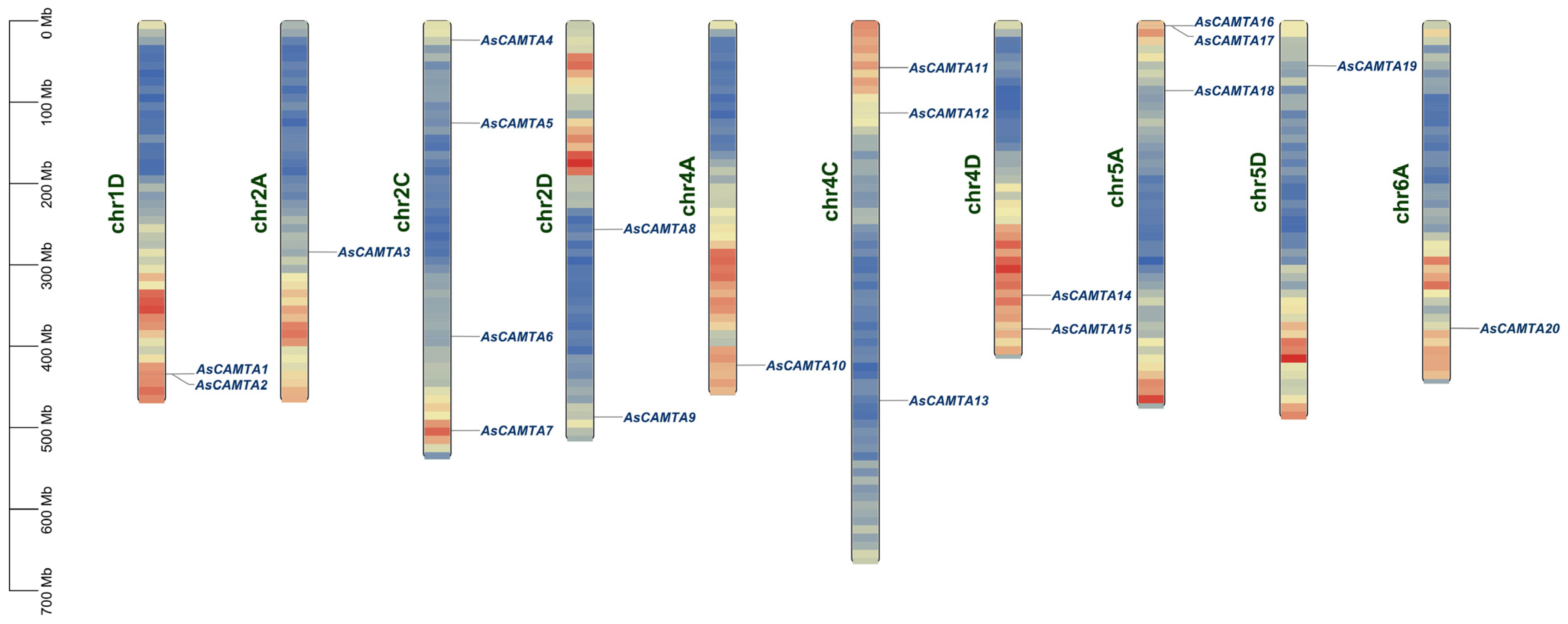
3.3. Chromosomal Location of AsCAMTAs
3.4. Gene Duplication and Collinearity Analysis of CAMTA Genes
3.5. Phylogenetic Analysis of CAMTA Members
3.6. Cis-Acting Element Analysis of AsCAMTA Genes
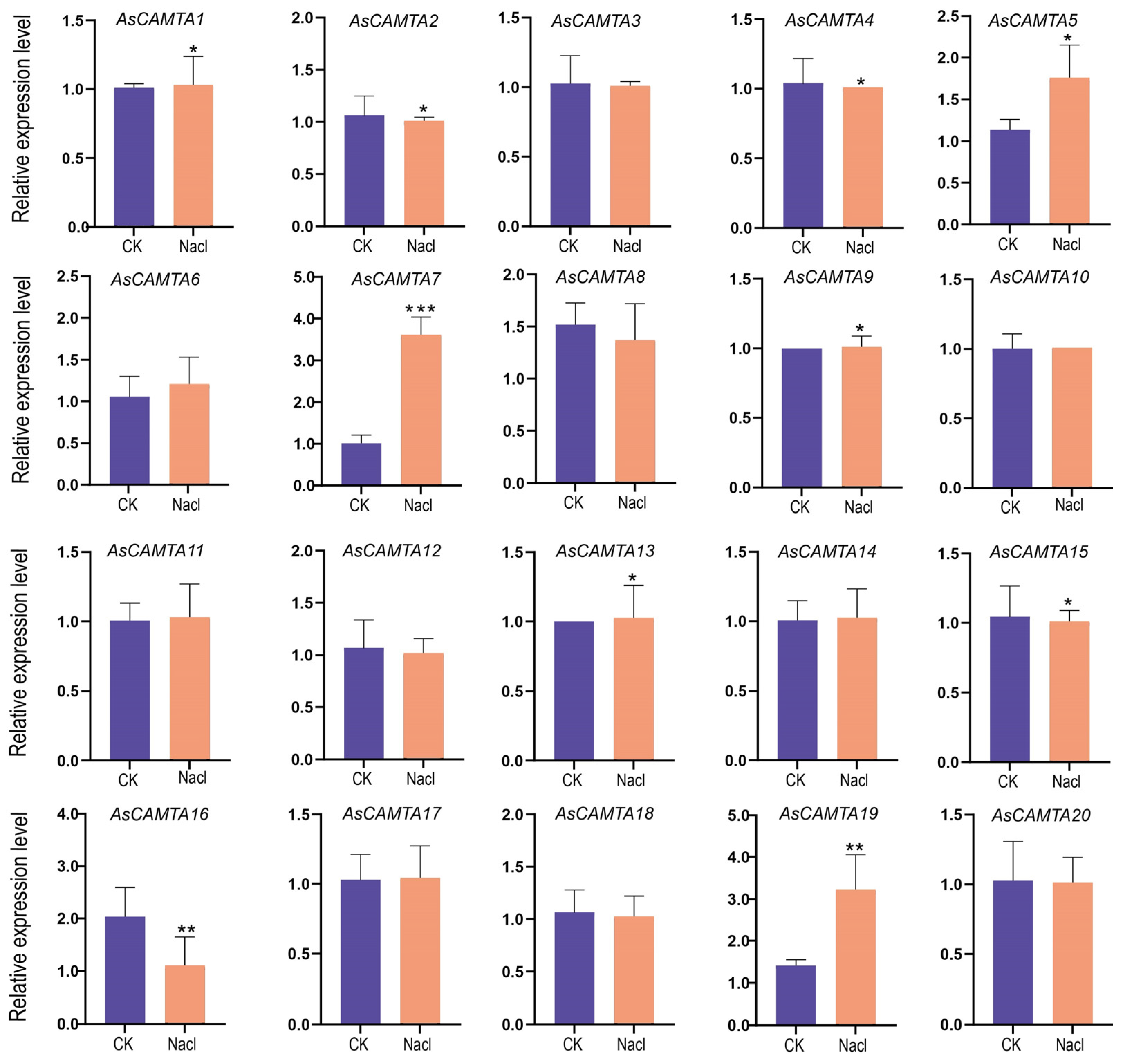
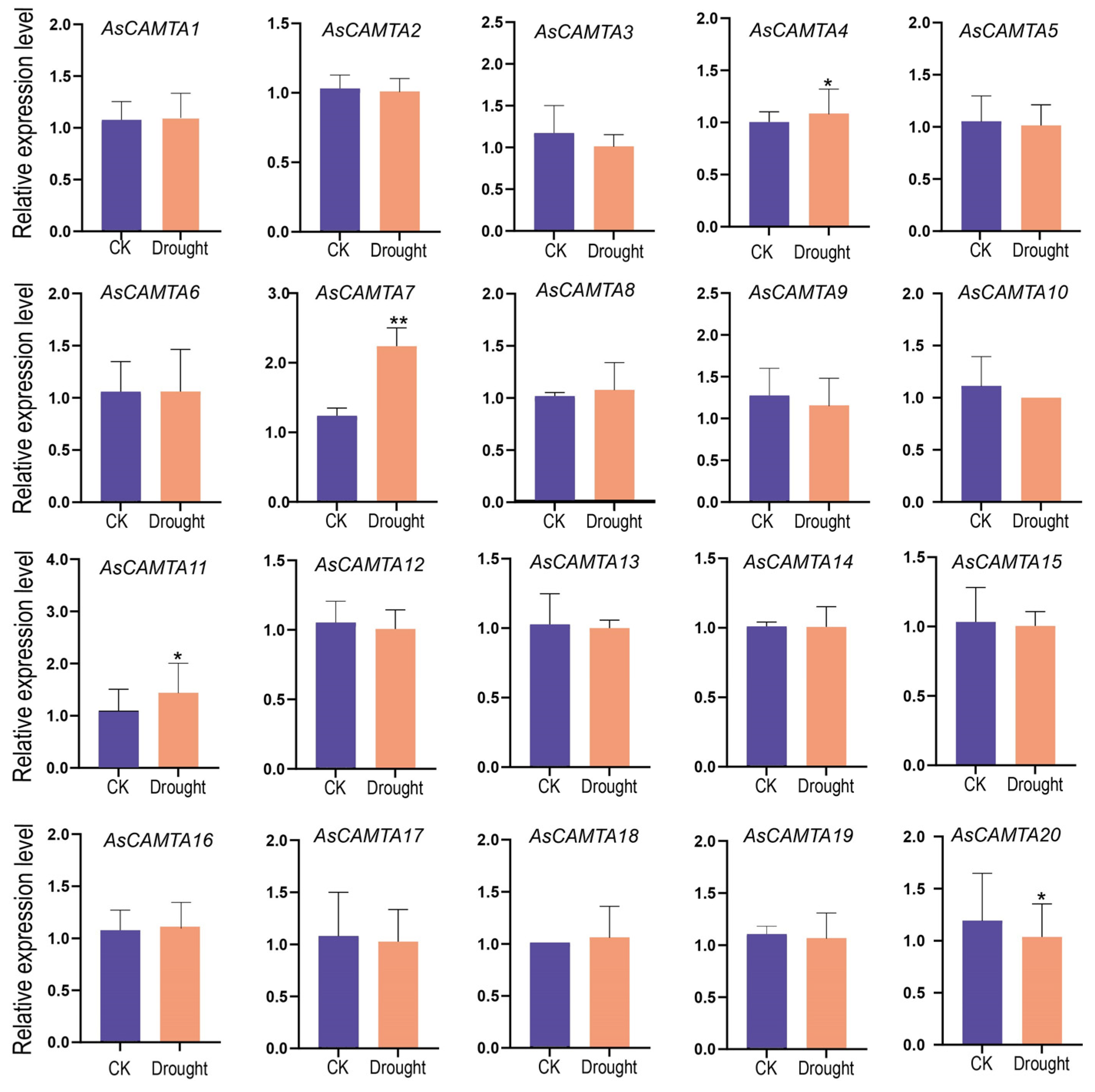
3.7. Expression Analysis of AsCAMTAs in A. sativa under Two Abiotic Stresses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fu, Y.B.; Peterson, G.W.; Williams, D.; Richards, K.W.; Fetch, J.M. Patterns of AFLP variation in a core subset of cultivated hexaploid oat germplasm. Theor. Appl. Genet. 2005, 111, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Doncheva, N.T.; Morris, J.H.; Gorodkin, J.; Jensen, L.J. Cytoscape stringApp: Network analysis and visualization of proteomics data. J. Proteome Res. 2019, 18, 623–632. [Google Scholar] [CrossRef]
- Bräutigam, M.; Lindlöf, A.; Zakhrabekova, S.; Gharti-Chhetri, G.; Olsson, B.; Olsson, O. Generation and analysis of 9792 EST sequences from cold acclimated oat, Avena sativa. BMC Plant Biol. 2005, 5, 18. [Google Scholar] [CrossRef]
- Zhao, D.; Wright, D.L.; Marois, J.J.; Mackowiak, C.L.; Brennan, M. Improved growth and nutrient status of an oat cover crop in sod-based versus conventional peanut-cotton Rotations. Agron. Sustain. Dev. 2010, 30, 497–504. [Google Scholar] [CrossRef]
- Xu, W.; Dai, W.; Yan, H.; Li, S.; Shen, H.; Chen, Y.; Xu, H.; Sun, Y.; He, Z.; Ma, M. Arabidopsis NIP3;1 Plays an important role in arsenic uptake and Root-to-Shoot translocation under arsenite stress conditions. Mol. Plant 2015, 8, 722–733. [Google Scholar] [CrossRef]
- Wu, B.; Munkhtuya, Y.; Li, J.; Hu, Y.; Zhang, Q.; Zhang, Z. Comparative transcriptional profiling and physiological responses of two contrasting oat genotypes under salt stress. Sci. Rep. 2018, 8, 16248. [Google Scholar] [CrossRef] [PubMed]
- Boczkowska, M.; Zebrowski, J.; Nowosielski, J.; Kordulasińska, I.; Nowosielska, D.; Podyma, W. Environmentally-related genotypic, phenotypic and metabolic diversity of oat (Avena sativa L.) landraces based on 67 Polish accessions. Genet. Resour. Crop Ev. 2017, 64, 1829–1840. [Google Scholar] [CrossRef]
- Shi, S.; Nan, L.; Smith, K. The current status, problems, and prospects of Alfalfa (Medicago sativa L.) breeding in China. Agronomy 2017, 7, 1. [Google Scholar] [CrossRef]
- Cui, X.Y.; Du, Y.T.; Fu, J.; Yu, T.F.; Wang, C.T.; Chen, M.; Chen, J.; Ma, Y.Z.; Xu, Z.S. Wheat CBL-interacting protein kinase 23 positively regulates drought stress and ABA responses. BMC Plant Biol. 2018, 18, 93. [Google Scholar] [CrossRef]
- Cheng, S.B.; Yang, X.Z.; Zou, L.; Wu, D.D.; Lu, J.L.; Cheng, Y.R.; Wang, Y.; Zeng, J.; Kang, H.Y.; Sha, L.N.; et al. Comparative physiological and root transcriptome analysis of two annual ryegrass cultivars under drought stress. J. Plant Physiol. 2022, 277, 153807. [Google Scholar] [CrossRef]
- Ling, L.; Li, M.; Chen, N.; Xie, X.; Han, Z.; Ren, G.; Yin, Y.; Jiang, H. Genome-wide identification of NAC gene family and expression analysis under abiotic stresses in Avena sativa. Genes 2023, 14, 1186. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Qiu, Q.; Zou, B.; Wu, Q.; Ye, X.; Wan, Y.; Huang, J.; Wu, X.; Sun, Y.; Yan, H.; et al. Comparative transcriptome and genome analysis unravels the response of tatary buckwheat root to nitrogen deficiency. Plant Physiol. Bioch. 2023, 196, 647–660. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, Z.; Shariq Iqbal, M.; Singh, S.P.; Buaboocha, T. Ca2+/calmodulin complex triggers CAMTA transcriptional machinery under stress in plants: Signaling cascade and molecular regulation. Front. Plant Sci. 2020, 11, 598327. [Google Scholar] [CrossRef]
- Xiao, P.; Feng, J.-W.; Zhu, X.-T.; Gao, J. Evolution analyses of CAMTA transcription factor in plants and its enhancing effect on cold-tolerance. Front. Plant Sci. 2021, 12, 758187. [Google Scholar] [CrossRef]
- Reddy, A.S.N. Calcium: Silver bullet in signaling. Plant Sci. 2001, 160, 381–404. [Google Scholar] [CrossRef]
- Snedden, W.A.; Fromm, H. Calmodulin as a versatile calcium signal transducer in plants. New Phytol. 2001, 151, 35–66. [Google Scholar] [CrossRef]
- Bouché, N.; Yellin, A.; Snedden, W.A.; Fromm, H. Plant-specific calmodulin-binding proteins. Annu. Rev. Plant Biol. 2005, 56, 435–466. [Google Scholar] [CrossRef]
- Yang, T.; Poovaiah, B.W. Calcium/calmodulin-mediated signal network in plants. Trends Plant Sci. 2003, 8, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.S.N.; Ali, G.S.; Celesnik, H.; Day, I.S. Coping with stresses: Roles of calcium- and calcium/calmodulin-regulated gene expression. Plant Cell 2011, 23, 2010–2032. [Google Scholar] [CrossRef]
- Poovaiah, B.W.; Du, L. Calcium signaling: Decoding mechanism of calcium signatures. New Phytol. 2018, 217, 1394–1396. [Google Scholar] [CrossRef]
- Yang, T.; Poovaiah, B.W. An early ethylene up-regulated gene encoding a calmodulin-binding protein pnvolved in plant senescence and death. J. Biol. Chem. 2000, 275, 38467–38473. [Google Scholar] [CrossRef] [PubMed]
- Noman, M.; Aysha, J.; Ketehouli, T.; Yang, J.; Du, L.; Wang, F.; Li, H. Calmodulin binding transcription activators: An interplay between calcium signalling and plant stress tolerance. J. Plant Physiol. 2021, 256, 153327. [Google Scholar] [CrossRef]
- Wang, D.; Wu, X.; Gao, S.; Zhang, S.; Wang, W.; Fang, Z.; Liu, S.; Wang, X.; Zhao, C.; Tang, Y. Systematic analysis and identification of drought-responsive genes of the CAMTA gene family in wheat (Triticum aestivum L.). Int. J. Mol. Sci. 2022, 23, 4542. [Google Scholar] [CrossRef]
- Cunha, S.R.; Mohler, P.J. Ankyrin protein networks in membrane formation and stabilization. J. Cell Mol. Med. 2009, 13, 4364–4376. [Google Scholar] [CrossRef]
- Bouché, N.; Scharlat, A.; Snedden, W.; Bouchez, D.; Fromm, H. A novel family of calmodulin-binding transcription activators in multicellular organisms. J. Biol. Chem. 2002, 277, 21851–21861. [Google Scholar] [CrossRef]
- Kim, Y.; Park, S.; Gilmour, S.J.; Thomashow, M.F. Roles of CAMTA transcription factors and salicylic acid in configuring the low-temperature transcriptome and freezing tolerance of Arabidopsis. Plant J. 2013, 75, 364–376. [Google Scholar] [CrossRef]
- Doherty, C.J.; Van Buskirk, H.A.; Myers, S.J.; Thomashow, M.F. Roles for Arabidopsis CAMTA transcription factors in cold-regulated gene expression and freezing tolerance. Plant Cell 2009, 21, 972–984. [Google Scholar] [CrossRef]
- Pandey, N.; Ranjan, A.; Pant, P.; Tripathi, R.K.; Ateek, F.; Pandey, H.P.; Patre, U.V.; Sawant, S.V. CAMTA 1 regulates drought responses in Arabidopsis thaliana. BMC Genom. 2013, 14, 216. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Ali, G.S.; Simons, K.A.; Hou, J.; Yang, T.; Reddy, A.S.N.; Poovaiah, B.W. Ca2+/calmodulin regulates salicylic-acid-mediated plant immunity. Nature 2009, 457, 1154–1158. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wei, F.; Zhou, H.; Liu, N.; Niu, X.; Yan, C.; Zhang, L.; Han, S.; Hou, C.; Wang, D. TaCAMTA4, a calmodulin-interacting protein, involved in defense response of wheat to Puccinia triticina. Sci. Rep. 2019, 9, 641. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Chou, K.-C.; Shen, H.-B. Cell-PLoc 2.0: An improved package of web-servers for predicting subcellular localization of proteins in various organisms. Nat. Sci. 2010, 2, 1090–1103. [Google Scholar] [CrossRef]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; DeBarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef]
- Wan, H.; Zhao, Z.; Qian, C.; Sui, Y.; Malik, A.A.; Chen, J. Selection of appropriate reference genes for gene expression studies by quantitative real-time polymerase chain reaction in cucumber. Anal. Biochem. 2010, 399, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Gamage, D.G.; Gunaratne, A.; Periyannan, G.R.; Russell, T.G. Applicability of instability index for in vitro protein stability prediction. Protein Pept. Lett. 2019, 26, 339–347. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhao, M.; Xing, F.; Mao, G.; Wang, Y.; Dai, Y.; Niu, M.; Yuan, H. Identification and expression analysis of CAMTA genes in tea plant reveal their complex regulatory role in stress responses. Front. Plant Sci. 2022, 13, 910768. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Li, Z.; Cao, X.; Duan, W.; Wei, C.; Zhang, C.; Jiang, D.; Li, M.; Chen, K.; Qiao, Y.; et al. Genome-wide analysis of calmodulin binding transcription activator (CAMTA) gene family in peach (Prunus persica L. Batsch) and ectopic expression of PpCAMTA1 in Arabidopsis camta2,3 mutant restore plant development. Int. J. Mol. Sci. 2022, 23, 10500. [Google Scholar] [CrossRef]
- Yang, F.; Dong, F.; Hu, F.; Liu, Y.; Chai, J.; Zhao, H.; Lv, M.; Zhou, S. Genome-wide identification and expression analysis of the calmodulin-binding transcription activator (CAMTA) gene family in wheat (Triticum aestivum L.). BMC Genet. 2020, 21, 105. [Google Scholar] [CrossRef]
- Rahman, H.; Xu, Y.P.; Zhang, X.R.; Cai, X.Z. Brassica napus genome possesses extraordinary high number of CAMTA genes and CAMTA3 contributes to PAMP triggered immunity and resistance to Sclerotinia sclerotiorum. Front. Plant Sci. 2016, 7, 581. [Google Scholar] [CrossRef]
- Kamal, N.; Tsardakas Renhuldt, N.; Bentzer, J.; Gundlach, H.; Haberer, G.; Juhász, A.; Lux, T.; Bose, U.; Tye-Din, J.A.; Lang, D.; et al. The Mosaic oat genome gives insights into a uniquely healthy cereal crop. Nature 2022, 606, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Yan, H.; Guo, L.; Deng, C.; Wang, C.; Wang, Y.; Kang, L.; Zhou, P.; Yu, K.; Dong, X.; et al. Reference genome assemblies reveal the origin and evolution of allohexaploid oat. Nat. Genet. 2022, 54, 1248–1258. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Shi, F.; Fukuda, K.; Yang, Y. Effects of salt and alkali stresses on germination, growth, photosynthesis and ion accumulation in alfalfa (Medicago sativa L.). Soil Sci. Plant Nutr. 2010, 56, 725–733. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, H.; Song, C.; Zhu, J.-K.; Shabala, S. Mechanisms of plant responses and adaptation to soil salinity. Ther. Innov. Regul. Sci. 2020, 1, 100017. [Google Scholar] [CrossRef]
- Zhu, T.; Wu, S.; Zhang, D.; Li, Z.; Xie, K.; An, X.; Ma, B.; Hou, Q.; Dong, Z.; Tian, Y.; et al. Genome-wide analysis of maize GPAT gene family and cytological characterization and breeding application of ZmMs33/ZmGPAT6 gene. Theor. Appl. Genet. 2019, 132, 2137–2154. [Google Scholar] [CrossRef]



| Gene Name | Gene ID | Length | MW | pI | Instability Index | GRAVY | Predicted Subcellular |
|---|---|---|---|---|---|---|---|
| CAMTA1 | AVESA.00010b.r2.1DG0131940 | 457 | 1,174,825.4 | 6.06 | 54.64 | −0.595 | Cytoplasm Nucleus Mitochondrion |
| CAMTA2 | AVESA.00010b.r2.1DG0131930 | 559 | 62,493.43 | 6.06 | 44.32 | −0.509 | Cytoplasm Nucleus |
| CAMTA3 | AVESA.00010b.r2.2AG0218090 | 1506 | 166,980.9 | 6.47 | 53.2 | −0.572 | Cytoplasm Nucleus |
| CAMTA4 | AVESA.00010b.r2.2CG0266790 | 1006 | 112,373 | 5.68 | 44.1 | −0.542 | Nucleus |
| CAMTA5 | AVESA.00010b.r2.2CG0277250 | 913 | 103,085.4 | 8.46 | 43.26 | −0.516 | Nucleus |
| CAMTA6 | AVESA.00010b.r2.2CG0297270 | 1194 | 132,918.5 | 5.72 | 50.97 | −0.53 | Nucleus |
| CAMTA7 | AVESA.00010b.r2.2CG0318680 | 858 | 98,133.49 | 6.45 | 44.36 | −0.571 | Nucleus |
| CAMTA8 | AVESA.00010b.r2.2DG0348770 | 893 | 100,442.5 | 6.77 | 44.31 | −0.444 | Cytoplasm Nucleus |
| CAMTA9 | AVESA.00010b.r2.2DG0332150 | 1027 | 114,492.8 | 5.49 | 49.47 | −0.527 | Cytoplasm Nucleus |
| CAMTA10 | AVESA.00010b.r2.4AG0644340 | 852 | 95,420.96 | 6.34 | 38.66 | −0.436 | Nucleus |
| CAMTA11 | AVESA.00010b.r2.4CG1266220 | 724 | 80,523.41 | 5.68 | 37.48 | −0.492 | Nucleus |
| CAMTA12 | AVESA.00010b.r2.4CG1279770 | 1039 | 116,689.5 | 5.68 | 50.36 | −0.551 | Nucleus |
| CAMTA13 | AVESA.00010b.r2.4CG1310880 | 1020 | 114,842.6 | 5.86 | 43.29 | −0.532 | Nucleus |
| CAMTA14 | AVESA.00010b.r2.4DG0771960 | 850 | 95,445.74 | 5.98 | 35.35 | −0.46 | Nucleus |
| CAMTA15 | AVESA.00010b.r2.4DG0783850 | 840 | 94,207.61 | 6.34 | 38.34 | −0.447 | Nucleus |
| CAMTA16 | AVESA.00010b.r2.5AG0794750 | 559 | 62,553.59 | 6.16 | 42.6 | −0.499 | Cytoplasm Nucleus |
| CAMTA17 | AVESA.00010b.r2.5AG0794760 | 403 | 62,247.36 | 5.68 | 61.97 | −0.675 | Nucleus |
| CAMTA18 | AVESA.00010b.r2.5AG0810750 | 674 | 76,305.21 | 5.36 | 45.51 | −0.519 | Nucleus |
| CAMTA19 | AVESA.00010b.r2.5DG0997260 | 1020 | 11,4890.9 | 6.04 | 43.69 | −0.522 | Nucleus |
| CAMTA20 | AVESA.00010b.r2.6AG1057720 | 1006 | 11,2506.2 | 5.61 | 44.81 | −0.544 | Nucleus |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Li, J.; Yao, M.; Chen, S. Genome-Wide Identification of CAMTA Gene Family in Oat (Avena sativa) and Expression Analysis under Various Abiotic Stresses. Agronomy 2024, 14, 2053. https://doi.org/10.3390/agronomy14092053
Yang Y, Li J, Yao M, Chen S. Genome-Wide Identification of CAMTA Gene Family in Oat (Avena sativa) and Expression Analysis under Various Abiotic Stresses. Agronomy. 2024; 14(9):2053. https://doi.org/10.3390/agronomy14092053
Chicago/Turabian StyleYang, Yanjiao, Jin Li, Mingjiu Yao, and Shiyong Chen. 2024. "Genome-Wide Identification of CAMTA Gene Family in Oat (Avena sativa) and Expression Analysis under Various Abiotic Stresses" Agronomy 14, no. 9: 2053. https://doi.org/10.3390/agronomy14092053
APA StyleYang, Y., Li, J., Yao, M., & Chen, S. (2024). Genome-Wide Identification of CAMTA Gene Family in Oat (Avena sativa) and Expression Analysis under Various Abiotic Stresses. Agronomy, 14(9), 2053. https://doi.org/10.3390/agronomy14092053




