Abstract
The transformation of spent coffee grounds (SCGs) into hydrochars has been extensively studied in recent years to explore their potential in biofortifying foods and mitigating the plant toxicity associated with SCGs. This study aimed to evaluate the effects of adding activated (ASCG and AH160) and functionalized SCGs, as well as SCG-derived hydrochars (ASCG-Fe and AH160-Fe), on cucumber production and plant iron content. To achieve this, SCGs and SCG-derived hydrochars activated and functionalized with Fe were incorporated into cucumber crops grown in a greenhouse over multiple harvests. Among the treatments, SCG-Fe proved to be the most promising for cucumber production, yielding an average of 25 kg of cumulative production per treatment across three harvests. Regarding iron content, the average results across all harvests showed that SCGs and functionalized SCG-hydrochars matched the performance of the commercial chelate (0.108 vs. 0.11 mg Fe/100 g fresh weight). However, in subsequent harvests, iron appeared to leach out, with the activated bio-products (ASCG and AH160) leaving the highest iron reserves in the soil. Additionally, the hydrochar activated at 160 °C demonstrated the highest utilization efficiency. In conclusion, the incorporation of SCG residues and second-generation residues (hydrochars) shows promise as agents for biofortifying cucumbers.
1. Introduction
Cucumber (Cucumis sativus L.), a member of the Cucurbitaceae family, is predominantly cultivated under greenhouse conditions [1]. Asia accounts for 90.1% of the world’s cucumber production, while Europe contributes 6% of the global output. In Spain, 8010 hectares are dedicated to cucumber cultivation, yielding 769,970 tons annually [2]. Various cultivation methods exist for cucumbers, with greenhouse production being particularly effective. Notably, the addition of organic amendments has been shown to boost yields. For example, Sallam et al. [3] reported a 74.6% increase in cucumber production in a greenhouse trial when organic amendments were combined with inorganic fertilizers.
In the context of organic amendments, agronomic biofortification is a process aimed at enhancing the levels of essential nutrients in crops, which helps address malnutrition in populations that depend on these crops as their primary food source [4]. This approach can increase the mineral content within the edible parts of crops, thereby improving the nutritional value of the food produced [5]. Additionally, micronutrient fertilization can positively impact other nutritional aspects of crops, including protein levels, amino acids, phenolic compounds, chlorophyll, carotenoids, and essential oils. Among the elements commonly used in agronomic biofortification, iron (Fe) is particularly important due to its critical role in both human and plant health [6].
Cucumber (Cucumis sativus) has long been utilized to study responses to iron deficiency, making it an ideal crop for testing the efficacy of organic amendments as bio-chelates in a greenhouse setting. Iron deficiency is a prevalent nutritional issue in many crop plants, leading to chlorosis, reduced yields, and diminished nutritional quality [7]. Improving the availability of iron in staple crops is a key strategy for combating iron deficiency in human populations. According to the World Health Organization (WHO), insufficient levels of micronutrients like iron and zinc present a significant threat to global health and development, with an estimated two billion people suffering from anemia, primarily due to iron deficiency [8].
The research group led by Cervera-Mata et al. [9] began investigating the use of spent coffee grounds (SCGs) as organic soil amendments. They studied the effects of this organic residue on the chemical and physicochemical properties of soil and its impact on lettuce growth in in vitro trials [9]. Similar experiments were conducted by other researchers [9,10,11,12,13,14]. The Cervera-Mata group also explored SCGs’ influence on soil physical properties, biological factors, organic matter content, and soil hydrophobicity [15]. Regarding plant growth, SCGs were found to have a phytotoxic effect [9,16], leading to further research that included nitrogen supplementation to assess its impact on the competition between soil microorganisms and plant roots for this essential macronutrient, as reviewed in [15]. Additionally, SCGs were functionalized with iron (Fe) and zinc (Zn) to investigate the biofortification potential of these bio-chelates [14]. However, all these experiments were conducted with lettuce in in vitro trials within a growth chamber, which differs significantly from a real production environment, such as greenhouses.
As mentioned previously, there has been a growing focus in recent years on converting spent coffee grounds (SCGs) into sustainable by-products through chemical, thermal, and biological processes [10]. Among these processes, the thermochemical conversion of SCGs has gained attention, transforming this waste into products like biochar and hydrochar [17]. Biochar is produced through slow/fast pyrolysis, torrefaction, or gasification in the absence of oxygen [18], while hydrochar is generated via hydrothermal carbonization (HTC), a process that occurs in water at moderate temperatures (160–250 °C) [19]. Once transformed or modified physically, biologically, or chemically, these by-products have been utilized for various applications, including water filtration, catalyst support, fermentation, soil conditioning, and carbon sequestration. Specifically, for hydrochars, studies by Afolabi et al. [20] and Kim et al. [21] reported that the hydrochar derived from SCGs is characterized by higher carbon and fixed carbon content due to dehydration and decarboxylation reactions. SCG-hydrochars have been applied to soil with various objectives [22,23]. Efficient utilization of SCGs could significantly reduce the carbon footprint associated with this waste by preventing its incineration or accumulation in landfills. Cervera-Mata et al. [24] characterized SCGs and SCG-hydrochars and found that these kinds of by-products had low contents of fixed C and higher levels of water-soluble C and N and volatile matter. On the other hand, Cervera-Mata et al. [22] reported that hydrochars contain high levels of polyphenols with respect to SCGs (186 vs. 77 mg GAE/g). This is the reason why hydrochars are more phytotoxic to the plant than the SCGs themselves [22,23]. The latest reported a stronger with high HTC temperatures (hydrochars at 200 °C).
Lara-Ramos et al. [23] investigated the application of SCGs and SCG-hydrochars at different temperatures (160 °C, 180 °C, and 200 °C) in a Mediterranean agricultural soil cultivated with lettuce. These by-products were previously activated and functionalized with iron. The study was conducted in an in vitro pot experiment with lettuce. The key findings were that all by-products or bio-chelates significantly increased the iron content in lettuce by 41% to 150% compared to the control.
Taking all these considerations into account, this study aims to demonstrate, through a greenhouse trial, that spent coffee grounds (SCGs) and SCG-derived hydrochar—both activated and functionalized—can effectively increase iron content in crops, building on previous studies that focused only on lettuce leaves. The potential for bio-chelates derived from SCGs to serve as alternatives to commercial chelates in agriculture is thereby explored. The primary value of this work lies in successfully transferring the promising results obtained from in vitro trials to a real greenhouse production system. This scaling process presents two major challenges: the first involves developing a method to manufacture activated bio-products on a semi-industrial scale, transitioning from a purely laboratory setting. The second challenge is the application of this bio-product at the field level, moving from a controlled pot system to a more complex greenhouse soil system. The significance of this article is in addressing these two critical issues. In summary, the study focuses on repurposing a waste product—unsuitable for large-scale use as an organic amendment due to its high phytotoxicity—into a functional product that acts as a carrier of essential elements, with the ability to scale its application to the greenhouse level.
2. Materials and Methods
2.1. SCG-Derived Bioproducts
Spent coffee grounds were obtained from the canteen of the School of Pharmacy (University of Granada). In order to decrease moisture levels, SCGs were spread into a thin layer over a large area and dried at 18–21 °C.
The methodology of Lara-Ramos et al. [23] (HTC process) was used to obtain SCG hydrochar at 160 °C (H160), and the later activated bio-products (AH160, ASCG), as well as the Fe bio-chelates (ASCG-Fe, AH160-Fe). Thirty grams of SCGs were mixed with deionized water at a solid–water ratio of 1:10 (w/w) in a 1 L reactor (Highpreactor™ BR-300, Berghof Ltd., Eningen, Germany) and hydrolyzed at 160 °C for 1 h at 300 rpm. After reactor cooling, vacuum filtration was used to recover hydrochars, which were then dried overnight at 60 °C. To activate SCGs and hydrochar, 40 g of the SCGs or hydrochars were suspended in 400 mL of NaOH 0.1 M and heated at 40 °C for one hour with stirring at 450 rpm. After cooling, the activated samples’ pH was adjusted to approximately 7 using 1 N HCl. The mixture was centrifuged at 3000 rpm, decanted, and solid residues were dried at 60 °C for 48 h in an oven, before being stored.
Iron Bio-chelates (ASCG-Fe and AH160-Fe) from activated SCG (ASCG) and hydrochars (AH160) were obtained by functionalization with Fe with the modified procedure by Morikawa and Saigusa [24]. Iron (II) sulfate heptahydrate (FeSO4 • 7H2O, MW 278.01) from Merck (Darmstadt, Germany) was used. For each sample, 40 g of bio-product, 10 g of the reagent, and 400 mL of deionized water were mixed and stored at room temperature in the dark with constant stirring for 24 h at 120 rpm. The resulting mixtures were centrifuged (2500 rpm, 20 °C, 10 min), decanted, and washed three times with 250 mL of deionized water using the same centrifugation method. The functionalized products were dried in an oven at 50 °C for 24 h and stored. The Fe content of the bio-chelates was determined by mineralizing 0.2 g of the bio-product with HNO3 (65%) and H2O2 at 185 °C for 20 min in a microwave digester (Multiwave 5000 with Rotor 24HVT50, Anton Parr GmbH, Graz, Austria). The analysis of the extracts of mineral elements was carried out with and ICP-MS/MS Agilent 8900 (Agilent Technologies Inc., Santa Clara, CA, USA).
2.2. Greenhouse Experiment
2.2.1. Greenhouse Conditions
The cultivation of cucumber was carried out in a greenhouse at the experimental station of Fundación Cajamar located in Almería, Spain (36°47′23″ N, 2°43′13″ W; 155 m a.s.l.) between October 2022–January 2023. The greenhouse consisted of a roof-end structure with the main axis of the house running west–east. The greenhouse cover consisted of a conventional three-layer plastic with anti-drip properties and a thickness of 200 μm. The experimental station is situated in place with a subtropical Mediterranean semi-arid climate (minimum and maximum temperatures in that period of 12.2 °C and 16.1 °C, average annual precipitation is 220.5 mm). The plot had an area of 255 m2 (30 m long × 8.5 m wide).
The soil of that plot was characterized by the staff of Fundación Cajamar, including the following properties: clay 12.64%, silt 17.57%, sand 69.79%, electrical conductivity 2.05 dS/m, pH 7.4, organic carbon 1.35%, carbonates as CaCO3 29.2%, total N 0.17%, C/N 8.06, soil NO3-N 46.05 mg/kg, available P 318.1 mg/kg. A dose of 0.2% was used for the four bio-products (ASCG, ASCG-Fe, AH160, AH160-Fe). The controls used were the commercial Fe chelate (concentration of 10 mg Fe /kg soil) and the soil without any bio-product. The dose of commercial chelate was calculated in order to obtain a final concentration of Fe of 10 mg/kg soil). Iron ethylenediamine-N,N′-bis (EDDHA-Fe, 6% w:w) was the commercial chelate supplied by Trade Corporation International, S.A.U. (Madrid, Spain) The experiment included six treatments as detailed in Table 1. A split-plots design was applied, including one replicate per treatment (4 plants) randomly distributed in each plot. Thus, each treatment included 16 plants (n = 16).

Table 1.
Description of the six treatments used in the greenhouse trial.
The distribution of planting points was as follows: 0.5 m between plants within each crop line and 1.5 m between crop lines (1.33 plants/m2 planting density). Bio-products were placed, for each planting point, in a hole 15 cm × 20 cm deep, equivalent to 4 kg of soil. Soils from those holes of the same treatment were mixed with their corresponding bio-products in a concrete mixer for 5 min. Then, holes were re-filled individually with the bio-product–soil mix. The same soil treatment was used for the holes of controls. In the case of the commercial chelate, it was dissolved in distilled water and spilled in the surface.
Acrena S.A.T 251 (Almería, Spain) supplied 21-day-old cucumber seedlings (Cucumis sativus L. var. ‘Almería’ cv. ‘Huracán’). Each planting point included one seedling planted after 6 days of soil preparation at 8 cm soil depth.
2.2.2. Maintenance of Crops
The trial was managed in an organic way, so that authorized fertilizers for organic cultivation were applied by drip irrigation. A polyethylene pipe was used to irrigate each row of cucumbers at a rate of 3 L/hm2, with emitters spaced at 50 cm. The cultivation was irrigated after 15 days post-transplant with a nutrient solution (5.56 mM K, 10 mM N, and 1.41 mM/P from an organic fertilizer with 6.5% K2O, 3.5% N, and 2.5% P2O5 richness diluted to a 3.2‰ concentration). The levels of Ca, Na, K, and nitrate were checked biweekly in the extracted soil solution by using suction probes to sustain nitrate levels ranging 3–10 mM and a K/Ca ratio 0.5–1. Fast analysis ionometers (LAQUAtwin, Horiba, Japan) were used to measure such concentrations.
2.2.3. Cucumber Sampling and Processing
Cucumbers were harvested three times. Harvest 1 (H1), 75 days after planting, when all individuals reached uniform maturity; then, harvest 2 and 3 (H2, H3), at intervals of 15 days. Cucumbers were cleaned, weighed (fresh weight), chopped, and dried for 48 h at room temperature in aluminum trays to allow water loss; then, cucumbers were totally dried at 50 °C for 72 h in an oven and re-weighed (dry weight). Dried cucumbers were milled and stored. The mineralization of cucumbers and subsequent analysis of mineral elements was performed with the same procedures used for the bio-chelates.
2.3. Iron Determination (in Bio-Products and Cucumbers)
In order to analyze Fe levels in the samples, 200 mg of sample were weighed in a precision balance (Ohaus, model PA224C, Europe GmbH, Greifensee, Switzerland) within borosilicate tubes. Then, 3 mL of 69% HNO3 (TraceSELECT, Merck; Darmstadt, Germany) and 3 mL of HNO3 11.5% solution were added. The borosilicate tubes were inserted into Teflon digestion vessels, placed in the microwave digester rotor, and mineralized (temperature range: 150–180 °C, 30 min).
The samples we diluted after mineralization with Milli-Q water to 50 mL, obtaining an analytical solution with a concentration of 4.14% HNO3. The analysis of iron was carried out with a calibration curve prepared from an Fe standard solution (1000 mg/L in HNO3 at 1%; Merck; Darmstadt, Germany). Serial dilutions (1000, 500, 50, 25, and 10 ng/L) from the stock solution (100 mg/L) were used to prepare different points of the linear calibration curve. The “Internal Standard Kit (Ge, Ir, Rh, Sc; ISC Science, Gijón, Spain, batch 20210712)”, was used to correct the counts per second of Fe (54Fe analyzed mass). Analyses were carried out in triplicate for each sample. The iron contents of the bio-products were (mg/kg): AH160: 60.2 ± 3.2; ASCG: 59.3 ± 1.2; AH160-Fe: 13,103.4 ± 316.1; ASCG-Fe: 12,082.5 ± 128.9.
2.4. Efficiency Parameters
The efficiency of iron utilization (UE) was calculated in cucumbers with respect to the total amount of Fe added to soil [25] as follows:
UE (%) = (Uptake in treatment − Uptake in control)/(Fe added) × 100
2.5. Statistical Analysis
The Tukey test was used to assess statistical differences among treatments through one-way analysis of variance (ANOVA). Ninety-five percent (p < 0.05) was set as the significance level. The statistical analysis was performed with the SPSS version 26.0 for Windows (IBM SPSS Inc., New York, NY, USA).
3. Results
3.1. Total Cumulative Production
The total cumulative cucumber production in the greenhouse trial was approximately 1.7 kg/m2 (132 kg total), with the greenhouse operators reporting an average production of around 2 kg/m2. Figure 1 illustrates the cumulative cucumber production per treatment during the trial. Notably, the cucumbers with the highest production were those from the control treatment, which did not include any bio-product additions. It is important to highlight that the cucumbers grown with the commercial chelate (control-Fe) exhibited the same phytotoxic effects as those treated with activated SCGs.
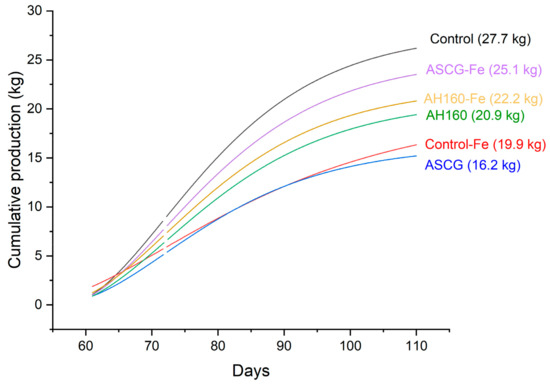
Figure 1.
Cumulative production of cucumbers during the trial per treatment. AH160: activated hydrochar at 160 °C; AH160-Fe: activated and functionalized hydrochar at 160 °C; ASCG: activated spent coffee grounds; ASCG-Fe: activated and functionalized spent coffee grounds.
Among the bio-products tested, the addition of SCG-A resulted in a decrease in total cucumber production. Similarly, both activated and functionalized hydrochars showed a comparable trend, with the best results for cucumber growth observed with the addition of ASCG-Fe. Although there was a decrease in production, it was not as pronounced as in other experiments involving bio-products derived from SCGs, suggesting that these treatments could still be viable for real agricultural use, as will be discussed later. In this study, the application of activated and functionalized residues at sub-toxic doses (0.2%) was tested, and the results were found to be better than those of the control-Fe treatment, although still lower than the control sample. Further research is needed to optimize the use of these bio-products in greenhouse trials.
3.2. Iron Biofortification
3.2.1. Average per Treatment
Figure 2 illustrates the distribution of iron (Fe) content across the different treatments applied to cucumbers, based on the average of the three harvests. The treatments that significantly increased Fe content in cucumbers were control-Fe, ASCG-Fe, and AH160-Fe, all of which were statistically significantly higher (p < 0.05) compared to the other treatments. This outcome is highly positive, as it demonstrates that adding activated and functionalized bio-products can achieve an iron content in the plant comparable to that obtained with the commercial iron chelate (Control-Fe). Additionally, the treatments with ASCG-Fe and AH160-Fe resulted in Fe content increases of 22% and 29%, respectively, compared to the control sample.
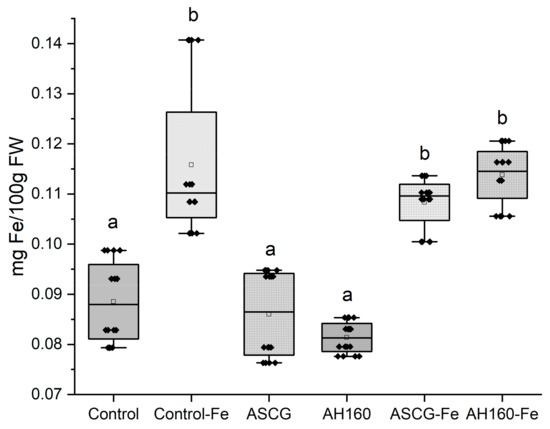
Figure 2.
Average Fe content in cucumbers per treatment. AH160: activated hydrochar at 160 °C; AH160-Fe: activated and functionalized hydrochar at 160 °C; ASCG: activated spent coffee grounds; ASCG-Fe: activated and functionalized spent coffee grounds. Different letters indicated statistically significant differences (p < 0.05).
According to the equation provided in Section 2, the utilization efficiency (UE) was calculated. This parameter measures the amount of iron (Fe) that the plant utilizes relative to the amount of Fe added. The UE values for the different treatments were as follows: ASCG (−2.394) < ASCG-Fe (0.094) < AH160-Fe (0.110) < Control-Fe (0.273) < AH160 (13.883). The most notable result from these data is the significantly higher UE value achieved with AH160 compared to the control-Fe. This indicates that plants appear to have a greater capacity to utilize Fe from the AH160 bio-product, even though the total Fe content in the cucumbers was not significantly higher.
3.2.2. Per Harvest
Figure 3 shows the distribution of iron content by both treatment and harvest.
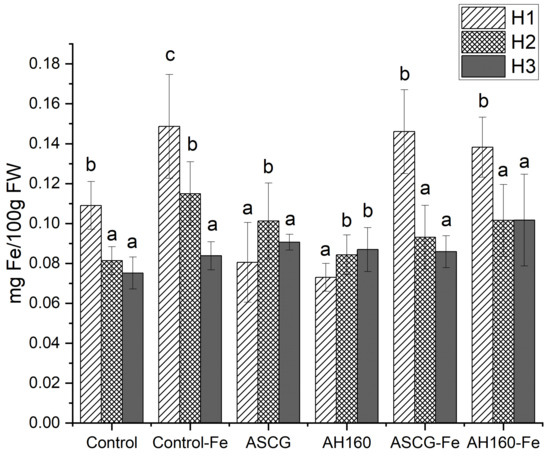
Figure 3.
Fe content in cucumbers per treatment, per harvest. AH160: activated hydrochar at 160 °C; AH160-Fe: activated and functionalized hydrochar at 160 °C; ASCG: activated spent coffee grounds; ASCG-Fe: activated and functionalized spent coffee grounds. Different letters indicated statistically significant differences between different harvests (p < 0.05).
In the first harvest, cucumbers with the highest iron content were those grown with the commercial chelate (control-Fe), followed by ASCG-Fe and AH160-Fe, which was an expected outcome. However, non-functionalized bio-products did not have a noticeable effect on iron mobilization in this initial harvest.
In the second harvest, an important trend emerged: the previously mobilized iron appeared to leach from the soil, leading to a decrease in iron content in cucumbers grown with all bio-chelates except ASCG and AH160. This suggests that ASCG and AH160 contributed to an iron reserve in the soil, allowing for continued mobilization of this micronutrient in subsequent harvests. A similar pattern was observed in the third harvest, where iron content decreased across all treatments compared to the second harvest, except for the hydrochars.
Table 2 presents the utilization efficiency (UE) parameter for the different harvests. In the first harvest, bio-products functionalized with Fe (ASCG-Fe and AH160-Fe), along with the commercial chelate, showed the highest UE. In the second harvest, the soil’s iron reserves appear to have increased, resulting in positive UE values for all treatments. This trend continued into the third harvest, with AH160 showing the greatest increase in Fe reserves in the soil.

Table 2.
Utilization efficiency (%) distributed by harvests.
4. Discussion
4.1. Production and Phytotoxicity Effect
During this trial, a control with commercial chelate (control-Fe) was used to compare its effects with those of the bioproducts. We also assessed the phytotoxic effects of the commercial product, as illustrated in Figure 1. Gregory et al. [26] and Mohammadi and Khoshgoftarmanesh [27] highlighted potential drawbacks of using salts for biofortification, including possible phytotoxicity and soil leaching issues. This contrasts with findings from Lara-Ramos et al. [22], who reported no significant impact on the total fresh or dry weight of lettuce when the same commercial iron chelate was used in an in vitro assay. The difference in results may stem from the closed soil–plant system used in Lara-Ramos et al. [22].
Regarding activated SCGs and hydrochars, these bio-chelates exhibited phytotoxic effects (Figure 1). Similar findings were reported by the group of Cervera-Mata et al., who noted phytotoxicity of SCGs in lettuce grown under in vitro conditions. Conversely, Cruz et al. [14] observed significant growth increases in lettuce grown with SCGs in a greenhouse trial, which may be attributed to the different soil type used, as their study utilized peat as a substrate. Limited plant growth with SCGs has been associated with the presence of caffeine, polyphenols, and tannins [28].
For hydrochars, Lara-Ramos et al. [22] and Cervera-Mata et al. [21] reported an increase in polyphenols following the hydrothermal carbonization process and observed a corresponding phytotoxic effect. Cervera-Mata et al. [23] also investigated various treatments to mitigate this toxicity, finding that only vermicomposting rendered SCGs a non-toxic organic amendment.
In other studies, phytotoxicity of these bio-products was attributed to competition between microorganisms and nitrogen in the soil [13]. However, this cannot be justified in our study due to fertigation, which prevents nitrogen blockage. In greenhouse production, nitrogen levels are carefully controlled to avoid such issues.
4.2. Effect on Iron Content in Cucumbers/Iron Biofortification
The treatments that most significantly increased iron (Fe) content in cucumbers were SCGs and H160 activated and functionalized with Fe. The metal-chelating activity of SCGs can likely be attributed to the presence of melanoidins and polyphenols [14]. Similarly, for H160-Fe, the hydrothermal carbonization process enhances the polyphenol content in these second-generation bioproducts [21], which may further improve their chelating and mobilizing capabilities for nutrients.
Our study achieved efficiency parameters significantly higher than those reported by Lara-Ramos et al. [22]. They reported a maximum utilization efficiency (UE) of 1.63 for AH160-Fe, whereas our study found a UE of 13.9 for the same bio-chelate. The control-Fe had a UE similar to our trial (0.24). However, Cervera-Mata et al. [21] reported UE parameters around 3.6, which are higher than those observed in our trial. For SCG-Fe, Cervera-Mata et al. found efficiency parameters exceeding those for activated SCGs in our study (approximately 0.25; [21]). These differences may be attributed to the type of crop used or the open system employed in their trials.
Figure 3 highlights the leaching of Fe, particularly in the commercial chelate and Fe-functionalized bioproducts. This leaching could be due to the intensive irrigation with water containing high salt concentrations. This is evidenced by the decrease in UE of the control-Fe between the first and third harvests (Table 2). The high solubility of salts in commercial chelates and their potential phytotoxicity [26,27] may contribute to this effect. The increased iron content in cucumbers during subsequent harvests could be attributed to the Fe reserves provided by these bio-products [21]. This trend is reflected in the UE parameter from the first to the third harvest for both activated and non-functionalized products (Table 2). This aspect warrants further investigation. As noted by the authors [21], the Fe reserve efficiency in the soil with SCGs functionalized with Fe is around 30%, which is promising for increasing Fe levels in cucumbers in future harvests.
Rousseau et al. [29] indicate that the bioaccessibility and bioavailability of iron can be negatively affected by polyphenols due to their strong affinity for the mineral. While this affinity is beneficial for designing bio-chelates to bind minerals [30], the formation of iron-polyphenol complexes can impede the release of iron into the medium for plant use [31]. Addressing this limiting effect of polyphenols is crucial, and methods such as encapsulation or the use of competing complexing agents are being explored to enhance iron bioavailability [32]. This is particularly relevant given the high polyphenol content in both SCGs and hydrochars [18].
4.3. Scalability of the Procedure
In this section, we will explore the potential applications of these bio-products in real crop production. This study marks a significant advancement in the commercial-scale development of the bio-products examined, addressing two key aspects: scaling up production to sufficient quantities for greenhouse trials and testing these products on crops cultivated under standard commercial conditions.
Agriculture plays a crucial role in Andalusia, contributing 23% to the region’s economy and highlighting its environmental significance. The area dedicated to greenhouse horticultural crops currently spans approximately 65,000 hectares [33], driven by the need for a consistent supply of fresh produce year-round. However, agricultural activities are associated with several environmental impacts, including substantial greenhouse gas emissions that contribute to global warming and climate change [34]. Notably, issues related to water consumption, fertilizer use, and phytosanitary products are of particular concern.
Recent studies have assessed the environmental impacts of various horticultural crop production systems, emphasizing these concerns [35,36]. Common issues include excessive fertilizer application, low efficiency in nutrient absorption by crops, and over-irrigation [37].
5. Conclusions
The addition of activated and functionalized SCGs and SCG-hydrochars in a greenhouse cucumber cultivation trial yielded promising results. Among the treatments tested, activating SCG-Fe notably increased cucumber production, surpassing even the commercial chelate addition. Except for activated and non-functionalized ASCG, none of the other bio-products led to a decrease in production compared to the commercial chelate. Regarding iron biofortification, the application of Fe-functionalized SCGs and SCG-hydrochars achieved iron content in cucumbers comparable to that of commercial chelate. This is a significant finding for agronomic biofortification. During the different harvests, the commercial chelate treatment initially resulted in the highest iron content in cucumbers, followed by the activated and functionalized bio-products. However, the bio-products that contributed the most to iron reserves in the soil for future harvests were the activated and non-functionalized SCGs and SCG-hydrochars. These results warrant further validation in subsequent harvests and with other types of greenhouse crops to obtain more definitive conclusions. The bio-products tested were produced at the laboratory scale, and their real-field application will require appropriate scaling studies, which will be the focus of future research. The preliminary results of Fe biofortification observed in vitro are promising but need additional research to facilitate simpler applications in greenhouse settings. Overall, these findings are significant for the greenhouse cultivation of vegetable products, especially in the critical production areas of southeastern Spain.
Author Contributions
Conceptualization, L.L.-R. and G.D.; methodology, L.L.-R., G.D. and A.F.-A.; software, L.L.-R.; validation, L.L.-R.; formal analysis, L.L.-R., G.D. and J.F.-B.; investigation, L.L.-R. and A.C.-M.; resources, L.L.-R. and G.D.; data curation, L.L.-R., J.F.-B.; writing—original draft preparation, L.L.-R., G.D.; writing—review and editing, J.F.-B. and A.F.-A.; visualization, J.F.-B.; supervision, J.F.-B., G.D., A.C.-M., J.Á.R.-H. and A.F.-A.; project administration, J.Á.R.-H. and A.F.-A.; funding acquisition, J.Á.R.-H. and A.F.-A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Plan propio de Investigación y Transferencia of the University of Granada under the program “Intensificación de la Investigación, modalidad B” and by the research project P20_00585 from Consejería de Economía, Conocimiento, Empresas y Universidad of the Andalusia Government.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
Acknowledgments
This paper is part of the doctoral thesis of Leslie Lara Ramos within the context of the “Chemistry Programme” at the University of Granada.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sharma, V.; Sharma, L.; Sandhu, K.S. Chapter 17. Cucumber (Cucumis sativus L.). In Antioxidants in Vegetables and Nuts—Properties and Health Benefits; Springer: Singapore, 2020; ISBN 978-981-15-7470-2. [Google Scholar]
- FAOSTAT. Crops and Livestock Products. 2022. Available online: https://www.fao.org/faostat/en/#data/QCL/visualize (accessed on 3 July 2024).
- Sallam, B.N.; Lu, T.; Yu, H.; Li, Q.; Sarfraz, Z.; Iqbal, M.S.; Khan, S.; Wang, H.; Liu, P.; Jiang, W. Productivity Enhancement of Cucumber (Cucumis sativus L.) through Optimized Use of Poultry Manure and Mineral Fertilizers under Greenhouse Cultivation. Horticulturae 2021, 7, 256. [Google Scholar] [CrossRef]
- Szerement, J.; Szatanik-Kloc, A.; Mokrzycki, J.; Mierzwa-Hersztek, M. Agronomic biofortification with Se, Zn, and Fe: An effective strategy to enhance crop nutritional quality and stress defense—A review. J. Soil. Sci. Plant Nutr. 2022, 22, 1129–1159. [Google Scholar] [CrossRef]
- Di Gioia, F.; Petropoulos, S.A.; Ozores-Hampton, M.; Morgan, K.; Rosskopf, E.N. Zn and iron agronomic biofortification of brassicaceae microgreens. Agronomy 2019, 9, 667. [Google Scholar] [CrossRef]
- Waters, B.M.; Troupe, G.C. Natural Variation in Iron Use Efficiency and Mineral Remobilization in Cucumber (Cucumis sativus). Plant Soil 2012, 352, 185–197. [Google Scholar] [CrossRef]
- Zuo, Y.; Zhang, F. Soil and Crop Management Strategies to Prevent Iron Deficiency in Crops. Plant Soil 2011, 339, 83–95. [Google Scholar] [CrossRef]
- WHO. Micronutrient Deficiency: Iron Deficiency Anaemia; WHO: Geneva, Switzerland, 2007; Available online: https://www.who.int/health-topics/anaemia#tab=tab_1 (accessed on 7 September 2024).
- Cervera-Mata, A.; Pastoriza, S.; Rufián-Henares, J.Á.; Párraga, J.; Martín-García, J.M.; Delgado, G. Impact of Spent Coffee Grounds as Organic Amendment on Soil Fertility and Lettuce Growth in Two Mediterranean Agricultural Soils. Arch. Agron. Soil Sci. 2018, 64, 790–804. [Google Scholar] [CrossRef]
- Cruz, R.; Baptista, P.; Cunha, S.; Pereira, J.A.; Casal, S. Carotenoids of lettuce (Lactuca sativa L.) grown on soil enriched with spent coffee grounds. Molecules 2012, 17, 1535–1547. [Google Scholar] [CrossRef]
- Cruz, R.; Gomes, T.; Ferreira, A.; Mendes, E.; Baptista, P.; Cunha, S.; Pereira, J.A.; Ramalhosa, E.; Casal, S. Antioxidant activity and bioactive compounds of lettuce improved by espresso coffee residues. Food Chem. 2014, 145, 95–101. [Google Scholar] [CrossRef]
- Cruz, R.; Morais, S.; Mendes, E.; Pereira, J.A.; Baptista, P.; Casal, S. Improvement of vegetables elemental quality by espresso coffee residues. Food Chem. 2014, 148, 294–299. [Google Scholar] [CrossRef]
- Cruz, S.; Cordovil, C.S.C. Espresso coffee residues as a nitrogen amendment for small-scale vegetable. J. Sci. Food Agric. 2015, 95, 3059–3066. [Google Scholar] [CrossRef]
- Cruz, R.; Mendes, E.; Torrinha, Á.; Morais, S.; Pereira, J.A.; Baptista, P.; Casal, S. Revalorization of spent coffee residues by a direct agronomic approach. Food Res. Int. 2015, 73, 190–196. [Google Scholar] [CrossRef]
- Pérez-Burillo, S.; Cervera-Mata, A.; Fernández-Arteaga, A.; Pastoriza, S.; Rufián-Henares, J.Á.; Delgado, G. Why Should We Be Concerned with the Use of Spent Coffee Grounds as an Organic Amendment of Soils? A Narrative Review. Agronomy 2022, 12, 2771. [Google Scholar] [CrossRef]
- Ciesielczuk, T.; Rosik-Dulewska, C.; Poluszyńska, J.; Sławińska, I. Acute toxicity of experimental fertilizers made of blood meal, spent coffee ground and biomass ash. J. Water Land Develop. 2017, 34, 95–102. [Google Scholar] [CrossRef]
- He, M.; Xu, Z.; Hou, D.; Gao, B.; Cao, X.; Ok, Y.S.; Rinklebe, J.; Bolan, N.S.; Tsang, D.C.W. Waste-Derived Biochar for Water Pollution Control and Sustainable Development. Nat. Rev. Earth Environ. 2022, 3, 444–460. [Google Scholar] [CrossRef]
- Sharma, H.B.; Vanapalli, K.R.; Bhatia, D.; Singh, S.; Arora, G.; Panigrahi, S.; Dubey, B.K.; Ramamurthy, P.C.; Mohanty, B. Engineered Biochar/Hydrochar Derived from Organic Wastes for Energy, Environmental, and Agricultural Applications; Springer: Berlin/Heidelberg, Germany, 2024; ISBN 1009802402. [Google Scholar]
- Xiao, L.P.; Shi, Z.J.; Xu, F.; Sun, R.C. Hydrothermal Carbonization of Lignocellulosic Biomass. Bioresour. Technol. 2012, 118, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Afolabi, O.O.D.; Sohail, M.; Cheng, Y.L. Optimisation and Characterisation of Hydrochar Production from Spent Coffee Grounds by Hydrothermal Carbonisation. Renew. Energy 2020, 147, 1380–1391. [Google Scholar] [CrossRef]
- Kim, D.; Lee, K.; Bae, D.; Park, K.Y. Characterizations of Biochar from Hydrothermal Carbonization of Exhausted Coffee Residue. J. Mater. Cycles Waste Manag. 2017, 19, 1036–1043. [Google Scholar] [CrossRef]
- Cervera-Mata, A.; Lara, L.; Fernández-Arteaga, A.; Ángel Rufián-Henares, J.; Delgado, G. Washed Hydrochar from Spent Coffee Grounds: A Second Generation of Coffee Residues. Evaluation as Organic Amendment. Waste Manag. 2021, 120, 322–329. [Google Scholar] [CrossRef]
- Lara-ramos, L.; Cervera-mata, A.; Navarro-alarc, M.; Delgado, G.; Fern, A. Hydrochars Derived from Spent Coffee Grounds as Zn Bio-Chelates for Agronomic Biofortification. Sustainability 2023, 15, 10700. [Google Scholar] [CrossRef]
- Morikawa, C.K.; Saigusa, M. Recycling Coffee and Tea Wastes to Increase Plant Available Fe in Alkaline Soils. Plant Soil 2008, 304, 249–255. [Google Scholar] [CrossRef]
- Zhao, A.; Yang, S.; Wang, B.; Tian, X. Effects of ZnSO4 and Zn-EDTA Applied by Broadcasting or by Banding on Soil Zn Fractions and Zn Uptake by Wheat (Triticum aestivum L.) under Greenhouse Conditions. J. Plant Nutr. Soil Sci. 2019, 182, 307–317. [Google Scholar] [CrossRef]
- Gregory, P.J.; Wahbi, A.; Adu-Gyamfi, J.; Heiling, M.; Gruber, R.; Joy, E.J.M.; Broadley, M.R. Approaches to Reduce Zinc and Iron Deficits in Food Systems. Glob. Food Sec. 2017, 15, 1–10. [Google Scholar] [CrossRef]
- Mohammadi, P.; Khoshgoftarmanesh, A.H. The Effectiveness of Synthetic Zinc(Zn)-Amino Chelates in Supplying Zn and Alleviating Salt-Induced Damages on Hydroponically Grown Lettuce. Sci. Hortic. 2014, 172, 117–123. [Google Scholar] [CrossRef]
- Leifa, F.; Pandey, A.; Soccol, C.R. Solid State Cultivation—An Efficient Method to Use Toxic Agro-Industrial Residues. J. Basic Microbiol. 2000, 40, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, S.; Kyomugasho, C.; Celus, M.; Hendrickx, M.E.G.; Grauwet, T. Barriers Impairing Mineral Bioaccessibility and Bioavailability in Plant-Based Foods and the Perspectives for Food Processing. Crit. Rev. Food Sci. Nutr. 2020, 60, 826–843. [Google Scholar] [CrossRef]
- Gulcin, İ.; Alwasel, S.H. Metal Ions, Metal Chelators and Metal Chelating Assay as Antioxidant Method. Processes 2022, 10, 132. [Google Scholar] [CrossRef]
- Cianciosi, D.; Forbes-Hernández, T.Y.; Regolo, L.; Alvarez-Suarez, J.M.; Navarro-Hortal, M.D.; Xiao, J.; Quiles, J.L.; Battino, M.; Giampieri, F. The Reciprocal Interaction between Polyphenols and Other Dietary Compounds: Impact on Bioavailability, Antioxidant Capacity and Other Physico-Chemical and Nutritional Parameters. Food Chem. 2022, 375, 131904. [Google Scholar] [CrossRef]
- McGee, E.J.T.; Diosady, L.L. Prevention of Iron-Polyphenol Complex Formation by Chelation in Black Tea. LWT 2018, 89, 756–762. [Google Scholar] [CrossRef]
- MAPA. Avance Anuario Estadístico, 2023; Ministerio de Agricultura, Pesca y Alimentación: Madrid, Spain, 2023. [Google Scholar]
- MITECO. Emisiones de Gases de efecto Invernadero en ESPAÑA. Año 2019; Ministerio para la Transición Ecológica y el reto Demográfico: Madrid, Spain, 2022. [Google Scholar]
- Romero-Gámez, M.; Audsley, E.; Suárez-Rey, E.M. Life Cycle Assessment of Cultivating Lettuce and Escarole in Spain. J. Clean. Prod. 2014, 73, 193–203. [Google Scholar] [CrossRef]
- Romero-Gámez, M.; Suárez-Rey, E.M. Environmental Footprint of Cultivating Strawberry in Spain. Int. J. Life Cycle Assess. 2020, 25, 719–732. [Google Scholar] [CrossRef]
- Thompson, R.B.; Martínez-Gaitan, C.; Gallardo, M.; Giménez, C.; Fernández, M.D. Identification of Irrigation and N Management Practices That Contribute to Nitrate Leaching Loss from an Intensive Vegetable Production System by Use of a Comprehensive Survey. Agric. Water Manag. 2007, 89, 261–274. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).