The Microbiological Activity of Soil in Response to Gliotoxin, the “Lethal Principle” of Trichoderma
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Sample Preparation and Experimental Design
2.2. Chemical Analysis
2.3. Extraction and Detection of GT in Soil
2.4. Microbiological Analysis
2.5. Enzymatic Analysis
2.6. Statistical Processing
3. Results
3.1. Soil Organic Carbon
3.2. GT Residues in Soils
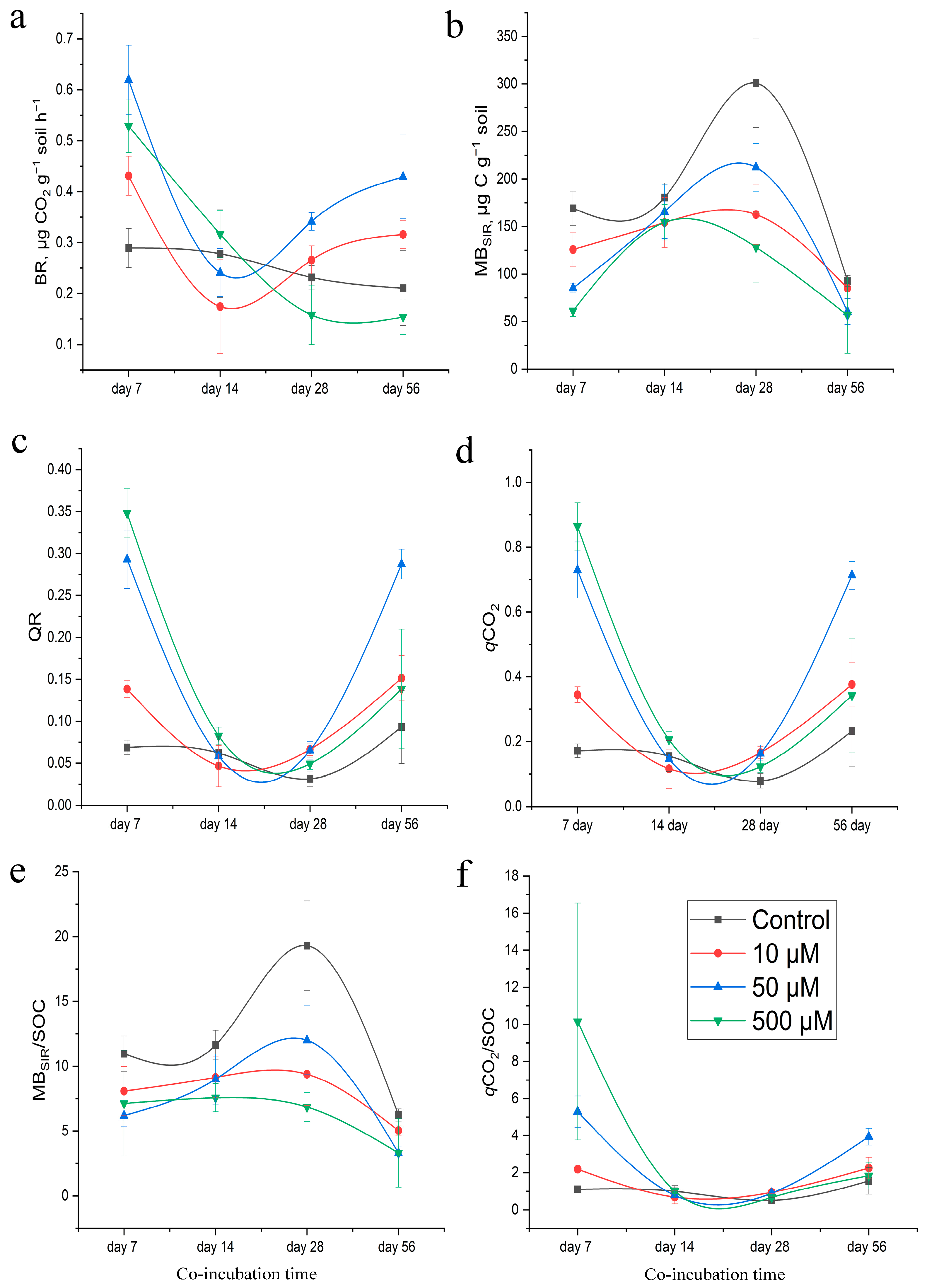
3.3. Microbial Activity of Soil under the Influence of GT
3.4. Eco-Physiological Changes in Soils under the Influence of GT
3.5. Enzymatic Activity of Soil under the Influence of Gliotoxin
4. Discussion
4.1. Effect of GT on Soil Microbial Activity
4.2. Changes in Enzymatic Activity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keswani, C.; Singh, H.B.; Hermosa, R.; García-Estrada, C.; Caradus, J.; He, Y.W.; Mezaache-Aichour, S.; Glare, T.R.; Borriss, R.; Vinale, F.; et al. Antimicrobial secondary metabolites from agriculturally important fungi as next biocontrol agents. Appl. Microbiol. Biotechnol. 2019, 103, 9287–9303. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, S.; García-Latorre, C.; Santamaria, O. Metabolites produced by fungi against fungal phytopathogens: Review, implementation and perspectives. Plants 2021, 11, 81. [Google Scholar] [CrossRef] [PubMed]
- Glare, T.; Caradus, J.; Gelernter, W.; Jackson, T.; Keyhani, N.; Köhl, J.; Marrone, P.; Morin, L.; Stewart, A. Have biopesticides come of age? Trends Biotechnol. 2012, 30, 250–258. [Google Scholar] [CrossRef]
- Deising, H.B.; Gase, I.; Kubo, Y. The unpredictable risk imposed by microbial secondary metabolites: How safe is biological control of plant diseases? J. Plant Dis. Prot. 2017, 124, 413–419. [Google Scholar] [CrossRef]
- Koch, E.; Becker, J.O.; Berg, G.; Hauschild, R.; Jehle, J.; Köhl, J.; Smalla, K. Biocontrol of plant diseases is not an unsafe technology! J. Plant Dis. Prot. 2018, 125, 121–125. [Google Scholar] [CrossRef]
- Cycoń, M.; Mrozik, A.; Piotrowska-Seget, Z. Antibiotics in the soil environment—Degradation and their impact on microbial activity and diversity. Front. Microbiol. 2019, 10, 338. [Google Scholar] [CrossRef]
- Davies, J. What are antibiotics? Archaic functions for modern activities. Mol. Microbiol. 1990, 4, 1227–1232. [Google Scholar] [CrossRef]
- Shahriar, S.A.; Islam, M.N.; Chun, C.N.W.; Kaur, P.; Rahim, M.A.; Islam, M.M.; Uddain, J.; Siddiquee, S. Microbial Metabolomics Interaction and Ecological Challenges of Trichoderma Species as Biocontrol Inoculant in Crop Rhizosphere. Agronomy 2022, 12, 900. [Google Scholar] [CrossRef]
- Tyśkiewicz, R.; Nowak, A.; Ozimek, E.; Jaroszuk-Ściseł, J. Trichoderma: The Current Status of Its Application in Agriculture for the Biocontrol of Fungal Phytopathogens and Stimulation of Plant Growth. Int. J. Mol. Sci. 2022, 23, 2329. [Google Scholar] [CrossRef]
- Smith, E.B.; Dolan, S.K.; Fitzpatrick, D.A.; Doyle, S.; Jones, G.W. Towards understanding the gliotoxin detoxification mechanism: In vivo thiomethylation protects yeast from gliotoxin cytotoxicity. Microb. Cell 2016, 3, 120–125. [Google Scholar] [CrossRef]
- Weindling, R. Studies on a lethal principle effective in the parasitic action of Trichoderma lignorum on Rhizoctonia solani and other soil fungi. Phytopathology 1934, 24, 1153–1179. [Google Scholar]
- Stanley, N.F.; Mills, J.A. The biological activity of a substance resembling gliotoxin produced by a strain of Aspergillus fumigatus. Aust. J. Exp. Biol. Med. Sci. 1946, 24, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.A.; Milstrey, K.P.; Trown, P.W. Specific inhibition of viral ribonucleic acid replication by Gliotoxin. Science 1968, 159, 431–432. [Google Scholar] [CrossRef] [PubMed]
- Waring, P.; Eichner, R.D.; Müllbacher, A. The chemistry and biology of the immunomodulating agent gliotoxin and related epipolythiodioxopiperazines. Med. Res. Rev. 1988, 4, 499–524. [Google Scholar] [CrossRef] [PubMed]
- Pahl, H.L.; Krauss, B.; Schulze-Osthoff, K.; Decker, T.; Traenckner, E.B.M.; Vogt, M.; Myers, C.; Parks, T.; Warring, P.; Mühlbacher, A.; et al. The immunosuppressive fungal metabolite gliotoxin specifically inhibits transcription factor NF-κB. J. Exp. Med. 1996, 183, 1829–1840. [Google Scholar] [CrossRef]
- Vigushin, D.M.; Mirsaidi, N.; Brooke, G.; Sun, C.; Pace, P.; Inman, L.; Moody, C.J.; Coombes, R.C. Gliotoxin is a dual inhibitor of farnesyltransferase and geranylgeranyltransferase I with antitumor activity against breast cancer in vivo. Med. Oncol. 2004, 21, 21–30. [Google Scholar] [CrossRef]
- Scharf, D.H.; Remme, N.; Heinekamp, T.; Hortschansky, P.; Brakhage, A.A.; Hertweck, C. Transannular disulfide formation in gliotoxin biosynthesis and its role in self-resistance of the human pathogen Aspergillus fumigatus. J. Am. Chem. Soc. 2010, 132, 10136–10141. [Google Scholar] [CrossRef]
- Kim, Y.S.; Park, S.J. Gliotoxin from the marine fungus Aspergillus fumigatus induces apoptosis in HT1080 fibrosarcoma cells by downregulating NF-κB. Fish. Aquat. Sci. 2016, 19, 35. [Google Scholar] [CrossRef]
- Wright, J.M. Production of Gliotoxin in Soils. Nature 1956, 177, 896. [Google Scholar] [CrossRef]
- Lumsden, R.D.; Locke, J.C.; Adkins, S.T.; Walter, J.F.; Ridout, C.J. Isolation and localization of the antibiotic gliotoxin produced by Gliocladium virens from alginate prill in soil and soilless media. Phytopathology 1992, 82, 230–235. [Google Scholar] [CrossRef]
- Aliaa, R.E.S. Control of root-rot diseases of Phaseolus vulgaris using gliotoxin. Malays J. 2008, 4, 40–43. [Google Scholar] [CrossRef]
- Tomah, A.A.; Abd Alamer, I.S.; Li, B.; Zhang, J.Z. A new species of Trichoderma and gliotoxin role: A new observation in enhancing biocontrol potential of T. virens against Phytophthora capsici on chili pepper. Biol. Control 2020, 145, 104261. [Google Scholar] [CrossRef]
- Jayalakshmi, R.; Oviya, R.; Premalatha, K.; Mehetre, S.T.; Paramasivam, M.; Kannan, R.; Theradimani, M.; Pallavi, M.S.; Mukherjee, P.K.; Ramamoorthy, V. Production, stability and degradation of Trichoderma gliotoxin in growth medium, irrigation water and agricultural soil. Sci. Rep. 2021, 11, 16536. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.A.; Roberts, D.P.; Hollenbeck, M.D. Induction of cytoplasmic leakage from Rhizoctonia solani hyphae by Gliocladium virens and partial characterization of a leakage factor. Biocontrol Sci. Technol. 1991, 1, 21–29. [Google Scholar] [CrossRef]
- Roberts, D.P.; Lumsden, R.D. Effect of extracellular metabolites from Gliocladium virens on germination of sporangia and mycelial growth of Pythium ultimum. Phytopathology 1990, 80, 461–465. [Google Scholar] [CrossRef]
- Vasilchenko, A.V.; Poshvina, D.V.; Semenov, M.V.; Timofeev, V.N.; Iashnikov, A.V.; Stepanov, A.A.; Pervushina, A.N.; Vasilchenko, A.S. Triazoles and Strobilurin Mixture Affects Soil Microbial Community and Incidences of Wheat Diseases. Plants 2023, 12, 660. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Guzmán, P.; Kumar, A.; de Los Santos-Villalobos, S.; Parra-Cota, F.I.; Orozco-Mosqueda, M.D.C.; Fadiji, A.E.; Hyder, S.; Babalola, O.O.; Santoyo, G. Trichoderma species: Our best fungal allies in the biocontrol of plant diseases—A review. Plants 2023, 12, 432. [Google Scholar] [CrossRef]
- Poveda Arias, J. Trichoderma as biocontrol agent against pests: New uses for a mycoparasite. Biol. Control 2021, 159, 104634. [Google Scholar] [CrossRef]
- Ferreira, F.V.; Musumeci, M.A. Trichoderma as biological control agent: Scope and prospects to improve efficacy. World J. Microbiol. Biotechnol. 2021, 37, 90. [Google Scholar] [CrossRef]
- Zaid, R.; Koren, R.; Kligun, E.; Gupta, R.; Leibman-Markus, M.; Mukherjee, P.K.; Kenerley, C.M.; Bar, M.; Horwitz, B.A. Gliotoxin, an immunosuppressive fungal metabolite, primes plant immunity: Evidence from Trichoderma virens-tomato interaction. mBio 2022, 13, e00389-22. [Google Scholar] [CrossRef]
- Vasilchenko, A.S.; Gurina, E.V.; Drozdov, K.A.; Vershinin, N.A.; Kravchenko, S.V.; Vasilchenko, A.V. Exploring the antibacterial action of gliotoxin: Does it induce oxidative stress or protein damage? Biochimie 2023, 214, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Pansu, M.; Gautheyrou, J. Handbook of Soil Analysis: Mineralogical, Organic and Inorganic Methods; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Vasilchenko, A.V.; Galaktionova, L.V.; Tretyakov, N.Y.; Dyachkov, S.M.; Vasilchenko, A.S. Impact of agricultural land use on distribution of microbial biomass and activity within soil aggregates. Soil Use Manag. 2023, 39, 618–633. [Google Scholar] [CrossRef]
- Anderson, T.H.; Domsch, K.H. Application of eco-physiological quotients (qCO2 and qD) on microbial biomasses from soils of different cropping histories. Soil Biol. Biochem. 1990, 22, 251–255. [Google Scholar] [CrossRef]
- Razavi, B.S.; Blagodatskaya, E.; Kuzyakov, Y. Nonlinear temperature sensitivity of enzyme kinetics explains canceling effect—A case study on loamy haplic Luvisol. Front. Microbiol. 2015, 6, 1126. [Google Scholar] [CrossRef]
- Teslya, A.V.; Gurina, E.V.; Poshvina, D.V.; Stepanov, A.A.; Iashnikov, A.A.; Vasilchenko, A.S. Fungal Secondary Metabolite Gliotoxin Enhances Enzymatic Activity in Soils by Reshaping Their Microbiome. 2024. Available online: https://ssrn.com/abstract=4824592 (accessed on 10 September 2024).
- German, D.P.; Weintraub, M.N.; Grandy, A.S.; Lauber, C.L.; Rinkes, Z.L.; Allison, S.D. Optimization of hydrolytic and oxidative enzyme methods for ecosystem studies. Soil Biol. Biochem. 2011, 43, 1387–1397. [Google Scholar] [CrossRef]
- Nieder, R.; Benbi, D.K.; Reichl, F.X.; Nieder, R.; Benbi, D.K.; Reichl, F.X. Health risks associated with pesticides in soils. Soil Compon. Hum. Health 2018, 503–573. [Google Scholar] [CrossRef]
- Koba, O.; Golovko, O.; Kodešová, R.; Fér, M.; Grabic, R. Antibiotics degradation in soil: A case of clindamycin, trimethoprim, sulfamethoxazole and their transformation products. Environ. Pollut. 2017, 220, 1251–1263. [Google Scholar] [CrossRef]
- Frkova, Z.; Vystavna, Y.; Koubová, A.; Kotas, P.; Grabicová, K.; Grabic, R.; Kodešová, R.; Chroňáková, A. Microbial responses to selected pharmaceuticals in agricultural soils: Microcosm study on the roles of soil, treatment and time. Soil Biol. Biochem. 2020, 149, 107924. [Google Scholar] [CrossRef]
- Moreno, J.L.; Aliaga, A.; Navarro, S.; Hernandez, T.; García, C. Effects of atrazine on microbial activity in semiarid soil. Appl. Soil Ecol. 2007, 35, 120–127. [Google Scholar] [CrossRef]
- Fenton, J.J.; Harsch, H.H.; Klein, D. Production of volatile nitrogenous compounds from the degradation of streptomycin by Pseudomonas maltophilia. J. Bacteriol. 1973, 116, 1267–1272. [Google Scholar] [CrossRef]
- Johnsen, J. Utilization of benzylpenicillin as carbon, nitrogen and energy source by a Pseudomonas fluorescens strain. Arch. Microbiol. 1977, 115, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Dantas, G.; Sommer, M.O.; Oluwasegun, R.D.; Church, G.M. Bacteria subsisting on antibiotics. Science 2008, 320, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Dantas, G.; Sommer, M.O. Ecological and clinical consequences of antibiotic subsistence by environmental microbes. In Antimicrobial Resistance in the Environment; Wiley-Blackwell: Hoboken, NJ, USA, 2012; pp. 29–41. [Google Scholar] [CrossRef]
- Perri, R.; Kolvenbach, B.A.; Corvini, P.F. Subsistence and complexity of antimicrobial resistance on a community-wide level. Environ. Microbiol. 2020, 22, 2463–2468. [Google Scholar] [CrossRef]
- Kumar, M.; Yadav, A.N.; Saxena, R.; Paul, D.; Tomar, R.S. Biodiversity of pesticides degrading microbial communities and their environmental impact. Biocatal. Agric. Biotechnol. 2021, 31, 101883. [Google Scholar] [CrossRef]
- Muñoz-Leoz, B.; Ruiz-Romera, E.; Antigüedad, I.; Garbisu, C. Tebuconazole application decreases soil microbial biomass and activity. Soil Biol. Biochem. 2011, 43, 2176–2183. [Google Scholar] [CrossRef]
- Bonfleur, E.J.; Tornisielo, V.L.; Regitano, J.B.; Lavorenti, A. The effects of glyphosate and atrazine mixture on soil microbial population and subsequent impacts on their fate in a tropical soil. Water Air Soil Pollut. 2015, 226, 21. [Google Scholar] [CrossRef]
- Chander, K.; Dyckmans, J.; Joergensen, R.; Meyer, B.; Raubuch, M. Different sources of heavy metals and their long-term effects on soil microbial properties. Biol. Fertil. Soils 2001, 34, 241–247. [Google Scholar] [CrossRef]
- Anderson, T.H. Microbial eco-physiological indicators to asses soil quality. Agric. Ecosyst. Environ. 2003, 98, 285–293. [Google Scholar] [CrossRef]
- Frische, T.; Höper, H. Soil microbial parameters and luminescent bacteria assays as indicators for in situ bioremediation of TNT-contaminated soils. Chemosphere 2003, 50, 415–427. [Google Scholar] [CrossRef]
- Rui, Y.; Murphy, D.V.; Wang, X.; Hoyle, F.C. Microbial respiration, but not biomass, responded linearly to increasing light fraction organic matter input: Consequences for carbon sequestration. Sci. Rep. 2016, 6, 35496. [Google Scholar] [CrossRef]
- Eisenhauer, N.; Klier, M.; Partsch, S.; Sabais, A.C.; Scherber, C.; Weisser, W.W.; Scheu, S. No interactive effects of pesticides and plant diversity on soil microbial biomass and respiration. Appl. Soil Ecol. 2009, 42, 31–36. [Google Scholar] [CrossRef]
- Anderson, T.H.; Domsch, K.H. Ratios of microbial biomass carbon to total organic carbon in arable soils. Soil Biol. Biochem. 1989, 21, 471–479. [Google Scholar] [CrossRef]
- Joergensen, R.G.; Castillo, X. Interrelationships between microbial and soil properties in young volcanic ash soils of Nicaragua. Soil Biol. Biochem. 2001, 33, 1581–1589. [Google Scholar] [CrossRef]
- Klose, S.; Wernecke, K.D.; Makeschin, F. Microbial activities in forest soils exposed to chronic depositions from a lignite power plant. Soil Biol. Biochem. 2004, 36, 1913–1923. [Google Scholar] [CrossRef][Green Version]
- Joergensen, R.G.; Emmerling, C. Methods for evaluating human impact on soil microorganisms based on their activity, biomass, and diversity in agricultural soils. J. Plant Nutr. Soil Sci. 2006, 169, 295–309. [Google Scholar] [CrossRef]
- Bedolla-Rivera, H.; Negrete-Rodríguez, M.X.; Medina-Herrera, M.; Gámez-Vázquez, F.; Álvarez-Bernal, D.; Samaniego-Hernández, M.; Gámez-Vázquez, A.; Conde-Barajas, E. Development of a soil quality index for soils under different agricultural management conditions in the Central Lowlands of Mexico: Physicochemical, biological and ecophysiological indicators. Sustainability 2020, 12, 9754. [Google Scholar] [CrossRef]
- Brian, P.W.; Hemming, H.G. Gliotoxin, a fungistatic metabolic product of Trichoderma viride. Ann. Appl. Biol. 1945, 32, 214–220. [Google Scholar] [CrossRef]
- Jefferys, E.G. The stability of antibiotics in soils. Microbiology 1952, 7, 295–312. [Google Scholar] [CrossRef][Green Version]
- Craig, W.A. The postantibiotic effect. Clin. Microbiol. Newsl. 1991, 13, 121–124. [Google Scholar] [CrossRef]
- Albert, J.; Muñoz, K. Kinetics of microbial and photochemical degradation of aflatoxin B1 in a sandy loam and clay soil. Sci. Rep. 2022, 12, 16849. [Google Scholar] [CrossRef]
- Albert, J.; More, C.; Korz, S.; Muñoz, K. Soil Microbial Responses to Aflatoxin Exposure: Consequences for Biomass, Activity and Catabolic Functionality. Soil Syst. 2023, 7, 23. [Google Scholar] [CrossRef]
- Dick, R.P. Soil enzyme activities as indicators of soil quality. Defin. Soil Qual. A Sustain. Environ. 1994, 35, 107–124. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Lauber, C.L.; Weintraub, M.N.; Ahmed, B.; Allison, S.D.; Crenshaw, C.; Contosta, A.R.; Cusack, D.; Frey, S.; Gallo, M.E.; et al. Stoichiometry of soil enzyme activity at global scale. Ecol. Lett. 2008, 11, 1252–1264. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.K.; Walker, A. Microbial degradation of organophosphorus compounds. FEMS Microbiol. Rev. 2006, 30, 428–471. [Google Scholar] [CrossRef]
- Doran, J.W. Soil microbial and biochemical changes associated with reduced tillage. Soil Sci. Soc. Am. J. 1980, 44, 765–771. [Google Scholar] [CrossRef]
- Trasar-Cepeda, C.; Leirós, M.C.; Gil-Sotres, F. Hydrolytic enzyme activities in agricultural and forest soils. Some implications for their use as indicators of soil quality. Soil Biol. Biochem. 2008, 40, 2146–2155. [Google Scholar] [CrossRef]
- Cenini, V.L.; Fornara, D.A.; McMullan, G.; Ternan, N.; Lajtha, K.; Crawley, M.J. Chronic nitrogen fertilization and carbon sequestration in grassland soils: Evidence of a microbial enzyme link. Biogeochemistry 2015, 126, 301–313. [Google Scholar] [CrossRef]
- Zeglin, L.H.; Stursova, M.; Sinsabaugh, R.L.; Collins, S.L. Microbial responses to nitrogen addition in three contrasting grassland ecosystems. Oecologia 2007, 154, 349–359. [Google Scholar] [CrossRef]
- Tabatabai, M.A. Soil enzymes. Methods Soil Anal. Part 2 Microbiol. Biochem. Prop. 1994, 5, 775–833. [Google Scholar] [CrossRef]
- Babaniyi, B.R.; Thompson, S.O.; Ogundele, O.D.; Oluwole, O.F. Effects of Agrochemicals on Soil Microbial Enzymes. In Ecological Interplays in Microbial Enzymology; Springer Nature: Singapore, 2022; pp. 353–377. [Google Scholar] [CrossRef]
- Feketeová, Z.; Hrabovský, A.; Šimkovic, I. Microbial features indicating the recovery of soil ecosystem strongly affected by mining and ore processing. Int. J. Environ. Res. Public Health 2021, 18, 3240. [Google Scholar] [CrossRef]
- Quiquampoix, H.; Burns, R.G. Interactions between proteins and soil mineral surfaces: Environmental and health consequences. Elements 2007, 3, 401–406. [Google Scholar] [CrossRef]
- Naidja, A.; Huang, P.M.; Bollag, J.M. Enzyme-clay interactions and their impact on transformations of natural and anthropogenic organic compounds in soil. J. Environ. Qual. 2000, 29, 677–691. [Google Scholar] [CrossRef]
- Zimmerman, A.R.; Ahn, M.-Y. Organo-mineral–enzyme interaction and soil enzyme activity. Soil Enzymol. 2011, 22, 271–292. [Google Scholar] [CrossRef]
- Silva-Olaya, A.M.; Mora-Motta, D.A.; Cherubin, M.R.; Grados, D.; Somenahally, A.; Ortiz-Morea, F.A. Soil enzyme responses to land use change in the tropical rainforest of the Colombian Amazon region. PLoS ONE 2021, 16, e0255669. [Google Scholar] [CrossRef]
- Li, C.; He, Y.Q.; Cui, L.Q.; Albuquerque, L.; Chen, R.W.; Long, L.J.; Tian, X.P. Miltoncostaea marina gen. nov. sp. nov., and Miltoncostaea oceani sp. nov., a novel deep branching phylogenetic lineage within the class Thermoleophilia isolated from marine environments, and proposal of Miltoncostaeaceae fam. nov. and Miltoncostaeales ord. nov. Syst. Appl. Microbiol. 2021, 44, 126216. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, L.; Zhang, H.; Deng, Y.; Hu, B.; Wang, W. Distinct roles of bacteria and fungi in mediating soil extracellular enzymes under long-term nitrogen deposition in temperate plantations. For. Ecol. Manag. 2023, 529, 120658. [Google Scholar] [CrossRef]
- Osorio, N.W.; Habte, M. Synergistic effect of a phosphate-solubilizing fungus and an arbuscular mycorrhizal fungus on leucaena seedlings in an Oxisol fertilized with rock phosphate. Botany 2013, 91, 274–281. [Google Scholar] [CrossRef]
- Phillips, L.A.; Ward, V.; Jones, M.D. Ectomycorrhizal fungi contribute to soil organic matter cycling in sub-boreal forests. ISME J. 2014, 8, 699–713. [Google Scholar] [CrossRef]
- Zhang, K.; Bonito, G.; Hsu, C.M.; Hameed, K.; Vilgalys, R.; Liao, H.L. Mortierella elongata increases plant biomass among non-leguminous crop species. Agronomy 2020, 10, 754. [Google Scholar] [CrossRef]
- Costa, O.Y.; Pijl, A.; Houbraken, J.; van Lith, W.; Kuramae, E.E. Soil substrate source drives the microbes involved in the degradation of gelatin used as a biostimulant. Appl. Soil Ecol. 2023, 189, 104906. [Google Scholar] [CrossRef]
- Liu, B.; Li, Y.; Zhang, X.; Wang, J.; Gao, M. Effects of chlortetracycline on soil microbial communities: Comparisons of enzyme activities to the functional diversity via Biolog EcoPlates™. Eur. J. Soil Biol. 2015, 68, 69–76. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, J.; Zhu, K.; Zhang, H. Influence of oxytetracycline on the structure and activity of microbial community in wheat rhizosphere soil. J. Environ. Sci. 2009, 21, 954–959. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Wu, S.C.; Nie, X.P.; Yediler, A.; Wong, M.H. The effects of residual tetracycline on soil enzymatic activities and plant growth. J. Environ. Sci. Health 2009, 44, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Schimel, J.; Becerra, C.A.; Blankinship, J. Estimating decay dynamics for enzyme activities in soils from different ecosystems. Soil Biol. Biochem. 2017, 114, 5–11. [Google Scholar] [CrossRef]
- Chen, H.; Li, D.; Zhao, J.; Xiao, K.; Wang, K. Effects of nitrogen addition on activities of soil nitrogen acquisition enzymes: A meta-analysis. Agric. Ecosyst. Environ. 2018, 252, 126–131. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Moorhead, D.L. Resource allocation to extracellular enzyme production: A model for nitrogen and phosphorus control of litter decomposition. Soil Biol. Biochem. 1994, 26, 1305–1311. [Google Scholar] [CrossRef]
- Allison, S.D. Cheaters, diffusion and nutrients constrain decomposition by microbial enzymes in spatially structured environments. Ecol. Lett. 2005, 8, 626–635. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, Y.; Tuo, Y.; Li, H.; Wang, Y. Effects of lime nitrogen on the fungi community of tobacco soil and its correlation analysis. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2022; Volume 1087, p. 012059. [Google Scholar] [CrossRef]
- Ma, C.; Hu, B.; Wei, M.B.; Zhao, J.H.; Zhang, H.Z. Influence of matured compost inoculation on sewage sludge composting: Enzyme activity, bacterial and fungal community succession. Bioresour. Technol. 2019, 294, 122165. [Google Scholar] [CrossRef]
- Dotaniya, M.L.; Aparna, K.; Dotaniya, C.K.; Singh, M.; Regar, K.L. Role of soil enzymes in sustainable crop production. In Enzymes in Food Biotechnology; Academic Press: Cambridge, MA, USA, 2019; pp. 569–589. [Google Scholar] [CrossRef]
- Kertesz, M.A.; Mirleau, P. The role of soil microbes in plant sulphur nutrition. J. Exp. Bot. 2004, 55, 1939–1945. [Google Scholar] [CrossRef] [PubMed]
- Katayama, Y.; Kuraishi, H. Characteristics of Thiobacillus thioparus and its thiocyanate assimilation. Can. J. Microbiol. 1978, 24, 804–810. [Google Scholar] [CrossRef]
- Kumar, M.; Zeyad, M.T.; Choudhary, P.; Paul, S.; Chakdar, H.; Rajawat, M.V. Thiobacillus. In Beneficial Microbes in Agro-Ecology; Academic Press: Cambridge, MA, USA, 2020; pp. 545–557. [Google Scholar] [CrossRef]
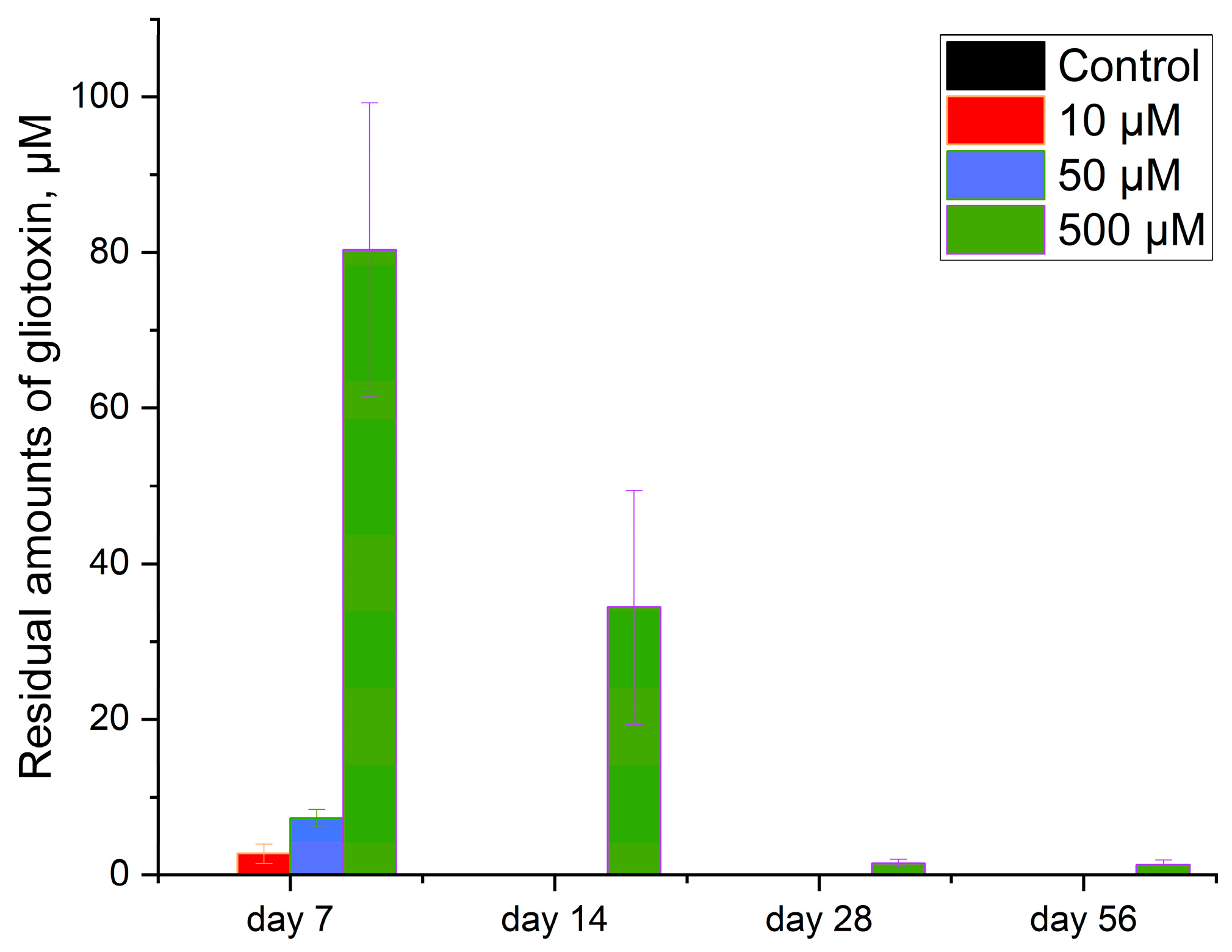

| GT Dose, µM kg−1 Soil | Soil Organic Carbon, g kg−1 | |||
|---|---|---|---|---|
| Incubation Time, Days | ||||
| 7 | 14 | 28 | 56 | |
| Control | 15.5 ± 0.58 | 15.5 ± 0.43 | 15.7 ± 1.36 | 14.9 ± 0.74 |
| 10 | 15.8 ± 1.38 | 16.9 ± 0.02 | 17.5 ± 1.23 | 16.9 ± 1.21 |
| 50 | 13.9 ± 1.11 | 18.5 ± 0.92 * | 17.9 ± 2.13 | 18.2 ± 1.19 * |
| 500 | 21.9 ± 1.63 ** | 20.5 ± 0.62 *** | 18.4 ± 2.49 | 18.1 ± 2.47 |
| Enzymes | Statistics | Factors | ||
|---|---|---|---|---|
| Sampling Time (A) | GT Concentration (B) | A × B | ||
| βG | F | 52.63 | 13.60 | 33.11 |
| p-value | 3.21 × 10−18 | 3.86 × 10−7 | 5.80 × 10−19 | |
| CB | F | 1.23 | 1.41 | 1.32 |
| p-value | 0.31 | 0.25 | 0.26 | |
| βX | F | 9.59 | 7.82 | 8.70 |
| p-value | 2.04 × 10−5 | 1.35 × 10−4 | 3.75 × 10−7 | |
| AP | F | 22.34 | 6.12 | 14.23 |
| p-value | 2.30 × 10−10 | 9.01 × 10−4 | 1.16 × 10−10 | |
| NAG | F | 246.10 | 7.11 | 126.60 |
| p-value | 4.41 × 10−38 | 2.97 × 10−4 | 1.57 × 10−36 | |
| LAP | F | 103.24 | 2.86 | 53.05 |
| p-value | 3.38 × 10−26 | 0.04284 | 1.17 × 10−24 | |
| ARS | F | 5.90 | 1.11 | 3.51 |
| p-value | 0.00116 | 0.35101 | 0.00421 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teslya, A.V.; Gurina, E.V.; Stepanov, A.A.; Iashnikov, A.V.; Vasilchenko, A.S. The Microbiological Activity of Soil in Response to Gliotoxin, the “Lethal Principle” of Trichoderma. Agronomy 2024, 14, 2084. https://doi.org/10.3390/agronomy14092084
Teslya AV, Gurina EV, Stepanov AA, Iashnikov AV, Vasilchenko AS. The Microbiological Activity of Soil in Response to Gliotoxin, the “Lethal Principle” of Trichoderma. Agronomy. 2024; 14(9):2084. https://doi.org/10.3390/agronomy14092084
Chicago/Turabian StyleTeslya, Anastasia V., Elena V. Gurina, Artyom A. Stepanov, Aleksandr V. Iashnikov, and Alexey S. Vasilchenko. 2024. "The Microbiological Activity of Soil in Response to Gliotoxin, the “Lethal Principle” of Trichoderma" Agronomy 14, no. 9: 2084. https://doi.org/10.3390/agronomy14092084
APA StyleTeslya, A. V., Gurina, E. V., Stepanov, A. A., Iashnikov, A. V., & Vasilchenko, A. S. (2024). The Microbiological Activity of Soil in Response to Gliotoxin, the “Lethal Principle” of Trichoderma. Agronomy, 14(9), 2084. https://doi.org/10.3390/agronomy14092084








